Microfluidic-Based Approaches for Foodborne Pathogen Detection
Abstract
1. Introduction
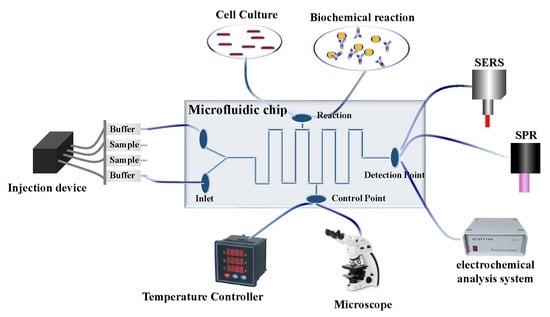
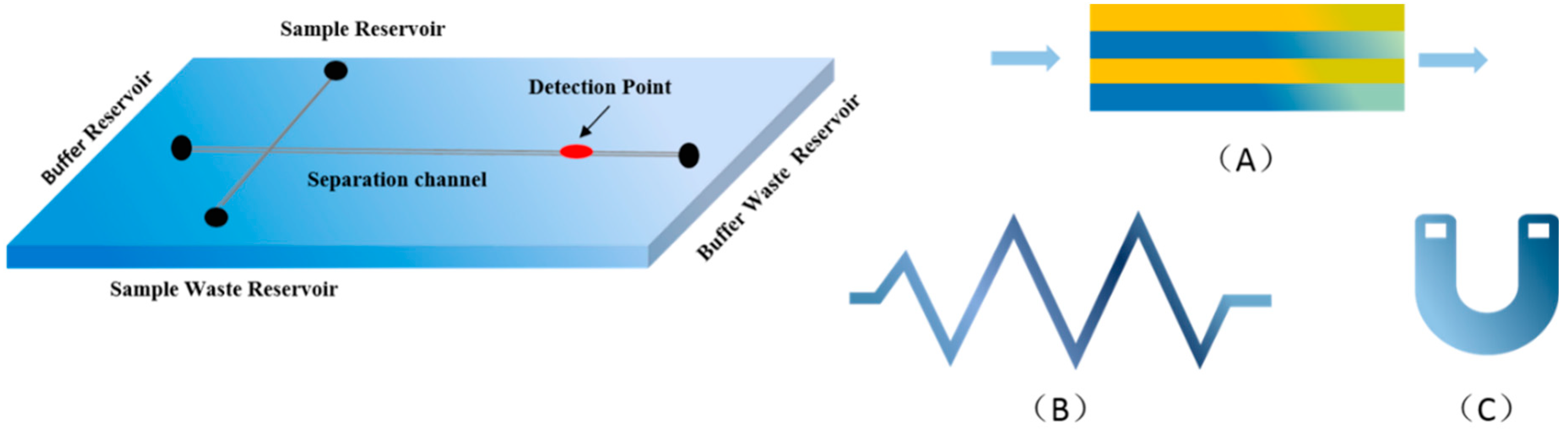
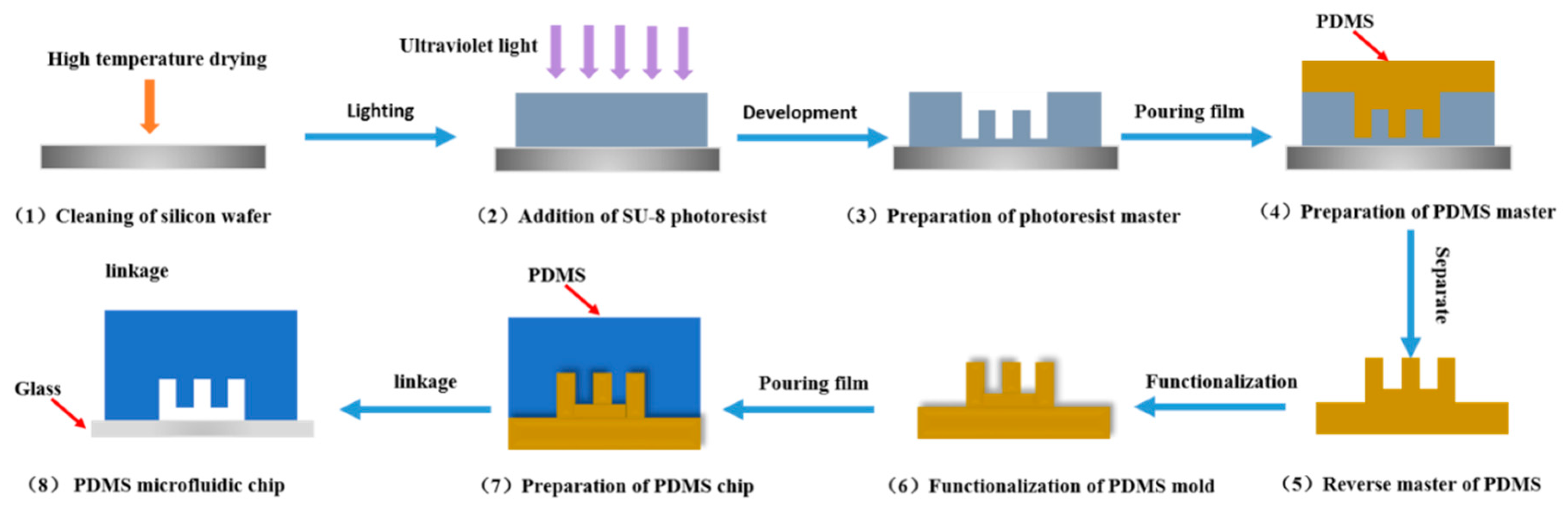
2. Microfluidic Chips
3. Sample Preparation in Microfluidics
3.1. For Single Component
3.2. Complex Components in Food Matrix
3.2.1. Special Materials and Sampling Methods
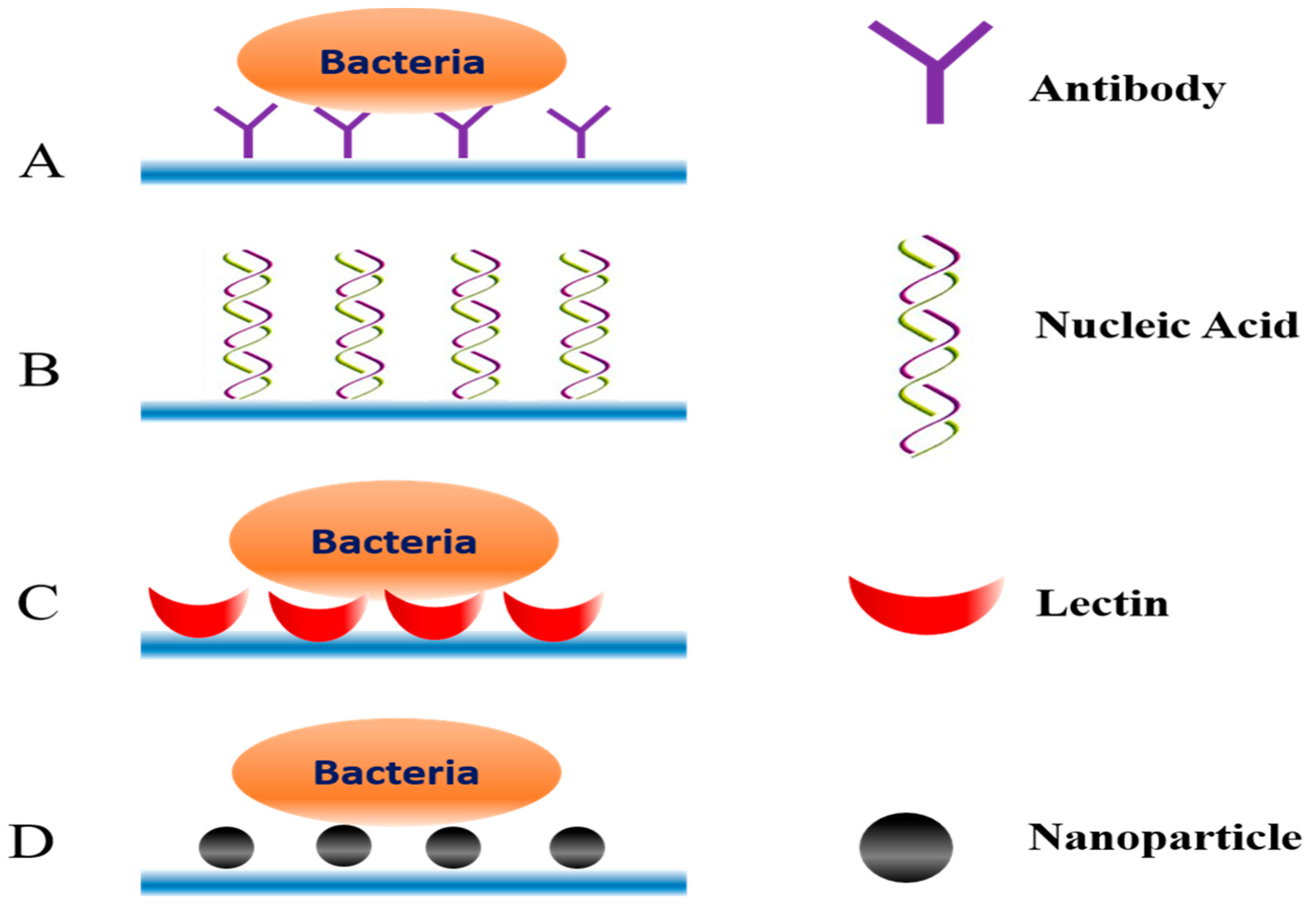
3.2.2. Bio-Recognition Molecules
4. Application of Microfluidic Combined with Different Technologies
4.1. Biosensor-Based Microfluidics for the Detection of Foodborne Pathogens
4.1.1. Microfluidic Chips with Optical Detection
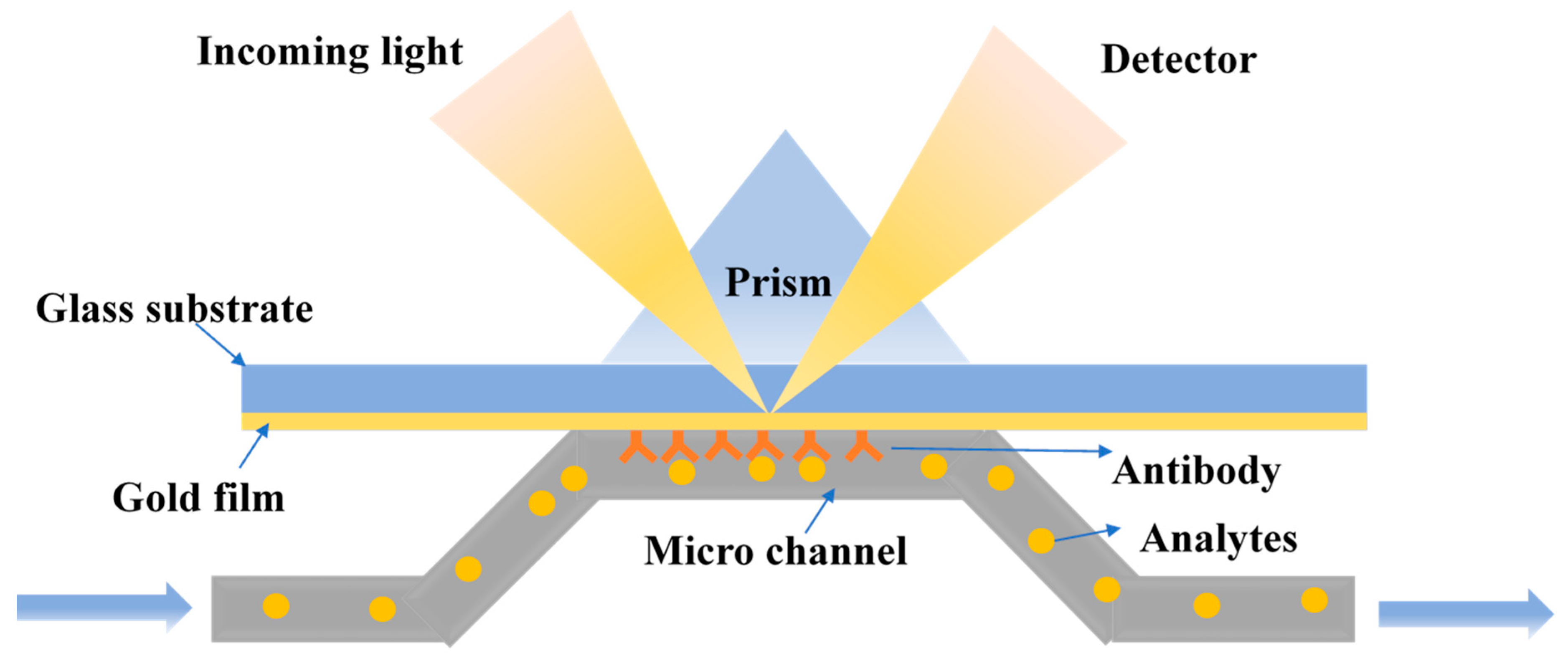
Surface Plasmon Resonance (SPR) Biosensors
Optical Fibre Biosensors
4.1.2. Microfluidic Chip with Electrochemical Detection
4.2. Immunoassay-Based Microfluidics for the Detection of Foodborne Pathogens
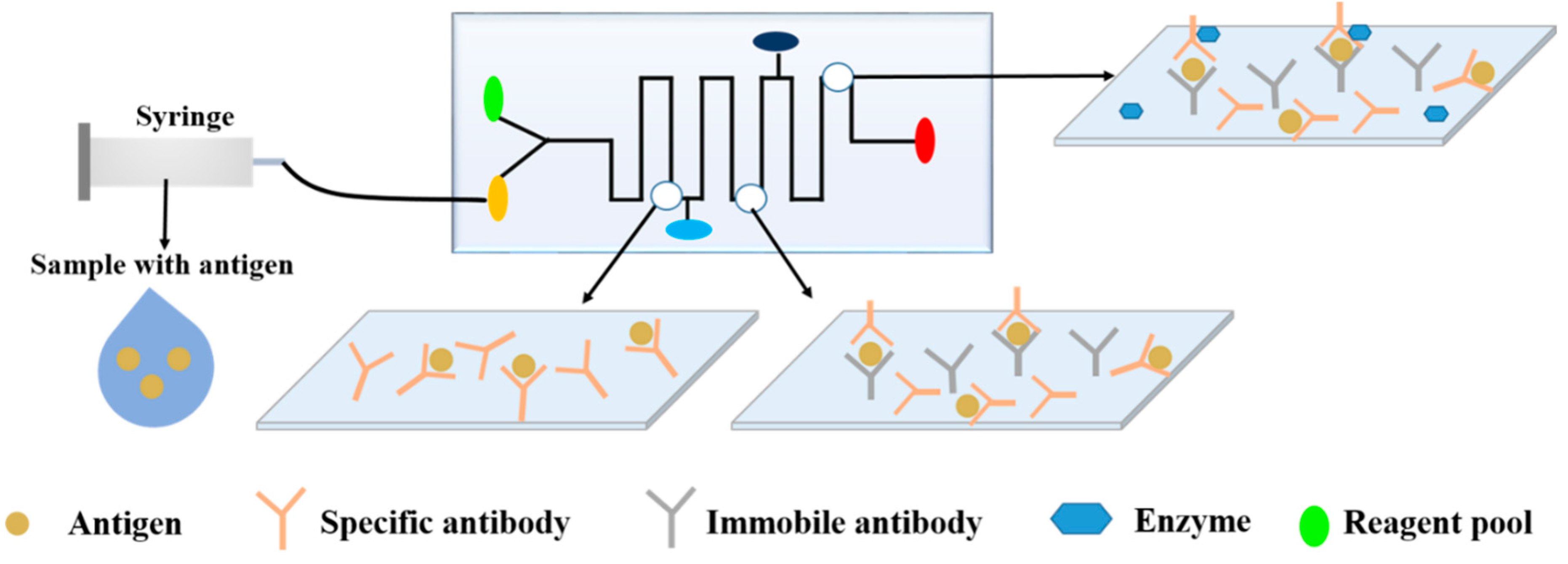
4.2.1. Enzyme-Linked Immunosorbent Assay (ELISA)
4.2.2. Immunomagnetic Fluorescence Assay (IMS)
4.3. Nucleic Acid-Based Microfluidics for the Detection of Foodborne Pathogens
4.3.1. Polymerase Chain Reaction (PCR)
4.3.2. Multiplex PCR
4.3.3. Loop-Mediated Isothermal Amplification (LAMP)
5. Challenges and Opportunities
6. Conclusions
Funding
Conflicts of Interest
References
- Chapman, B.; Gunter, C. Local Food Systems Food Safety Concerns. Microbiol. Spectr. 2018, 6, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Xu, Z. Detection of Foodborne Pathogens by Surface Enhanced Raman Spectroscopy. Front. Microbiol. 2018, 9, 1236. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.F.; Yackley, J. Foodborne Disease Outbreaks in the United States: A Historical Overview. Foodborne Pathog. Dis. 2018, 15, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Crump, J.A.; Glass, K.; Howden, B.P.; Furuya-Kanamori, L.; Vilkins, S.; Gray, D.J.; Kirk, M.D. Health Outcomes from Multidrug-Resistant Salmonella Infections in High-Income Countries: A Systematic Review and Meta-Analysis. Foodborne Pathog. Dis. 2018, 15, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lin, C.W.; Wang, J.; Oh, D.H. Advances in rapid detection methods for foodborne pathogens. J. Microbiol. Biotechnol. 2014, 24, 297. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Zhou, R.; Li, L.; Peters, B.M.; Li, B.; Lin, C.W.; Peters, B.M.; Chuang, T.L.; Chen, D.Q.; Zhao, X.H.; et al. Viable but non-culturable state and toxin gene expression of enterohemorrhagic Escherichia coli O157 under cryopreservation. Res. Microbiol. 2017, 168, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fèvre, E.M.; Sripa, B.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Wei, C.J.; Zhong, J.L.; Jin, S.W. Research advance in rapid detection of foodborne Staphylococcus aureus. Biotechnol. Biotechnol. Equip. 2016, 30, 827–833. [Google Scholar] [CrossRef]
- Umesha, S.; Manukumar, H.M. Advanced Molecular Diagnostic Techniques for Detection of Food-borne Pathogens: Current Applications and Future Challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 84–104. [Google Scholar] [CrossRef]
- Wei, C.J.; Zhong, J.L.; Hu, T.; Zhao, X.H. Simultaneous detection of Escherichia coli O157:H7, Staphylococcus aureus and Salmonella by multiplex PCR in milk. 3 Biotech 2018, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, X. Isothermal amplification technologies for the detection of foodborne pathogens. Food Anal. Methods 2018, 11, 1543–1560. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.W.; Ding, T. Current perspectives on viable but non-culturable state in foodborne pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.; Gawad, S. The application of microfluidics in biology. Methods Mol. Biol. 2010, 583, 55–80. [Google Scholar] [PubMed]
- Yujie, L.I.; Huo, Y.; Di, L.I.; Tang, X.; Shi, F.; Wang, C. Technology, application and development of microfluidics. J. Hebei Univ. Sci. Technol. 2014, 35, 11. [Google Scholar]
