Genetic Modification of mfsT Gene Stimulating the Putative Penicillin Production in Monascus ruber M7 and Exhibiting the Sensitivity towards Precursor Amino Acids of Penicillin Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. DNA Extraction
2.3. mfsT Gene Cloning and Computational Analysis
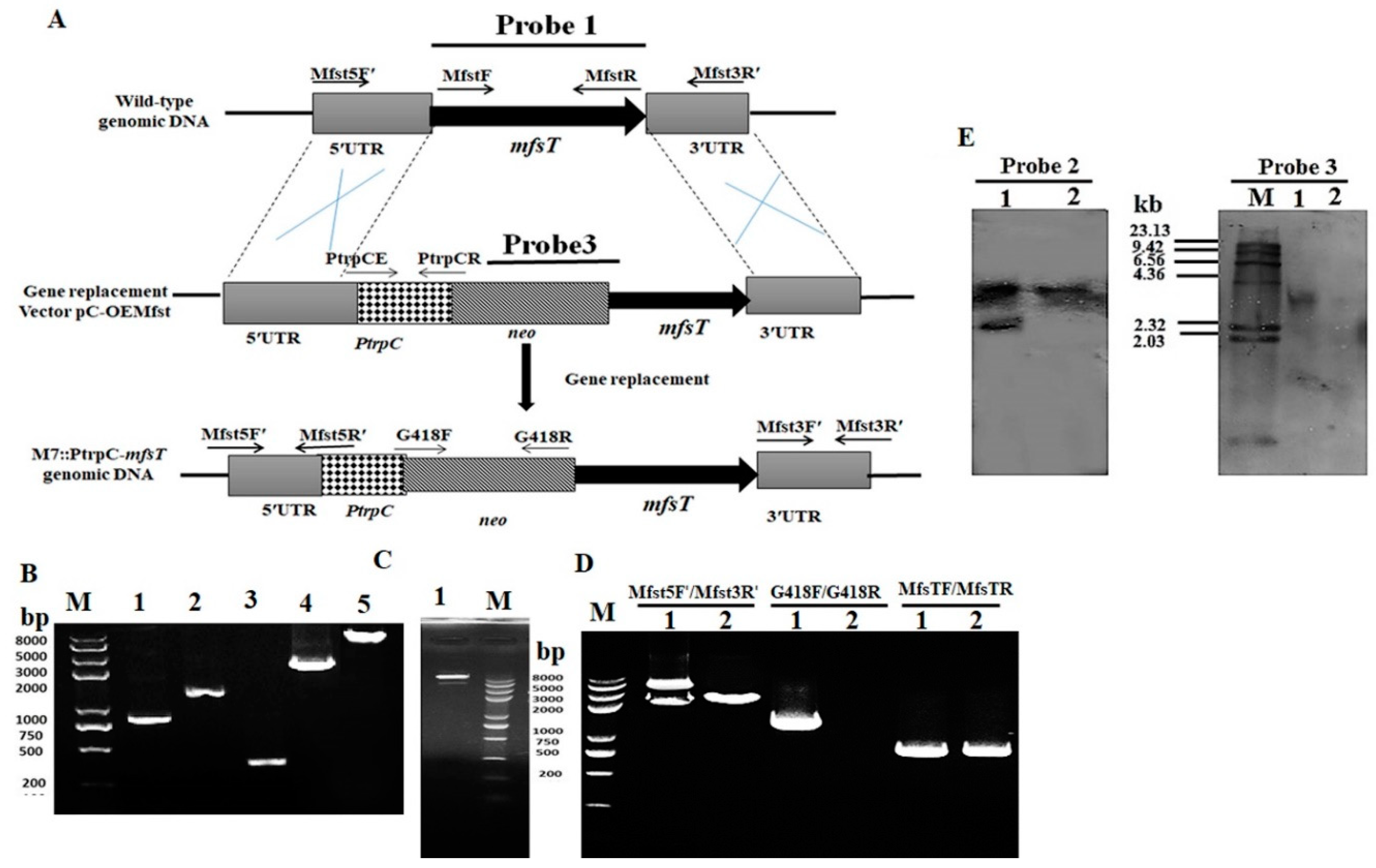
2.4. Construction of the mfsT Gene Deletion, Complementation, and Overexpression Strains
2.5. Southern Hybridization Analysis
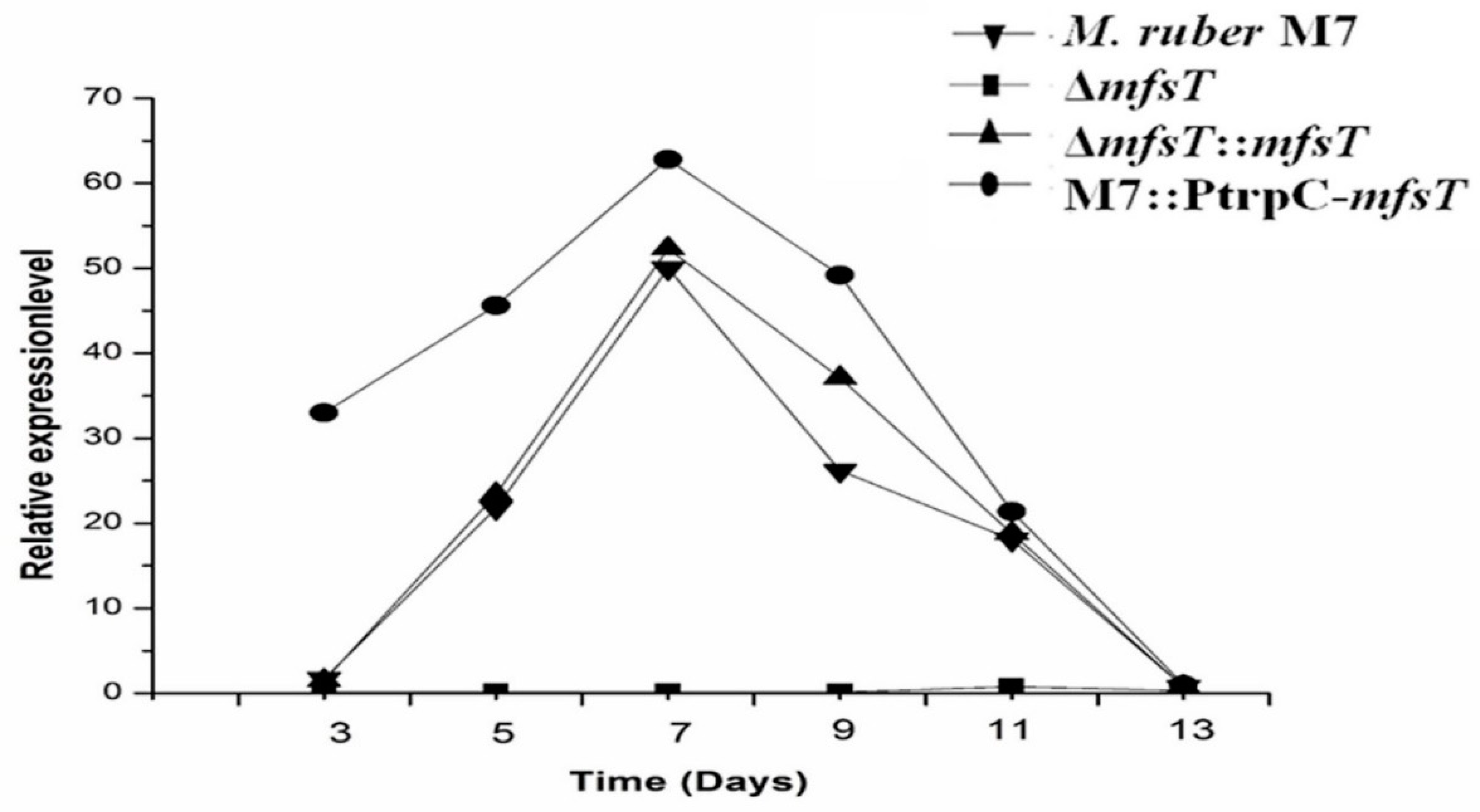
2.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
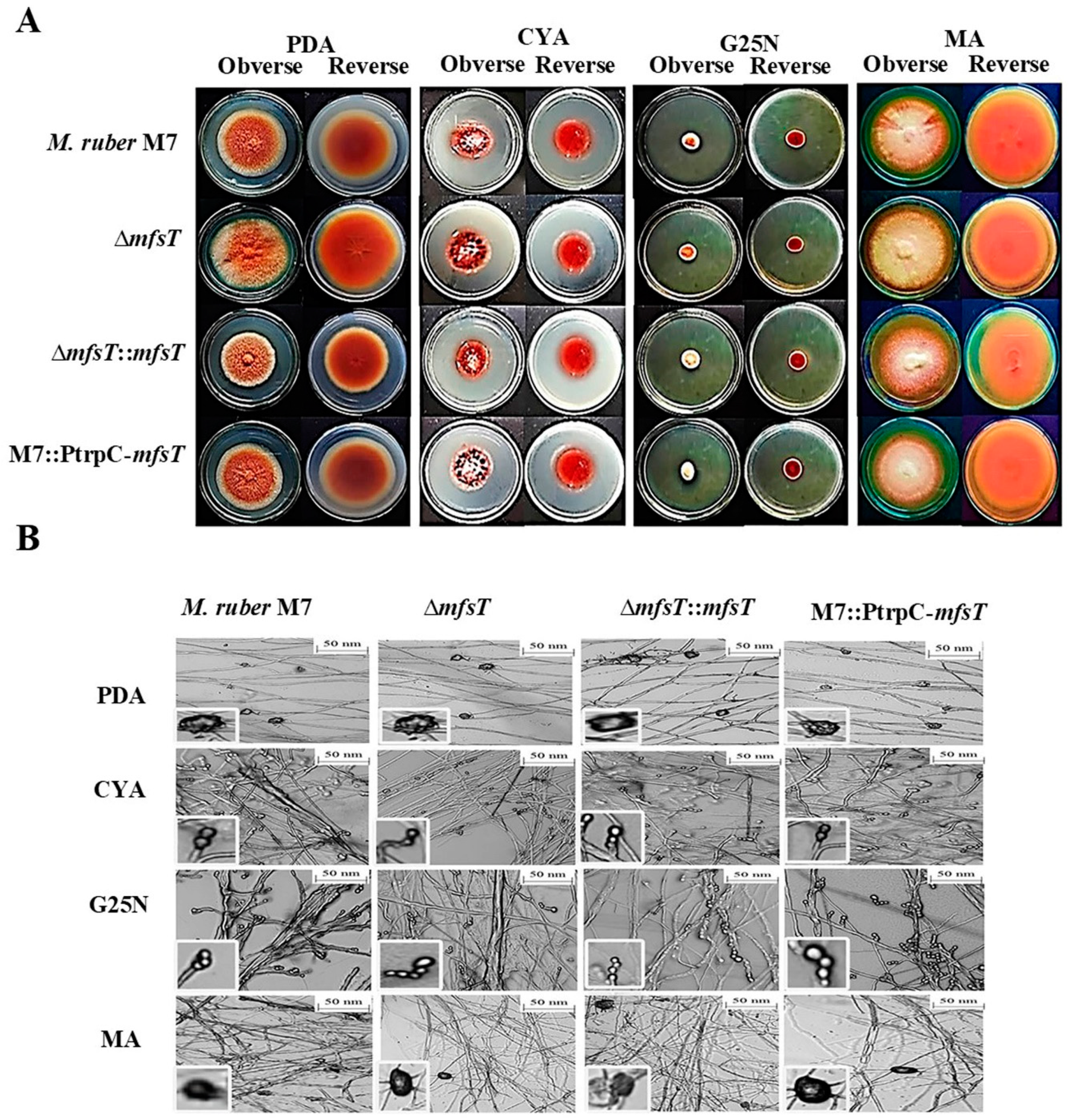
2.7. Phenotypic Characterization
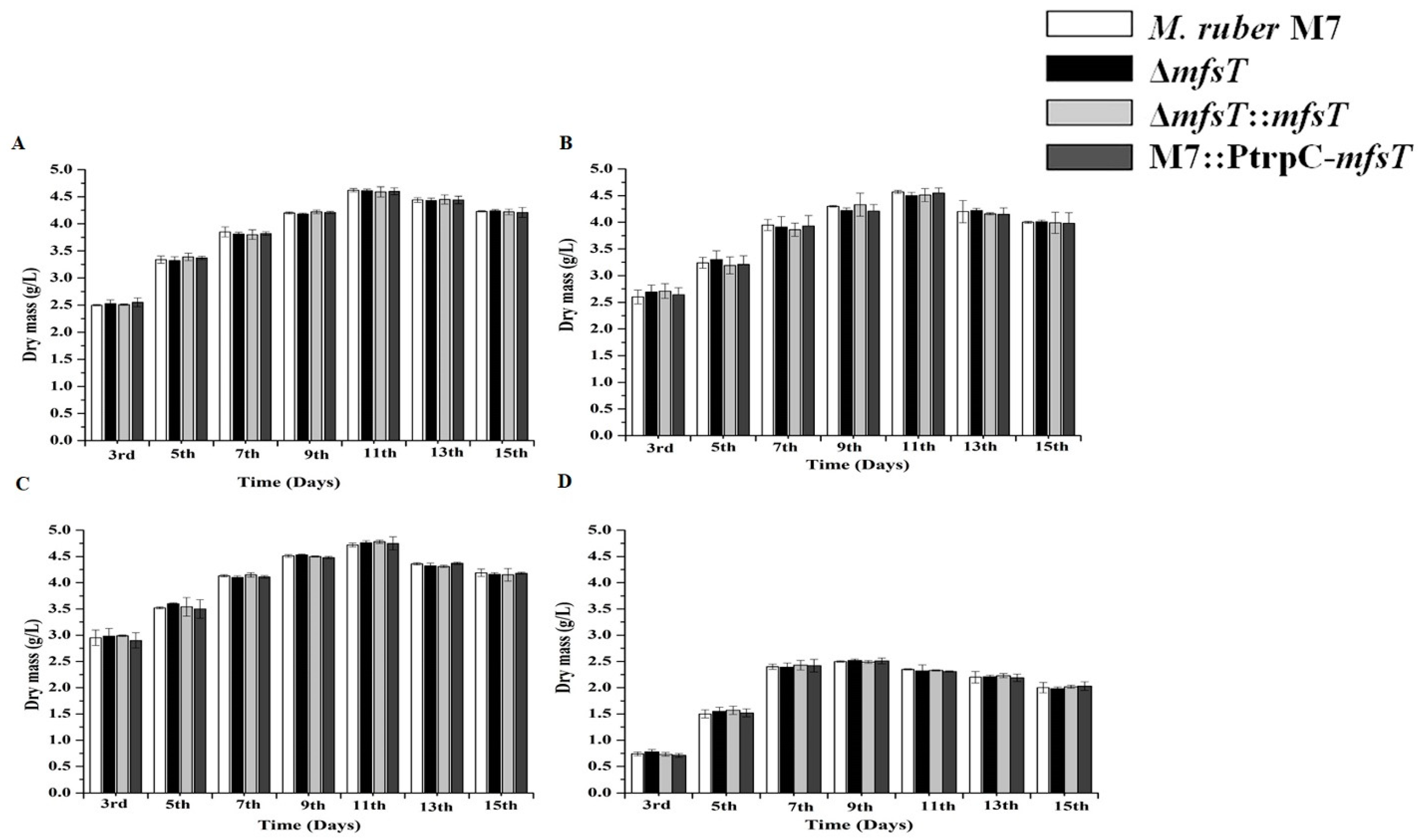
2.8. Biomass Estimation
2.9. Extraction and Measurement of the PG Contents
2.9.1. PG Extraction
2.9.2. PG Detected by HPLC
2.9.3. PG and IPN Verified by UPLC-MS/MS
2.10. Feeding of Precursor Amino Acids
2.11. Statistical Analyses
3. Results
3.1. mfsT Gene Sequence Analysis in M. ruber M7
3.2. Genetic Engineering of mfsT Gene
3.3. Real-Time PCR Analysis of ΔmfsT, ΔmfsT::mfsT and M7::PtrpC-mfsT
3.4. Phenotypic Characterization of ΔmfsT, ΔmfsT::mfsT and M7::PtrpC-mfsT
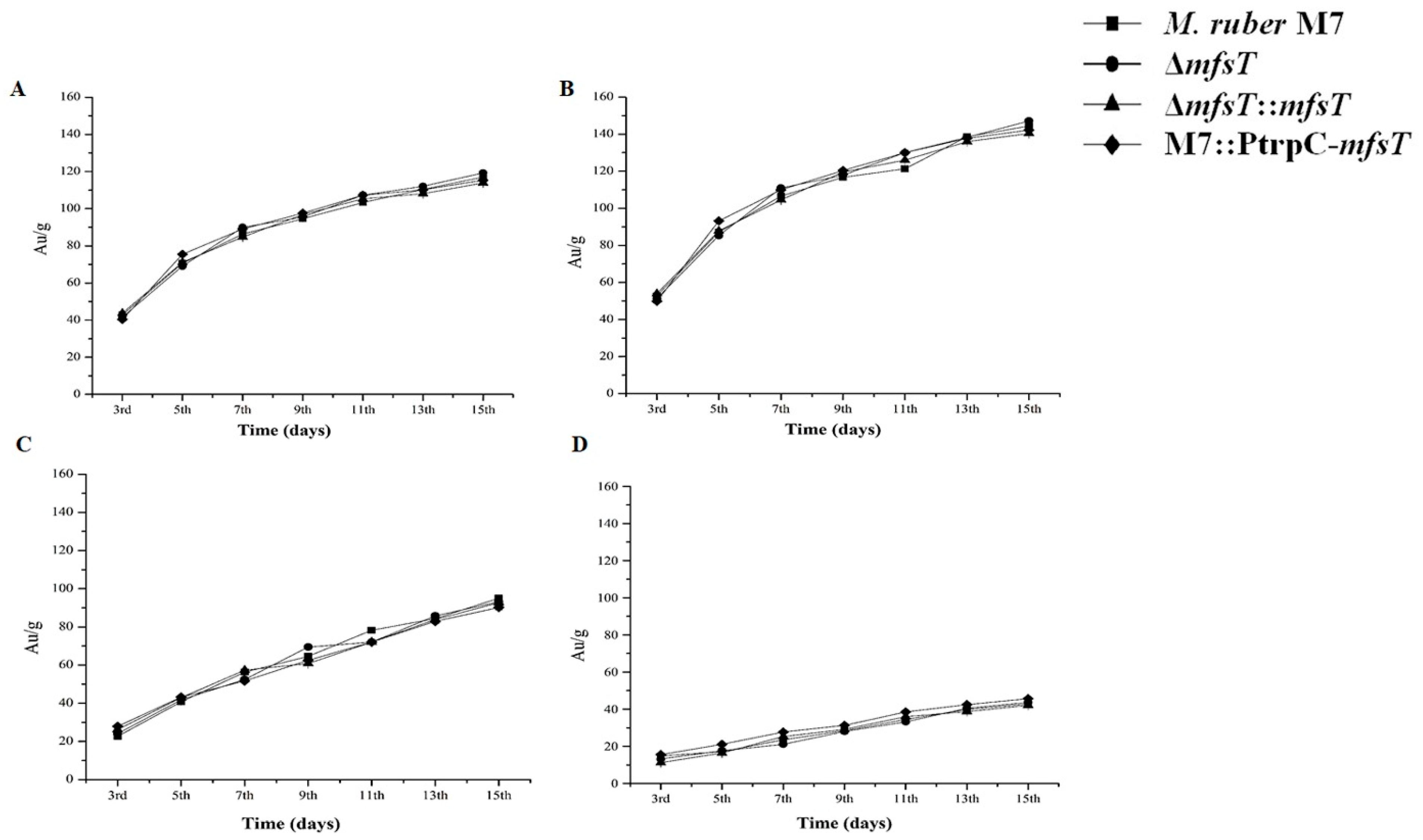
3.5. Biomass
3.6. Analyses of MP Production ΔmfsT, ΔmfsT::mfsT and M7::PtrpC-mfsT
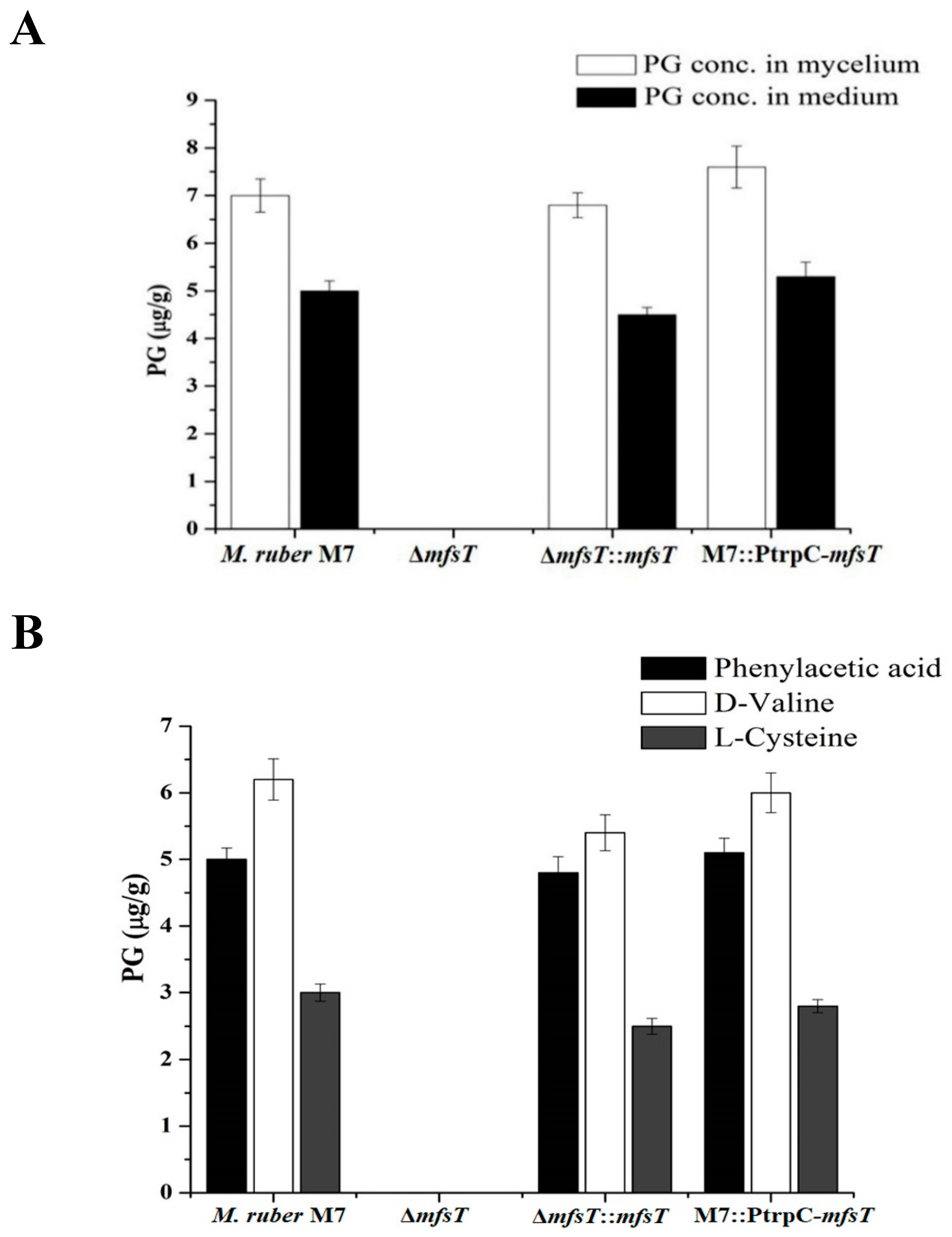
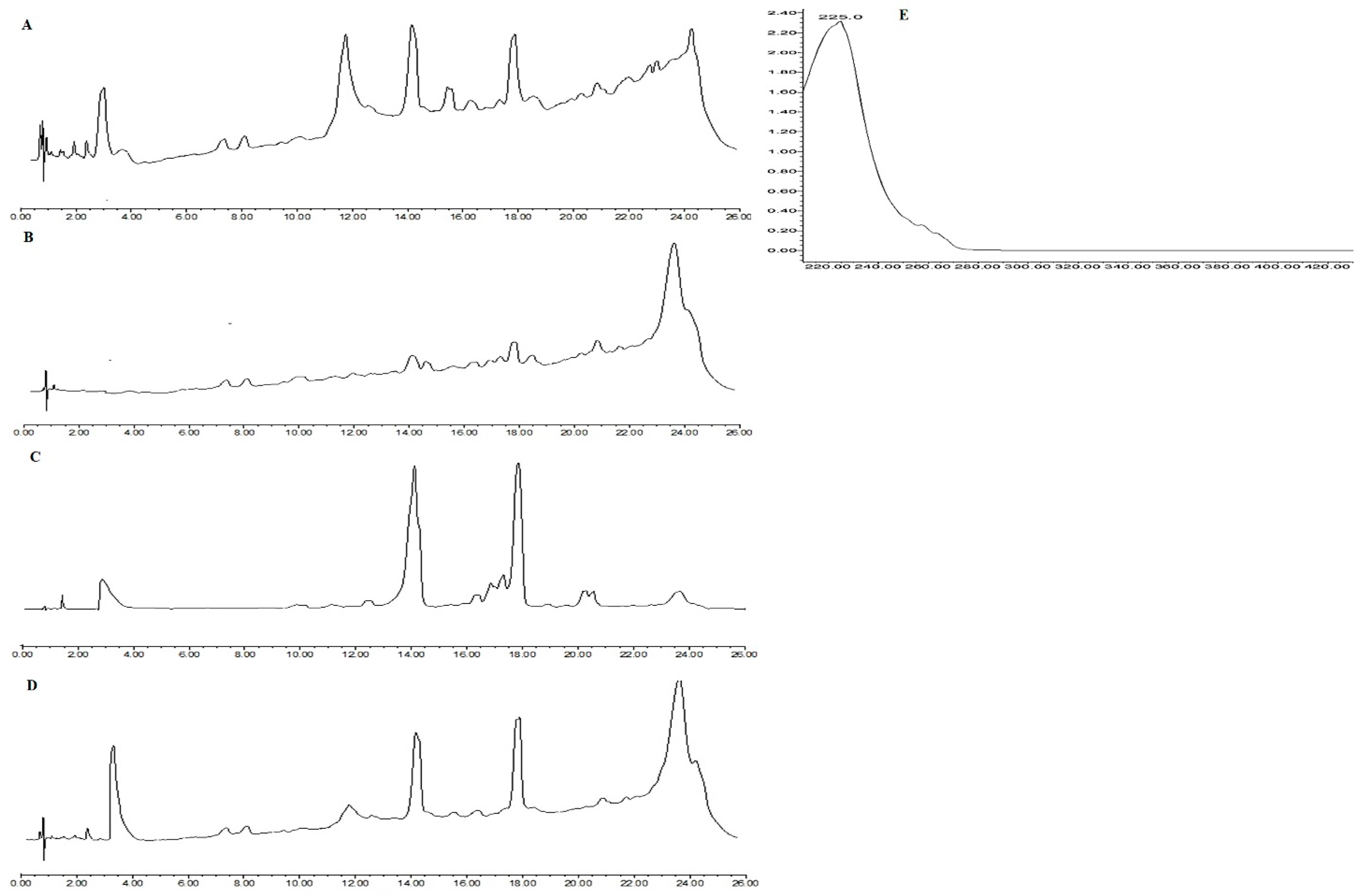
3.7. Detection and Production of PG by HPLC and UPLC
3.8. Effect of Feeding of Precursor Amino Acids
3.9. Detection of Beta Lactam Metabolites by UPLC-MS Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shi, Y.C.; Pan, T.M. Beneficial effects of Monascus purpureus NTU 568-fermented products: A review. Appl. Microbiol. Biotechnol. 2011, 90, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Kiyota, A.; Yasukawa, K.; Sakamoto, N.; Kimura, Y.; Suzuki, T.; Takayasu, J.; Nishino, H. Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem. Biodivers. 2005, 2, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jung, H.; Kim, Y.O.; Shin, C.S. Antimicrobial activities of amino acid derivatives of monascus pigments. FEMS Microbiol. Lett. 2006, 264, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Pan, T.M. Benefit of Monascus-fermented products for hypertension prevention: A review. Appl. Microbiol. Biotechnol. 2012, 94, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Pan, T.M. Development of Monascus fermentation technology for high hypolipidemic effect. Appl. Microbiol. Biotechnol. 2012, 264, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Li, H.; Hong, J.; Cho, H.Y.; Bae, K.S.; Kim, M.A.; Kim, D.K.; Jung, J.H. