Volatile Composition and Sensory Properties of Mead
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Honey
2.2. Immobilization of Yeast Cells
2.3. Honey-Must and Fermentation Conditions
2.4. General Oenological Parameters
2.5. HPLC Determination of Glucose, Fructose, Glycerol, Acetic Acid and Ethanol
2.6. Analysis of Mead Volatile Compounds
2.6.1. Chromatographic Analysis of Major Volatile Compounds
2.6.2. Chromatographic Analysis of Minor Volatile Compounds
2.7. Odour Activity Values
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. General Physicochemical Characterization of Mead
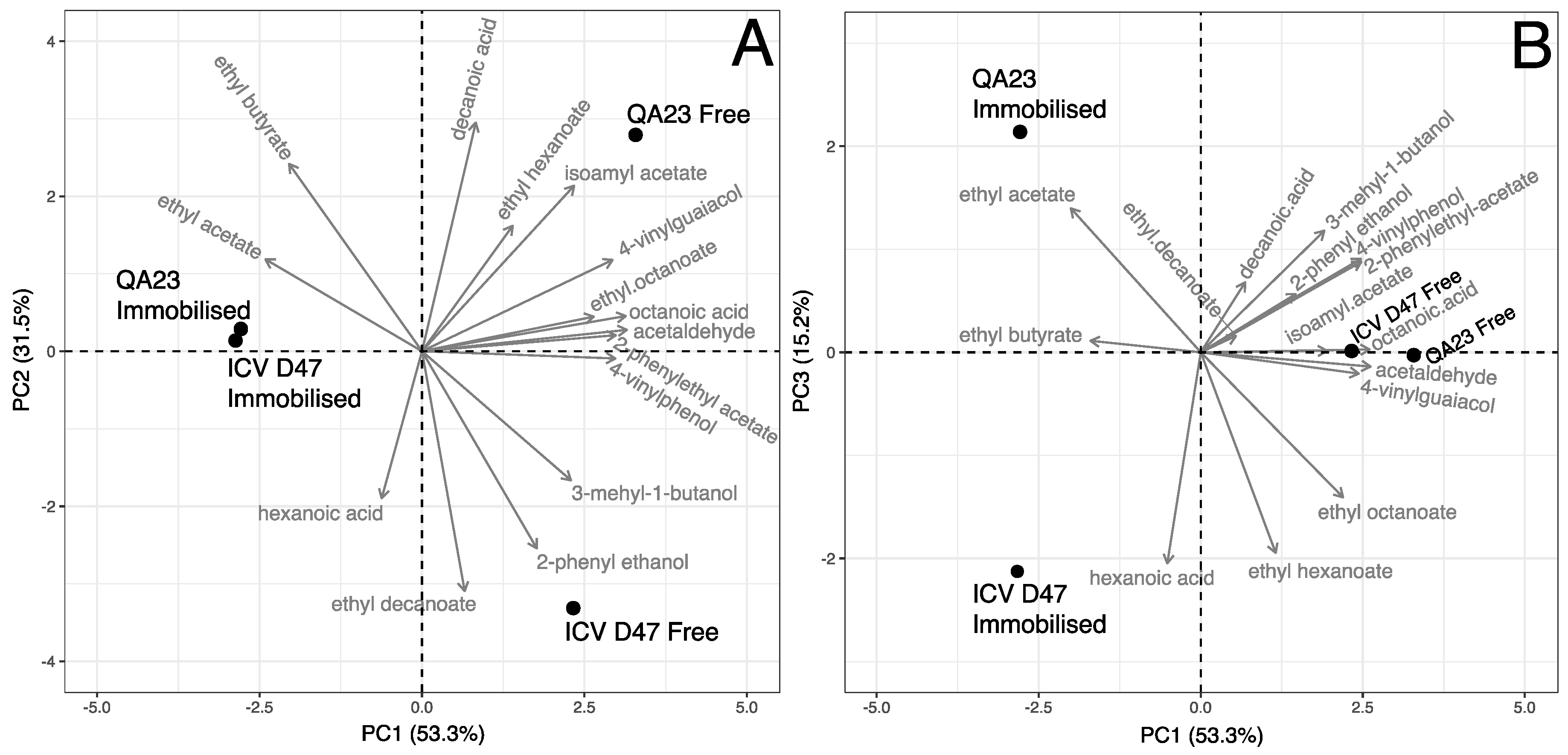
3.2. Mead Volatile Compounds
3.3. Odour Activity Values
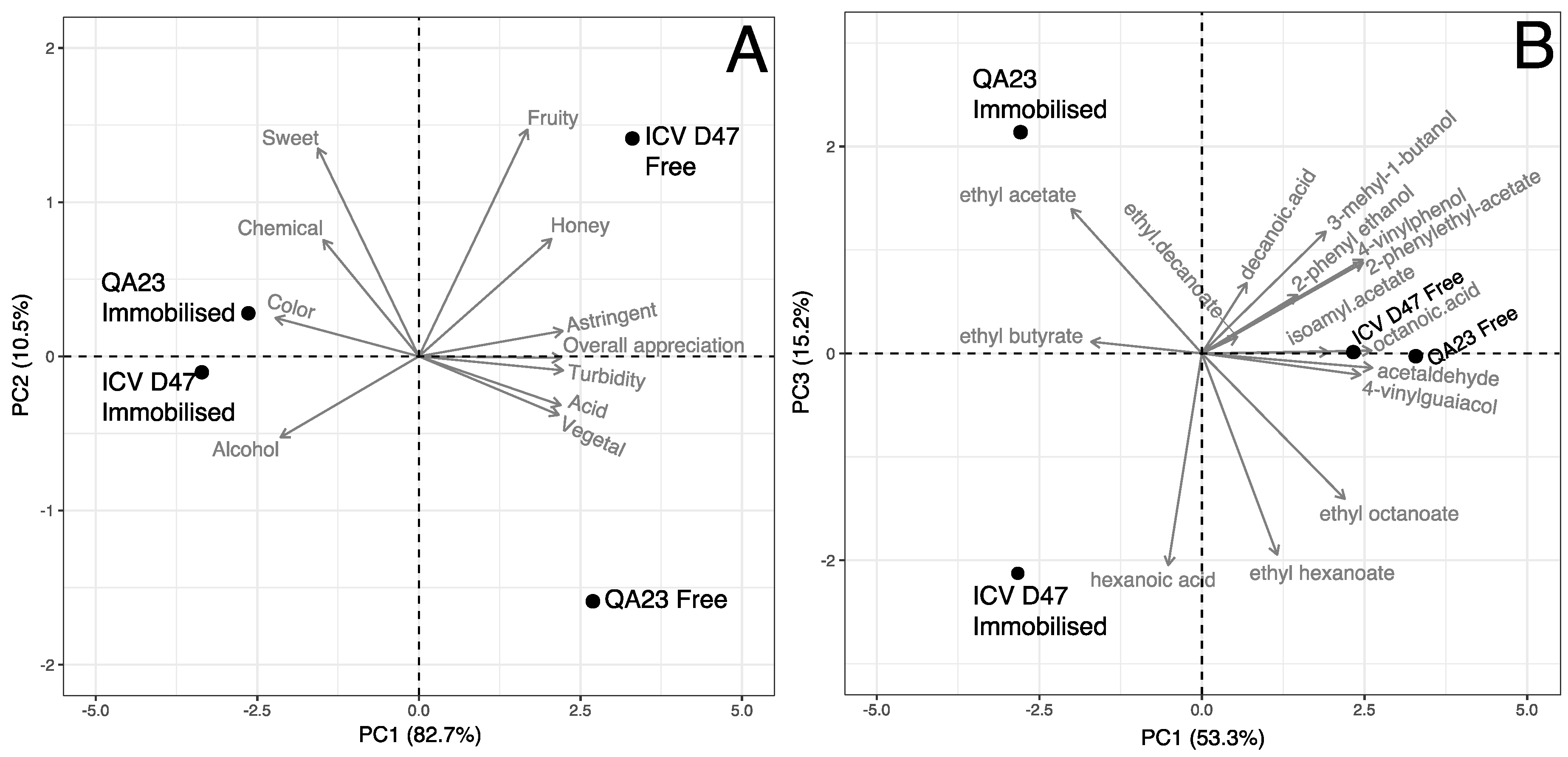
3.4. Mead Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pascoal, A.; Anjos, O.; Feás, X.; Oliveira, J.M.; Estevinho, L.M. Impact of fining agents on the volatile composition of sparkling mead. J. Inst. Brew. 2018, 125, 125–133. [Google Scholar] [CrossRef]
- Mendes-Ferreira, A.; Barbosa, C.; Lage, P.; Mendes-Faia, A. The impact of nitrogen on yeast fermentation and wine quality. Cienc. Tecn. Vitivinic. 2011, 26, 17–32. [Google Scholar]
- Robinson, A.L.; Boss, P.K.; Heymann, H.; Soloman, P.S.; Trengove, R.D. Influence of yeast strain, canopy management, and site on the volatile composition and sensory attributes of Cabernet Sauvignon wines from Western Australia. J. Agric. Food Chem. 2011, 59, 3273–3284. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Vilanova, M.; Oliveira, J.M. Application of Gas Chromatography on the Evaluation of Grape and Wine Aroma in Atlantic Viticulture (NW Iberian Peninsula). In Gas Chromatography in Plant Science, Wine technology, Toxicology and Some Specific Applications; Salih, B., Çelikbiçak, O., Eds.; Intech Open: London, UK, 2012; pp. 109–146. [Google Scholar]