- Wen, N.; Zhao, Z.; Fan, B.; Chen, D.; Men, D.; Wang, J.; Chen, J. Development of Droplet Microfluidics Enabling High-Throughput Single-Cell Analysis. Molecules 2016, 21, 881. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-K.; Ke, Y.; Jun, Z.; Can-Can, Z.; Ling, Z.; Yong, L. Rapid Detection of Hepatitis B Virus Nucleic Acid Based on Microfluidic Chip Using Fluorescence Quantitative PCR. J. Anal. Sci. 2018, 34, 11–15. [Google Scholar]
- Wang, C.; Madiyar, F.; Yu, C.; Li, J. Detection of extremely low concentration waterborne pathogen using a multiplexing self-referencing SERS microfluidic biosensor. J. Biol. Eng. 2017, 11, 9. [Google Scholar] [CrossRef]
- Wan, L.; Chen, T.; Gao, J.; Dong, C.; Wong, A.H.; Jia, Y.; Mak, P.; Deng, C.X.; Martins, R.P. A digital microfluidic system for loop-mediated isothermal amplification and sequence specific pathogen detection. Sci. Rep. 2017, 7, 14586. [Google Scholar] [CrossRef]
- Jokerst, J.C.; Adkins, J.A.; Bisha, B.; Mentele, M.M.; Goodridge, L.D.; Henry, C.S. Development of a Paper-Based Analytical Device for Colorimetric Detection of Select Foodborne Pathogens. Anal. Chem. 2012, 84, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Bao, L.J.; Habeeb, A.A.; Lu, P.X. Effects of doping concentration on the surface plasmonic resonances and optical nonlinearities in AGZO nano-triangle arrays. Opt. Quantum Electron. 2017, 49, 345. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, D.M.; Lu, P.; Sun, Q.Z.; Yang, W.; Wang, S.; Liu, L.; Zhang, J.S. Dual-Parameters Optical Fiber Sensor with Enhanced Resolution Using Twisted MMF Based on SMS Structure. IEEE Sens. J. 2017, 17, 3045–3051. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Neethirajan, S. Paper-based microfluidic aptasensor for food safety. J. Food Saf. 2017, 38, e12412. [Google Scholar] [CrossRef]
- Xu, J.; Kawano, H.; Liu, W.W.; Hanada, Y.; Lu, P.X.; Miyawaki, A.; Midorikawa, K.; Sugioka, K. Controllable alignment of elongated microorganisms in 3D microspace using electrofluidic devices manufactured by hybrid femtosecond laser microfabrication. Microsyst. Nanoeng. 2017, 3, 16078. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Q.; He, X.Q.; Liew, K.M. A sensitive interval of imperfect interface parameters based on the analysis of general solution for anisotropic matrix containing an elliptic inhomogeneity. Int. J. Solids Struct. 2015, 73–74, 67–77. [Google Scholar] [CrossRef]
- Hou, M.; Wang, Y.; Liu, S.; Guo, J.; Li, Z.; Lu, P. Sensitivity-Enhanced Pressure Sensor with Hollow-Core Photonic Crystal Fiber. J. Lightwave Technol. 2014, 32, 4637–4641. [Google Scholar]
- Manz, A.; Graber, N.; Widmer, H.M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1990, 1, 244–248. [Google Scholar] [CrossRef]
- Manz, A.; Harrison, D.J.; Verpoorte, E.M.J.; Fettinger, J.C.; Paulus, A.; Lüdi, H.; Widmer, H.M. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems: Capillary electrophoresis on a chip. J. Chromatogr. A 1992, 593, 253–258. [Google Scholar] [CrossRef]
- Woolley, A.T.; Mathies, R.A. Ultra-high-speed DNA sequencing using capillary electrophoresis chips. Anal. Chem. 1995, 67, 3676–3680. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.T.; Hadley, D.; Landre, P.; Demello, A.J.; Mathies, R.A.; Northrup, M.A. Functional integration of PCR amplification and capillary electrophoresis in a microfabricated DNA analysis device. Anal. Chem. 1996, 68, 4081–4086. [Google Scholar] [CrossRef] [PubMed]
- Brahmasandra, S.N.; Johnson, B.N.; Webster, J.R.; Burke, D.T.; Mastrangelo, C.H.; Burns, M.A. On-chip DNA band detection in microfabricated separation systems. Proc. Spie—Int. Soc. Opt. Eng. 1998, 3515, 242–251. [Google Scholar]