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch. Pharm. Res. 2010, 33, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shao, Y.; Zhou, Y.; Chen, W.; Chen, F. Monascus Pigments. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Vandamme, E.J., Revuelta, J.L., Eds.; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Dhakal, R.; Bajpai, V.K.; Baek, K.H. Production of GABA (γ-aminobutyric acid) by microorganisms: A review. Brazilian J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef]
- Kistler, H.C.; Broz, K. Cellular compartmentalization of secondary metabolism. Front. Microbiol. 2015, 6, 68. [Google Scholar] [CrossRef]
- Wang, W.; Gao, M.; Shao, Z.; Li, F.; Zhang, B.; Ke, W.; Liao, Y. Citrinin Monomer and Dimer Derivatives with Antibacterial and Cytotoxic Activities Isolated from the Deep Sea-Derived Fungus Penicillium citrinum NLG-S01-P1. Mar. Drugs 2019, 17, 46. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.W.G.; Islam, M.T.; Ali, E.S.; Uddin, S.J.; de Oliveira Santos, J.V.; de Alencar, M.V.O.B.; Júnior, A.L.G.; Paz, M.F.C.J.; de Brito, M.D.R.M.; e Sousa, J.M.D.C.; et al. A comprehensive review on biological properties of citrinin. Food Chem. Toxicol. 2017, 110, 130–141. [Google Scholar] [CrossRef]
- Ozcengiz, G.; Demain, A.L. Recent advances in the biosynthesis of penicillins, cephalosporins and clavams and its regulation. Biotechnol. Adv. 2013, 31, 287–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Insights Into Biological Characteristics Of Monascus Ruber M7 by Genomics Approaches. Doctor Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Wei, W.; Lin, S.; Chen, M.; Liu, T.; Wang, A.; Li, J.; Guo, Q.; Shang, X. Monascustin, an unusual γ-lactam from red yeast rice. J. Nat. Prod. 2017, 80, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Lobanovska, M.; Pilla, G. Penicillin’s discovery and antibiotic resistance: Lessons for the future? Yale J. Biol. Med. 2017, 90, 135–145. [Google Scholar] [PubMed]
- Lamas-Maceiras, M.; Vaca, I.; Rodríguez, E.; Casqueiro, J.; Martín, J.F. Amplification and disruption of the phenylacetyl-CoA ligase gene of Penicillium chrysogenum encoding an aryl-capping enzyme that supplies phenylacetic acid to the isopenicillin N-acyltransferase. Biochem. J. 2006, 395, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aguado, M.; Martín, J.F.; Rodríguez-Castro, R.; García-Estrada, C.; Albillos, S.M.; Teijeira, F.; Ullán, R.V. New insights into the isopenicillin N transport in Penicillium chrysogenum. Metab. Eng. 2014, 22, 89–103. [Google Scholar] [CrossRef]