- Chen, C.-H.; Wu, Y.-L.; Lo, D.; Wu, M.-C. Physicochemical property changes during the fermentation of longan (Dimocarpus longan) mead and its aroma composition using multiple yeast inoculations. J. Inst. Brew. 2013, 119, 303–308. [Google Scholar] [CrossRef]
- Gupta, J.K.; Sharma, R. Production technology and quality characteristics of mead and fruit-honey wines: A review. Nat. Prod. Radiance 2009, 8, 345–355. [Google Scholar]
- Andreu-Sevilla, A.J.; Mena, P.; Martí, N.; Viguera, C.G.; Carbonell-Barrachina, A.A. Volatile composition and descriptive sensory analysis of pomegranate juice and wine. Food Res. Int. 2013, 54, 246–254. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Rudnitskaya, A.; Saliba, A.J.; Blackman, J.W.; Scollary, G.R.; Clarck, A.C.; Rutledge, D.N.; Delgadillo, I.; Legin, A. Sensory, chemical, and electronic tongue assessment of micro-oxygenated wines and oak chip maceration: Assessing the commonality of analytical techniques. J. Agric. Food Chem. 2010, 58, 5026–5033. [Google Scholar] [CrossRef] [PubMed]
- Smyth, H.; Cozzolino, D. Instrumental methods (spectroscopy, electronic nose, and tongue) as tools to predict taste and aroma in beverages: Advantages and limitations. Chem. Rev. 2013, 113, 1429–1440. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef]
- Gomes, T.; Barradas, C.; Dias, T.; Verdial, J.; Morais, J.S.; Ramalhosa, E.; Estevinho, L.M. Optimization of mead production using Response Surface Methodology. Food Chem. Toxicol. 2013, 59, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Ferreira, A.; Cosme, F.; Barbosa, C.; Falco, V.; Inês, A.; Mendes-Faia, A. Optimization of honey-must preparation and alcoholic fermentation by Saccharomyces cerevisiae for mead production. Int. J. Food Microbiol. 2010, 144, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Navrátil, M.; Šturdík, E.; Gemeiner, P. Batch and continuous mead production with pectate immobilised, ethanol-tolerant yeast. Biotechnol. Lett. 2001, 23, 977–982. [Google Scholar] [CrossRef]