- Anderson, J.R.; Chiu, D.T.; Jackman, R.J.; Cherniavskaya, O.; Mcdonald, J.C.; Wu, H.; Whitesides, S.H.; Whitesides, G.M. Fabrication of topologically complex three-dimensional microfluidic systems in PDMS by rapid prototyping. Anal. Chem. 2000, 72, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Ghaemmaghami, A.M.; Hancock, M.J.; Harrington, H.; Kaji, H.; Khademhosseini, A. Biomimetic tissues on a chip for drug discovery. Drug Discov. Today 2012, 17, 173–181. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zheng, W.; Sun, J.; Zhang, W.; Jiang, X. Microfluidics for Manipulating Cells. Small 2013, 9, 9–21. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, W.W.; Ke, S.L.; Liu, Q.J. Large lateral shift in complex dielectric multilayers with nearly parity-time symmetry. Opt. Quantum Electron. 2018, 50, 323. [Google Scholar] [CrossRef]
- Chuang, T.L.; Chang, C.C.; Chu-Su, Y.; Wei, S.C.; Zhao, X.H.; Hsueh, P.R.; Lin, C.W. Disposable surface plasmon resonance aptasensor with membranebased sample handling design for quantitative interferon-gamma detection. Lab Chip 2014, 14, 2968–2977. [Google Scholar] [CrossRef]
- Atsushi, K.; Akiko, I.; Tamotsu, Y.; Yoshiaki, U.; Eiichi, T.; Yuzuru, T. Highly sensitive elemental analysis for Cd and Pb by liquid electrode plasma atomic emission spectrometry with quartz glass chip and sample flow. Anal. Chem. 2011, 83, 9424–9430. [Google Scholar]
- Francisca, A.; Neha, S.; Mohammad-Ali, S.; Dongfei, L.; Bárbara, H.B.; Makila, E.M.; Jarno, J.S.; Jouni, T.H.; Pedro, L.G.; Bruno, S.; et al. Microfluidic Assembly of a Multifunctional Tailorable Composite System Designed for Site Specific Combined Oral Delivery of Peptide Drugs. Acs Nano 2015, 9, 8291–8302. [Google Scholar]
- Xuan, T.V.; Stockmann, R.; Wolfrum, B.; Offenhäusser, A.; Ingebrandt, S. Fabrication and application of a microfluidic-embedded silicon nanowire biosensor chip. Phys. Status Solidi 2010, 207, 850–857. [Google Scholar]
- Zhang, H.; Dongfei, L.; Mohammad-Ali, S.; Ermei, M.K.; Bárbara, H.B.; Jarno, S.; Hirvonen, J.; Santos, H.A. Fabrication of a multifunctional nano-in-micro drug delivery platform by microfluidic templated encapsulation of porous silicon in polymer matrix. Adv. Mater. 2014, 26, 4497–4503. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, H.J.; Morrissey, Y.C.; Taylor, B.J.; Liang, T.; Johnstone, R.W.; Stickel, A.J.; Manage, P.; Atrazhev, A.; Backhouse, C.J.; Pilarski, L.M. Inhibition of on-chip PCR using PDMS–glass hybrid microfluidic chips. Microfluid. Nanofluid. 2012, 13, 383–398. [Google Scholar] [CrossRef]
- Tan, F.; Leung, P.H.M.; Liu, Z.B.; Zhang, Y.; Xiao, L.; Ye, W.; Zhang, X.; Yi, L.; Yang, M. A PDMS microfluidic impedance immunosensor for E. coli O157:H7 and Staphylococcus aureus detection via antibody-immobilized nanoporous membrane. Sens. Actuators B Chem. 2011, 159, 328–335. [Google Scholar] [CrossRef]
- Al-Shehri, S.; Palitsin, V.; Webb, R.P.; Grime, G.W. Fabrication of three-dimensional SU-8 microchannels by proton beam writing for microfluidics applications: Fluid flow characterisation. Nucl. Instrum. Methods Phys. Res. B 2015, 348, 223–228. [Google Scholar] [CrossRef]
- Dy, A.J.; Cosmanescu, A.; Sluka, J.; Glazier, J.A.; Stupack, D.; Amarie, D. Fabricating microfluidic valve master molds in SU-8 photoresist. J. Micromech. Microeng. 2014, 24, 057001. [Google Scholar] [CrossRef]
- Floquet, C.F.A.; Sieben, V.J.; Milani, A.; Joly, E.P.; Ogilvie, I.R.G.; Morgan, H.; Mowlem, M.C. Nanomolar detection with high sensitivity microfluidic absorption cells manufactured in tinted PMMA for chemical analysis. Talanta 2011, 84, 235–239. [Google Scholar] [CrossRef]
- Wu, N.; Zhu, Y.G.; Brown, S.; Oakeshott, J.; Peat, T.; Surjadi, R.; Easton, C.; Leech, P.W.; Sexton, B.A. A PMMA microfluidic droplet platform for in Vitro protein expression using crude E. Coli S30 extract. Lab Chip 2009, 9, 3391–3398. [Google Scholar] [CrossRef]
- Stojkovič, G.; Krivec, M.; Vesel, A.; Marinšek, M.; Žnidaršič-Plazl, P. Surface cell immobilization within perfluoroalkoxy microchannels. Appl. Surf. Sci. 2014, 320, 810–817. [Google Scholar] [CrossRef]
- Detlev, B.; Alfred, D.; Frank, K.; Martin, L. Poly (vinyl alcohol)-coated microfluidic devices for high-performance microchip electrophoresis. Electrophoresis 2015, 23, 3567–3573. [Google Scholar]
