Molecular Epidemiological Characterization of Staphylococcus argenteus Clinical Isolates in Japan: Identification of Three Clones (ST1223, ST2198, and ST2550) and a Novel Staphylocoagulase Genotype XV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates, Species Identification
2.2. Antimicrobial Susceptibility Testing
2.3. Genetic Typing, Detection of Virulence Factors and Drug Resistance Genes
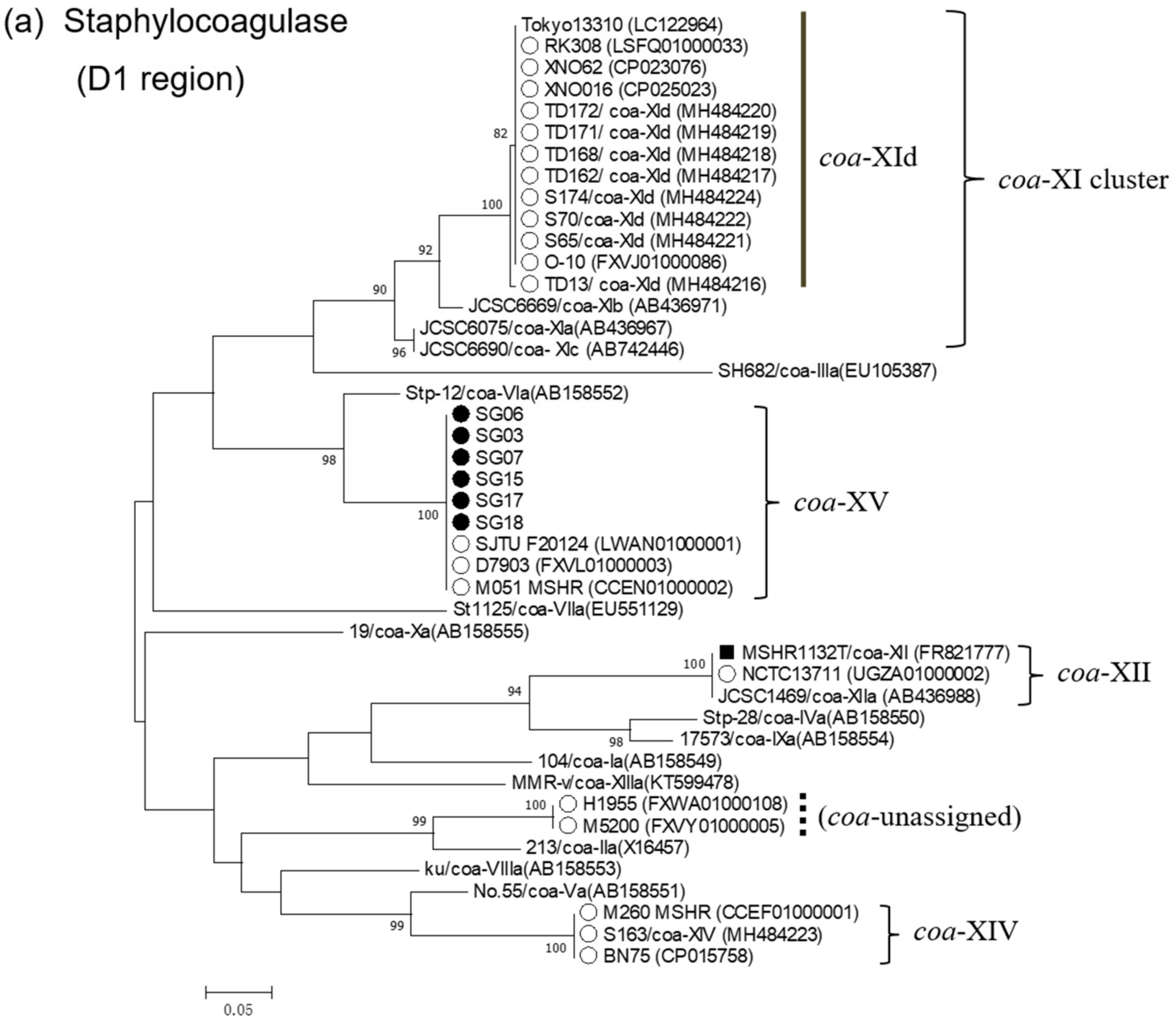
2.4. Phylogenetic Analysis of Virulence Factors
3. Results
3.1. Identification and Prevalence of S. argenteus
3.2. Genotypes (ST, spa Type, coa-Type)
3.3. Prevalence of Virulence Factors, Drug Resistance Genes and Antimicrobial Susceptibility
3.4. Phylogenetic Analysis of Virulence Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tong, S.Y.; Schaumburg, F.; Ellington, M.J.; Corander, J.; Pichon, B.; Leendertz, F.; Bentley, S.D.; Parkhill, J.; Holt, D.C.; Peters, G.; et al. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: The non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.C.; Holden, M.T.; Tong, S.Y.; Castillo-Ramirez, S.; Clarke, L.; Quail, M.A.; Currie, B.J.; Parkhill, J.; Bentley, S.D.; Feil, E.J.; et al. A very early-branching Staphylococcus aureus lineage lacking the carotenoid pigment staphyloxanthin. Genome Biol. Evol. 2011, 3, 881–895. [Google Scholar] [CrossRef]
- Hansen, T.A.; Bartels, M.D.; Høgh, S.V.; Dons, L.E.; Pedersen, M.; Jensen, T.G.; Kemp, M.; Skov, M.N.; Gumpert, H.; Worning, P.; et al. Whole genome sequencing of Danish Staphylococcus argenteus reveals a genetically diverse collection with clear separation from Staphylococcus aureus. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Zhang, D.F.; Xu, X.; Song, Q.; Bai, Y.; Zhang, Y.; Song, M.; Shi, C.; Shi, X. Identification of Staphylococcus argenteus in Eastern China based on a nonribosomal peptide synthetase (NRPS) gene. Future Microbiol. 2016, 11, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; San, T.; San, N.; Oo, W.M.; Ko, P.M.; Thet, K.T.; Urushibara, N.; Kawaguchiya, M.; Sumi, A.; Kobayashi, N. Molecular characterization of Staphylococcus argenteus in Myanmar: Identification of novel genotypes/clusters in staphylocoagulase, protein Aalpha-haemolysin and other virulence factors. J. Med. Microbiol. 2019, 68, 95–104. [Google Scholar]
- McDonald, M.; Dougall, A.; Holt, D.; Huygens, F.; Oppedisano, F.; Giffard, P.M.; Inman-Bamber, J.; Stephens, A.J.; Towers, R.; Carapetis, J.R.; et al. Use of a single-nucleotide polymorphism genotyping system to demonstrate the unique epidemiology of methicillin-resistant Staphylococcus aureus in remote aboriginal communities. J. Clin. Microbiol. 2006, 44, 3720–3727. [Google Scholar] [CrossRef] [PubMed]
- Okuma, K.; Iwakawa, K.; Turnidge, J.D.; Grubb, W.B.; Bell, J.M.; O’Brien, F.G.; Coombs, G.W.; Pearman, J.W.; Tenover, F.C.; Kapi, M.; et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J. Clin. Microbiol. 2002, 40, 4289–4294. [Google Scholar] [CrossRef]