- Pereira, A.P.; Dias, T.; Andrade, J.; Ramalhosa, E.; Estevinho, L.M. Mead production: Selection and characterization assays of Saccharomyces cerevisiae strains. Food Chem. Toxicol. 2009, 47, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Mendes-Ferreira, A.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. High-cell-density fermentation of Saccharomyces cerevisiae for the optimisation of mead production. Food Microbiol. 2013, 33, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Mendes-Ferreira, A.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. Effect of Saccharomyces cerevisiae cells immobilisation on mead production. LWT-Food Sci. Technol. 2014, 56, 21–30. [Google Scholar] [CrossRef]
- Roldán, A.; van Muiswinkel, G.C.J.; Lasanta, C.; Palacios, V.; Caro, I. Influence of pollen addition on mead elaboration: Physicochemical and sensory characteristics. Food Chem. 2011, 126, 574–582. [Google Scholar] [CrossRef]
- Kahoun, D.; Řezková, S.; Královský, J. Effect of heat treatment and storage conditions on mead composition. Food Chem. 2017, 219, 357–363. [Google Scholar] [CrossRef]
- Czabaj, S.; Kawa-Rygielska, J.; Kucharska, A.Z.; Kliks, J. Effects of mead wort heat treatment on the mead fermentation process and antioxidant activity. Molecules 2017, 22, 803. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Szatkowska, K. Fruit and herbal meads–chemical composition and antioxidant properties. Food Chem. 2019, 283, 19–27. [Google Scholar] [CrossRef]
- Šmogrovičová, D.; Nádaský, P.; Tandlich, R.; Wilhelmi, B.S.; Cambray, G. Analytical and aroma profiles of Slovak and South African meads. Czech J. Food Sci. 2012, 30, 241–246. [Google Scholar] [CrossRef]
- Sroka, P.; Tuszyński, T. Changes in organic acid contents during mead wort fermentation. Food Chem. 2007, 104, 1250–1257. [Google Scholar] [CrossRef]
