Fusarium Species and Mycotoxins Contaminating Veterinary Diets for Dogs and Cats
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbiological Fungal Species Identification
2.2. Molecular Analysis
2.3. Ergosterol and Mycotoxins Analysis
2.3.1. Standards and Chemical Reagents
2.3.2. Extraction and Purification Procedure
Ergosterol
Deoxynivalenol, Nivalenol, and Zearalenone
Fumonisin B1
2.3.3. HPLC Analysis
- Waters 2996 Photodiode Array Detector with Nova Pak C-18 column (150 × 3.9 mm) and methanol:acetonitrile (90:10, v/v) as a mobile phase for ERG (λmax = 282 nm) analysis and with Nova Pak C-18 column (300 × 3.9 mm) and methanol:water (25:75, v/v) as a mobile phase for DON and NIV analysis (λmax = 224 nm),
- Waters 2475 Multi λ Fluorescence Detector (λex = 274 nm, λem = 440 nm) and Waters 2996 Photodiode Array Detector with Nova Pak C-18 column (150 × 3.9 mm) and acetonitrile:water:methanol (46:46:8, v/v/v) as a mobile phase for ZON analysis,
- Waters 2475 Multi λ Fluorescence Detector (λex = 335 nm, λem = 440 nm) with an XBridge column (3.0 × 100 mm) and methanol:sodium dihydrogen phosphate (0.1 M in water) solution (77:23, v/v) adjusted to pH 3.35 with o-phosphoric acid as a mobile phase for FB1 analysis after pre-column derivatization with o-phthaldialdehyde (OPA) reagent.
2.4. Statistical Analysis
3. Results
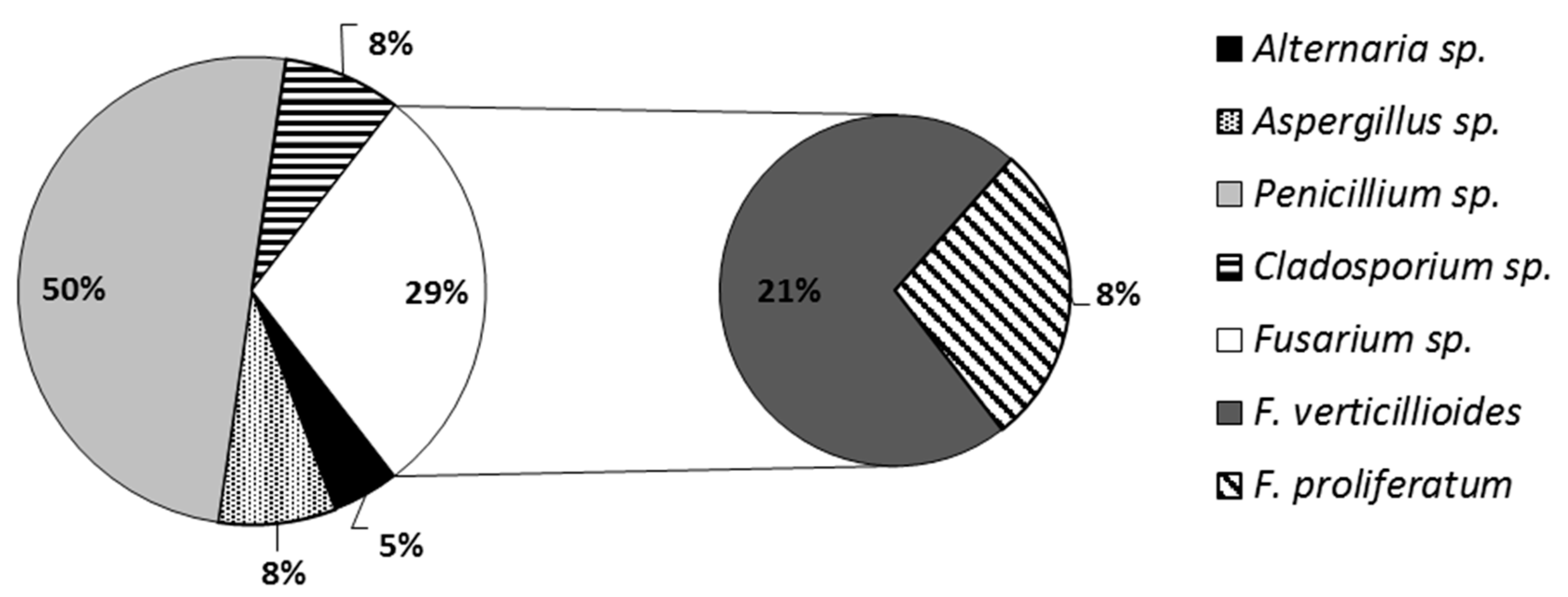
3.1. Microbiological and Molecular Fungal Species Identification
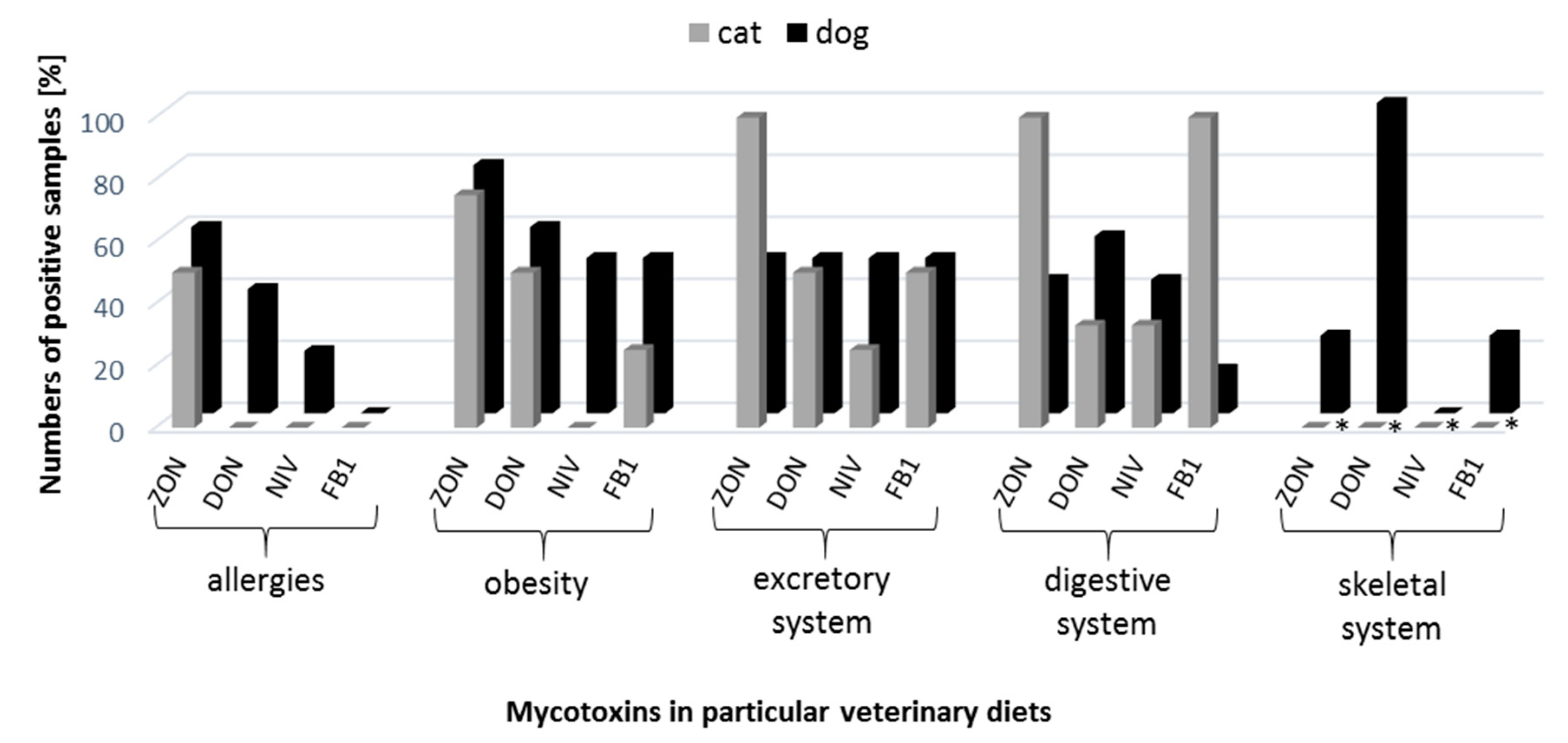
