Molecular Basis of The Retinal Pigment Epithelial Changes That Characterize The Ocular Lesion in Toxoplasmosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Primary Antibodies, Recombinant Proteins, and Enzyme-Linked Immunosorbent Assay Kits
2.3. Cells
2.4. Parasites
2.5. Infection Protocols
2.6. Antigen KI-67 Immunolabelling
2.7. VEGF and IGF1 Blockade Assays and TSP1 Supplementation Assay
2.8. RNA Extraction and Reverse Transcription
2.9. Quantitative Real-Time Polymerase Chain Reaction
2.10. Statistical Analysis
2.11. Research Ethics and Biosafety
3. Results
3.1. Typical Retinal Lesions with Hyperpigmentation Represent the Majority of Ocular Toxoplasmosis
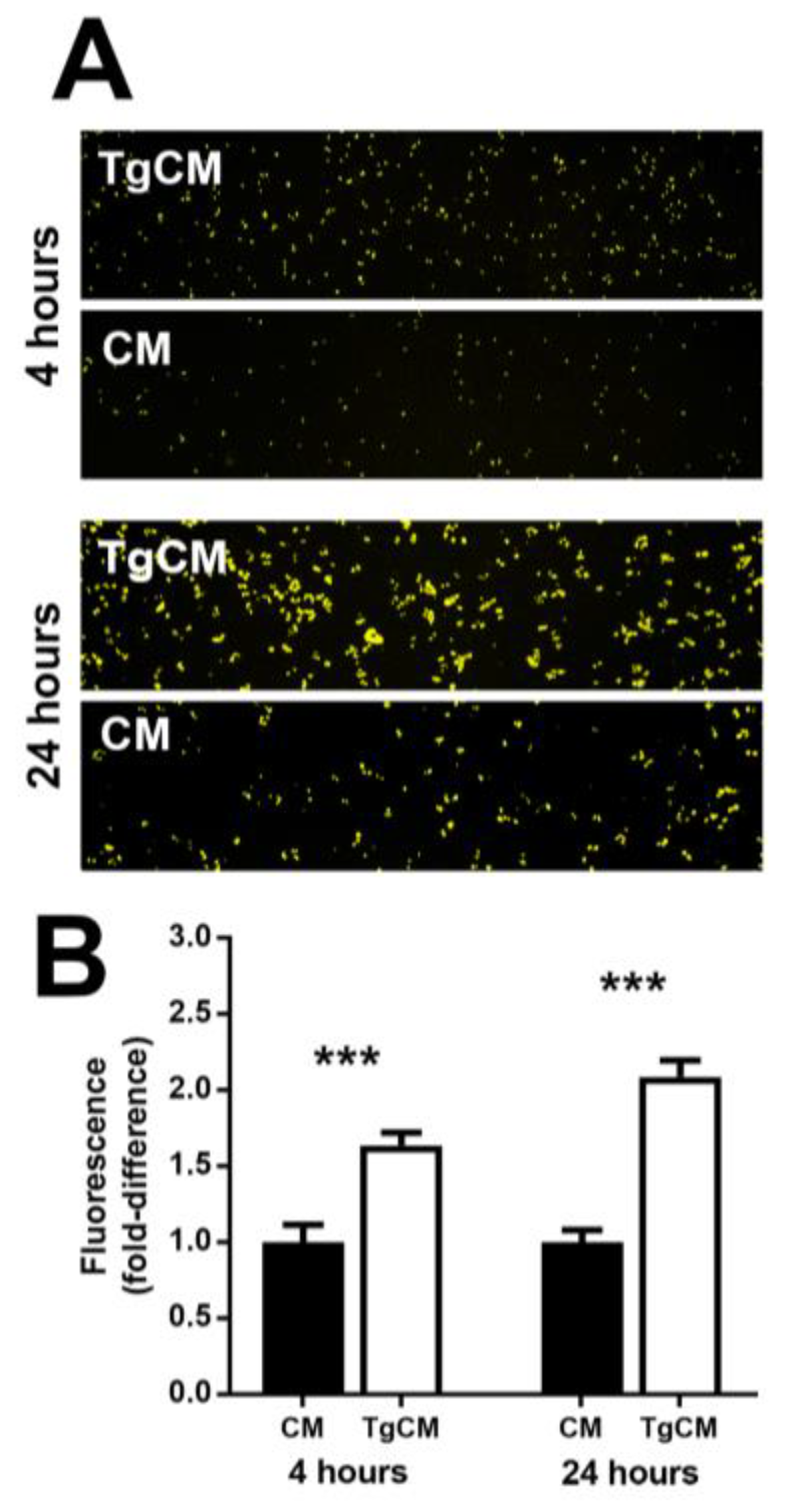
3.2. Infection with T. gondii Proliferates Human Retinal Pigment Epithelial Cells
3.3. Infection of Human Retinal Pigment Epithelial Cells with T. gondii Induces Changes in the Levels of Some Growth Factors
3.4. Increase in Vascular Endothelial Growth Factor and Insulin-like Growth Factor 1—and Decrease in Thrombospondin 1—by T. gondii-Infected Retinal Pigment Epithelial Cells Promote Proliferation of Uninfected Cells
3.5. Proliferating Retinal Pigment Epithelial Cells are Relatively Susceptible to Infection with T. gondii Tachyzoites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butler, N.J.; Furtado, J.M.; Winthrop, K.L.; Smith, J.R. Ocular toxoplasmosis II: Clinical features, pathology and management. Clin. Exp. Ophthalmol. 2013, 41, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Furtado, J.M.; Winthrop, K.L.; Butler, N.J.; Smith, J.R. Ocular toxoplasmosis I: Parasitology, epidemiology and public health. Clin. Exp. Ophthalmol. 2013, 41, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.N. Ocular toxoplasmosis: A global reassessment. Part II: Disease manifestations and management. Am. J. Ophthalmol. 2004, 137, 1–17. [Google Scholar] [CrossRef]
