Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway
Abstract
:1. Introduction
2. Results
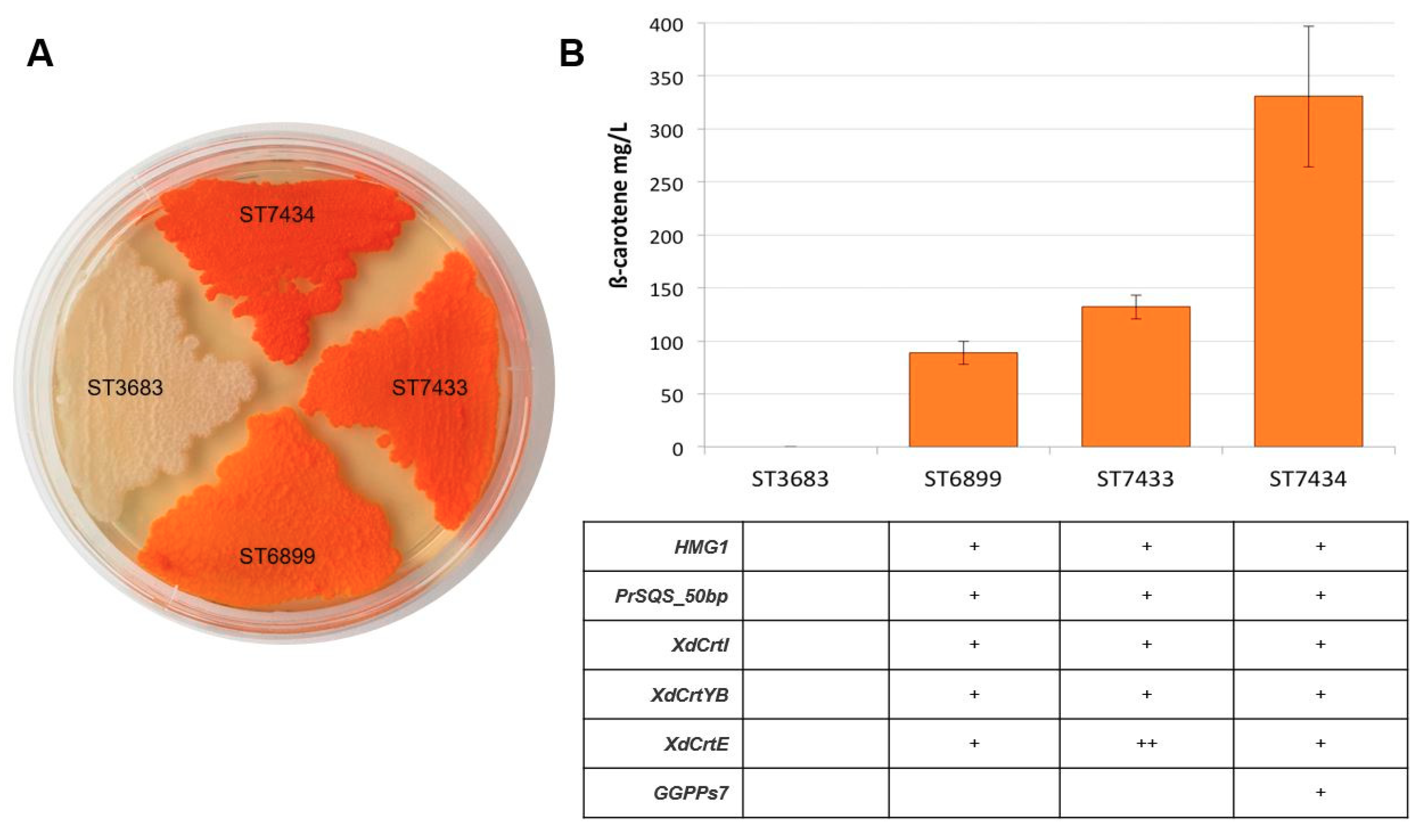
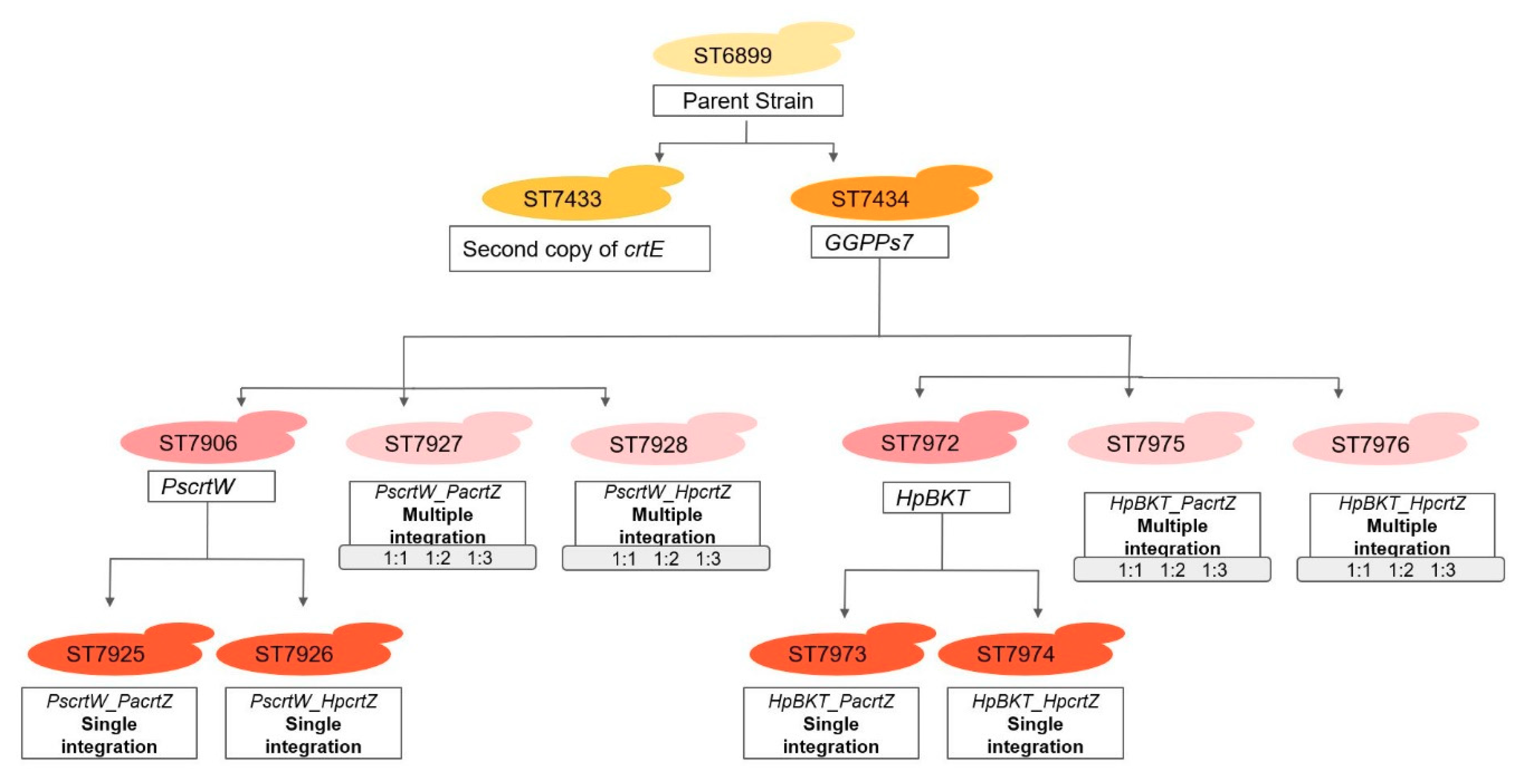
2.1. Enhancement of Beta-Carotene Production by the Introduction of crtE and GGPPs7
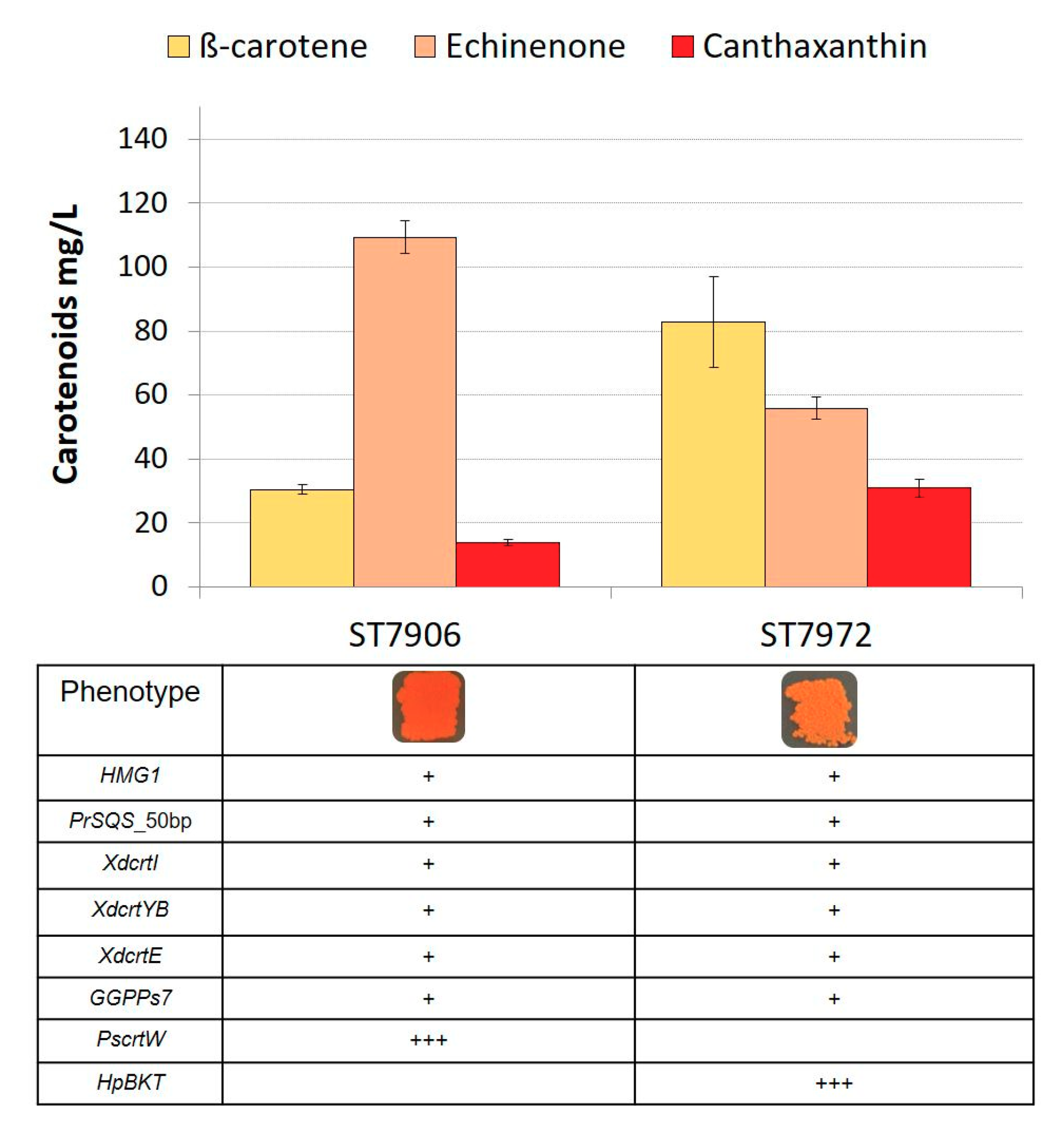
2.2. Expression of Heterologous β-ketolases for the Biosynthesis of Astaxanthin Intermediates
2.3. Single-Copy Expression of β-Hydroxylase for Production of Astaxanthin
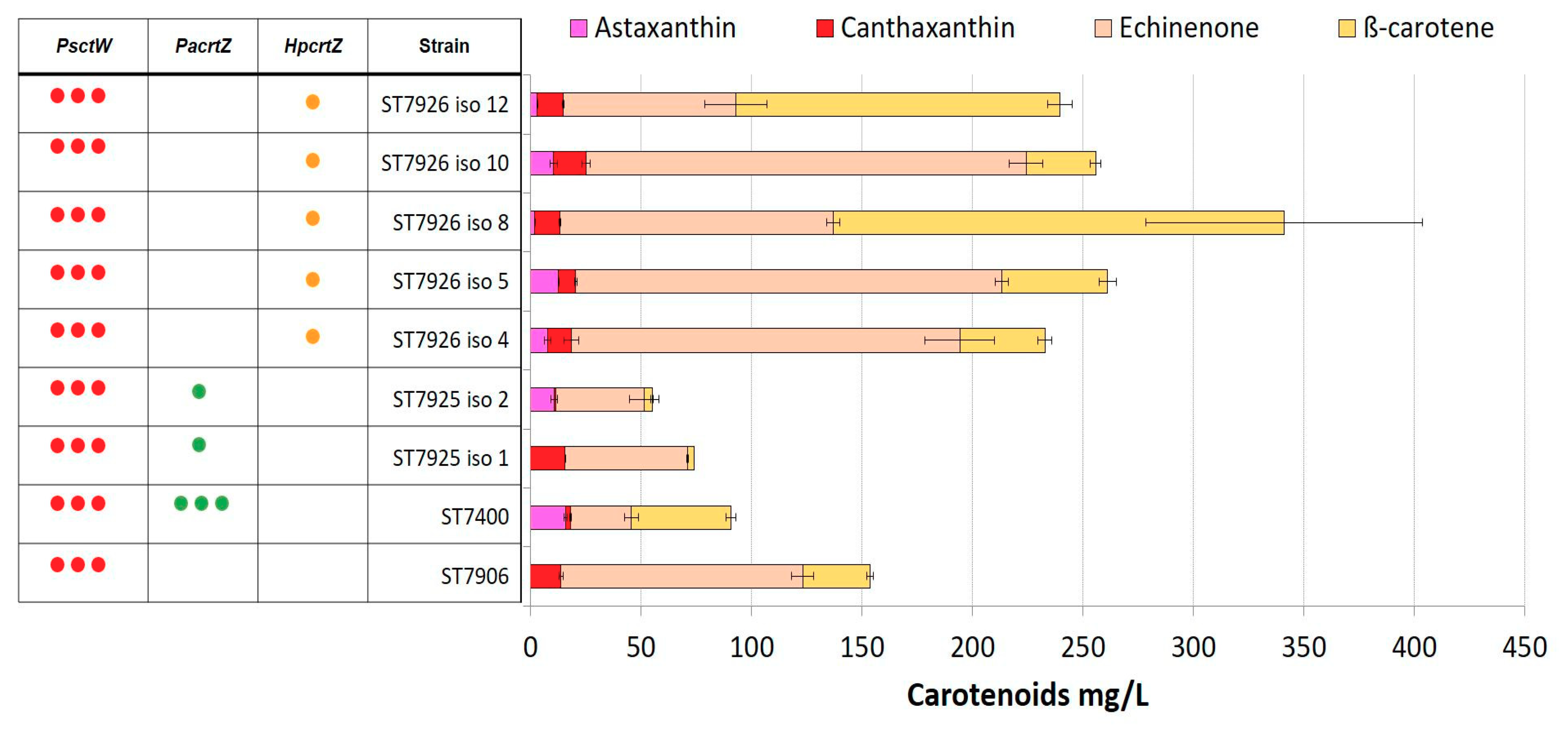
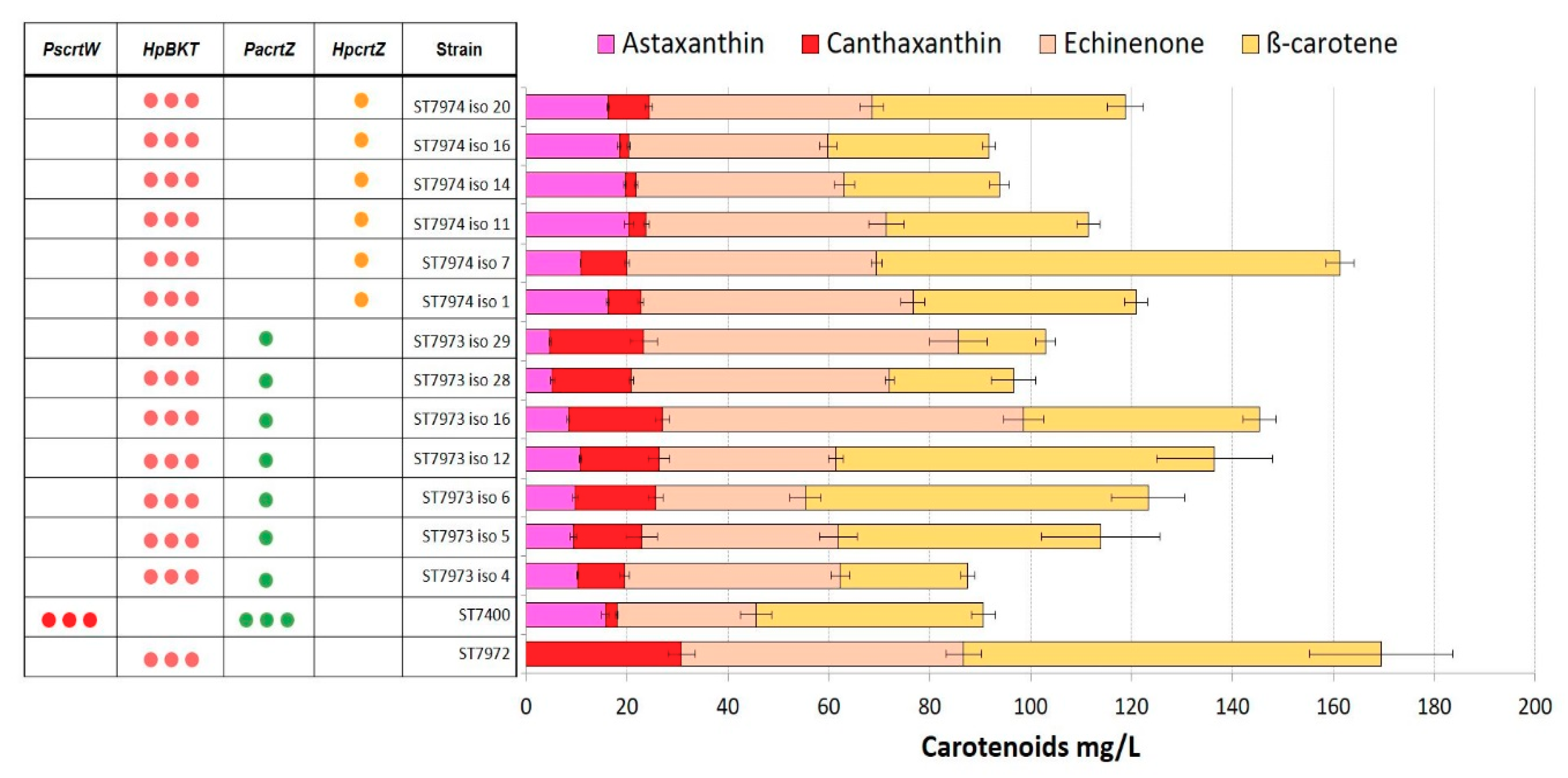
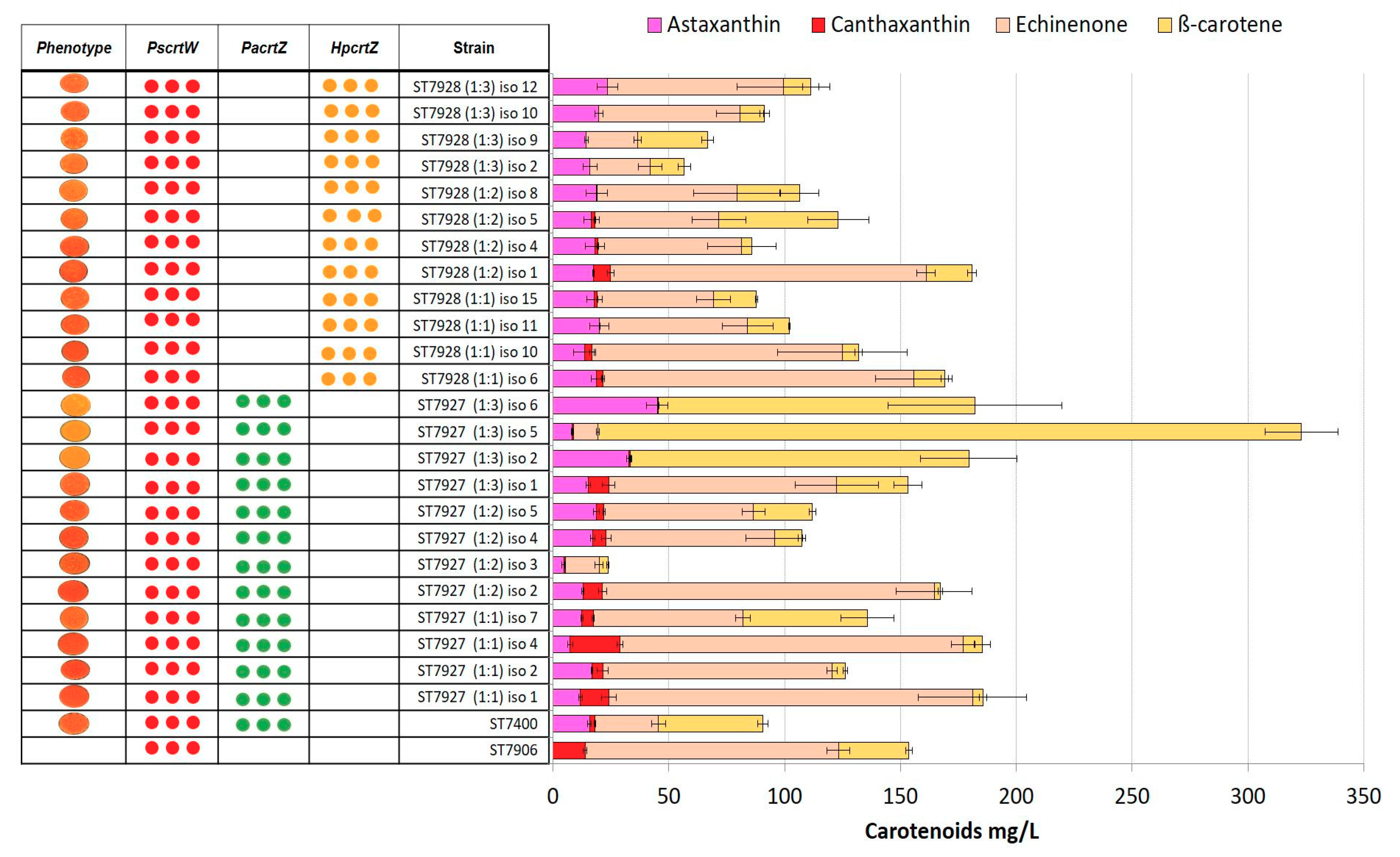
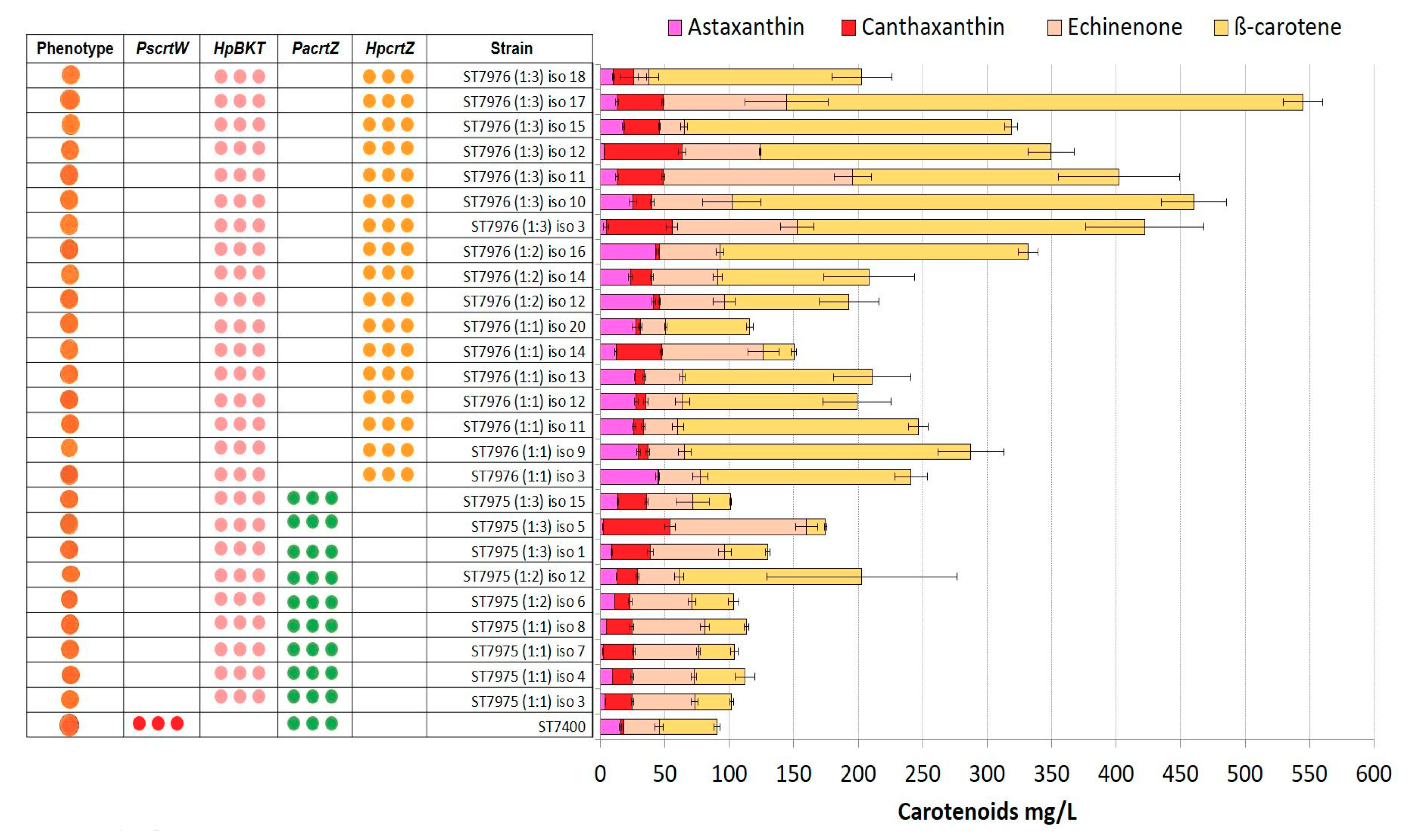
2.4. Integration of Multiple Copies of β-ketolase and β-hydroxylase Increases Astaxanthin Production
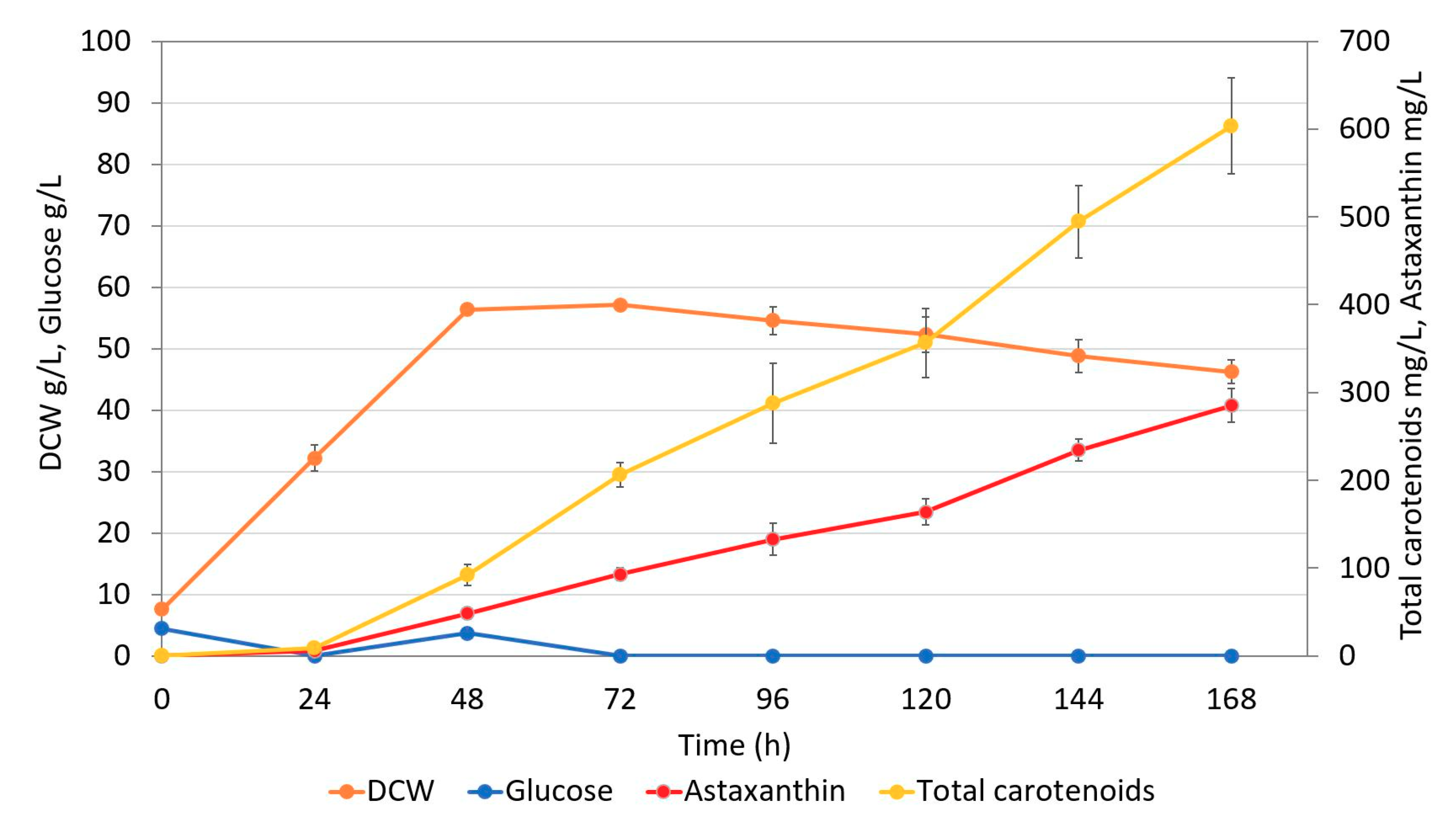
