Experimental In-Field Transfer and Survival of Escherichia coli from Animal Feces to Romaine Lettuce in Salinas Valley, California
Abstract
:1. Introduction
2. Materials and Methods
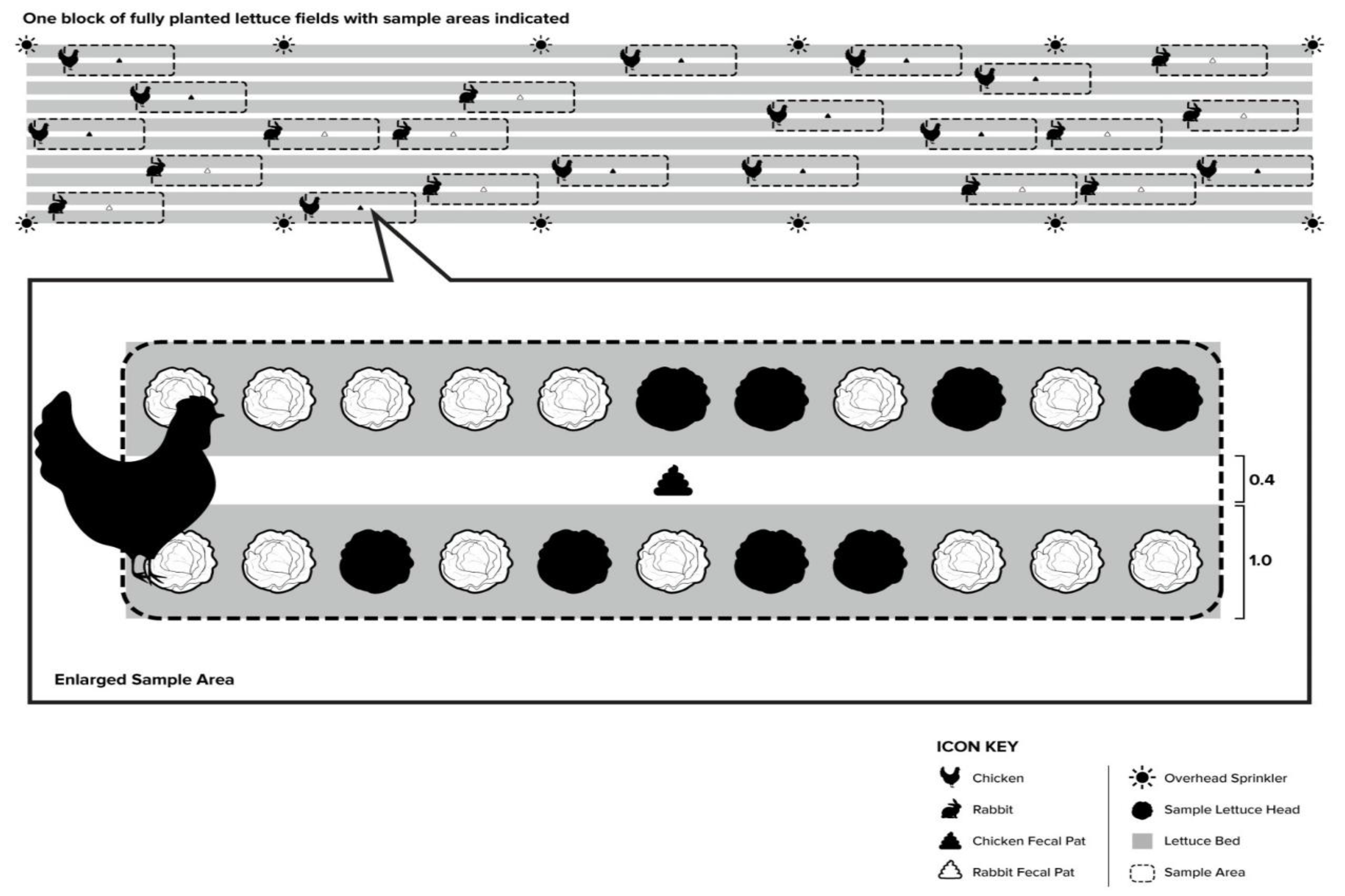
2.1. Lettuce Field Conditions
2.2. Bacterial Strain and Inoculum Preparation
2.3. E. coli Transfer (Experiment A)
2.4. Fecal Slurry Inoculation onto the Upper Surface of Lettuce Leaves (Experiment B)
2.5. Harvest
2.6. Indicator E. coli Rifr Most Probable Number (MPN) Determination (Experiment A)
2.7. Indicator E. coli Rifr Most Probable Number (MPN) Determination (Experiment B)
2.8. Bacterial Concentrations and Confirmatory Test
2.9. Statistical Analysis
3. Results
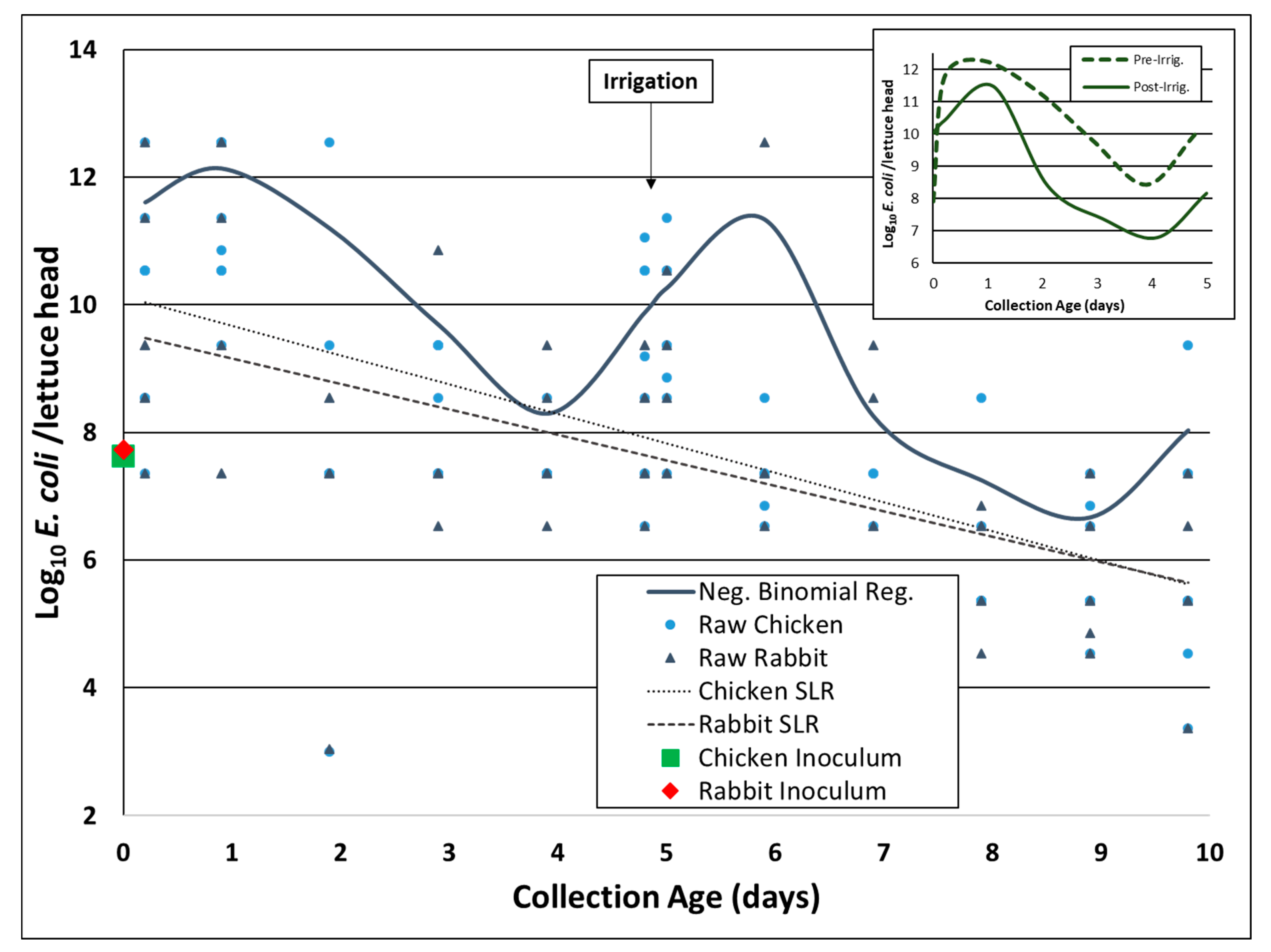
3.1. E. coli Rifr Concentration on Romaine Lettuce after Irrigation (Experiment A)
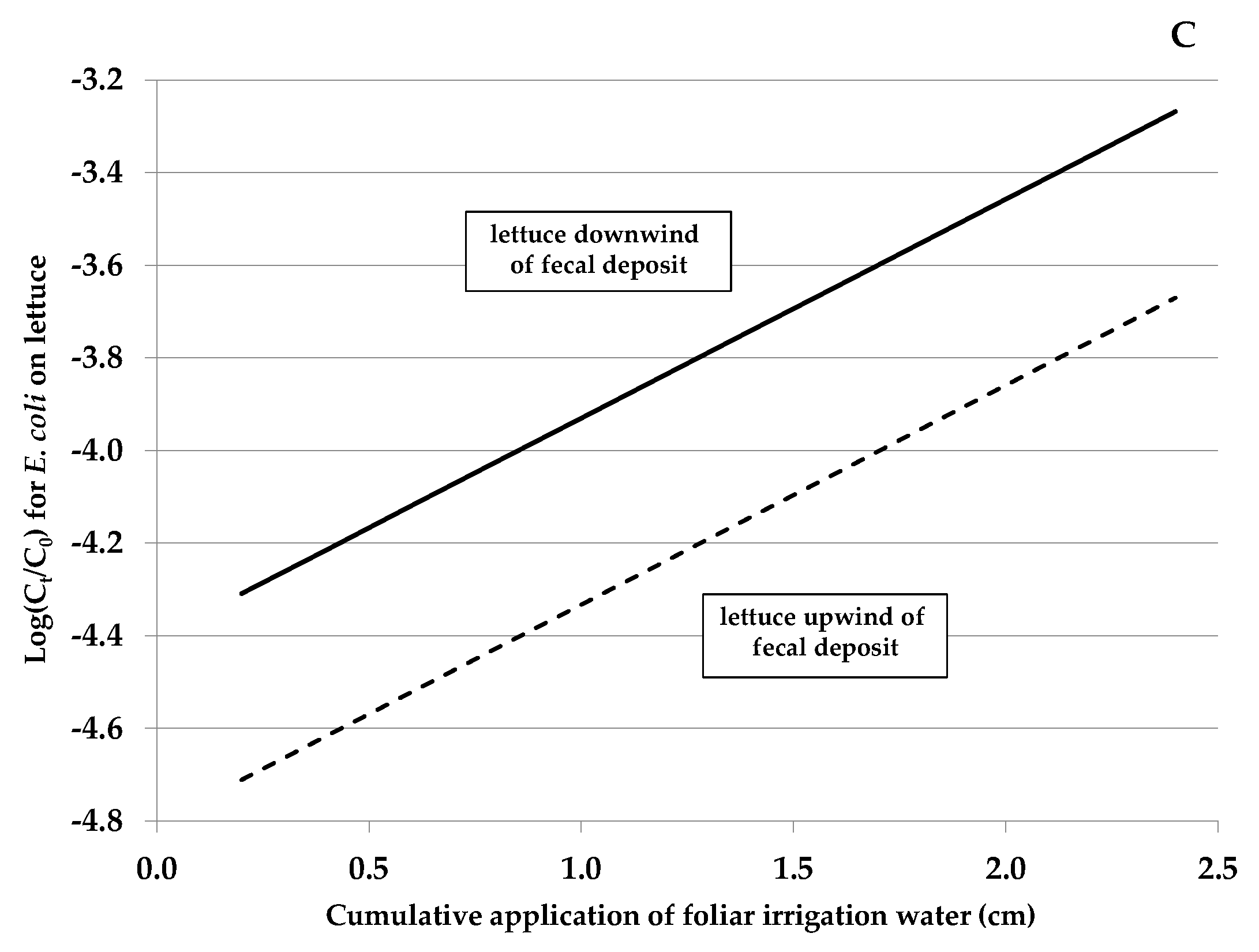
3.2. Contributing Factors Associated and Not Associated with E. coli Rifr Transfer from Feces to Lettuce (Experiment A)
3.3. Environmental Inactivation of E. coli Rifr in Chicken and Rabbit Fecal Slurries on Romaine Lettuce Surfaces (Experiment B)
3.4. Environmental Data (Experiment B)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doyle, M.P.; Erickson, M.C. Summer meeting 2007-the problems with fresh produce an overview. J. Appl. Microbiol. 2008, 105, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.H.; Walsh, K.A.; Vieira, A.R.; Herman, K.; Williams, I.T.; Hall, A.J.; Cole, D. Surveillance for foodborne disease outbreaks-United States, 1998–2008. MMWR Surveill Summ. 2013, 62, 1–34. [Google Scholar] [PubMed]
- Lynch, M.F.; Tauxe, R.V.; Hedberg, C.W. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 2009, 137, 307–315. [Google Scholar] [CrossRef]
- Griffin, P.M.; Tauxe, R.V. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 1991, 13, 60–98. [Google Scholar]