- Ozgonul, C.; Besirli, C.G. Recent developments in the diagnosis and treatment of ocular toxoplasmosis. Ophthalmic Res. 2017, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 2011. [Google Scholar]
- Nicholson, D.H.; Wolchok, E.B. Ocular toxoplasmosis in an adult receiving long-term corticosteroid therapy. Arch. Ophthalmol. 1976, 94, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.N.; Engstrom R.E., Jr.; Glasgow, B.J.; Berger, B.B.; Daniels, S.A.; Sidikaro, Y.; Harmon, J.A.; Fischer, D.H.; Boyer, D.S.; Rao, N.A.; et al. Ocular toxoplasmosis in patients with the acquired immunodeficiency syndrome. Am. J. Ophthalmol. 1988, 106, 653–667. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Specht, C.S.; Allaire, G.; Leavitt, J.A. Toxoplasma gondii retinochoroiditis and optic neuritis in acquired immune deficiency syndrome. Report of a case. Ophthalmology 1990, 97, 1342–1346. [Google Scholar] [CrossRef]
- Yeo, J.H.; Jakobiec, F.A.; Iwamoto, T.; Richard, G.; Kreissig, I. Opportunistic toxoplasmic retinochoroiditis following chemotherapy for systemic lymphoma. A light and electron microscopic study. Ophthalmology 1983, 90, 885–898. [Google Scholar] [CrossRef]
- Parke, D.W., 2nd; Font, R.L. Diffuse toxoplasmic retinochoroiditis in a patient with AIDS. Arch. Ophthalmol. 1986, 104, 571–575. [Google Scholar] [CrossRef]