3.2. Ergosterol and Mycotoxins Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Richard, J.L.; Payne, G.A. CAST Report Mycotoxins: Risk in Plant, Animal, and Human Systems; Council for Agricultural Science and Technology Task Force Report, No. 139; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J. Limiting mycotoxins in stored wheat. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 644–650. [Google Scholar] [CrossRef]
- Hope, R.; Aldred, D.; Magan, N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett. Appl. Microbiol. 2005, 40, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Magan, N.; Ramos, A.J.; Sanchis, V. Fumonisin-producing strains of Fusarium: A review of their ecophysiology. J. Food Prot. 2004, 67, 1792–1805. [Google Scholar] [CrossRef]
- Popovski, S.; Celar, F.A. The impact of environmental factors on the infection of cereals with Fusarium species and mycotoxin production—A review. Acta Agric. Slov. 2013, 101, 105–116. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Perkowski, J.; Buśko, M.; Stuper, K.; Kostecki, M.; Matysiak, A.; Szwajkowska-Michałek, L. Concentration of ergosterol in small-grained naturally contaminated and inoculated cereals. Biologia 2008, 63, 542–547. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Stępień, Ł. Mycotoxins biosynthesized by plant-derived Fusarium isolates. Arh. Hig. Rada Toksikol. 2012, 63, 437–445. [Google Scholar] [CrossRef]