- Ackers, M.-L.; Mahon, B.E.; Leahy, E.; Goode, B.; Damrow, T.; Hayes, P.S.; Bibb, W.F.; Rice, D.H.; Barrett, T.J.; Hutwagner, L.; et al. An outbreak of Escherichia coli O157:H7 infections associated with leaf lettuce consumption. J. Infect. Dis. 1998, 177, 1588–1593. [Google Scholar] [CrossRef]
- Hilborn, E.D.; Mermin, J.H.; Mshar, P.A.; Hadler, J.L.; Voetsch, A.; Wojtkunski, C.; Swartz, M.; Mshar, R.; Lambert-Fair, M.A.; Farrar, J.A.; et al. A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch. Intern Med. 1999, 159, 1758–1764. [Google Scholar] [CrossRef]
- Besser, R.E.; Lett, S.M.; Weber, J.T.; Doyle, M.P.; Barrett, T.J.; Wells, J.G.; Griffin, P.M. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. J. Am. Vet. Med. Assoc. 1993, 269, 2217–2220. [Google Scholar] [CrossRef]
- Breuer, T.; Benkel, D.H.; Shapiro, R.L.; Hall, W.N.; Winnett, M.M.; Linn, M.J.; Neimann, J.; Barrett, T.J.; Dietrich, S.; Downes, F.P.; et al. A multistate outbreak of Escherichia coli O157:H7 infections linked to alfalfa sprouts grown from contaminated seeds. Emerg. Infect. Dis. 2001, 7, 977. [Google Scholar] [CrossRef]
- Laidler, M.R.; Tourdjman, M.; Buser, G.L.; Hostetler, T.; Repp, K.K.; Leman, R.; Samadpour, M.; Keene, W.E. Escherichia coli O157:H7 infections associated with consumption of locally grown strawberries contaminated by deer. Clin. Infect. Dis. 2013, 57, 1129–1134. [Google Scholar] [PubMed]
- Marder, E.P.; Garman, K.N.; Ingram, L.A.; Dunn, J.R. Multistate outbreak of Escherichia coli O157:H7 associated with bagged salad. Foodborne Pathog. Dis. 2014, 11, 593–595. [Google Scholar] [CrossRef]
- McCollum, J.T.; Cronquist, A.B.; Silk, B.J.; Jackson, K.A.; O’Connor, K.A.; Cosgrove, S.; Gossack, J.P.; Parachini, S.S.; Jain, N.S.; Ettestad, P. Multistate outbreak of Listeriosis associated with cantaloupe. N. Engl. J. Med. 2013, 369, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A.M.; Johnson, D.H.; Sharapov, U.; Grant, J.; Archer, J.R.; Monson, T.; Koschmann, C.; Davis, J.P. Multistate outbreak of Escherichia coli O157:H7 infection associated with consumption of packaged spinach, August-September 2006: the Wisconsin investigation. Clin. Infect. Dis. 2009, 48, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Cooley, M.B.; Carychao, D.; Crawford-Miksza, L.; Jay-Russell, M.T.; Myers, C.; Rose, C.; Keys, C.; Farrar, J.; Mandrell, R.E. Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS ONE 2007, 2, e1159. [Google Scholar] [CrossRef] [PubMed]
- Jay-Russell, M.T.; Cooley, M.; Carychao, D.; Wiscomb, G.W.; Sweitzer, R.A.; Crawford-Miksza, L.; Farrar, J.A.; Lau, D.K.; O’Connell, J.; Millington, A.; et al. Escherichia coli O157:H7 in feral swine near spinach fields and cattle, central California coast. Emerg. Infect. Dis. 2007, 13, 1908. [Google Scholar]
- Jay-Russell, M.T. What is the risk from wild animals in food-borne pathogen contamination of plants? CAB Rev. 2013, 8, 1–16. [Google Scholar] [CrossRef]
- LanghoLz, J.A.; Jay-Russell, M.T. Potential role of wildlife in pathogenic contamination of fresh produce. Hum. Wildl. Interact. 2013, 7, 140–157. [Google Scholar]
- Buck, J.W.; Walcott, R.R.; Beuchat, L.R. Recent trends in microbiological safety of fruits and vegetables. Plant Health Progress. 2003, 10, 1094. [Google Scholar] [CrossRef]
- Islam, M.; Doyle, M.P.; Phatak, S.C.; Millner, P.; Jiang, X. Persistence of enterohemorrhagic Escherichia coli O157:H7 in soil and on leaf lettuce and parsley grown in fields treated with contaminated manure composts or irrigation water. J. Food Prot. 2004, 67, 1365–1370. [Google Scholar] [CrossRef]
- Islam, M.; Morgan, J.; Doyle, M.P.; Phatak, S.C.; Millner, P.; Jiang, X. Persistence of Salmonella enterica serovar Typhimurium on lettuce and parsley and in soils on which they were grown in fields treated with contaminated manure composts or irrigation water. Foodborne Pathog. Dis. 2004, 1, 27–35. [Google Scholar] [CrossRef]
- Atwill, E.R.; Chase, J.A.; Oryang, D.; Bond, R.F.; Koike, S.T.; Cahn, M.D.; Anderson, M.; Mokhtari, A.; Dennis, S. Transfer of Escherichia coli O157:H7 from simulated wildlife scat onto romaine lettuce during foliar irrigation. J. Food Prot. 2015, 78, 240–247. [Google Scholar] [CrossRef]