- Orefice, J.L.; Costa, R.A.; Scott, I.U.; Calucci, D.; Orefice, F.; Grupo Mineiro de Pesquisa em Doenças Oculares Inflamatórias (MINAS). Spectral optical coherence tomography findings in patients with ocular toxoplasmosis and active satellite lesions (MINAS Report 1). Acta Ophthalmol. 2013, 91, e41–e47. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, R.C.; Smith, R.L.; Corte-Real, S.; Calabrese, K.S. Ocular toxoplasmosis: The role of retinal pigment epithelium migration in infection. Parasitol. Res. 2004, 92, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Passos, A.D.C.; Bollela, V.R.; Furtado, J.M.F.; Lucena, M.M.; Bellissimo-Rodrigues, F.; Paula, J.S.; Melo, L.V.L.; Rodrigues, M.L.V. Prevalence and risk factors of toxoplasmosis among adults in a small Brazilian city. Rev. Soc. Bras. Med. Trop. 2018, 51, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef]
- Lie, S.; Rochet, E.; Segerdell, E.; Ma, Y.; Ashander, L.M.; Shadforth, A.M.A.; Blenkinsop, T.A.; Michael, M.Z.; Appukuttan, B.; Wilmot, B.; et al. Immunological molecular responses of human retinal pigment epithelial cells to infection with Toxoplasma gondii. Front. Immunol. 2019, 10, 708. [Google Scholar] [CrossRef]
- Furtado, J.M.; Bharadwaj, A.S.; Ashander, L.M.; Olivas, A.; Smith, J.R. Migration of Toxoplasma gondii-infected dendritic cells across human retinal vascular endothelium. Invest. Ophthalmol. Vis. Sci. 2012, 53, 6856–6862. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Behnke, M.S.; Dunay, I.R.; White, M.W.; Sibley, L.D. Phenotypic and gene expression changes among clonal type I strains of Toxoplasma gondii. Eukaryot. Cell 2009, 8, 1828–1836. [Google Scholar] [CrossRef]