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Fan, Y.; Sun, L.; Ma, S.; Ji, C.; Ma, Q.; Zhang, J. Occurrence of mycotoxins in feed ingredients and complete feeds obtained from the Beijing region of China. J. Anim. Sci. Biotechnol. 2014, 5, 37–43. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Li, Y.; Li, T.; He, Q.; Tang, Y.; Liu, H.; Su, Y.; Yin, Y.; Liao, P. Aflatoxin B1, zearalenone and deoxynivalenol in feed ingredients and complete feed from different Province in China. J. Anim. Sci. Biotechnol. 2016, 7, 63–72. [Google Scholar] [CrossRef]
- Haschek, W.M.; Gumprecht, L.A.; Smith, G.; Tumbleson, M.E.; Constable, P.D. Fumonisin toxicosis in swine: AN overview of porcine pulmonary edema and current perspectives. Environ. Health Perspect. 2001, 109, 251–257. [Google Scholar]
- Marasas, W.F.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.A.; et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef]
- Marasas, W.F.; Kellerman, T.S.; Gelderblom, W.C.; Coetzer, J.A.; Thiel, P.G.; van der Lugt, J.J. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J. Vet. Res. 1988, 55, 197–203. [Google Scholar] [PubMed]
- Chanemougasoundharam, A.; Doohan, M. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 2013, 217, 149–158. [Google Scholar]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- COMMISSION RECOMMENDATION of 17 August 2006 on the Prevention and Reduction of Fusarium Toxins in Cereals and Cereal Products (2006/583/EC); European Commission: Brussels, Belgium, 2006.