- Huang, K.W.; Wu, Y.C.; Lee, J.A.; Chiou, P.Y. Microfluidic integrated optoelectronic tweezers for single-cell preparation and analysis. Lab Chip 2013, 13, 3721–3727. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Xue, P.; Wu, Y.; Bao, J.; Chuah, Y.J.; Kang, Y. A concentration gradient generator on a paper-based microfluidic chip coupled with cell culture microarray for high-throughput drug screening. Biomed. Microdevices 2016, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Wu, A.; Li, Z.C.; Zhang, G.; Yu, W.Y. A new calibration method between an optical sensor and a rotating platform in turbine blade inspection. Meas. Sci. Technol. 2017, 28, 035009. [Google Scholar] [CrossRef]
- Zeng, D.; Chen, Z.; Jiang, Y.; Xue, F.; Li, B. Advances and Challenges in Viability Detection of Foodborne Pathogens. Front. Microbiol. 2016, 7, 1833. [Google Scholar] [CrossRef]
- Zhang, R.; Hai-Qing, G.; Xudong, Z.; Chaoping, L.; Chunchau, S. A microfluidic liquid phase nucleic acid purification chip to selectively isolate DNA or RNA from low copy/single bacterial cells in minute sample volume followed by direct on-chip quantitative PCR assay. Anal. Chem. 2013, 85, 1484–1491. [Google Scholar] [CrossRef]
- Wu, J.; Kodzius, R.; Cao, W.; Wen, W. Extraction, amplification and detection of DNA in microfluidic chip-based assays. Microchim. Acta 2014, 181, 1611–1631. [Google Scholar] [CrossRef]
- Tachibana, H.; Saito, M.; Shibuya, S.; Tsuji, K.; Miyagawa, N.; Yamanaka, K.; Tamiya, E. On-chip quantitative detection of pathogen genes by autonomous microfluidic PCR platform. Biosens. Bioelectron. 2015, 74, 725–730. [Google Scholar] [CrossRef]
- Wang, Y.; Jianfeng, P.; Zunzhong, Y.; Jian, W.; Yibin, Y. Impedimetric immunosensor based on gold nanoparticles modified graphene paper for label-free detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2013, 4, 492–498. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S.; Chem, A. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Wang, Y.N.; Fu, L.M.; Chen, K.L. Microfluidic paper-based chip platform for benzoic acid detection in food. Food Chem. 2018, 249, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.F.; Juan, H.U.; Zheng, G.; Zhao, G.H. Application of microfluidic chip in food safety analysis. Sci. Technol. Food Ind. 2011, 32, 401–404. [Google Scholar]
- Zheng, Y.; Mao, S.; Liu, S.; Wong, S.H.; Wang, Y.W. Normalized Relative RBC-Based Minimum Risk Bayesian Decision Approach for Fault Diagnosis of Industrial Process. IEEE Trans. Ind. Electron. 2016, 63, 7723–7732. [Google Scholar] [CrossRef]
- Ganesh, I.; Tran, B.M.; Kim, Y.; Kim, J.; Cheng, H.; Lee, N.Y.; Park, S. An integrated microfluidic PCR system with immunomagnetic nanoparticles for the detection of bacterial pathogens. Biomed. Microdevices 2016, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Park, B.H.; Choi, G.; Seo, J.H.; Jung, J.H.; Choi, J.S.; Kim, D.H.; Seo, T.S. Fully automated and colorimetric foodborne pathogen detection on an integrated centrifugal microfluidic device. Lab Chip 2016, 16, 1917–1926. [Google Scholar] [CrossRef]
- Sayad, A.; Ibrahim, F.; Mukim, S.U.; Cho, J.; Madou, M.; Thong, K.L. A microdevice for rapid, monoplex and colorimetric detection of foodborne pathogens using a centrifugal microfluidic platform. Biosens. Bioelectron. 2017, 100, 96–104. [Google Scholar] [CrossRef]
- Li, X.; Ximenes, E.; Amalaradjou, M.A.; Vibbert, H.B.; Foster, K.; Jones, J.; Liu, X.Y.; Bhunia, A.K.; Ladisch, M.R. Rapid sample processing for detection of food-borne pathogens via cross-flow microfiltration. Appl. Environ. Microbiol. 2013, 79, 7048–7054. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, C.; Xing, D. Segmented continuous-flow multiplex polymerase chain reaction microfluidics for high-throughput and rapid foodborne pathogen detection. Anal. Chim. Acta 2014, 826, 51–60. [Google Scholar] [CrossRef]