- Gubbels, M.J.; Li, C.; Striepen, B. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob. Agents Chemother. 2003, 47, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Yoshimura, N.; Tanihara, H.; Honda, Y. Insulin-like growth factor-related genes, receptors, and binding proteins in cultured human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1994, 35, 916–923. [Google Scholar] [PubMed]
- Guerrin, M.; Moukadiri, H.; Chollet, P.; Moro, F.; Dutt, K.; Malecaze, F.; Plouet, J. Vasculotropin/vascular endothelial growth factor is an autocrine growth factor for human retinal pigment epithelial cells cultured in vitro. J. Cell. Physiol. 1995, 164, 385–394. [Google Scholar] [CrossRef]
- Schlingemann, R.O. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Leschey, K.H.; Hackett, S.F.; Singer, J.H.; Campochiaro, P.A. Growth factor responsiveness of human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1990, 31, 839–846. [Google Scholar] [PubMed]
- Farnoodian, M.; Kinter, J.B.; Yadranji Aghdam, S.; Zaitoun, I.; Sorenson, C.M.; Sheibani, N. Expression of pigment epithelium-derived factor and thrombospondin-1 regulate proliferation and migration of retinal pigment epithelial cells. Physiol. Rep. 2015, 3, e12266. [Google Scholar] [CrossRef]
- Molestina, R.E.; El-Guendy, N.; Sinai, A.P. Infection with Toxoplasma gondii results in dysregulation of the host cell cycle. Cell. Microbiol. 2008, 10, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- London, N.J.; Hovakimyan, A.; Cubillan, L.D.; Siverio, C.D., Jr.; Cunningham, E.T., Jr. Prevalence, clinical characteristics, and causes of vision loss in patients with ocular toxoplasmosis. Eur. J. Ophthalmol. 2011, 21, 811–819. [Google Scholar] [CrossRef]