- COMMISSION REGULATION (EC) No 1126/2007 of 28 September 2007 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Fusarium Toxins in Maize and Maize Products; European Commission: Brussels, Belgium, 2007.
- Leung, M.C.; Díaz-Llano, G.; Smith, T.K. Mycotoxins in pet food: A review on worldwide prevalence and preventative strategies. J. Agric. Food Chem. 2006, 54, 9623–9635. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Dalcero, A.; Magnoli, C.; Luna, M.; Ancasi, G.; Reynaro, M.M.; Chiacchiera, S.; Miazzo, R.; Palacio, G. Mycoflora and naturally occurring mycotoxins in poultry feeds in Argentina. Mycopathologia 1998, 141, 37–43. [Google Scholar] [CrossRef] [PubMed]
- González Pereyra, M.L.; Chiacchiera, S.M.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. Fungal and mycotoxin contamination in mixed feeds: Evaluating risk in cattle intensive rearing operations (feedlots). Rev. Bio Ciencias 2012, 2, 68–80. [Google Scholar]
- González Pereyra, M.L.; Keller, K.M.; Keller, L.A.M.; Cavaglieri, L.R.; Queiroz, B.; Tissera, J. Mycobiota and mycotoxins of equine feedstuffs in the central region of Argentina. Rev. Bras. Med. Vet. 2009, 31, 24–29. [Google Scholar]
- González Pereyra, M.L.; Pereyra, C.M.; Ramirez, M.L.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. Determination of mycobiota and mycotoxins in pig feed in central Argentina. Lett. Appl. Microbiol. 2008, 46, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Grajewski, J.; Błajet-Kosicka, A.; Twarużek, M.; Kosicki, R. Occurrence of mycotoxins in Polish animal feed in years 2006–2009. J. Anim. Physiol. Anim. Nutr. 2012, 96, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Krnjaja, V.; Stojanović, L.; Ćmiljanić, R.; Trenkovski, S.; Tomsević, D. The presence of potentially toxigenic fungi in poultry feed. Biotechnol. Anim. Husb. 2008, 24, 87–93. [Google Scholar] [CrossRef]
- Labuda, R.; Tančinová, D. Fungi recovered from Slovakian poultry feed mixtures and their toxinogenity. Ann. Agric. Environ. Med. 2006, 13, 193–200. [Google Scholar]
- Basalan, M.; Hismiogullari, S.E.; Hismiogullari, A.A.; Filazi, A. Fungi and aflatoxin B1 in horse and dog feeds in Western Turkey. Rev. Med. Vet. 2004, 156, 248–252. [Google Scholar]
- Błajet-Kosicka, A.; Kosicki, R.; Twarużek, M.; Grajewski, J. Determination of moulds and mycotoxins in dry dog and cat food using liquid chromatography with mass spectrometry and fluorescence detection. Food Addit. Contam. Part B Surveill. 2014, 7, 302–308. [Google Scholar]
- Gazzotti, T.; Biagi, G.; Pagliuca, G.; Pinna, C.; Scardilli, M.; Grandi, M.; Zaghini, G. Occurrence of mycotoxins in extruded commercial dog food. Anim. Feed Sci. Technol. 2015, 202, 81–89. [Google Scholar] [CrossRef]
- Martins, M.L.; Martins, H.M.; Bernardo, F. Fungal flora and mycotoxins detection in commercial pet food. Rev. Port. Cienc. Vet. 2003, 98, 179–183. [Google Scholar]