- Barreiro, C.; García-Estrada, C. Proteomics and Penicillium chrysogenum: Unveiling the secrets behind penicillin production. J. Proteomics 2019, 198, 119–131. [Google Scholar] [CrossRef]
- Van Den Berg, M.A.; Albang, R.; Albermann, K.; Badger, J.H.; Daran, J.M.; Driessen, A.J.; Garcia-Estrada, C.; Fedorova, N.D.; Harris, D.M.; Heijne, W.H.M.; et al. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat. Biotechnol. 2008, 26, 1161–1168. [Google Scholar] [CrossRef]
- Ávalos, J.; Díaz-Sánchez, V.; García-Martínez, J.; Castrillo, M.; Ruger-Herreros, M.; Limón, M.C. Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites, Fungal Biology; Springer: Berlin, Germany, 2014. [Google Scholar]
- García-Estrada, C.; Vaca, I.; Fierro, F.; Sjollema, K.; Veenhuis, M.; Martín, J.F. The unprocessed preprotein form IATC103S of the isopenicillin N acyltransferase is transported inside peroxisomes and regulates its self-processing. Fungal Genet. Biol. 2008, 45, 1043–1052. [Google Scholar] [CrossRef][Green Version]
- Evers, M.E.; Trip, H.; van den Berg, M.A.; Bovenberg, R.A.L.; Driessen, A.J.M. Compartmentalization and Transport in β-Lactam Antibiotics Biosynthesis. In Molecular Biotechnolgy of Fungal beta-Lactam Antibiotics and Related Peptide Synthetases; Springer: Berlin, Germany, 2012; Volume 88. [Google Scholar]
- Martín, J.F.; Ullán, R.V.; García-Estrada, C. Regulation and compartmentalization of β-lactam biosynthesis. Microb. Biotechnol. 2010, 3, 285–299. [Google Scholar] [CrossRef]
- García-Estrada, C.; Vaca, I.; Ullán, R.V.; Van Den Berg, M.A.; Bovenberg, R.A.; Martín, J.F. Molecular characterization of a fungal gene paralogue of the penicillin penDE gene of Penicillium chrysogenum. BMC Microbiol. 2009, 9, 104. [Google Scholar] [CrossRef]
- Martín, J.F.; Ullán, R.V.; García-Estrada, C. Role of peroxisomes in the biosynthesis and secretion of β-lactams and other secondary metabolites. J. Ind. Microbiol. Biotechnol. 2012, 39, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Casqueiro, J.; Liras, P. Secretion systems for secondary metabolites: How producer cells send out messages of intercellular communication. Curr. Opin. Microbiol. 2005, 8, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aguado, M.; Ullán, R.V.; Teijeira, F.; Rodríguez-Castro, R.; Martín, J.F. The transport of phenylacetic acid across the peroxisomal membrane is mediated by the PaaT protein in Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 2013, 97, 3073–3084. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sands, Z.A.; Biggin, P.C. A Numbering System for MFS Transporter Proteins. Front. Mol. Biosci. 2016, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, G.; Wolff, N.A. Structure of renal organic anion and cation transporters. Am. J. Physiol. Physiol. 2017, 278, F853–F866. [Google Scholar] [CrossRef]