- Bosch-Driessen, L.E.; Berendschot, T.T.; Ongkosuwito, J.V.; Rothova, A. Ocular toxoplasmosis: Clinical features and prognosis of 154 patients. Ophthalmology 2002, 109, 869–878. [Google Scholar] [CrossRef]
- de-la-Torre, A.; Pfaff, A.W.; Grigg, M.E.; Villard, O.; Candolfi, E.; Gomez-Marin, J.E. Ocular cytokinome is linked to clinical characteristics in ocular toxoplasmosis. Cytokine 2014, 68, 23–31. [Google Scholar] [CrossRef]
- Wiertz, K.; De Visser, L.; Rijkers, G.; De Groot-Mijnes, J.; Los, L.; Rothova, A. Intraocular and serum levels of vascular endothelial growth factor in acute retinal necrosis and ocular toxoplasmosis. Retina 2010, 30, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.; Schlickeiser, S.; Metzner, S.; Dames, C.; Pleyer, U. Immune mediator profile in aqueous humor differs in patients with primary acquired ocular toxoplasmosis and recurrent acute ocular toxoplasmosis. Mediators Inflamm. 2019, 2019, 9356728. [Google Scholar] [CrossRef]
- Behnke, M.S.; Dubey, J.P.; Sibley, L.D. Genetic mapping of pathogenesis determinants in Toxoplasma gondii. Annu. Rev. Microbiol. 2016, 70, 63–81. [Google Scholar] [CrossRef]
- Xia, J.; Cheng, X.Y.; Wang, X.J.; Peng, H.J. Association between Toxoplasma gondii types and outcomes of human infection: A meta-analysis. Acta Microbiol. Immunol. Hung. 2017, 64, 229–244. [Google Scholar] [CrossRef]