- Scudamore, K.A.; Hetmanski, M.T.; Nawaz, S.; Naylor, J.; Rainbird, S. Determination of mycotoxins in pet foods sold for domestic pets and wild birds using linked-column immunoassay clean-up and HPLC. Food Addit. Contam. 1997, 14, 175–186. [Google Scholar] [CrossRef]
- Bailey, W.S.; Groth, A.H. The relationship of hepatitis X in dogs and moldy corn poisoning of swine. J. Am. Vet. Med. Assoc. 1959, 134, 483–486. [Google Scholar]
- Garland, T.; Reagor, J. Chronic canine aflatoxicosis and management of an epidemic. In Mycotoxins and Phycotoxins in Perspective at the Turn of the Millennium; deKoe, W., Samson, R., van Egmond, H., Gilbert, J., Sabino, M., Eds.; Ponsen and Looven: Wageningen, The Netherlands, 2001; pp. 231–236. [Google Scholar]
- Stenske, K.A.; Smith, J.R.; Newman, S.J.; Newman, L.B.; Kirk, C.A. Aflatoxicosis in dogs and dealing with suspected contaminated commercial foods. J. Am. Vet. Med. Assoc. 2006, 228, 1686–1691. [Google Scholar] [CrossRef]
- Bastianello, S.S.; Nesbit, J.W.; Williams, M.C.; Lange, A.L. Pathological findings in a natural outbreak of aflatoxicosis in dogs. Onderstepoort J. Vet. Res. 1987, 54, 635–640. [Google Scholar]
- Krishnamachari, K.; Bhat, R.; Nagarajan, V.; Tilak, T. Hepatitis due to aflatoxicosis: An outbreak in western India. Lancet 1975, 305, 1061–1063. [Google Scholar] [CrossRef]
- Gareis, M.; Reubel, G.; Kröning, T.; Porzig, R. Ein Fall von infektiosem Welpensterben bei Afaghanen in Verbindüng mit der Verfutterung von Ochratoxin A - haltigem Milchpulver. Tieraerztl. Umsch. 1987, 42, 77–80. [Google Scholar]
- Jeong, W.I.; Do, S.H.; Jeong, D.H.; Chung, J.Y.; Yang, H.J.; Yuan, D.W.; Hong, I.H.; Park, J.K.; Goo, M.J.; Jeong, K.S. Canine renal failure syndrome in three dogs. J. Vet. Sci. 2006, 7, 299–301. [Google Scholar] [CrossRef]
- Little, C.J.L.; McNeil, P.E.; Robb, J. Hepatopathy and dermatitis in a dog associated with the ingestion of mycotoxins. J. Small Anim. Pract. 1991, 32, 23–26. [Google Scholar] [CrossRef]
- Newman, S.J.; Smith, J.R.; Stenske, K.A.; Newman, L.B.; Dunlap, J.R.; Imerman, P.M.; Kirk, C.A. Aflatoxicosis in nine dogs after exposure to contaminated commercial dog food. J. Vet. Diagn. Investig. 2007, 19, 168–175. [Google Scholar] [CrossRef]
- Stępień, Ł.; Jestoi, M.; Chełkowski, J. Cyclic hexadepsipeptides in wheat field samples and esyn1 gene divergence among enniatin producing Fusarium avenaceum strains. World Mycotoxin J. 2013, 6, 399–409. [Google Scholar] [CrossRef]
- .Waśkiewicz, A.; Morkunas, I.; Bednarski, W.; Mai, V.C.; Formela, M.; Beszterda, M.; Wiśniewska, H.; Goliński, P. Deoxynivalenol and oxidative stress indicators in winter wheat inoculated with Fusarium graminearum. Toxins 2014, 6, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. FUM cluster divergence in fumonisins-producing Fusarium species. Fungal Biol. 2011, 115, 112–123. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Cegielska-Radziejewska, R.; Stuper-Szablewska, K.; Szablewski, T. Microflora and mycotoxin contamination in poultry feed mixtures from western Poland. Ann. Agric. Environ. Med. 2013, 20, 30–35. [Google Scholar] [PubMed]