- Mootian, G.; Wu, W.H.; Matthews, K.R. Transfer of Escherichia coli O157:H7 from soil, water, and manure contaminated with low numbers of the pathogen to lettuce plants. J. Food Prot. 2009, 72, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, L.A.; Jay-Russell, M.T.; Atwill, E.R.; Cooley, M.B.; Carychao, D.; Larsen, R.E.; Mandrell, R.E. Risk factors for Escherichia coli O157 on beef cattle ranches located near a major produce production region. Epidemiol. Infect. 2015, 143, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Kilonzo, C.; Li, X.; Vivas, E.J.; Jay-Russell, M.T.; Fernandez, K.L.; Atwill, E.R. Fecal shedding of zoonotic food-borne pathogens by wild rodents in a major agricultural region of the central California coast. Appl. Environ. Microbiol. 2013, 79, 6337–6344. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.C.; Webb, C.C.; Diaz-Perez, J.C.; Phatak, S.C.; Silvoy, J.J.; Davey, L.; Payton, A.S.; Liao, J.; Ma, L.; Doyle, M.P. Surface and internalized Escherichia coli O157:H7 on field-grown spinach and lettuce treated with spray-contaminated irrigation water. J. Food Prot. 2010, 73, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.B.; Pang, H.J.; Matthews, K.R. Persistence of Escherichia coli O157:H7 on lettuce plants following spray irrigation with contaminated water. J. Food Prot. 2003, 66, 2198–2202. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.B.; Yaron, S.; Matthews, K.R. Transmission of Escherichia coli O157: H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 2002, 68, 397–400. [Google Scholar] [CrossRef]