- Ukpabi, U.J. Quality evaluation of meads produced with Cassava (Manihot esculenta) floral honey under farm conditions in Nigeria. Trop. Subtrop. Agroecosyst. 2006, 6, 37–41. [Google Scholar]
- OIV—The International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2015; Volume 1, OIV-MA-AS1-03. [Google Scholar]
- Aerny, J. Composés azotés des moûts et des vins. Rev. Suisse Vitic. Arboric. Hortic. 1996, 28, 161–165. [Google Scholar]
- Genisheva, Z.; Vilanova, M.; Mussatto, S.I.; Teixeira, J.A.; Oliveira, J.M. Consecutive alcoholic fermentations of white grape musts with yeasts immobilized on grape skins-effect of biocatalyst storage and SO2 concentration on wine characteristic. LWT-Food Sci. Technol. 2014, 59, 1114–1122. [Google Scholar] [CrossRef]
- Boidron, J.N.; Chatonnet, P.; Pons, M. Influence du bois sur certaines substances odorantes des vins. Connaiss. J. Int. Sci. Vigne Vin 1988, 22, 275–294. [Google Scholar] [CrossRef]
- Escudero, A.; Gogorza, B.; Melús, M.A.; Ortín, N.; Cacho, J.; Ferreira, V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. J. Agric. Food Chem. 2004, 52, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Guth, H. Quantification and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Moreno, J.A.; Zea, L.; Moyano, L.; Medina, M. Aroma compounds as markers of the changes in sherry wines subjected to biological ageing. Food Control 2005, 16, 333–338. [Google Scholar] [CrossRef]
- Oliveira, M.E.S.; Pantoja, L.; Duarte, W.F.; Collela, C.F.; Valarelli, L.T.; Schwan, R.F.; Dias, D.R. Fruit wine produced from cagaita (Eugenia dysenterica DC) by both free and immobilised yeast cell fermentation. Food Res. Int. 2011, 44, 2391–2400. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.5.999. 2017. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 10 February 2019).