- Saier, M.H.; Paulsen, I.T. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 2001, 12, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Kaback, H.R. Lessons from lactose permease. Annu. Rev. Biophys. Biomol. Struct. 2005, 35, 67–91. [Google Scholar] [CrossRef]
- Newstead, S.; Drew, D.; Cameron, A.D.; Postis, V.L.G.; Xia, X.; Fowler, P.W.; Ingram, J.C.; Carpenter, E.P.; Sansom, M.S.P.; McPherson, M.J.; et al. Crystal structure of a prokaryotic homologue of the mammalian oligopeptide-proton symporters, PepT1 and PepT2. EMBO J. 2011, 30, 417–426. [Google Scholar] [CrossRef]
- Ullán, R.V.; Godio, R.P.; Teijeira, F.; Vaca, I.; García-Estrada, C.; Feltrer, R.; Kosalkova, K.; Martín, J.F. RNA-silencing in Penicillium chrysogenum and Acremonium chrysogenum: Validation studies using β-lactam genes expression. J. Microbiol. Methods 2008, 75, 209–218. [Google Scholar] [CrossRef]
- Ullán, R.V.; Teijeira, F.; Guerra, S.M.; Vaca, I.; Martín, J.F. Characterization of a novel peroxisome membrane protein essential for conversion of isopenicillin N into cephalosporin C. Biochem. J. 2010, 432, 227–236. [Google Scholar] [CrossRef]
- Ullán, R.V.; Teijeira, F.; Martín, J.F. Expression of the Acremonium chrysogenum cefT gene in Penicillum chrysogenum indicates that it encodes an hydrophilic β-lactam transporter. Curr. Genet. 2008, 54, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ullán, R.; Liu, G.; Casqueiro, J.; Gutiérrez, S.; Bañuelos, O.; Martín, J. The cefT gene of Acremonium chrysogenum C10 encodes a putative multidrug efflux pump protein that significantly increases cephalosporin C production. Mol. Genet. Genomics 2002, 267, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aguado, M.; Teijeira, F.; Martín, J.F.; Ullán, R.V. A vacuolar membrane protein affects drastically the biosynthesis of the ACV tripeptide and the beta-lactam pathway of Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 2013, 97, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hu, X. Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. Int. J. Food Microbiol. 2005, 103, 331–337. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, Q.; Shao, Y.; Chen, F. ku70 and ku80 null mutants improve the gene targeting frequency in Monascus ruber M7. Appl. Microbiol. Biotechnol. 2013, 97, 4965–4976. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ding, Y.; Zhao, Y.; Yang, S.; Xie, B.; Chen, F. Characteristic analysis of transformants in T-DNA mutation library of Monascus ruber. World J. Microbiol. Biotechnol. 2009, 25, 989–995. [Google Scholar] [CrossRef]
- Chen, W.; Xie, T.; Shao, Y.; Chen, F. Genomic characteristics comparisons of 12 food-related filamentous fungi in tRNA gene set, codon usage and amino acid composition. Gene 2012, 497, 116–124. [Google Scholar] [CrossRef]
- Chen, W.; Xie, T.; Shao, Y.; Chen, F. Phylogenomic Relationships between Amylolytic Enzymes from 85 Strains of Fungi. PLoS ONE 2012, 7, e49679. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Xu, G. Promotion of Monacolin K production by Agrobacterium tumefaciens-mediated transformation in Monascus albidus 9901. Curr. Microbiol. 2011, 62, 501–507. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, N.; He, Y.; Wang, L.; Shao, Y.; Zhao, H.; Chen, F. MpigE, a gene involved in pigment biosynthesis in Monascus ruber M7. Appl. Microbiol. Biotechnol. 2014, 98, 285–296. [Google Scholar] [CrossRef]
- Chen, G.; Bei, Q.; Huang, T.; Wu, Z. Tracking of pigment accumulation and secretion in extractive fermentation of Monascus anka GIM 3.592. Microb. Cell Fact. 2017, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, Y.; Chen, W.; Shao, Y.; Chen, F. Effects of Light Intensity and Color on the Biomass, Extracellular Red Pigment, and Citrinin Production of Monascus ruber. J. Agric. Food Chem. 2016, 64, 9506–9514. [Google Scholar] [CrossRef]
- Şenyuva, H.Z.; Gilbert, J.; Öztürkoǧlu, Ş. Rapid analysis of fungal cultures and dried figs for secondary metabolites by LC/TOF-MS. Anal. Chim. Acta 2008, 617, 97–106. [Google Scholar] [CrossRef]
- Bi, P.; Li, D.Q.; Dong, H.R. A novel technique for the separation and concentration of penicillin G from fermentation broth: Aqueous two-phase flotation. Sep. Purif. Technol. 2009, 69, 205–209. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Yi, T.; Zhao, M.; Xie, N.; Lei, M.; Liu, Q.; Shao, Y.; Chen, F. Identification and role analysis of an intermediate produced by a polygenic mutant of Monascus pigments cluster in Monascus ruber M7. Appl. Microbiol. Biotechnol. 2016, 100, 7037–7049. [Google Scholar] [CrossRef]