- Wachtel, M.R.; Whitehand, L.C.; Mandrell, R.E. Association of Escherichia coli O157: H7 with preharvest leaf lettuce upon exposure to contaminated irrigation water. J. Food Prot. 2002, 65, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.A.; Atwill, E.R.; Partyka, M.L.; Bond, R.F.; Oryang, D. Inactivation of Escherichia coli O157: H7 on romaine lettuce when inoculated in a fecal slurry matrix. J. Food Prot. 2017, 80, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.M.; Fallon, S.D.; Sanchez, C.A.; Nolte, K.D. Escherichia coli survival in lettuce fields following its introduction through different irrigation systems. J. Appl. Microbiol. 2011, 110, 893–902. [Google Scholar] [CrossRef]
- Weller, D.L.; Kovac, L.J.; Kent, D.J.; Roof, S.; Tokman, J.I.; Mudrak, E.; Kowalcyk, B.; Oryang, D.; Aceituno, A.; Wiedmann, M. Escherichia coli transfer from simulated wildlife feces to lettuce during foliar irrigation: A field study in the Northeastern United States. Food Microbiol. 2017, 68, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.L.; Kovac, L.J.; Roof, S.; Kent, D.J.; Tokman, J.I.; Kowalcyk, B.; Oryang, D.; Ivanek, R.; Aceituno, A.; Sroka, C.; et al. Survival of Escherichia coli on lettuce under field conditions encountered in the Northeastern United States. J. Food Prot. 2017, 80, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption. 2015. Available online: https://federalregister.gov/documents/2015/11/27/2015-28159/standards-for-the-growing-harvesting.packing-and-holding-of-produce-for-human-consumption (accessed on 27 November 2018).
- Leafy Green Marketing Agreement. Commodity Specific Food Safety Guidelines for the Production and Harvest of Lettuce and Leafy Greens. 2016. Available online: http://lgma.ca.gov/wp-content/uploads/2014/09/California-LGMA-metrics-01-29-16-Final1.pdf (accessed on 29 January 2018).
- Chase, J.A.; Partyka, M.L.; Bond, R.F.; Atwill, E.R. Environmental inactivation and irrigation-mediated regrowth of Escherichia coli O157: H7 on romaine lettuce when inoculated in a fecal slurry matrix. Peer J. 2019, 7, e6591. [Google Scholar] [CrossRef] [PubMed]