- Ugliano, M.; Henschke, P.A. Yeasts and wine flavour. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer Science + Business Media: New York, NY, USA, 2009; pp. 313–392. [Google Scholar]
- Bartowsky, E.J.; Pretorius, I.S. Microbial formation and modification of flavor and off-flavor compounds in wine. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 209–231. [Google Scholar]
- Genisheva, Z.; Macedo, S.; Mussatto, S.I.; Teixeira, J.A.; Oliveira, J.M. Production of white wine by Saccharomyces cerevisiae immobilized on grape pomace. J. Inst. Brew. 2012, 118, 163–173. [Google Scholar] [CrossRef]
- Genisheva, Z. Development of an integrated process for continuous winemaking. Ph.D. Thesis, University of Minho, Braga, Portugal, 2013. [Google Scholar]
- Boulton, B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Chapman and Hall: New York, NY, USA, 1996. [Google Scholar]
- Tomás-Barberán, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Baumes, R. Wine aroma precursors. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer Science + Business Media: New York, NY, USA, 2009; pp. 251–274. [Google Scholar]

| Parameters | QA23–F | QA 23–I | ICV D47–F | ICV D47–I | Significance | ||
|---|---|---|---|---|---|---|---|
| Strain | Condition | S × C | |||||
| pH | 3.48 ± 0.01 | 3.53 ± 0.01 | 3.46 ± 0.02 | 3.47 ± 0.01 | 0.021 | 0.047 | ns |
| VA (g·L−1) | 0.57 ± 0.04 | 0.69 ± 0.04 | 0.51 ± 0.04 | 0.54 ± 0.00 | 0.016 | 0.045 | ns |
| TA (g·L−1) | 5.79 ± 0.03 | 5.38 ± 0.19 | 5.87 ± 0.29 | 5.53 ± 0.29 | ns | ns | ns |
| YAN (mg·L−1) | 31.50 ± 4.95 | 29.75 ± 2.47 | 33.25 ± 2.47 | 36.75 ± 2.47 | ns | ns | ns |
| CSO2 (mg·L−1) | 29.44 ± 1.81 | 24.32 ± 1.81 | 26.88 ± 1.81 | 25.60 ± 0.00 | ns | 0.045 | ns |
| ASv (%) | 11.38 ± 0.18 | 11.13 ± 0.18 | 11.13 ± 0.18 | 11.00 ± 0.35 | ns | ns | ns |
| CRS (g·L−1) | 24.31 ± 5.88 | 24.66 ± 0.98 | 25.70 ± 3.43 | 21.71 ± 0.49 | ns | ns | ns |
| Parameters | QA23–F | QA23–I | ICV D47–F | ICV D47–I | Significance | ||
|---|---|---|---|---|---|---|---|
| Strain | Condition | S × C | |||||
| Glucose (g·L−1) | 1.78 ± 0.53 | 1.72 ± 0.03 | 1.84 ± 0.23 | 1.69 ± 0.07 | ns | ns | ns |
| Fructose (g·L−1) | 2.72 ± 0.06 | 2.66 ± 0.16 | 3.67 ± 0.14 | 3.05 ± 0.14 | 0.002 | 0.021 | 0.040 |
| Glycerol (g·L−1) | 5.23 ± 0.19 | 5.14 ± 0.08 | 5.07 ± 0.21 | 4.43 ± 0.25 | 0.032 | ns | ns |
| Acetic acid (g·L−1) | 0.30 ± 0.02 | 0.39 ± 0.03 | 0.21 ± 0.01 | 0.29 ± 0.01 | 0.001 | 0.002 | ns |
| Ethanol (%) | 9.63 ± 0.05 | 10.12 ± 0.06 | 10.36 ± 0.15 | 9.54 ± 0.78 | ns | ns | ns |
| Compounds | QA23–F | QA23–I | ICV D47–F | ICV D47–I | Significance | ||
|---|---|---|---|---|---|---|---|
| Strain | Condition | S × C | |||||