- Ritchie, S.R.; Thomas, M.G.; Rainey, P.B. The genetic structure of Staphylococcus aureus populations from the Southwest Pacific. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Jenney, A.; Holt, D.; Ritika, R.; Southwell, P.; Pravin, S.; Buadromo, E.; Carapetis, J.; Tong, S.; Steer, A. The clinical and molecular epidemiology of Staphylococcus aureus infections in Fiji. BMC Infect. Dis. 2014, 14. [Google Scholar] [CrossRef]
- Thaipadungpanit, J.; Amornchai, P.; Nickerson, E.K.; Wongsuvan, G.; Wuthiekanun, V.; Limmathurotsakul, D.; Peacock, S.J. Clinical and molecular epidemiology Staphylococcus argenteus infections in Thailand. J. Clin. Microbiol. 2015, 53, 1005–1008. [Google Scholar] [CrossRef]
- Chantratita, N.; Wikraiphat, C.; Tandhavanant, S.; Wongsuvan, G.; Ariyaprasert, P.; Suntornsut, P.; Thaipadungpanit, J.; Teerawattanasook, N.; Jutrakul, Y.; Srisurat, N.; et al. Comparison of community-onset Staphylococcus argenteus and Staphylococcus aureus sepsis in Thailand: A prospective multicentre observational study. Clin. Microbiol. Infect. 2016, 22. [Google Scholar] [CrossRef]
- Yeap, A.D.; Woods, K.; Dance, D.A.B.; Pichon, B.; Rattanavong, S.; Davong, V.; Phetsouvanh, R.; Newton, P.N.; Shetty, N.; Kearns, A.M. Molecular epidemiology of Staphylococcus aureus skin and soft tissue infections in the Lao People’s Democratic Republic. Am. J. Trop. Med. Hyg. 2017, 97, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Ruimy, R.; Armand-Lefevre, L.; Barbier, F.; Ruppe, E.; Cocojaru, R.; Mesli, Y.; Maiga, A.; Benkalfat., M.; Benchouk, S.; Hassaine, H.; et al. Comparisons between geographically diverse samples of carried Staphylococcus aureus. J. Bacteriol. 2009, 191, 5577–5583. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; San, T.; Aye, M.M.; Mya, S.; Maw, W.W.; Zan, K.N.; Htut, W.H.W.; Kawaguchiya, M.; Urushibara, N.; Kobayashi, N. Prevalence and genetic characteristics of Staphylococcus aureus and Staphylococcus argenteus isolates harboring Panton-Valentine leukocidin, enterotoxins, and TSST-1 genes from food handlers in Myanmar. Toxins 2017, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, Y.; Umeda, K.; Yonog, I.S.; Nakamura, H.; Yamamoto, K.; Kumeda, Y.; Kawatsu, K. Staphylococcal food poisoning caused by Staphylococcus argenteus harboring staphylococcal enterotoxin genes. Int. J. Food Microbiol. 2018, 265, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kubota, H.; Ono, H.K.; Kobayashi, M.; Murauchi, K.; Kato, R.; Hirai, A.; Sadamas, K. Food poisoning outbreak in Tokyo, Japan caused by Staphylococcus argenteus. Int. J. Food Microbiol. 2017, 262, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Ohge., H.; Hisatsune, J.; Masuda, K.; Aziz, F.; Hara, T.; Kuroo, Y.; Sugai, M. Low incidence of Staphylococcus argenteus bacteremia in Hiroshima, Japan. J. Infect. Chemother. 2019. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lee, H.; Wang, X.M.; Lee, T.F.; Liao, C.H.; Teng., L.J.; Hsueh, P.R. High mortality impact of Staphylococcus argenteus on patients with community-onset staphylococcal bacteraemia. Int. J. Antimicrob. Agents 2018, 52, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Dodémont, M.; Vandendriessche, S.; Rottiers, S.; Tribes, C.; Roisin, S.; de Mendonça, R.; Nonhoff, C.; Deplano, A.; Denis, O. Low occurrence of the new species Staphylococcus argenteus in a Staphylococcus aureus collection of human isolates from Belgium. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1017–1022. [Google Scholar] [CrossRef]