- Ribot, E.M.; Fair, M.A.; Gautom, R.; Cameron, D.N.; Hunter, S.B.; Swaminathan, B.; Barrett, T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157: H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006, 3, 59–67. [Google Scholar] [CrossRef]
- Moyne, A.L.; Harris, L.J.; Marco, M.L. Assessments of total and viable Escherichia coli O157: H7 on field and laboratory grown lettuce. PLoS ONE 2013, 8, e70643. [Google Scholar] [CrossRef] [PubMed]
- Barker-Reid, F.; Harapas, D.; Engleitner, S.; Kreidl, S.; Holmes, R.; Faggian, R. Persistence of Escherichia coli on injured iceberg lettuce in the field, overhead irrigated with contaminated water. J. Food Prot. 2009, 72, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Pang, H.; Buchanan, R.L.; Schaffner, D.W.; Pradhan, A.K.A. System Model for Understanding the Role of Animal Feces as a Route of Contamination of Leafy Greens before Harvest. Appl. Environ. Microbiol. 2016, 83, e02775-16. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Atwill, E.R.; Lennox, M.S.; Pereira, M.D.G.; Miller, W.A.; Conrad, P.A.; Tate, K.W. Management of microbial contamination in storm runoff from California coastal dairy pastures. J. Env. Qual. 2010, 39, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Franz, E.; Semenov, A.V.; Van Bruggen, A.H.C. Modelling the contamination of lettuce with Escherichia coli O157: H7 from manure-amended soil and the effect of intervention strategies. J. Appl. Microbio. 2008, 105, 1569–1584. [Google Scholar] [CrossRef]
- Pielaat, A.; van den Bosch, F. A model for dispersal of plant pathogens by rainsplash. Math Med. Biol. 1998, 15, 117–134. [Google Scholar] [CrossRef]
- Kincaid, D.; Solomon, K.; Oliphant, J. Drop size distributions for irrigation sprinklers. Trans. ASAE 1996, 39, 839–845. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Ravishankar, S.; Sanchez, C. Estimation of the Area Affected by Animal Feces in Vegetable Field under Overhead Sprinkle Irrigation System. 2008. Available online: https://calgreens.org/control/uploads/Food_Safety_-_Fonseca.pdf (accessed on 7 January 2018).