- Brunet, J.; Pfaff, A.W.; Abidi, A.; Unoki, M.; Nakamura, Y.; Guinard, M.; Klein, J.P.; Candolfi, E.; Mousli, M. Toxoplasma gondii exploits UHRF1 and induces host cell cycle arrest at G2 to enable its proliferation. Cell. Microbiol. 2008, 10, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, M.B.; Silva, N.M.; Castro, A.S.; Gomes, A.O.; Silva, D.A.; Mineo, J.R.; Ferro, E.A. Apoptosis and S phase of the cell cycle in BeWo trophoblastic and HeLa cells are differentially modulated by Toxoplasma gondii strain types. Placenta 2009, 30, 785–791. [Google Scholar] [CrossRef]
- Lavine, M.D.; Arrizabalaga, G. Induction of mitotic S-phase of host and neighboring cells by Toxoplasma gondii enhances parasite invasion. Mol. Biochem. Parasitol. 2009, 164, 95–99. [Google Scholar] [CrossRef]
- Wang, G.; Gao, M. Influence of Toxoplasma gondii on in vitro proliferation and apoptosis of hepatoma carcinoma H7402 cell. Asian Pac. J. Trop. Med. 2016, 9, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, M.A.; Bougdour, A. Toxoplasma’s ways of manipulating the host transcriptome via secreted effectors. Curr. Opin. Microbiol. 2015, 26, 24–31. [Google Scholar] [CrossRef]
- Spear, W.; Chan, D.; Coppens, I.; Johnson, R.S.; Giaccia, A.; Blader, I.J. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell. Microbiol. 2006, 8, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Lis, A.; Wiley, M.; Vaughan, J.; Gray, P.C.; Blader, I.J. The activin receptor, Activin-Like Kinase 4, mediates Toxoplasma gondii activation of hypoxia inducible factor-1. Front. Cell. Infect. Microbiol. 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Koshy, A.A.; Dietrich, H.K.; Christian, D.A.; Melehani, J.H.; Shastri, A.J.; Hunter, C.A.; Boothroyd, J.C. Toxoplasma co-opts host cells it does not invade. PLoS Pathog. 2012, 8, e1002825. [Google Scholar] [CrossRef]