| Alcohols (mg L−1) | |||||||
| methanol | 3.25 ± 0.66 | 4.82 ± 1.01 | 1.09 ± 0.24 | 4.01 ± 0.24 | 0.028 | 0.007 | ns |
| 1-propanol | 32.28 ± 6.04 | 41.87 ± 1.14 | 40.15 ± 8.98 | 65.37 ± 18.23 | ns | ns | ns |
| 2-methyl-1-propanol | 15.73 ± 2.04 | 14.74 ± 0.69 | 18.27 ± 1.05 | 10.77 ± 0.13 | ns | 0.007 | 0.018 |
| 2-methyl-1-butanol | 13.36 ± 0.47 | 11.04 ± 1.79 | 16.61 ± 0.32 | 8.79 ± 0.35 | ns | 0.002 | 0.015 |
| 3-methyl-1-butanol | 104.72 ± 3.49 | 99.65 ± 14.05 | 125.18 ± 9.75 | 78.79 ± 6.16 | ns | 0.017 | 0.034 |
| 3-ethoxy-1-propanol | 0.18 ± 0.01 | 0.16 ± 0.01 | 0.02 ± 0.01 | 0.009 ± 0.004 | 0.000 | ns | ns |
| 3-(methylthio)-1-propanol | 0.0136 ± 0.0003 | 0.010 ± 0.004 | 0.04 ± 0.01 | 0.005 ± 0.002 | ns | 0.008 | 0.018 |
| 2-phenylethanol | 24.59 ± 5.19 | 24.78 ± 6.48 | 35.50 ± 3.29 | 21.64 ± 5.68 | ns | ns | ns |
| Total | 194.12 ± 8.96 | 197.07 ± 15.67 | 236.85 ± 13.71 | 189.39 ±20.07 | |||
| Esters (mg L−1) | |||||||
| ethyl acetate | 34.28 ± 0.81 | 50.07 ± 11.83 | 28.02 ± 1.61 | 38.03 ± 2.94 | ns | 0.041 | ns |
| ethyl butyrate | 0.15 ± 0.04 | 0.167 ± 0.003 | 0.08 ± 0.01 | 0.16 ± 0.06 | ns | ns | ns |
| isoamyl acetate | 1.49 ± 0.16 | 1.13 ± 0.33 | 1.16 ± 0.40 | 1.12 ± 0.27 | ns | ns | ns |
| ethyl hexanoate | 0.47 ± 0.05 | 0.31 ± 0.10 | 0.37 ± 0.13 | 0.44 ± 0.08 | ns | ns | ns |
| ethyl lactate | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.03 | 0.04 ± 0.01 | ns | ns | ns |
| ethyl octanoate | 0.92 ± 0.21 | 0.45 ± 0.01 | 0.80 ± 0.08 | 0.71 ± 0.23 | ns | ns | ns |
| ethyl decanoate | nd | 0.24 ± 0.02 | 0.82 ± 0.25 | 0.19 ± 0.01 | 0.013 | ns | 0.009 |
| ethyl phenylacetate | 0.017 ± 0.005 | 0.007 ± 0.001 | 0.013 ± 0.001 | 0.0033 ± 0.0003 | ns | 0.005 | ns |
| 2-phenylethyl acetate | 0.74 ± 0.22 | 0.51 ± 0.09 | 0.67 ± 0.13 | 0.37 ± 0.06 | ns | ns | ns |
| ethyl dodecanoate | 0.08 ± 0.03 | 0.007 ± 0.002 | 0.12 ± 0.07 | 0.007 ± 0.004 | ns | 0.030 | ns |
| Total | 38.21 ± 1.20 | 52.94 ± 11.83 | 32.14 ± 1.69 | 41.07 ± 2.97 | |||
| Volatile phenols (μg·L−1) | |||||||
| 4-vinylguaiacol | 128.11 ± 27.76 | 53.15 ± 3.72 | 87.76 ± 9.67 | 59.03 ± 4.80 | ns | 0.008 | ns |
| 4-vinylphenol | 183.67 ± 28.65 | 157.34 ± 8.41 | 179.66 ± 5.62 | 139.85 ± 16.90 | ns | ns | ns |
| Total | 311.78 ± 39.89 | 210.48 ± 9.20 | 267.42 ± 11.18 | 198.89 ± 17.57 | |||
| Volatile fatty acids (μg L−1) | |||||||
| isobutyric acid | 25.80 ± 0.71 | 19.41 ± 0.43 | 40.12 ± 19.15 | 17.06 ± 4.90 | ns | ns | ns |
| butanoic acid | 20.89 ± 2.68 | 15.07 ± 3.03 | 29.13 ± 11.76 | 15.52 ± 5.44 | ns | ns | ns |
| hexanoic acid | 714.12 ± 95.56 | 713.94 ± 14.99 | 757.47 ± 98.22 | 769.58 ± 296.92 | ns | ns | ns |