- Ferens, W.A.; Hovde, C.J. Escherichia coli O157: H7: animal reservoir and sources of human infection. Foodborne Pathog. Dis. 2011, 8, 465–487. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.H.; Hancock, D.D.; Besser, T.E. Faecal culture of wild animals for Escherichia coli O157: H7. Vet. Rec. 2003, 152, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Fegan, N.; Vanderlinde, P.; Higgs, G.; Desmarchelier, P. The prevalence and concentration of Escherichia coli O157 in faeces of cattle from different production systems at slaughter. J. Appl. Microbiol. 2004, 97, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Omisakin, F.; MacRae, M.; Ogden, I.D.; Strachan, N.J.C. Concentration and prevalence of Escherichia coli O157 in cattle feces at slaughter. Appl. Environ. Microbiol. 2003, 69, 2444–2447. [Google Scholar] [CrossRef] [PubMed]
- Cooley, M.B.; Jay-Russell, M.; Atwill, E.R.; Carychao, D.; Nguyen, K.; Quiñones, B.; Patel, R.; Walker, S.; Swimley, M.; Pierre-Jerome, E.; et al. Development of a robust method for isolation of shiga toxin-positive Escherichia coli (STEC) from fecal, plant, soil and water samples from a leafy greens production region in California. PLoS ONE 2013, 8, e65716. [Google Scholar] [CrossRef] [PubMed]
- Worley, J.N.; Flores, K.A.; Yang, X.; Chase, J.A.; Cao, G.; Tang, S.; Meng, J.; Atwill, E.R. Prevalence and Genomic Characterization of Escherichia coli O157: H7 in Cow-Calf Herds throughout California. Appl. Environ. Microbiol. 2017, 83, e00734-17. [Google Scholar] [CrossRef]
- Nielsen, E.M.; Skov, M.N.; Madsen, J.J.; Lodal, J.; Jespersen, J.B.; Baggesen, D.L. Verocytotoxin-producing Escherichia coli in wild birds and rodents in close proximity to farms. Appl. Environ. Microbiol. 2004, 70, 6944–6947. [Google Scholar] [CrossRef]
- Cernicchiaro, N.; Pearl, D.L.; McEwen, S.A.; Harpster, L.; Homan, H.J.; Linz, G.M.; Lejeune, J.T. Association of wild bird density and farm management factors with the prevalence of E. coli O157 in dairy herds in Ohio (2007–2009). Zoonoses Public Health 2012, 59, 320–329. [Google Scholar] [CrossRef]
- Himathongkham, S.; Riemann, H.; Bahari, S.; Nuanualsuwan, S.; Kass, P.; Cliver, D.O. Survival of Salmonella typhimurium and Escherichia coli O157: H7 in Poultry Manure and Manure Slurry at Sublethal Temperatures. Avian Dis. 2000, 44, 853–860. [Google Scholar] [CrossRef]
- Chase-Topping, M.; Gally, D.; Low, C.; Matthews, L.; Woolhouse, M. Super-shedding and the link between human infection and livestock carriage of Escherichia coli O157. Nat. Rev. Microbiol. 2008, 6, 904–912. [Google Scholar] [CrossRef]
- Stephens, T.P.; McAllister, T.A.; Stanford, K. Development of an experimental model to assess the ability of Escherichia coli O157: H7-inoculated fecal pats to mimic a super shedder within a feedlot environment. J. Food Prot. 2008, 71, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Cornick, N.A.; Helgerson, A.F. Transmission and infectious dose of Escherichia coli O157: H7 in swine. Appl. Environ. Microbiol. 2004, 70, 5331–5335. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G. Verocytoxigenic Escherichia coli in animal faeces, manures and slurries. J. Appl. Microbiol. 2003, 94, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Himathongkham, S.; Bahari, S.; Riemann, H.; Cliver, D. Survival of Escherichia coli O157: H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol. Lett. 1999, 178, 251–257. [Google Scholar] [CrossRef]