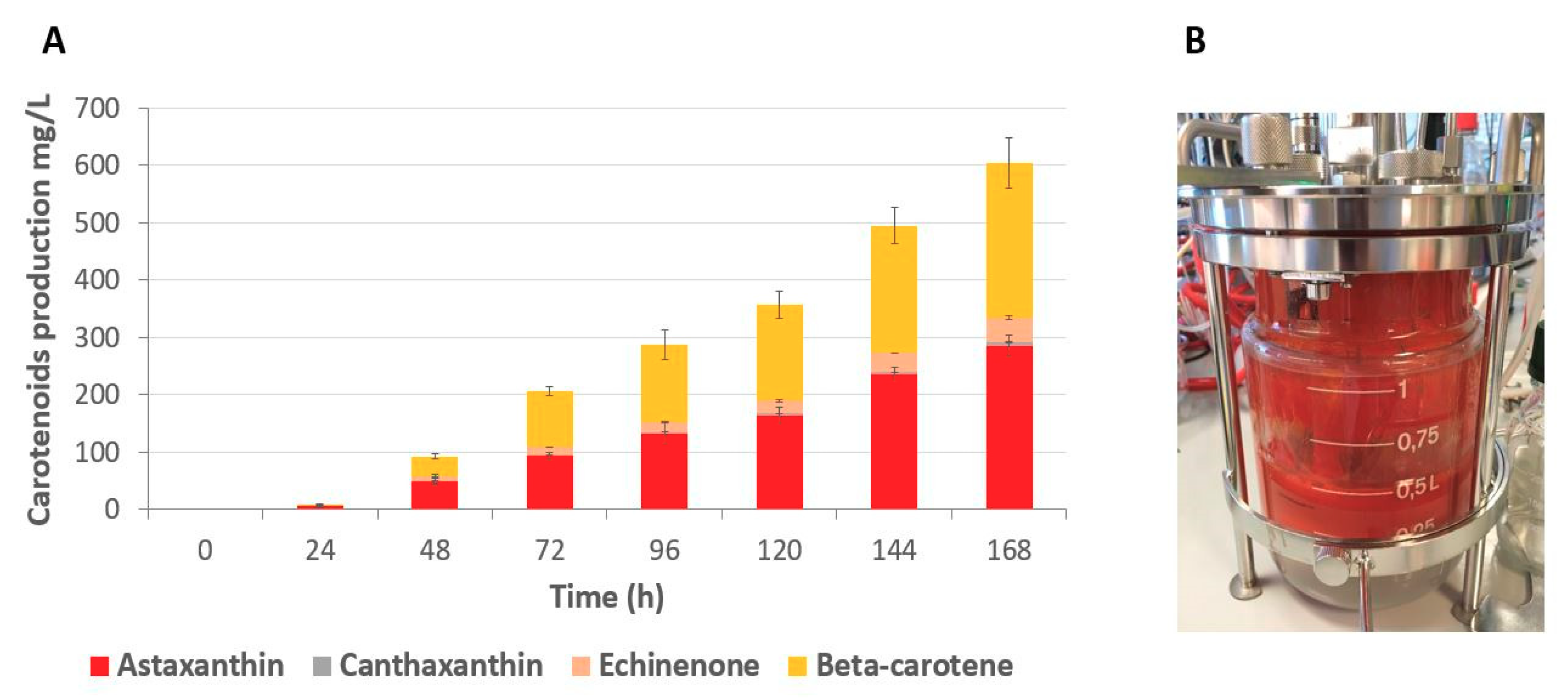
2.5. Fed-batch Fermentation of Astaxanthin Producer Strain
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Plasmid Construction
4.3. Construction and Cultivation of Y. lipolytica
4.4. Carotenoid Extraction
4.5. Carotenoid Quantification by HPLC
4.6. Fermentation Procedures
4.7. Biomass and Glucose Quantification in Bioreactors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant J. 2008, 54, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Paul, S.B. Microbial xanthophylls. Appl. Microbiol. Biotechnol. 2005, 68, 445–455. [Google Scholar]
- Mann, V.; Harker, M.; Pecker, I.; Hirschberg, J. Metabolic engineering of astaxanthin production in tobacco flowers. Nat. Biotechnol. 2000, 18, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar]