- Boermans, H.J.; Leung, M.C.K. Mycotoxins and the pet food industry: Toxicological evidence and risk assessment. Int. J. Food Microbiol. 2007, 119, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Fandohan, P.; Hell, K.; Marasas, W.F.O.; Wingfield, M.J. Infection of maize by Fusarium species and contamination with fumonisin in Africa. Afr. J. Biotechnol. 2003, 2, 570–579. [Google Scholar]
- Milićević, D.R.; Škrinjar, M.; Baltić, T. Real and perceived risks for mycotoxin contamination in foods and feeds: Challenges for food safety control. Toxins 2010, 2, 572–592. [Google Scholar] [CrossRef] [PubMed]
- Milićević, D.; Nikšic, M.; Baltić, T.; Vranic, D.; Stefanovic, S.; Janković, S. A Survey of occurrence of toxigenic fungi and mycotoxins in pig feed samples-Use in evaluation of risk assessment. Vet. World 2010, 3, 305–311. [Google Scholar] [CrossRef]
- Bueno, D.J.; Silva, J.O.; Oliver, G. Mycoflora in commercial pet foods. J. Food Prot. 2001, 64, 741–743. [Google Scholar] [CrossRef]
- Campos, S.G.; Cavaglieri, L.R.; Fernández Juri, M.G.; Dalcero, A.M.; Krüger, C.; Keller, L.A.M.; Rosa, C.A.R. Mycobiota and aflatoxins in raw materials and pet food in Brazil. J. Anim. Physiol. Anim. Nutr. 2008, 92, 377–383. [Google Scholar] [CrossRef]
- Copetti, M.V.; Santurio, J.M.; Cavalheiro, A.S.; Alves, S.H.; Ferreiro, L. Comparison of different culture media for mycological evaluation of commercial pet food. Act. Sci. Vet. 2009, 37, 329–335. [Google Scholar]
- Ismaiel, A.A.; Papenbrock, J. Mycotoxins: Producing fungi and mechanisms of phytotoxicity. Agricuture 2015, 5, 492–537. [Google Scholar] [CrossRef]
- Greco, M.V.; Franchi, M.L.; Rcio Golba, S.L.; Pardo, A.G.; Pose, G.N. Mycotoxins and mycotoxigenic fungi in poultry feed for food-producing animals. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of climatic factors on Fusarium species pathogenic to cereals. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins in maize ears epidemiology of mycotoxin producing fungi. Eur. J. Plant Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Czembor, E.; Stępień, Ł.; Waśkiewicz, A. The impact of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in Poland. PLoS ONE 2015, 10, e0133644. [Google Scholar] [CrossRef]
- Xu, X.M.; Parry, D.W.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Edwards, S.G.; Cooke, B.M.; Doohan, F.M.; Brennan, J.M.; Moretti, A.; et al. Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. Eur. J. Plant Pathol. 2005, 112, 143–154. [Google Scholar] [CrossRef]
- Gimeno, A.; Martins, M.L. Los hongos y las micotoxinas en la alimentaci´animal; conceptos, problemas, control y recomendaciones. 2002. Available online: https://www.engormix.com/micotoxinas/articulos/los-hongos-micotoxinas-alimentacion-t26085.htm (accessed on 28 December 2018).