| octanoic acid | 3224.03 ± 282.58 | 2825.68 ± 293.58 | 3094.58 ± 758.90 | 2817.21 ± 335.32 | ns | ns | ns |
| decanoic acid | 1263.80 ± 71.73 | 1178.30 ± 178.95 | 1081.01 ± 354.72 | 1126.96 ± 204.77 | ns | ns | ns |
| dodecanoic acid | 3.48 ± 1.80 | 10.69 ± 1.26 | 2.55 ± 0.90 | 8.72 ± 1.69 | ns | 0.003 | ns |
| Total | 5252.12 ± 306.82 | 4763.09 ± 344.16 | 5004.86 ± 843.74 | 4755.06 ± 492.53 | |||
| Carbonyl compounds (mg L−1) | |||||||
| Acetaldehyde | 15.80 ± 2.25 | 3.63 ± 0.28 | 12.72 ± 2.33 | 4.32 ± 0.53 | ns | 0.001 | ns |
| Compounds | Odour Descriptor a | Odour Threshold (μg·L−1) | QA23-F | QA23-I | ICV D47-F | ICV D47-I |
|---|---|---|---|---|---|---|
| 3-methyl-1-butanol | cheese; nail polish | 30,000 | 3.5 | 3.3 | 4.2 | 2.6 |
| 2-phenyl-ethanol | roses; flowery | 14,000 | 1.8 | 1.8 | 2.5 | 1.5 |
| ethyl acetate | solvent; nail polish | 12,300 | 2.8 | 4.1 | 2.3 | 3.1 |
| ethyl butyrate | fruity; sweet | 20 | 7.4 | 8.3 | 4.2 | 8.1 |
| isoamyl acetate | banana | 30 | 49.7 | 37.5 | 38.6 | 37.3 |
| ethyl hexanoate | fruity; aniseed | 14 | 33.6 | 22.4 | 26.7 | 31.3 |
| ethyl octanoate | fruity; sweet | 5 | 183.5 | 89.9 | 159.7 | 141.3 |
| ethyl decanoate | pleasant; soap | 200 | nd | 1.2 | 4.1 | < 1 |
| 2-phenylethyl acetate | flowery; roses | 250 | 3.0 | 2.0 | 2.7 | 1.5 |
| 4-vinylguaicol | clove | 130 | 1.0 | < 1 | < 1 | < 1 |
| 4-vinylphenol | almond shell | 180 | 1.0 | < 1 | 1.0 | < 1 |
| hexanoic acid | cheese; sweaty | 420 | 1.7 | 1.7 | 1.8 | 1.8 |
| octanoic acid | fatty; rancid | 500 | 6.4 | 5.7 | 6.2 | 5.6 |
| decanoic acid | fatty; soap | 1000 | 1.3 | 1.2 | 1.1 | 1.1 |
| Acetaldehyde | fresh; green | 500 | 31.6 | 7.3 | 25.4 | 8.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.P.; Mendes-Ferreira, A.; Dias, L.G.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. Volatile Composition and Sensory Properties of Mead. Microorganisms 2019, 7, 404. https://doi.org/10.3390/microorganisms7100404
Pereira AP, Mendes-Ferreira A, Dias LG, Oliveira JM, Estevinho LM, Mendes-Faia A. Volatile Composition and Sensory Properties of Mead. Microorganisms. 2019; 7(10):404. https://doi.org/10.3390/microorganisms7100404
Chicago/Turabian StylePereira, Ana Paula, Ana Mendes-Ferreira, Luís G. Dias, José M. Oliveira, Leticia M. Estevinho, and Arlete Mendes-Faia. 2019. "Volatile Composition and Sensory Properties of Mead" Microorganisms 7, no. 10: 404. https://doi.org/10.3390/microorganisms7100404
APA StylePereira, A. P., Mendes-Ferreira, A., Dias, L. G., Oliveira, J. M., Estevinho, L. M., & Mendes-Faia, A. (2019). Volatile Composition and Sensory Properties of Mead. Microorganisms, 7(10), 404. https://doi.org/10.3390/microorganisms7100404