- Dupieux, C.; Blonde, R.; Bouchiat, C.; Meugnier, H.; Bes, M.; Laurent, S.; Vandenesch, F.; Laurent, F.; Tristan, A. Community-acquired infections due to Staphylococcus argenteus lineage isolates harboring the Panton-Valentine leucocidin, France, 2014. Euro. Surveill. 2015, 20. [Google Scholar] [CrossRef]
- Tang Hallbäck, E.; Karami, N.; Adlerberth, I.; Cardew, S.; Ohlén, M. Methicillin-resistant Staphylococcus argenteus misidentified as methicillin-resistant Staphylococcus aureus emerging in western Sweden. J. Med. Microbiol. 2018, 67, 968–971. [Google Scholar] [CrossRef]
- Ruimy, R.; Angebault, C.; Djossou, F.; Dupont, C.; Epelboin, L.; Jarraud, S.; Lefevre, L.A.; Bes, M.; Lixandru, B.E.; Bertine, M.; et al. Are host genetics the predominant determinant of persistent nasal Staphylococcus aureus carriage in humans? J. Infect. Dis. 2010, 202, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Monecke, S.; Stieber, B.; Roberts, R.; Akpaka, P.E.; Slickers, P.; Ehricht, R. Population structure of Staphylococcus aureus from Trinidad & Tobago. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Moradigaravand, D.; Jamrozy, D.; Mostowy, R.; Anderson, A.; Nickerson, E.K.; Thaipadungpanit, J.; Wuthiekanun, V.; Limmathurotsakul, D.; Tandhavanant, S.; Wikraiphat, C.; et al. Evolution of the Staphylococcus argenteus ST2250 clone in Northeastern Thailand is linked with the acquisition of livestock-associated staphylococcal genes. MBIO 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Schaumburg, F.; Kearns, A.; Larsen, A.R.; Lindsay, J.A.; Skov, R.L.; Westh, H. Implications of identifying the recently defined members of the Staphylococcus aureus complex S. argenteus and S. schweitzeri: A position paper of members of the ESCMID Study Group for Staphylococci and Staphylococcal Diseases (ESGS). Clin. Microbiol. Infect. 2019, 25, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Shinjoh, M.; Ohara, H.; Kawai, T.; Kamimaki, I.; Mizushima, R.; Kamada, K.; Itakura, Y.; Iguchi, S.; Uzawa, Y.; et al. Purulent lymphadenitis caused by Staphylococcus argenteus, representing the first Japanese case of Staphylococcus argenteus (multilocus sequence type 2250) infection in a 12-year-old boy. J. Infect. Chemother. 2018, 24, 925–927. [Google Scholar] [CrossRef]
- Schuster, D.; Rickmeyer, J.; Gajdiss, M.; Thye, T.; Lorenzen, S.; Reif, M.; Josten, M.; Szekat, C.; Melo, L.D.R.; Schmithausen, R.M.; et al. Differentiation of Staphylococcus argenteus (formerly: Staphylococcus aureus clonal complex 75) by mass spectrometry from S. aureus using the first strain isolated from a wild African great ape. Int. J. Med. Microbiol. 2017, 307, 57–63. [Google Scholar] [CrossRef]