- Kubizna, J.; Jamroz, D.; Kubizna, J.K. Contamination of feed mixtures with mycoflora in south-western Poland. EJPAU 2011, 14, 8. [Google Scholar]
- De Castro, M.F.P.M.; Bragagnolo, N.; de Toledo Valentini, S.R. The relationship between fungi growth and aflatoxin production with ergosterol content of corn grains. Braz. J. Microbiol. 2002, 33, 22–26. [Google Scholar] [CrossRef]
- Stanisz, E.; Zgoła-Grześkowiak, A.; Waśkiewicz, A.; Stępień, Ł.; Beszterda, M. Can ergosterol be an indicator of Fusarium fungi and mycotoxins in cereal products? J. Braz. Chem. Soc. 2015, 26, 705–712. [Google Scholar]
- Ng, H.E.; Raj, S.S.A.; Wong, S.H.; Tey, D.; Tan, H.M. Estimation of fungal growth using the ergosterol assay: A rapid tool in assessing the microbiological status of grains and feeds. Lett. Appl. Microbiol. 2008, 46, 113–118. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Mari, N.; Goto, T. The relationship between ergosterol and mycotoxin contamination in maize from various countries. Mycotoxin Res. 2015, 31, 91–99. [Google Scholar] [CrossRef]
- Bissoqui, L.Y.; Frehse, M.S.; Freire, R.L.; Ono, M.A.; Bordini, J.G.; Hirozawa, M.T.; de Oliveira, A.J.; Ono, E.Y.S. Exposure assessment of dogs to mycotoxins through consumption of dry feed. J. Sci. Food Agric. 2016, 96, 4135–4142. [Google Scholar] [CrossRef]
- Gutarowska, B.; Żakowska, Z. Estimation of fungal contamination of various plant materials with UV-determination of fungal ergosterol. Ann. Microbiol. 2010, 60, 415–422. [Google Scholar] [CrossRef]
- Böhm, J.; Koinig, L.; Razzazi-Fazeli, E.; Błajet-Kosicka, A.; Twarużek, M.; Grajewski, J.; Lang, C. Survey and risk assessment of the mycotoxins deoxynivalenol, zearalenone, fumonisins, ochratoxin A, and aflatoxins in commercial dry dog food. Mycotoxin Res. 2010, 26, 147–153. [Google Scholar] [CrossRef]
- Hołda, K.; Głogowski, R. A survey of deoxynivalenol and zearalenone content in commercial dry foods for growing dogs. Ann. Warsaw Univ. Life Sci. SGGW Anim. Sci. 2014, 53, 111–117. [Google Scholar]
- Mulunda, M.; Ndou, R.V.; Dzoma, B.; Nyirenda, M.; Bakunzi, F. Canine aflatoxicosis outbreak in South Africa (2011): A possible multi-mycotoxins aetiology. J. S. Afr. Vet. Assoc. 2013, 84, 1–5. [Google Scholar] [CrossRef]
- Songsermsakul, P.; Razzazi-Fazeli, E.; Böhm, J.; Zentek, J. Occurrence of deoxynivalenol (DON) and ochratoxin A (OTA) in dog foods. Mycotoxin Res. 2007, 23, 65–67. [Google Scholar] [CrossRef]
- Zwierzchowski, W.; Gajęcki, M.; Obremski, K.; Zielonka, Ł.; Baranowski, M. The occurrence of zearalenone and its derivatives in standard and therapeutic feeds for companion animals. Pol. J. Vet. Sci. 2004, 7, 289–293. [Google Scholar]
- Hughes, D.M.; Gahl, M.J.; Graham, C.H.; Grieb, S.L. Overt signs of toxicity to dogs and cats of dietary deoxynivalenol. J. Anim. Sci. 1999, 77, 693–700. [Google Scholar] [CrossRef]