- Yang, J.; Guo, L. Biosynthesis of β-carotene in engineered E. coli using the MEP and MVA pathways. Microb. Cell Fact. 2014, 13, 160. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Schmidt, I.; Schewe, H.; Gassel, S.; Jin, C.; Buckingham, J.; Hümbelin, M.; Sandmann, G.; Schrader, J. Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2011, 89, 555–571. [Google Scholar] [CrossRef]
- Sanchez, S.; Ruiz, B.; Rodríguez-Sanoja, R.; Flores-Cotera, L.B. Microbial production of carotenoids. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; McNeil, B., Archer, D., Giavasis, I., Harvey, L., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013. [Google Scholar]
- Shah, M.M.R.; Liang, Y.; Jay, J.C.; Maurycy, D. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Gassel, S.; Schewe, H.; Schmidt, I.; Schrader, J.; Sandmann, G. Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering. Biotechnol. Lett. 2013, 35, 565–569. [Google Scholar] [CrossRef]
- Gassel, S.; Breitenbach, J.; Sandmann, G. Genetic engineering of the complete carotenoid pathway towards enhanced astaxanthin formation in Xanthophyllomyces dendrorhous starting from a high-yield mutant. Appl. Microbiol. Biotechnol. 2014, 98, 345–350. [Google Scholar] [CrossRef]
- Steinbrenner, J.; Sandmann, G. Transformation of the green alga Haematococcus pluvialis with a phytoene desaturase for accelerated astaxanthin biosynthesis. Appl. Environ. Microbiol. 2006, 72, 7477–7484. [Google Scholar] [CrossRef] [PubMed]