- Naemat, A.; Elsheikha, H.M.; Boitor, R.A.; Notingher, I. Tracing amino acid exchange during host-pathogen interaction by combined stable-isotope time-resolved Raman spectral imaging. Sci. Rep. 2016, 6, 20811. [Google Scholar] [CrossRef]
- Nogueira, A.R.; Leve, F.; Morgado-Diaz, J.; Tedesco, R.C.; Pereira, M.C. Effect of Toxoplasma gondii infection on the junctional complex of retinal pigment epithelial cells. Parasitology 2016, 143, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Song, H.B.; Jun, H.O.; Kim, J.H.; Lee, Y.H.; Choi, M.H.; Kim, J.H. Disruption of outer blood-retinal barrier by Toxoplasma gondii-infected monocytes is mediated by paracrinely activated FAK signaling. PLoS ONE 2017, 12, e0175159. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Guidry, C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest. Ophthalmol. Vis. Sci. 1995, 36, 391–405. [Google Scholar] [PubMed]
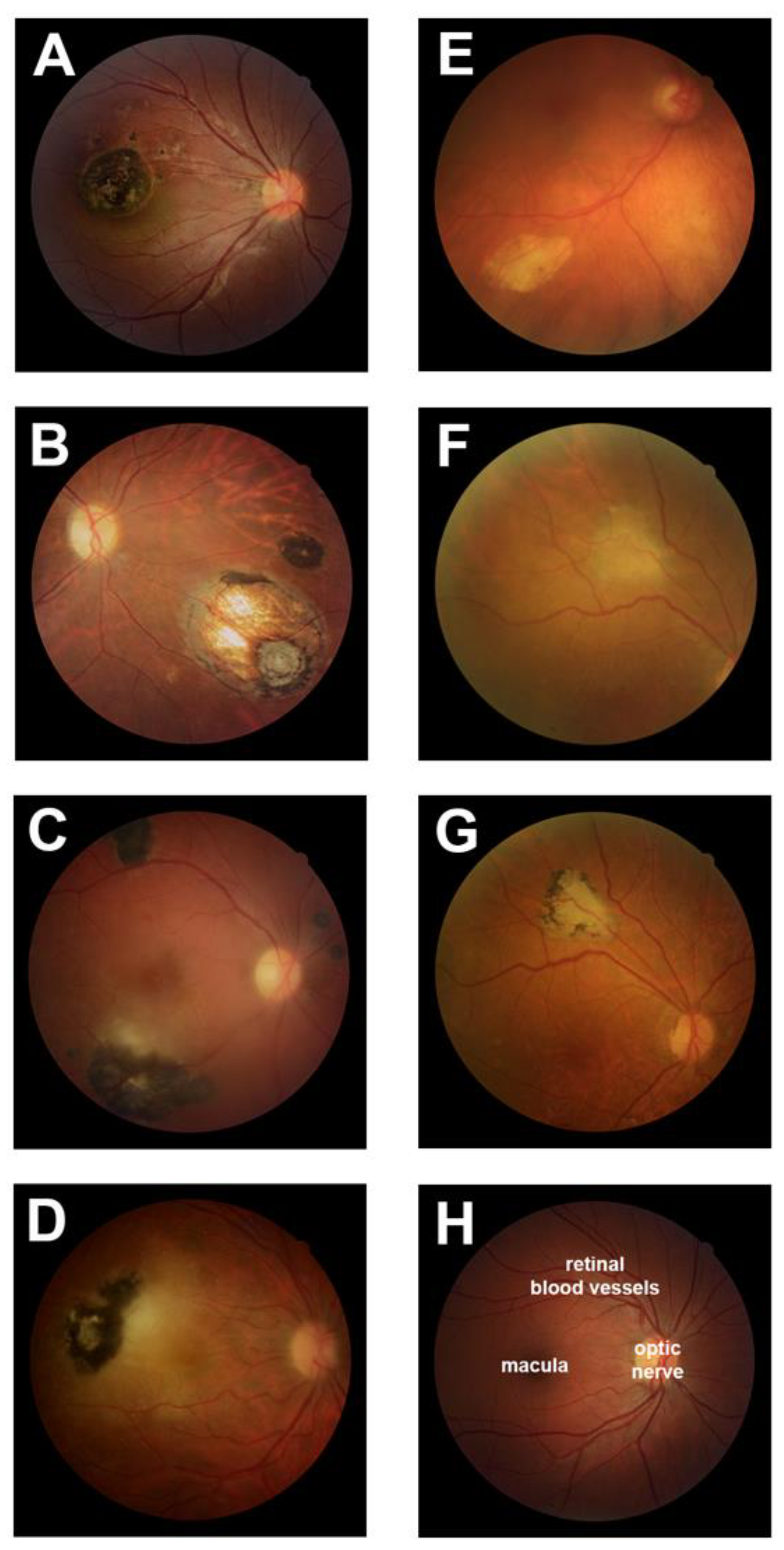
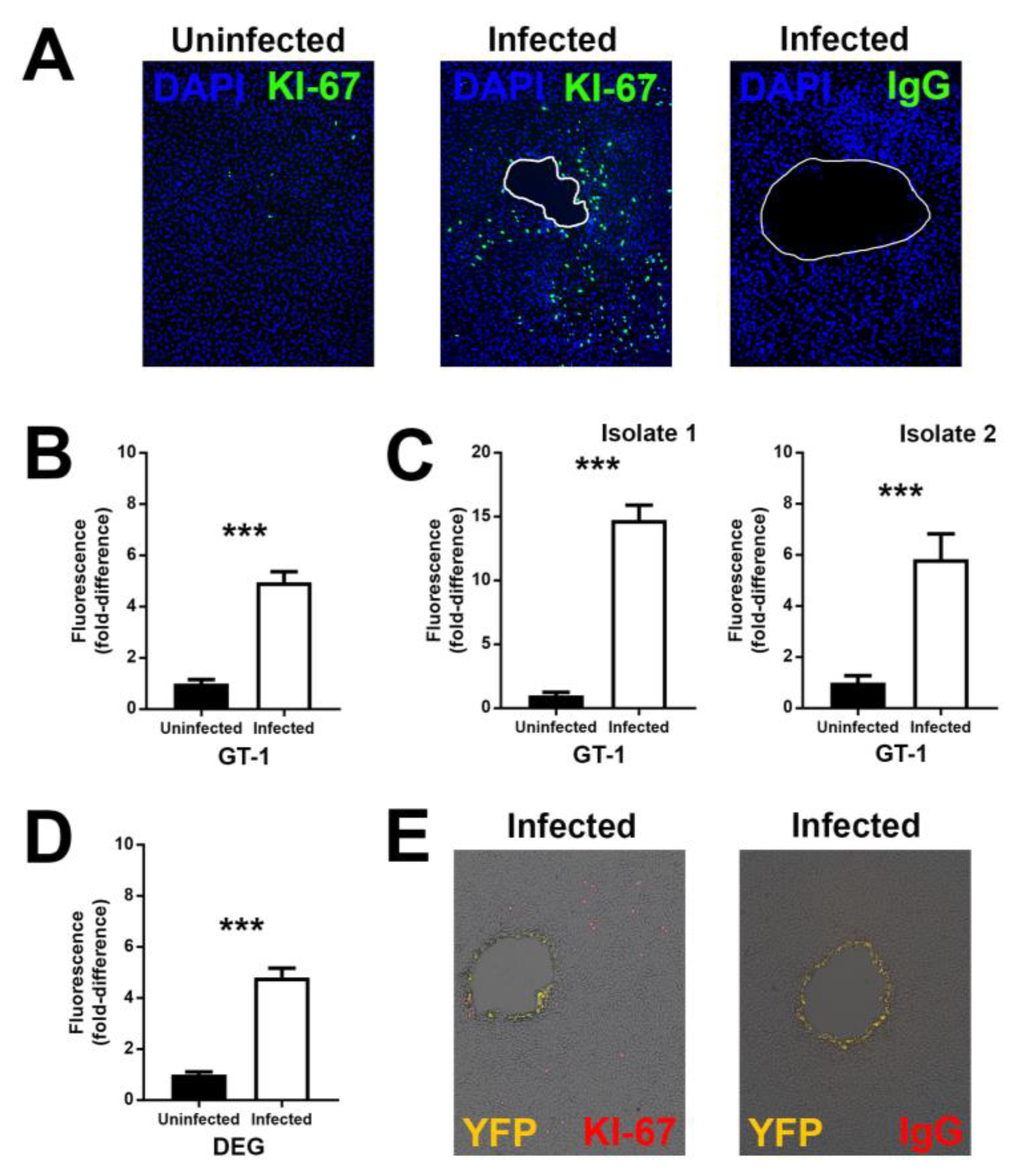
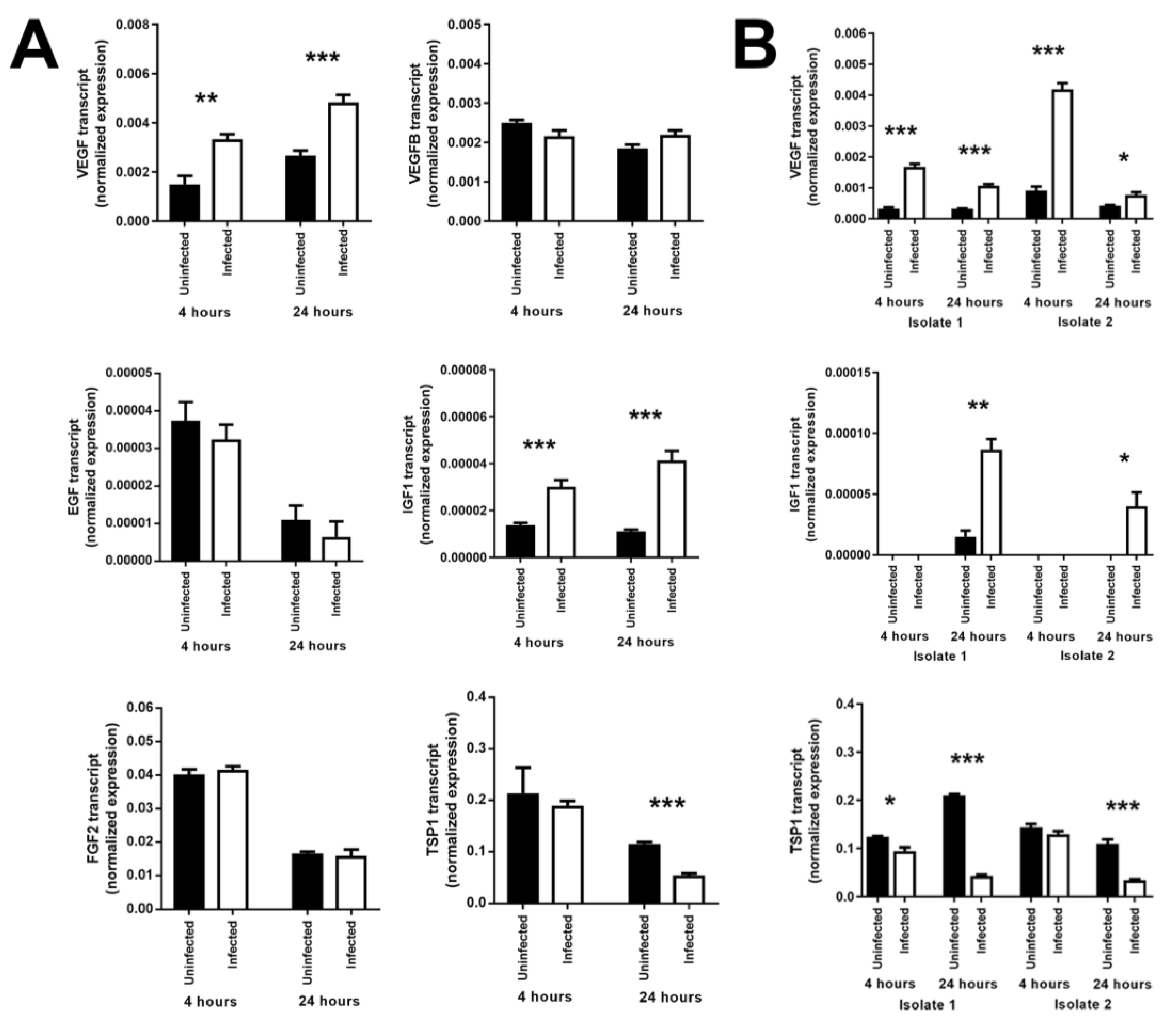
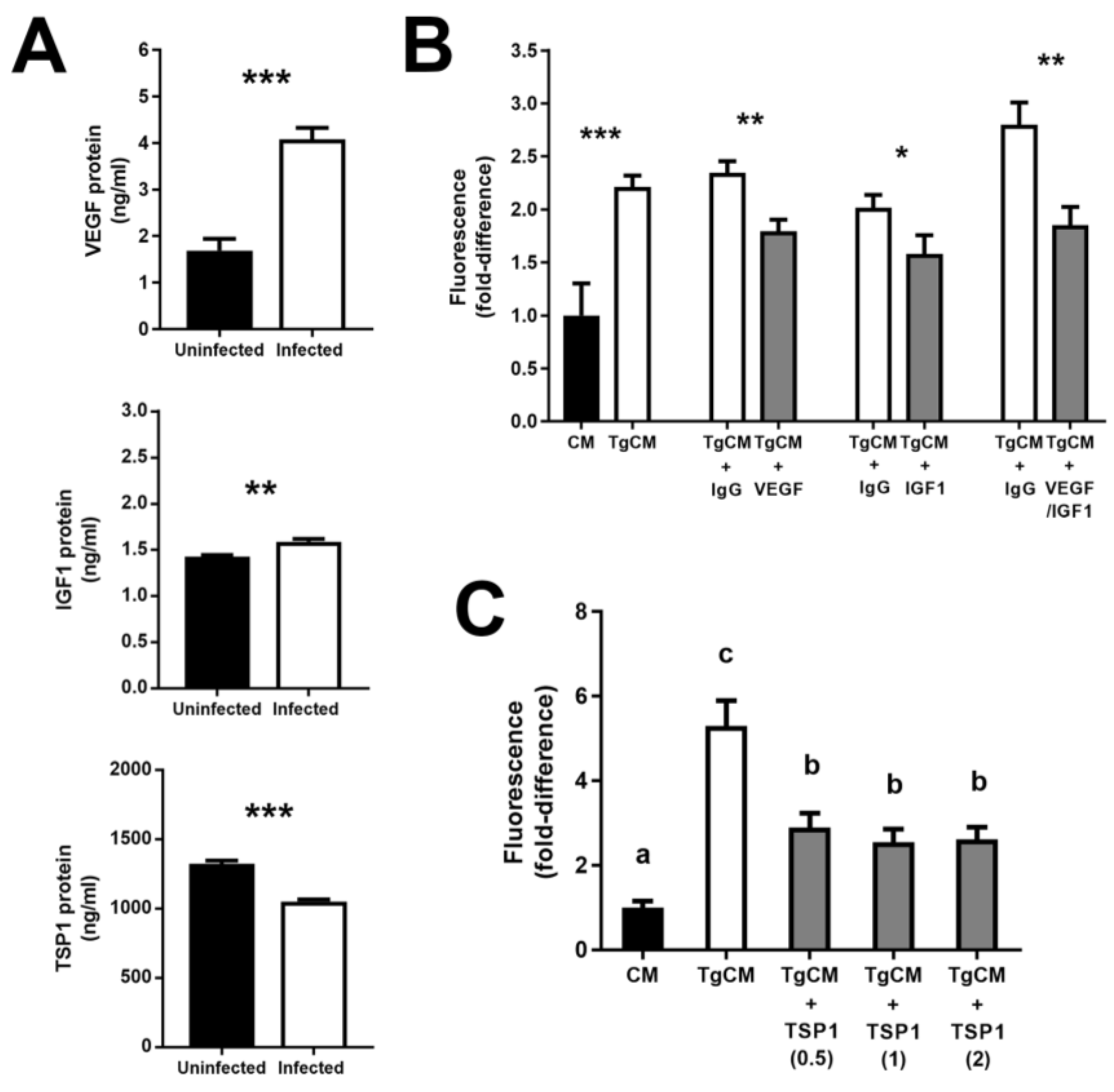
| Human Subjects, n (%) | |
| Gender | |
| Male | 124 (47.1%) |
| Female | 139 (52.9%) |
| Age (years) | |
| ≤17 | 29 (11.0%) |
| 18–64 | 210 (79.8%) |
| ≥65 | 24 (9.1%) |
| Self-reported ethnicity | |
| Multiracial | 124 (47.1%) |
| Caucasian | 101 (38.4%) |
| Afro-Brazilian | 35 (13.3%) |
| Unknown | 3 (1.1%) |
| Form of ocular toxoplasmosis | |
| Primary active | 49 (18.6%) |
| Recurrent active | 88 (33.4%) |
| Inactive | 126 (47.9%) |
| Mode of infection | |
| Congenital | 24 (9.1%) |
| Acquired | 13 (4.9%) |
| Unknown | 226 (85.9%) |
| Results of serological studies | |