- Kudva, I.T.; Blanch, K.; Hovde, C.J. Analysis of Escherichia coli O157: H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 1998, 64, 3166–3174. [Google Scholar]

| Days Prior to Irrigation | The Mean Bacterial Inoculum as MPN/g, (Standard Deviation) | |||
|---|---|---|---|---|
| Chicken Feces (n = 3) | Rabbit Feces (n = 3) | |||
| Initial Concentration * | After Irrigation | Initial Concentration * | After Irrigation | |
| Day −4 | 1.06 × 108 | 2.01 × 108 (1.92 × 108) | 1.48 × 107 | 2.58 × 109 (3.00 × 109) |
| Day −2 | 7.72 × 107 | 2.33 × 1011 (3.25 × 1011) | 9.42 × 107 | 2.49 × 1011 (3.15 × 1011) |
| Day −1 | 2.00 × 108 | 2.33 × 1011 (3.25 × 1011) | 5.44 × 107 | 4.17 × 109 (3.00 × 109) |
| Day 0 | 1.69 × 108 | 2.58 × 109 (3.00 × 109) | 5.65 × 107 | 4.64 × 1011 (3.24 × 1011) |
| Overall mean | 1.38 × 108 | 1.17 × 1011 (2.79 × 1011) | 5.50 × 107 | 1.79 × 1011 (2.65 × 1011) |
| In-Field Factor | Coefficient | 95% C.I. * | P-Value * |
|---|---|---|---|
| Mean distance from feces to four sprinklers (cm) | 0.0137 | 0.0048 to 0.022 | 0.002 |
| Distance from feces to head of lettuce (cm) | −0.0109 | −0.021 to −0.0007 | 0.037 |
| Cumulative application of foliar irrigation (cm) | 1.0895 | 0.068 to 2.11 | 0.037 |
| Head of lettuce downwind of the fecal deposit ⊥ | 0.9265 | 0.029 to 1.82 | 0.043 |
| Intercept | −0.0328 | −7.35 to 6.69 | 0.927 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeamsripong, S.; Chase, J.A.; Jay-Russell, M.T.; Buchanan, R.L.; Atwill, E.R. Experimental In-Field Transfer and Survival of Escherichia coli from Animal Feces to Romaine Lettuce in Salinas Valley, California. Microorganisms 2019, 7, 408. https://doi.org/10.3390/microorganisms7100408
Jeamsripong S, Chase JA, Jay-Russell MT, Buchanan RL, Atwill ER. Experimental In-Field Transfer and Survival of Escherichia coli from Animal Feces to Romaine Lettuce in Salinas Valley, California. Microorganisms. 2019; 7(10):408. https://doi.org/10.3390/microorganisms7100408
Chicago/Turabian StyleJeamsripong, Saharuetai, Jennifer A. Chase, Michele T. Jay-Russell, Robert L. Buchanan, and Edward R. Atwill. 2019. "Experimental In-Field Transfer and Survival of Escherichia coli from Animal Feces to Romaine Lettuce in Salinas Valley, California" Microorganisms 7, no. 10: 408. https://doi.org/10.3390/microorganisms7100408
APA StyleJeamsripong, S., Chase, J. A., Jay-Russell, M. T., Buchanan, R. L., & Atwill, E. R. (2019). Experimental In-Field Transfer and Survival of Escherichia coli from Animal Feces to Romaine Lettuce in Salinas Valley, California. Microorganisms, 7(10), 408. https://doi.org/10.3390/microorganisms7100408