- Savas, S.; Ersoy, A.; Gulmez, Y.; Kilic, S.; Levent, B.; Altintas, Z. Nanoparticle Enhanced Antibody and DNA Biosensors for Sensitive Detection of Salmonella. Materials 2018, 11, 1541. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Shi, Z.; Fang, C.; Wang, Z. Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels. Anal. Chem. 2014, 86, 3100–3107. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Um, E.; Diaz, A.; Driscoll, H.; Rodas, M.J.; Domansky, K.; Rodas, M.J.; Watters, A.L.; Super, M.; Stone, H.A.; et al. Optimization of Pathogen Capture in Flowing Fluids with Magnetic Nanoparticles. Small 2015, 11, 5657–5666. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.N.T.; Yoon, J.; Jin, C.E.; Koo, B.; Han, K.; Yong, S.; Lee, T.Y. Rapid and Sensitive Detection of Salmonella based on Microfluidic Enrichment with a Label-free Nanobiosensing Platform. Sens. Actuators B Chem. 2017, 262, 588–594. [Google Scholar] [CrossRef]
- Deshmukh, R.A.; Joshi, K.; Bhand, S.; Roy, U. Recent developments in detection and enumeration of waterborne bacteria: A retrospective minireview. Microbiologyopen 2016, 5, 901–922. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.; Ab Mutalib, N.S.; Chan, K.G.; Lee, L.H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar] [CrossRef] [PubMed]
- Ríos, Á.; Zougagh, M. Modern qualitative analysis by miniaturized and microfluidic systems. Trends Anal. Chem. 2015, 69, 105–113. [Google Scholar] [CrossRef]
- Kant, K.; Shahbazi, M.A.; Dave, V.P.; Ngo, T.A.; Chidambara, V.A.; Linh, Q.T.; Dang, D.B.; Anders, W. Microfluidic devices for sample preparation and rapid detection of foodborne pathogens. Biotechnol. Adv. 2018, 36, 1003–1024. [Google Scholar] [CrossRef]
- Chi, L.W.; Olivo, M. Surface Plasmon Resonance Imaging Sensors: A Review. Plasmonics 2014, 9, 809–824. [Google Scholar]
- Lee, H.; Xu, L.; Koh, D.; Nyayapathi, N.; Oh, K.W. Various on-chip sensors with microfluidics for biological applications. Sensors 2014, 14, 17008–17036. [Google Scholar] [CrossRef]
- Liu, S.H.; Tian, J.; Liu, N.L.; Lu, P.X. Temperature Insensitive Liquid Level Sensor Based on Antiresonant Reflecting Guidance in Silica Tube. J. Lightwave Technol. 2016, 34, 5239–5243. [Google Scholar] [CrossRef]
- Safavieh, M.; Ahmed, M.U.; Tolba, M.; Zourob, M. Microfluidic electrochemical assay for rapid detection and quantification of Escherichia coli. Biosens. Bioelectron. 2012, 31, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance-a review. J. Food Sci. Technol. 2012, 49, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Lin, Z. Recent developments and applications of surface plasmon resonance biosensors for the detection of mycotoxins in foodstuffs. Food Chem. 2012, 132, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Fan, S.K. Microfluidic Surface Plasmon Resonance Sensors: From Principles to Point-of-Care Applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef] [PubMed]
- Pennacchio, A.; Ruggiero, G.; Staiano, M.; Piccialli, G.; Oliviero, G.; Lewkowicz, A.; Synak, A.; Bojarski, P.; D’Auriaa, S. A surface plasmon resonance based biochip for the detection of patulin toxin. Opt. Mater. 2014, 36, 1670–1675. [Google Scholar] [CrossRef]
- Zordan, M.D.; Grafton, M.M.G.; Acharya, G.; Reece, L.M.; Cooper, C.L.; Aronson, A.I.; Park, K.; Leary, J.F. Detection of pathogenic E. coli O157:H7 by a hybrid microfluidic SPR and molecular imaging cytometry device. Cytom. Part A 2010, 75, 155–162. [Google Scholar]
- Zordan, M.D.; Grafton, M.M.G.; Acharya, G.; Reece, L.M.; Aronson, A.I.; Park, K.; Leary, J.F. A microfluidic-based hybrid SPR/molecular imaging biosensor for the multiplexed detection of foodborne pathogens. Proc. Spie—Int. Soc. Opt. Eng. 2009, 7167, 1–10. [Google Scholar]
- Tokel, O.; Yildiz, U.H.; Inci, F.; Durmus, N.G.; Ekiz, O.O.; Turker, B.; Cetin, C.; Rao, S.; Sridhar, K.; Natarajan, N.; et al. Portable Microfluidic Integrated Plasmonic Platform for Pathogen Detection. Sci. Rep. 2015, 5, 9152. [Google Scholar] [CrossRef] [PubMed]