- Sharon-gojman, R.; Maimon, E.; Leu, S.; Zarka, A.; Boussiba, S. Advanced methods for genetic engineering of Haematococcus pluvialis. Algal Res. 2015, 10, 8–15. [Google Scholar] [CrossRef]
- Gutiérrez, C.L.; Gimpel, J.; Escobar, C.; Marshall, S.H.; Henríquez, V. chloroplast genetic tool for the green microalgae Haematococcus pluvialis (chlorophyceae, volvocales). J. Phycol. 2012, 48, 976–983. [Google Scholar]
- Park, S.Y.; Binkley, R.M.; Kim, W.J.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Seow, V.Y.; Chen, X.; Too, H.P. Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat. Commun. 2018, 9, 1858. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Yao, M.; Gu, X.; Li, B.; Liu, H.; Ding, M.; Xiao, W.; Yuan, Y. Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol. Biofuels 2018, 11, 230. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; Li, A.; Wang, F.; Yao, Z.; Bian, Q.; Zhu, Y.; Yu, H.; Ye, L. Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzyme Microb. Technol. 2017, 100, 28–36. [Google Scholar] [CrossRef]
- Zhou, P.; Ye, L.; Xie, W.; Lv, X.; Yu, H. Highly efficient biosynthesis of astaxanthin in Saccharomyces cerevisiae by integration and tuning of algal crtZ and bkt. Appl. Microbiol. Biotechnol. 2015, 99, 8419–8428. [Google Scholar] [CrossRef]
- Zieniuk, B.; Fabiszewska, A. Yarrowia lipolytica: A beneficious yeast in biotechnology as a rare opportunistic fungal pathogen: A minireview. World J. Microbiol. Biotechnol. 2019, 35, 10. [Google Scholar] [CrossRef]
- Gao, S.; Han, L.; Zhu, L.; Ge, M.; Yang, S.; Jiang, Y.; Chen, D. One-step integration of multiple genes into the oleaginous yeast Yarrowia lipolytica. Biotechnol. Lett. 2014, 36, 2523–2528. [Google Scholar]
- Tai, M.; Stephanopoulos, G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kildegaard, K.R.; Adiego-Pérez, B.; Doménech Belda, D.; Khangura, J.K.; Holkenbrink, C.; Borodina, I. Engineering of Yarrowia lipolytica for production of astaxanthin. Synth. Syst. Biotechnol. 2017, 2, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kishore, G.M.; Motion, M.; Hicks, P.M.; Hansen, J.; Houghton-larsen, J.; Hansen, E.H.; Mikkelsen, M.D.; Tavares, S.; Blom, C. Production of Steviol Glycosides in Microorganisms. U.S. Patent No. 9,562,251, 7 February 2017. [Google Scholar]
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/astaxanthin-market-162119410.html (accessed on 11 August 2019).