- Indrawattana, N.; Pumipuntu, N.; Suriyakhun, N.; Jangsangthong, A.; Kulpeanprasit, S.; Chantratita, N.; Sookrung, N.; Chaicumpa, W.; Buranasinsup, S. Staphylococcus argenteus from rabbits in Thailand. Microbiologyopen 2019, 8. [Google Scholar] [CrossRef]
- Pumipuntu, N.; Tunyong, W.; Chantratita, N.; Diraphat, P.; Pumirat, P.; Sookrung, N.; Chaicumpa, W.; Indrawattana, N. Staphylococcus spp. associated with subclinical bovine mastitis in central and northeast provinces of Thailand. Peer J. 2019, 14. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, Wayne, PA, USA, 2007. Twenty-Seven Informational Supplement, M100-S27. Available online: https//clisi.org>media>catalog2017_web (accessed on 9 August 2019).
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 7.1. 2017, pp. 21–25. Available online: www.eucast.org>clinical_breakpoints (accessed on 9 August 2019).
- Watanabe, A.; Yanagihara, K.; Matsumoto, T.; Kohno, S.; Aoki, N.; Oguri, T.; Sato, J.; Muratani, T.; Yagisawa, M.; Ogasawara, K.; et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the Surveillance Committee of Japanese Society of Chemotherapy, Japanese Association for Infectious Diseases, and Japanese Society for Clinical Microbiology in 2009: General view of the pathogens’ antibacterial susceptibility. J. Infect. Chemother. 2012, 18, 609–620. [Google Scholar]
- Zhang, K.; McClure, J.A.; Elsayed, S.; Louie, T.; Conly, J.M. Novel multiplex PCR assay for simultaneous identification of community-associated methicillin-resistant Staphylococcus aureus strains USA300 and USA400 and detection of mecA and panton-valentine leukocidin genes, with discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J. Clin. Microbiol. 2018, 46, 1118–1122. [Google Scholar]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [PubMed]
- Shopsin, B.; Gomez, M.; Montgomery, S.O.; Smith, D.H.; Waddington, M.; Dodge, D.E.; Bost, D.A.; Riehman, M.; Naidich, S.; Kreiswirth, B.N. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 1999, 37, 3556–3563. [Google Scholar] [PubMed]
- Hirose, M.; Kobayashi., N.; Ghosh, S.; Paul, S.K.; Shen, T.; Urushibara, N.; Kawaguchiya, M.; Shinagawa, M.; Watanabe, N. Identification of staphylocoagulase genotypes I-X and discrimination of type IV and V subtypes by multiplex PCR assay for clinical isolates of Staphylococcus Aureus. Jpn. J. Infect. Dis. 2010, 63, 257–263. [Google Scholar] [PubMed]
- Kinoshita, M.; Kobayashi, N.; Nagashima, S.; Ishino, M.; Otokozawa, S.; Mise, K.; Sumi, A.; Tsutsumi, H.; Uehara, N.; Watanabe, N.; et al. Diversity of staphylocoagulase and identification of novel variants of staphylocoagulase gene in Staphylococcus Aureus. Microbiol. Immunol. 2008, 52, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Aung, T.S.; Mya, S.; San, T.; Nwe, K.M.; Kobayashi, N. Virulence factors and genetic characteristics of methicillin-resistant and -susceptible Staphylococcus aureus isolates in Myanmar. Microb. Drug Resist. 2011, 17, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Sumi, A.; Shinagawa, M.; Takahashi, S.; Kobayashi, N. Clonal Diversity and Genetic Characteristics of Methicillin-Resistant Staphylococcus aureus Isolates from a Tertiary Care Hospital in Japan. Microb. Drug Resist. 2019. [Google Scholar] [CrossRef]