- Lopes, F.C.; Tichota, D.M.; Sauter, I.P.; Meira, S.M.M.; Segalin, J.; Rott, M.B.; Rios, A.O.; Brandelli, A. Active metabolites produced by Penicillium chrysogenum IFL1 growing on agro-industrial residues. Ann. Microbiol. 2013, 63, 771–778. [Google Scholar] [CrossRef]
- Yang, J.; Xu, X.; Liu, G. Amplification of an MFS transporter encoding gene penT significantly stimulates penicillin production and enhances the sensitivity of Penicillium chrysogenum to phenylacetic acid. J. Genet. Genomics 2012, 39, 593–602. [Google Scholar] [CrossRef]
- Altschul, S.F.; Wootton, J.C.; Gertz, E.M.; Agarwala, R.; Morgulis, A.; Schäffer, A.A.; Yu, Y.K. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 2005, 272, 5101–5109. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Liu, J.; Lei, M.; Zhou, Y.; Chen, F. A comprehensive analysis of the small GTPases Ypt7 involved in the regulation of fungal development and secondary metabolism in monascus ruber M7. Front. Microbiol. 2019, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Aldeek, F.; Canzani, D.; Standland, M.; Crosswhite, M.R.; Hammack, W.; Gerard, G.; Cook, J.M. Identification of Penicillin G Metabolites under Various Environmental Conditions Using UHPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 6100–6107. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T. Multidrug efflux pumps and resistance: Regulation and evolution. Curr. Opin. Microbiol. 2003, 6, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Shlykov, M.A.; Castillo, R.; Sun, E.I.; Saier, M.H. The major facilitator superfamily (MFS) revisited. FEBS J. 2012, 279, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Yan, N. Structural advances for the major facilitator superfamily (MFS) transporters. Trends Biochem. Sci. 2013, 38, 151–159. [Google Scholar] [CrossRef]
- Teijeira, F.; Ullán, R.V.; Fernández-Aguado, M.; Martín, J.F. CefR modulates transporters of beta-lactam intermediates preventing the loss of penicillins to the broth and increases cephalosporin production in Acremonium chrysogenum. Metab. Eng. 2011, 13, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Hamari, Z.; Han, K.H.; Seo, J.A.; Reyes-Domínguez, Y.; Scazzocchio, C. Double-joint PCR: A PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 2004, 41, 973–981. [Google Scholar] [CrossRef]
- Chang, P.K.; Yu, J.; Yu, J.H. aflT, a MFS transporter-encoding gene located in the aflatoxin gene cluster, does not have a significant role in aflatoxin secretion. Fungal Genet. Biol. 2004, 41, 911–920. [Google Scholar] [CrossRef]