- Reig, B.; Bardinal, V.; Camps, T.; Doucet, J.B. A miniaturized VCSEL-based system for optical sensing in a microfluidic channel. Sensors 2012, 23, 1–4. [Google Scholar]
- Ohk, S.H.; Koo, O.K.; Sen, T.; Yamamoto, C.M.; Bhunia, A.K. Antibody–aptamer functionalized fibre-optic biosensor for specific detection of Listeria monocytogenes from food. J. Appl. Microbiol. 2010, 109, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, W.; Wang, Z.; Liu, T.; Zhang, Y. Progress on fiber-optic evanescent wave biosensor technique in food safety detection. J. Food Saf. Qual. 2014, 5, 3971–3974. [Google Scholar]
- Li, M.; Zhao, F.; Zeng, J.; Qi, J.; Lu, J.; Shih, W.C. Microfluidic surface-enhanced Raman scattering sensor with monolithically integrated nanoporous gold disk arrays for rapid and label-free biomolecular detection. J. Biomed. Opt. 2014, 19, 111611. [Google Scholar] [CrossRef] [PubMed]
- Mungroo, N.A.; Oliveira, G.; Neethirajan, S. SERS based point-of-care detection of food-borne pathogens. Microchim. Acta 2016, 183, 697–707. [Google Scholar] [CrossRef]
- Gilli, E. Optical biosensor system with integrated microfluidic sample preparation and TIRF based detection. Proc. Spie—Int. Soc. Opt. Eng. 2013, 8774, 140–144. [Google Scholar]
- Setterington, E.B.; Alocilja, E.C. Electrochemical Biosensor for Rapid and Sensitive Detection of Magnetically Extracted Bacterial Pathogens. Biosensors 2012, 2, 15–31. [Google Scholar] [CrossRef]
- Campuzano, S.; Yanez-Sedeno, P.; Pingarron, J.M. Molecular Biosensors for Electrochemical Detection of Infectious Pathogens in Liquid Biopsies: Current Trends and Challenges. Sensors 2017, 17, 2533. [Google Scholar] [CrossRef]
- Dong, S.; Zhou, J.; Hui, D.; Pang, X.; Wang, Q.; Zhang, S.; Wang, L. Interaction between edge dislocations and amorphous interphase in carbon nanotubes reinforced metal matrix nanocomposites incorporating interface effect. Int. J. Solids Struct. 2014, 51, 1149–1163. [Google Scholar] [CrossRef][Green Version]
- Ligaj, M.; Tichoniuk, M.; Gwiazdowska, D.; Filipiak, M. Electrochemical DNA biosensor for the detection of pathogenic bacteria Aeromonas hydrophila. Electrochim. Acta 2014, 128, 67–74. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Cai, G.; Xiong, Y.; Li, Y.; Wang, M.; Hou, H.; Lin, J. Fast and sensitive detection of foodborne pathogen using electrochemical impedance analysis, urease catalysis and microfluidics. Biosens. Bioelectron. 2016, 86, 770–776. [Google Scholar] [CrossRef]
- Liu, H.T.; Wen, Z.Y.; Xu, Y.; Shang, Z.G.; Peng, J.L.; Tian, P. An integrated microfluidic analysis microsystems with bacterial capture enrichment and in-situ impedance detection. Mod. Phys. Lett. B 2017, 31, 196–199. [Google Scholar] [CrossRef]
- Wang, X.; Niessner, R.; Tang, D.; Knopp, D. Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal. Chim. Acta 2016, 912, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Jing, H.; Cao, X.; Huang, K.; Luo, Y.; Xu, W. Development of a double-antibody sandwich ELISA for rapid detection of Bacillus Cereus in food. Sci. Rep. 2016, 6, 16092. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, A.; Bruck, H.A.; Kostov, Y. An ELISA Lab-on-a-Chip (ELISA-LOC). Humana Press 2013, 949, 451–471. [Google Scholar]
- Thaitrong, N.; Charlermroj, R.; Himananto, O.; Seepiban, C.; Karoonuthaisiri, N. Implementation of microfluidic sandwich ELISA for superior detection of plant pathogens. PLoS ONE 2013, 8, e83231. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Ma, Y.D.; Chung, Y.D.; Lee, G.B. An integrated microfluidic system for dual aptamer assay utilizing magnetic-composite-membranes. IEEE Int. Conf. Nano/Micro Eng. Mol. Syst. 2017, 4, 438–441. [Google Scholar]
- Yanagisawa, N.; Dutta, D. Enhancement in the Sensitivity of Microfluidic Enzyme-Linked Immunosorbent Assays through Analyte Preconcentration. Anal. Chem. 2012, 84, 7029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Liu, S.L.; Zhao, W.; Zhang, W.P.; Yu, X.; Li, Y.; Li, A.J.; Pang, D.W.; Zhang, Z.L. A Simple Point-of-Care Microfluidic Immunomagnetic Fluorescence Assay for Pathogens. Anal. Chem. 2013, 85, 2645–2651. [Google Scholar] [CrossRef]