- Aung, M.S.; Kawaguchiya, M.; Urushibara, N.; Sumi, A.; Ito, M.; Kudo, K.; Morimoto, S.; Hosoya, S.; Kobayashi, N. Molecular Characterization of Methicillin-Resistant Staphylococcus aureus from Outpatients in Northern Japan: Increasing Tendency of ST5/ST764 MRSA-IIa with Arginine Catabolic Mobile Element. Microb. Drug Resist. 2017, 23, 616–625. [Google Scholar] [CrossRef]
- Wilson, G.J.; Tuffs, S.W.; Wee, B.A.; Seo, K.S.; Park, N.; Connelley, T.; Guinane, C.M.; Morrison, W.I.; Fitzgerald, J.R. Bovine Staphylococcus aureus Superantigens Stimulate the Entire T Cell Repertoire of Cattle. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Zhang, D.F.; Yang, X.Y.; Zhang, J.; Qin, X.; Huang, X.; Cui, Y.; Zhou, M.; Shi, C.; French, N.P.; Shi, X. Identification and characterization of two novel superantigens among Staphylococcus aureus complex. Int. J. Med. Microbiol. 2018, 308, 438–446. [Google Scholar] [CrossRef]
- Watanabe, S.; Ito, T.; Sasaki, T.; Li, S.; Uchiyama, I.; Kishii, K.; Kikuchi, K.; Skov, R.L.; Hiramatsu, K. Genetic diversity of staphylocoagulase genes (coa): Insight into the evolution of variable chromosomal virulence factors in Staphylococcus aureus. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Downer, R.; Roche, F.; Park, P.W.; Mecham, R.P.; Foster, T.J. The elastin-binding protein of Staphylococcus aureus (EbpS) is expressed at the cell surface as an integral membrane protein and not as a cell wall-associated protein. J. Biol. Chem. 2002, 277, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, E.; McCrea, K.W.; Ní Eidhin, D.; O’Connell, D.; Cox, J.; Höök, M.; Foster, T.J. Three new members of the serine-aspartate repeat protein multigene family of Staphylococcus aureus. Microbiology 1998, 144, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu., M.; Zhuo, W.; Gu, J.; Zhang, S.; Ge, J.; Yang, M. Crystal structures of Bbp from Staphylococcus aureus reveal the ligand binding mechanism with Fibrinogen α. Protein Cell 2015, 6, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Becherelli, M.; Prachi, P.; Viciani, E.; Biagini, M.; Fiaschi, L.; Chiarot, E.; Nosari, S.; Brettoni, C.; Marchi, S.; Biancucci, M.; et al. Protective activity of the CnaBE3 domain conserved among Staphylococcus aureus Sdr proteins. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Zhi, X.Y.; Zhang, J.; Paoli, G.C.; Cui, Y.; Shi, C.; Shi, X. Preliminary comparative genomics revealed pathogenic potential and international spread of Staphylococcus argenteus. BMC Genom. 2017, 18, 808. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ito, T.; Takeuchi, F.; Endo, M.; Okuno, E.; Hiramatsu, K. Structural comparison of ten serotypes of staphylocoagulases in Staphylococcus aureus. J. Bacteriol. 2005, 187, 3698–3707. [Google Scholar] [CrossRef] [PubMed]