- Weber, S.S.; Kovalchuk, A.; Bovenberg, R.A.L.; Driessen, A.J.M. The ABC transporter ABC40 encodes a phenylacetic acid export system in Penicillium chrysogenum. Fungal Genet. Biol. 2012, 49, 915–921. [Google Scholar] [CrossRef]
- Zhang, W.; Hunter, I.; Tham, R. Microbial and Plant Cell Synthesis of Secondary Metabolites and Strain Improvement. In Fermentation Microbiology and Biotechnology; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Parameswari, S.; Sivasankari, S. Execution of Enriched Rice Bran Medium in Hyper Production of Penicillin V by Penicillium chrysogenum. Waste and Biomass Valorization 2018, 9, 1559–1565. [Google Scholar] [CrossRef]
- Rajendran, K.; Mahadevan, S.; Jeyaprakash, R.; Paramasamy, G.; Mandal, A.B. Strategies for enhancing the production of penicillin G acylase from bacillus badius: Influence of phenyl acetic acid dosage. Appl. Biochem. Biotechnol. 2013, 171, 1328–1338. [Google Scholar] [CrossRef] [PubMed]




| Name | Sequence 5′⟶3′ | Description |
|---|---|---|
| MfsT5F | GCTCTAGAAGTCCGAGCGCTGCAGC | For the amplification of the 759 bp of the 5′ flanking region of the mfsT gene |
| MfsT5R | CAATATCATCTTCTGTCGACTCGGTCTCGCGTGCTTG | |
| MfsT3F | GAGGTAATCCTTCTTTCTAGTTACCCATCAAGGCAGGC | For the amplification of the 677 bp of the 3′ flanking region of the mfsT gene |
| MfsT3R | GGGGTACCCCGGAAAGCAGAAGACCA | |
| hphF | GTCGACAGAAGATGATATTG | For the amplification of the 2317 bp of the hph cassette from the plasmid pSKH |
| hphR | CTAGAAAGAAGGATTACCTC | |
| MfsTF | GCCCTGTGTGTATTGCTC | For the amplification of the 700 bp of the partial mfsT gene which was used as probe 1 |
| MfsTR | GTAACCACGGTCCATGAG | |
| MfsT5F′ | GCTCTAGATGGCTGGTGCTGAGGGTAG | For the amplification of the 761 bp of the 5′ flanking region for overexpression and complementation of the mfsT gene |
| MfsT5R′ | CAATATCATCTTCTGTCGACTAGGGCTAAGGGCAAGGGC | |
| PtrpCF | GTCGACAGAAGATGATATTG | For the amplification of the 373 bp of the trpC promoter from the plasmid pSKH |
| PtrpCR | GGTTCGGTTCGCATATCGATGCTTGGGTAGAATA | |
| G418F | CCAACTCAACCCCATCGAACCGTAACC | For the amplification of the 1221 bp of the neo cassette from the plasmid pKN1 |
| G418R | ACGATGCTGGACGGGGACATCATCATGCAACATGCATG | |
| MfsT3F′ | GTCCCCGTCCAGCATCGT | For the amplification of the 2312 bp of the 3′ flanking region + ORF for overexpression and complementation of the mfsT gene |
| MfsT3R′ | GGGGTACCAGCGGCTGGGTAGAGTCC | |
| GAPDHF | CTATGCGTGTGCCTACTTCCA | For the real-time RT-PCR analysis of gpd |
| GAPDHR | GAGTTGAGGGCGATACCAGC | |
| MfsTF | TCGTGCTCTCCTTGGGCTTC | For the real-time RT-PCR analysis of mfsT |
| MfsTR | TGACGAGAGAGCGGATGAGATT |