- Kanayeva, D.A.; Wang, R.; Rhoads, D.; Erf, G.F.; Slavik, M.F.; Tung, S.; Li, Y. Efficient separation and sensitive detection of Listeria monocytogenes using an impedance immunosensor based on magnetic nanoparticles, a microfluidic chip, and an interdigitated microelectrode. J. Food Prot. 2012, 75, 1951–1959. [Google Scholar] [CrossRef]
- Dector, A.; Galindo-De-La-Rosa, J.; Amaya-Cruz, D.M.; Ortíz-Verdín, A.; Guerra-Balcázar, M.; Olivares-Ramírez, J.M.; Arriaga, L.G.; Ledesma-García, J. Towards autonomous lateral flow assays: Paper-based microfluidic fuel cell inside an HIV-test using a blood sample as fuel. Int. J. Hydrog. Energy 2017, 42, 29–32. [Google Scholar] [CrossRef]
- Hsieh, H.; Dantzler, J.; Weigl, B. Analytical Tools to Improve Optimization Procedures for Lateral Flow Assays. Diagnostics 2017, 7, 29. [Google Scholar] [CrossRef]
- Doller, C.; Jakubik, J. Direct solid-phase radioimmunoassay for the detection of Aujeszky’s disease antibodies. Zent. Bakteriol. A 1980, 247, 1–7. [Google Scholar]
- Yao, L.; Wang, L.; Huang, F.; Cai, G.; Xi, X.; Lin, J. A microfluidic impedance biosensor based on immunomagnetic separation and urease catalysis for continuous-flow detection of E. coli O157:H7. Sens. Actuators B Chem. 2018, 259, 2657. [Google Scholar] [CrossRef]
- Mangal, M.; Bansal, S.; Sharma, S.K.; Gupta, R.K. Molecular Detection of Food Borne Pathogens: A Rapid and Accurate Answer to Food Safety. Crit. Rev. Food Sci. Nutr. 2016, 56, 1568–1584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.H. An automated all-in-one microfludic device for parallel solid phase DNA extraction and droplet-inoil PCR analysis. IEEE Int. Conf. Micro Electro Mech. Syst. 2010, 9, 971–974. [Google Scholar]
- Zhang, C.; Wang, H.; Xing, D. Multichannel oscillatory-flow multiplex PCR microfluidics for high-throughput and fast detection of foodborne bacterial pathogens. Biomed. Microdevices 2011, 13, 885. [Google Scholar] [CrossRef]
- Tourlousse, D.M.; Ahmad, F.; Stedtfeld, R.D.; Seyrig, G.; Tiedje, J.M.; Hashsham, S.A. A polymer microfluidic chip for quantitative detection of multiple water- and foodborne pathogens using real-time fluorogenic loop-mediated isothermal amplification. Biomed. Microdevices 2012, 14, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.M.; Ibrahim, F.; Sayad, A.A.; Thiha, A.; Pei, K.X.; Mohktar, M.S.; Hashim, U.; Cho, J.M.; Thong, K.L. A portable automatic endpoint detection system for amplicons of loop mediated isothermal amplification on microfluidic compact disk platform. Sensors 2015, 15, 5376–5389. [Google Scholar] [CrossRef]
- Sun, Y.; Quyen, T.L.; Hung, T.Q.; Chin, W.H.; Wolff, A.; Bang, D.D. A lab-on-a-chip system with integrated sample preparation and loop-mediated isothermal amplification for rapid and quantitative detection of Salmonella spp. in food samples. Lab Chip 2015, 15, 1898–1904. [Google Scholar] [CrossRef]
- Lutz, S.; Weber, P.; Focke, M.; Faltin, B.; Hoffmann, J.; Müller, C.; Mark, D.; Roth, G.; Munday, P.; Armes, N.; et al. Microfluidic lab-on-a-foil for nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA). Lab Chip 2010, 10, 887–893. [Google Scholar] [CrossRef]
- Tortajada-Genaro, L.A.; Santiago-Felipe, S.; Amasia, M.; Russom, A.; Maquieira, Á. Isothermal solid-phase recombinase polymerase amplification on microfluidic digital versatile discs (DVDs). RSC Adv. 2015, 5, 29987–29995. [Google Scholar] [CrossRef]