| Isolate ID | Age/Sex | Specimen | City/Town a | Inpatient/Outpatient | coa Genotype | ST | spa Type b | spa Repeat Profile | Leukocidins, Haemolysins, Enterotoxins, TSST-1 c,d | Adhesins, Modulators of Host Defense c,d | Drug Resistance Genes e | Antimicrobial Resistance Profile f |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SG01 | 81/F | sputum | A | Inpatient | XI-d | ST2250 | t5078 | 299-31-25-17-17-16-16-16-16 | sec2, sey, selz, sel26, sel27 | sdrC, sdrD | ||
| SG04 | 30/F | urine | B | Outpatient | XI-d | ST2250 | NT | 299-31-25-17-16-16-16 | sec3, sei, sel, sem, sey, selz, sel26, sel27, tst-1 | sdrC, sak | ||
| SG05-1 | 46/F | ear discharge | A | Outpatient | XI-d | ST2250 | NT | 299-31-25-17-17-16 | sey, selz, sel26, sel27 | sdrC, sdrD, sak | ||
| SG05-2 | 46/F | ear discharge | A | Outpatient | XI-d | ST2250 | NT | 299-31-25-17-17-16 | sey, selz, sel26, sel27 | sdrC, sdrD, sak | ||
| SG09 | 14/M | nasal discharge | B | Outpatient | XI-d | ST2250 | NT | N-17-17-16-16-16-16 | sey, selz, sel26, sel27 | sdrC, sdrD | ||
| SG11 | 70/F | vaginal discharge | A | Outpatient | XI-d | ST2250 | t17928 | 299-31-25-17-17-16-16-16 | sey, selz, sel26, sel27 | sdrC, sdrD | ||
| SG16 | N g/F | stool | C | Outpatient | XI-d | ST2250 | t5078 | 299-31-25-17-17-16-16-16-16 | selx, sey, sel26, sel27 | sdrC, sdrD, sak | ||
| SG19 | 68/F | sputum | A | Inpatient | XI-d | ST2250 | t5787 | 299-31-31-25-17-17-16-16-16-16 | sec3, sey, sel26, sel27, tst-1 | sdrD | ||
| SG21 | 81/M | sputum | A | Outpatient | XI-d | ST2250 | t7960 | 299-25-17-17-16-16-16-16 | sey | sdrC, sdrD | ||
| SG22 | 83/F | pharynx | A | Inpatient | XI-d | ST2250 | t5078 | 299-31-25-17-17-16-16-16-16 | sey | sak, sdrC, sdrD | ||
| SG23 | 83/F | blood | A | Inpatient | XI-d | ST2250 | t5078 | 299-31-25-17-17-16-16-16-16 | sey | sak, sdrC, sdrD | tet(L) | TET, DOX |
| SG25 | 94/F | sputum | A | Inpatient | XI-d | ST2250 | t17928 | 299-31-25-17-17-16-16-16 | sey | sdrC, sdrD | ||
| SG13 | 88/M | sputum | D | Inpatient | XI-d | ST3951 (ST2250 SLV) | t5078 | 299-31-25-17-17-16-16-16-16 | sey, selz, sel26, sel27 | sdrD | ||
| SG02 | 75/F | urine | E | Outpatient | XIV | ST2198 | NT | 259-23-23-23-23-17-17-16 | selx | sdrC, sdrD | ||
| SG08 | 73/M | skin | F | Outpatient | XIV | ST2198 | t7959 | 259-23-23-17-16 | selx, selz | sdrC | ||
| SG10 | 81/M | sputum | A | Inpatient | XIV | ST2198 | t9385 | 259-366-23-17-17-17-23-23-368-17-16 | selx, selz | sdrC, sak | ||
| SG12 | 82/M | pharynx | G | Inpatient | XIV | ST2198 | NT | 259-17-16 | selx, selz | sdrC | ||
| SG14 | 75/M | skin | A | Outpatient | XIV | ST2198 | NT | 259-23-23-23-23-17-16 | selx | sdrC | ||
| SG20 | 74/M | pus | H | Outpatient | XIV | ST2198 | NT | 259-23-23-23-17-16 | selx | sdrC, sdrD | blaZ | AMP |
| SG03 | 65/M | subdural abscess | D | Inpatient | XV | ST1223 | t5142 | 259-25-17-17-16-16-16-17-16-16-16-16-16 | seg, sei, sem, sen, seo, selw, selz | sdrC, sdrD | ||