| Protein Number | Strain | Function | Positive Amino Acid (%) |
|---|---|---|---|
| XP_001270324.1 | Aspergillus clavatus NRRL 1 | MFS transporter putative | 83 |
| RDW58813.1 | Coleophoma cylindrospora | MFS transporter-10 | 74 |
| GAD96209.1 | Byssochlamys spectabilis No. 5 | MFS transporter putative | 57 |
| XP_016598554.1 | Penicillium expansum | Major facilitator superfamily domain, general substrate transporter | 54 |
| KGO74874.1 | Penicillium italicum | Major facilitator superfamily domain, general substrate transporter | 53 |
| XP_014538707.1 | Penicillium digitatum Pd1 | MFS multidrug transporter, putative | 55 |
| KZN94365.1 | Penicillium chrysogenum | Proton-coupled folate transporter | 53 |
| KXG49310.1 | Penicillium griseofulvum | Major facilitator superfamily domain, general substrate transporter | 52 |
| KFH48645.1 | Acremonium chrysogenum | Putative transporter like protein | 52 |
| PCG89412.1 | Penicillium sp. ‘occitanis’ | Major facilitator superfamily domain, general substrate transporter | 51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramzan, R.; Safiullah Virk, M.; Muhammad, Z.; Ahmed, A.M.M.; Yuan, X.; Chen, F. Genetic Modification of mfsT Gene Stimulating the Putative Penicillin Production in Monascus ruber M7 and Exhibiting the Sensitivity towards Precursor Amino Acids of Penicillin Pathway. Microorganisms 2019, 7, 390. https://doi.org/10.3390/microorganisms7100390
Ramzan R, Safiullah Virk M, Muhammad Z, Ahmed AMM, Yuan X, Chen F. Genetic Modification of mfsT Gene Stimulating the Putative Penicillin Production in Monascus ruber M7 and Exhibiting the Sensitivity towards Precursor Amino Acids of Penicillin Pathway. Microorganisms. 2019; 7(10):390. https://doi.org/10.3390/microorganisms7100390
Chicago/Turabian StyleRamzan, Rabia, Muhammad Safiullah Virk, Zafarullah Muhammad, Amani Mohedein Mohammed Ahmed, Xi Yuan, and Fusheng Chen. 2019. "Genetic Modification of mfsT Gene Stimulating the Putative Penicillin Production in Monascus ruber M7 and Exhibiting the Sensitivity towards Precursor Amino Acids of Penicillin Pathway" Microorganisms 7, no. 10: 390. https://doi.org/10.3390/microorganisms7100390
APA StyleRamzan, R., Safiullah Virk, M., Muhammad, Z., Ahmed, A. M. M., Yuan, X., & Chen, F. (2019). Genetic Modification of mfsT Gene Stimulating the Putative Penicillin Production in Monascus ruber M7 and Exhibiting the Sensitivity towards Precursor Amino Acids of Penicillin Pathway. Microorganisms, 7(10), 390. https://doi.org/10.3390/microorganisms7100390