- Ma, T.; Zhou, Y.; Li, X.; Zhu, F.; Cheng, Y.; Liu, Y.; Deng, Z.; Liu, T. Genome mining of astaxanthin biosynthetic genes from Sphingomonas sp. ATCC 55669 for heterologous overproduction in Escherichia coli. Biotechnol. J. 2016, 11, 228–237. [Google Scholar] [CrossRef]
- Misawa, N.; Shimada, H. Metabolic engineering for the production of carotenoids in non- carotenogenic bacteria and yeasts. J. Biotechnol. 1998, 59, 169–181. [Google Scholar] [CrossRef]
- Ukibe, K.; Hashida, K.; Yoshida, N.; Takagi, H. Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Appl. Environ. Microbiol. 2009, 75, 7205–7211. [Google Scholar] [CrossRef]
- Yokoyama, A.; Shizuri, Y.; Misawa, N. Production of new carotenoids, astaxanthin glucosides, by Escherichia coli transformants carrying carotenoid biosynthetic genes. Tetrahedron Lett. 1998, 39, 3709–3712. [Google Scholar] [CrossRef]
- Jensen, N.B. Methods and Materials for Biosynthesis of Manoyl Oxide. U.S. Patent No. 10,208,326, 19 February 2019. [Google Scholar]
- Verwaal, R.; Wang, J.; Meijnen, J.; Visser, H.; Sandmann, G.; Berg, J.; Ooyen, A. High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2007, 73, 4342–4350. [Google Scholar] [CrossRef]
- Xie, W.; Lv, X.; Ye, L.; Zhou, P.; Yu, H. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab. Eng. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Saito, T.; Shimada, H.; Misawa, N.; Kondo, K.; Nakamura, K.; Miura, Y. Production of lycopene by the food yeast, Candida utilis that does not naturally synthesize carotenoid. Biotechnol. Bioeng. 2002, 58, 306–308. [Google Scholar]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.T.; Hernandez, R.; Holmes, W.E.; Claupein, W. Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Larroude, M.; Celinska, E.; Back, A.; Thomas, S.; Nicaud, J.M.; Ledesma-Amaro, R. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol. Bioeng. 2018, 115, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tong, Y.; Zhu, L.; Ge, M.; Zhang, Y.; Chen, D.; Jiang, Y.; Yang, S. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production. Metab. Eng. 2017, 41, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Seraphim, P.; Aggelis, G. Lipids of oleaginous yeast. Part I. Biochemistry related with single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar]
- Ye, R.W.; Stead, K.J.; Yao, H.; He, H. Mutational and functional analysis of the β-carotene ketolase involved in the production of canthaxanthin and astaxanthin. Appl. Environ. Microbiol. 2006, 72, 5829–5837. [Google Scholar] [CrossRef]
- Holkenbrink, C.; Dam, M.I.; Kildegaard, K.R.; Beder, J.; Dahlin, J.; Doménech Belda, D.; Borodina, I. EasyCloneYALI: CRISPR/Cas9-Based Synthetic Toolbox for Engineering of the Yeast Yarrowia lipolytica. Biotechnol. J. 2018, 13, 1700543. [Google Scholar] [CrossRef]
- Chen, D.C.; Beckerich, J.M.; Gaillardin, C. One-step transformation of the dimorphic yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 1997, 48, 232–235. [Google Scholar] [CrossRef]

| Organism | Genotype | Astaxanthin Titer and Content | Reference |
|---|---|---|---|
| X. dendrorhous | crtYB and asy (native genes) | 9.7 mg/g DCW (bioreactor) | [10] |
| X. dendrorhous | crtYB, asy, crtE and trHMG (native genes) | 9 mg/g DCW (shake-flasks) | [11] |
| H. pluvialis | site-directed mutagenesis of PDS (native gene) | 11.4 mg/g DCW (shake-flasks) | [12] |
| E. coli | crtE, crtY, crtI, crtB, crtZ (from P. ananatis); trBKT (from C. reinhardtii); ispD and ispF (native genes) | 432 mg/L, 7 mg/g DCW (bioreactor) | [15] |
| E. coli | Module 1: atoB (native), hmgS (S. cerevisiae), and thmgR (S. cerevisiae); module 2: mevk (S. cerevisiae), pmk (S. cerevisiae), pmd (S. cerevisiae), and idi (native); module 3: crtEBI (amplified from pAC-LYC plasmid) and ispA (native); crtY (P. ananatis), crtZ (from P. ananatis), crtW (Brevundimonas sp.) | 320 mg/L, 2 mg/g DCW (SFE) | [16] |
| S. cerevisiae | crtW (from Brevundimonas vesicularis),crtZ (from Agrobacterium aurantiacum), and mutagenesis of CSS1, YBR012W-B and DAN4 | 217.9 mg/L, 13.8 mg/g DCW (bioreactor) | [17] |
| S. cerevisiae | crtE, crtI, crtYB (from X. dendrorhous); trHMG1 (native gene); BKT and crtZ (from H. pluvialis) | 47 mg/L, 8 mg/g DCW (shake-flasks) | [18] |
| S. cerevisiae | BKT and crtZ (from H. pluvialis) | 4.7 mg/g DCW (shake-flasks) | [19] |
| Y. lipolytica | crtYB, crtI, crtE (from X. dendrorhous); HMG1 (native gene); ↓SQS1; crtW (from Paracoccus sp.) and crtZ (from P. ananatis) | 54.6 mg/L, 3.5 mg/g DCW (microtiter plates) | [23] |
| Y. lipolytica | GGPPs7 (from Synechococcus sp.), HpBKT, HpcrtZ (from H. pluvialis) | 285 mg/L, 6 mg/g DCW (bioreactor) | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramontin, L.R.R.; Kildegaard, K.R.; Sudarsan, S.; Borodina, I. Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway. Microorganisms 2019, 7, 472. https://doi.org/10.3390/microorganisms7100472
Tramontin LRR, Kildegaard KR, Sudarsan S, Borodina I. Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway. Microorganisms. 2019; 7(10):472. https://doi.org/10.3390/microorganisms7100472
Chicago/Turabian StyleTramontin, Larissa Ribeiro Ramos, Kanchana Rueksomtawin Kildegaard, Suresh Sudarsan, and Irina Borodina. 2019. "Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway" Microorganisms 7, no. 10: 472. https://doi.org/10.3390/microorganisms7100472
APA StyleTramontin, L. R. R., Kildegaard, K. R., Sudarsan, S., & Borodina, I. (2019). Enhancement of Astaxanthin Biosynthesis in Oleaginous Yeast Yarrowia lipolytica via Microalgal Pathway. Microorganisms, 7(10), 472. https://doi.org/10.3390/microorganisms7100472