| SG06 | 73/M | nasal discharge | I | Outpatient | XV | ST1223 | NT | 259-25-16-16-16-16-16 | seg, sei, sem, sen, seo, selw, selz | sdrC, sdrD | ||
| SG07 | 17/M | stool | J | Outpatient | XV | ST1223 | NT | 259-25 | seg, sei, sem, sen, seo, selw, selz | sdrC | ||
| SG15 | N/F | stool | A | Outpatient | XV | ST1223 | t9791 | 259-25-17-17-16-16-17-16-16-16-16-16 | seg, sei, sem, sen, seo, selx, selw | sdrC, sdrD | ||
| SG17 | 32/M | stool | K | Outpatient | XV | ST1223 | t7463 | 259-25-17-17-16-16-16-17-16-16-16-16 | seg, sei, sem, sen, seo, selw | sdrC, sdrD | ||
| SG18 | N/F | vaginal discharge | A | Outpatient | XV | ST1223 | t5142 | 259-25-17-17-16-16-16-17-16-16-16-16-16 | seg, sei, sem, sen, seo, selw | sdrC, sdrD |
| Strain | Species | GenBank Accession No. | coa Type a | SG03 | SG06 | SG17 | SG18 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2C | D1 | D2C | D1 | D2C | D1 | D2C | ||||
| 104 | S. aureus | AB158549 | Ia | 68.8 | 69.1 | 68.8 | 68.8 | 68.8 | 69.1 | 68.8 | 69.2 |
| 213 | S. aureus | X16457 | IIa | 66.5 | 70.6 | 66.5 | 70.3 | 66.5 | 70.6 | 66.5 | 70.7 |
| SH682 | S. aureus | EU105387 | IIIa | 66.0 | 71.4 | 66.0 | 71.1 | 66.0 | 71.4 | 66.0 | 71.5 |
| Stp-28 | S. aureus | AB158550 | IVa | 64.9 | 71.8 | 64.9 | 71.5 | 64.9 | 71.8 | 64.9 | 71.9 |
| No.55 | S. aureus | AB158551 | Va | 66.5 | 65.6 | 66.5 | 65.3 | 66.5 | 65.6 | 66.5 | 65.7 |
| Stp-12 | S. aureus | AB158552 | VIa | 88.3 | 76.8 | 88.3 | 76.5 | 88.3 | 76.8 | 88.3 | 76.9 |
| St-1125 | S. aureus | EU551129 | VIIa | 67.0 | 67.7 | 67.0 | 67.4 | 67.0 | 67.7 | 67.0 | 67.8 |
| Ku | S. aureus | AB158553 | VIIIa | 69.3 | 68.8 | 69.3 | 68.5 | 69.3 | 68.8 | 69.3 | 68.9 |
| 17573 | S. aureus | AB158554 | IXa | 66.1 | 70.6 | 66.1 | 70.3 | 66.1 | 70.6 | 66.1 | 70.7 |
| 19 | S. aureus | AB158555 | Xa | 65.8 | 66.0 | 65.8 | 65.7 | 65.8 | 66.0 | 65.8 | 66.1 |
| JCSC6075 | S. aureus | AB436967 | XIa | 71.9 | 70.3 | 71.9 | 70.0 | 71.9 | 70.3 | 71.9 | 70.4 |
| JCSC6669 | S. aureus | AB436971 | XIb | 71.6 | 69.0 | 71.6 | 68.7 | 71.6 | 69.0 | 71.6 | 69.1 |
| JCSC6990 | S. aureus | AB742446 | XIc | 72.1 | 65.8 | 72.1 | 65.5 | 72.1 | 65.8 | 72.1 | 65.9 |
| Tokyo 13310 | S. aureus | LC122964 | XI-variant | 70.7 | 82.9 | 70.7 | 82.6 | 70.7 | 82.7 | 70.7 | 82.8 |
| JCSC1469 | S. aureus | AB436988 | XIIa | 67.7 | 78.7 | 67.7 | 78.4 | 67.7 | 78.7 | 67.7 | 78.8 |
| mmr-v | S. aureus | KT599478 | XIIIa | 64.9 | 68.3 | 64.9 | 68.0 | 64.9 | 68.3 | 64.9 | 68.4 |
| MSHR1132T | S. argenteus | FR821777 | XII | 67.7 | 78.7 | 67.7 | 78.4 | 67.7 | 78.7 | 67.7 | 78.8 |
| S174 | S. argenteus | MH484224 | XId | 70.7 | 82.9 | 70.7 | 82.6 | 70.7 | 82.7 | 70.7 | 82.8 |
| RK308 | S. argenteus | LSFQ01000033 | XId | 70.7 | 82.9 | 70.7 | 82.6 | 70.7 | 82.7 | 70.7 | 82.8 |
| O-10 | S. argenteus | FXVJ01000086 | XId | 70.7 | 82.9 | 70.7 | 82.6 | 70.7 | 82.7 | 70.7 | 82.8 |
| XNO016 | S. argenteus | CP025023 | XId a | 70.7 | 82.9 | 70.7 | 82.6 | 70.7 | 82.7 | 70.7 | 82.8 |