- Mauk, M.G.; Liu, C.; Song, J.; Bau, H.H. Integrated Microfluidic Nucleic Acid Isolation, Isothermal Amplification, and Amplicon Quantification. Microarrays 2015, 4, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Fu, K.; Liu, Y.; Ding, X.; Hu, J.; Wu, W.; Xu, K.; Song, X.L.; Wang, J.; Mu, Y.; et al. Development of a self-priming PDMS/paper hybrid microfluidic chip using mixed-dye-loaded loop-mediated isothermal amplification assay for multiplex foodborne pathogens detection. Anal. Chim. Acta 2018, 1040, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.L.; Zhao, X.H. Detection of viable but non-culturable Escherichia coli O157:H7 by PCR in combination with propidium monoazide. 3 Biotech 2017, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Li, L.M.; Liu, R.Y. Application of paper based microfluidic chip technology in food safety detection. J. Food Saf. Qual. 2018, 38, e12412. [Google Scholar]
| Material Type | Classification | Representative | Methods of Preparation | Advantages | Disadvantages | Application | References |
|---|---|---|---|---|---|---|---|
| organic material | ------- | glass/quartz | photolithography and etching techniques | cheap and easy to obtain, reusable, good light transmission and electroosmosis, good electrical insulation and corrosion resistance | complex manufacturing process, time-consuming and high cost, fragile | gas chromatography and capillary electrophoresis (CE) and electrochemical detection, organic synthesis and droplet formation, PCR | [41,42] |
| silicon material | silicon/silicon dioxide | etching techniques | mature process, good thermal stability and inertness. | high cost of materials, opaque, brittle, poor electrical insulation, and low adhesion coefficient | organic synthesis and droplet formation, PCR and CE | [43,44] | |
| elastomers | polydimethylsiloxane (PDMS) | molding and soft lithography | Low cost and easy to use, non-toxic and transparent, excellent chemical inertness and light transmission | Incompatibility of organic solvents and poor pressure resistance, low thermal conductivity and immature processing technology | protein crystallization and bioculture, PCR | [45,46] | |
| Polymer materials | thermosets | SU-8 photoresist and polyimide | photopolymerization and casting | High resistance of temperature and most solvents, transparent and reusable | high cost of materials | CE, organic synthesis and droplet formation, PCR | [47,48] |
| thermoplastics | poly (methyl methacrylate (PMMA) polystyrene (PS) and polycarbonate (PC) | hot embossing and laser ablation | good electrical insulation and light transmission, low cost and easy to use, simple preparation and high precision | Non-breathable, high-cost preparation equipment and rough process | CE and PCR, droplet formation | [49,50] | |
| perfluoropolymers | perfluoroalkoxy (PFA) and fluorinated ethylene propylene | photolithography | Good inertness and antifouling properties, transparent and soft | poor adhesion | environmental monitoring and food analysis | [51] | |
| Special materials | hydrogels | polyvinyl alcohol (PVA) | photopolymerization, casting | high permeability and controllable aperture, allowing small molecules or even biological particles to diffuse, and biocompatible | difficult to store | 3D bioculture | [52] |
| ceramics | polysiloxane | soft lithography and laser ablation | high resistance of temperature and pressure | poor light transmission, fragile | suitable for applications under harsh conditions | [53] | |
| paper | analysis filter paper | photolithography and printing | high permeability and low cost, portable and easy to use | easy to damage and disposable | bioculture | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Li, M.; Liu, Y. Microfluidic-Based Approaches for Foodborne Pathogen Detection. Microorganisms 2019, 7, 381. https://doi.org/10.3390/microorganisms7100381
Zhao X, Li M, Liu Y. Microfluidic-Based Approaches for Foodborne Pathogen Detection. Microorganisms. 2019; 7(10):381. https://doi.org/10.3390/microorganisms7100381
Chicago/Turabian StyleZhao, Xihong, Mei Li, and Yao Liu. 2019. "Microfluidic-Based Approaches for Foodborne Pathogen Detection" Microorganisms 7, no. 10: 381. https://doi.org/10.3390/microorganisms7100381
APA StyleZhao, X., Li, M., & Liu, Y. (2019). Microfluidic-Based Approaches for Foodborne Pathogen Detection. Microorganisms, 7(10), 381. https://doi.org/10.3390/microorganisms7100381