| Toxoplasma gondii IgG+ IgM + | 20 (7.6%) |
| T. gondii IgG+ IgM- | 243 (92.4%) |
| Eyes, n (%) | |
| Number of ocular lesion(s) | |
| 1 | 166 (48.1%) |
| 2–4 | 147 (42.6%) |
| ≥5 | 32 (9.2%) |
| Location of retinal lesion(s) | |
| Posterior pole | 124 (35.9%) |
| Periphery | 176 (51.0%) |
| Posterior pole and periphery | 45 (13.0%) |
| Hyperpigmented scars | |
| Present | 325 (94.2%) |
| Absent | 20 (5.8%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lie, S.; Vieira, B.R.; Arruda, S.; Simões, M.; Ashander, L.M.; Furtado, J.M.; Smith, J.R. Molecular Basis of The Retinal Pigment Epithelial Changes That Characterize The Ocular Lesion in Toxoplasmosis. Microorganisms 2019, 7, 405. https://doi.org/10.3390/microorganisms7100405
Lie S, Vieira BR, Arruda S, Simões M, Ashander LM, Furtado JM, Smith JR. Molecular Basis of The Retinal Pigment Epithelial Changes That Characterize The Ocular Lesion in Toxoplasmosis. Microorganisms. 2019; 7(10):405. https://doi.org/10.3390/microorganisms7100405
Chicago/Turabian StyleLie, Shervi, Bárbara R. Vieira, Sigrid Arruda, Milena Simões, Liam M. Ashander, João M. Furtado, and Justine R. Smith. 2019. "Molecular Basis of The Retinal Pigment Epithelial Changes That Characterize The Ocular Lesion in Toxoplasmosis" Microorganisms 7, no. 10: 405. https://doi.org/10.3390/microorganisms7100405
APA StyleLie, S., Vieira, B. R., Arruda, S., Simões, M., Ashander, L. M., Furtado, J. M., & Smith, J. R. (2019). Molecular Basis of The Retinal Pigment Epithelial Changes That Characterize The Ocular Lesion in Toxoplasmosis. Microorganisms, 7(10), 405. https://doi.org/10.3390/microorganisms7100405