| XNO62 | S. argenteus | CP023076 | XId a | 70.7 | 82.9 | 70.7 | 82.6 | 70.7 | 82.7 | 70.7 | 82.8 |
| BN75 | S. argenteus | CP015758 | XIV | 67.0 | 82.8 | 67.0 | 82.5 | 67.0 | 82.6 | 67.0 | 82.7 |
| S163 | S. argenteus | MH484223 | XIV | 67.0 | 82.8 | 67.0 | 82.5 | 67.0 | 82.6 | 67.0 | 82.7 |
| M260_MSHR | S. argenteus | CCEF01000001 | XIV | 67.0 | 82.8 | 67.0 | 82.5 | 67.0 | 82.5 | 67.0 | 82.6 |
| SJTU F20124 | S. argenteus | LWAN01000001 | XV | 100 | 100 | 100 | 99.7 | 100 | 99.8 | 100 | 99.9 |
| D7903 | S. argenteus | FXVL01000003 | XV | 100 | 100 | 100 | 99.7 | 100 | 99.8 | 100 | 99.9 |
| M051_MSHR | S. argenteus | CCEN01000002 | XV | 100 | 100 | 100 | 99.7 | 100 | 99.8 | 100 | 99.9 |
| SG03 | S. argenteus | MN166536 | XV | 100 | 100 | 100 | 99.7 | 100 | 99.8 | 100 | 99.9 |
| SG06 | S. argenteus | MN166537 | XV | 100 | 99.7 | 100 | 100 | 100 | 99.4 | 100 | 99.5 |
| SG17 | S. argenteus | MN166540 | XV | 100 | 99.8 | 100 | 99.4 | 100 | 100 | 100 | 99.9 |
| SG18 | S. argenteus | MN166541 | XV | 100 | 99.9 | 100 | 99.5 | 100 | 99.9 | 100 | 100 |
| NCTC13711 | S. argenteus | UGZA01000002 | NA | 67.7 | 78.7 | 67.7 | 78.4 | 67.7 | 78.7 | 67.7 | 78.8 |
| H1955 | S. argenteus | FXWA01000108 | NA | 68.9 | 86.5 | 68.9 | 86.2 | 68.9 | 86.5 | 68.9 | 86.6 |
| M5200 | S. argenteus | FXVY01000005 | NA | 67.4 | 86.3 | 67.4 | 86.0 | 67.4 | 67.3 | 67.4 | 86.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Sumi, A.; Takahashi, S.; Ike, M.; Ito, M.; Habadera, S.; Kobayashi, N. Molecular Epidemiological Characterization of Staphylococcus argenteus Clinical Isolates in Japan: Identification of Three Clones (ST1223, ST2198, and ST2550) and a Novel Staphylocoagulase Genotype XV. Microorganisms 2019, 7, 389. https://doi.org/10.3390/microorganisms7100389
Aung MS, Urushibara N, Kawaguchiya M, Sumi A, Takahashi S, Ike M, Ito M, Habadera S, Kobayashi N. Molecular Epidemiological Characterization of Staphylococcus argenteus Clinical Isolates in Japan: Identification of Three Clones (ST1223, ST2198, and ST2550) and a Novel Staphylocoagulase Genotype XV. Microorganisms. 2019; 7(10):389. https://doi.org/10.3390/microorganisms7100389
Chicago/Turabian StyleAung, Meiji Soe, Noriko Urushibara, Mitsuyo Kawaguchiya, Ayako Sumi, Seika Takahashi, Miyo Ike, Masahiko Ito, Satoshi Habadera, and Nobumichi Kobayashi. 2019. "Molecular Epidemiological Characterization of Staphylococcus argenteus Clinical Isolates in Japan: Identification of Three Clones (ST1223, ST2198, and ST2550) and a Novel Staphylocoagulase Genotype XV" Microorganisms 7, no. 10: 389. https://doi.org/10.3390/microorganisms7100389
APA StyleAung, M. S., Urushibara, N., Kawaguchiya, M., Sumi, A., Takahashi, S., Ike, M., Ito, M., Habadera, S., & Kobayashi, N. (2019). Molecular Epidemiological Characterization of Staphylococcus argenteus Clinical Isolates in Japan: Identification of Three Clones (ST1223, ST2198, and ST2550) and a Novel Staphylocoagulase Genotype XV. Microorganisms, 7(10), 389. https://doi.org/10.3390/microorganisms7100389