- Pestka, J.J. Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 2010, 3, 323–347. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Pandey, A.; Goyary, D.; Chaurasia, A.; Singh, L.; Veer, V. Technetium-99m-labeled deoxynivalenol from Fusarium mycotoxin alters organ toxicity in BALB/c mice by oral and intravenous route. J. Venom. Anim. Incl. Trop. Dis. 2012, 18, 258–263. [Google Scholar] [CrossRef]
- De Souza, K.K.; Scussel, V.M. Occurrence of dogs and cats diseases records in the veterinary clinics routine in South Brazil and its relationship to mycotoxins. Int. J. Appl. Sci. Technol. 2012, 2, 129–134. [Google Scholar]
- Kempe, R.; Saastamoinen, M.; Hyyppä, S. Composition digestibility and nutritive value of cereals for dogs. Agric. Food Sci. 2004, 13, 5–17. [Google Scholar] [CrossRef]
| Specification of Diet | Sample No. | Cereal Component | ||
|---|---|---|---|---|
| Rice | Maize | Wheat | ||
| Allergies | cD21 | + | - | - |
| cD22 | + | - | - | |
| dD34 | + | - | - | |
| dD35 | + | - | - | |
| dD68 | - | - | - | |
| dD69 | - | - | - | |
| dD77 | + | - | - | |
| Overweight/obesity | cD3 | - | - | + |
| cD10 | + | + | - | |
| cD11 | + | + | - | |
| cD20 | + | + | - | |
| dD31 | - | + | + | |
| dD32 | - | + | + | |
| dD62 | + | + | - | |
| dD64 | - | + | + | |
| dD74 | - | + | - | |
| Excretory system diseases | cD5 | - | + | - |
| cD7 | + | + | + | |
| cD8 | + | + | - | |
| cD9 | - | + | + | |
| cD17 | + | + | - | |
| cD19 | + | + | - | |
| cD27 | + | + | + | |
| cD28 | + | - | - | |
| dD38 | - | + | + | |
| dD44 | - | + | + | |
| dD61 | + | + | - | |
| dD73 | + | + | - | |
| Digestive system diseases | cD6 | + | + | + |
| cD18 | + | + | - | |
| cD23 | + | + | - | |
| dD37 | - | + | + | |
| dD63 | + | + | - | |
| dD66 | - | + | - | |
| dD70 | - | - | + | |
| dD71 | + | + | - | |
| dD72 | + | - | - | |
| dD75 | + | + | - | |
| Skeletal system diseases | dD41 | + | + | + |
| dD65 | + | + | - | |
| dD67 | + | + | - | |
| dD76 | + | + | - | |
| Specification of Diet | Sample No. | Fusarium | ERG [μg/g] | Mycotoxins (ng/g) | ||||
|---|---|---|---|---|---|---|---|---|
| Fp | Fv | ZON | DON | NIV | FB1 | |||
| Allergies | ||||||||
| Food allergy | cD21 | - | - | 1.04 ± 0.06 | n.d. | n.d. | n.d. | n.d. |
| cD22 | - | - | 0.79 ± 0.08 | 1.71 ± 0.43 | n.d. | n.d. | n.d. | |
| dD34 | - | + | 0.42 ± 0.03 | 45.84 ± 5.79 | n.d. | n.d. | n.d. | |
| dD35 | - | - | 0.06 ± 0.01 | 11.88 ± 3.24 | n.d. | n.d. | n.d. | |
| dD69 | - | - | 0.32 ± 0.03 | n.d. | 61.90 ± 3.48 | 38.92 ± 8.68 | n.d. | |
| Skin allergy | dD77 | - | - | 2.44 ± 0.21 | n.d. | n.d. | n.d. | n.d. |
| Other allergy | dD68 | - | + | 0.12 ± 0.01 | 2.07 ± 0.40 | 185.40 ± 19.01 | n.d. | n.d. |
| Overweight/obesity | ||||||||
| Obesity management | cD3 | - | - | 0.36 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| cD10 | - | + | 3.03 ± 0.13 | 24.81 ± 4.64 | n.d. | n.d. | n.d. | |
| cD11 | - | - | 1.46 ± 0.03 | 7.79 ± 0.28 | n.d. | n.d. | n.d. | |
| cD20 | - | + | 2.16 ± 0.10 | 7.64 ± 0.34 | 351.02 ± 17.82 | n.d. | 5.02 ± 0.12 | |
| dD31 | - | - | 1.10 ± 0.17 | 7.72 ± 1.28 | n.d. | n.d. | n.d. | |
| dD32 | - | + | 0.57 ± 0.04 | 7.80 ± 0.36 | n.d. | 21.33 ± 4.11 | 15.84 ± 4.43 | |
| dD62 | - | + | 0.72 ± 0.03 | 6.41 ± 1.03 | 103.33 ± 11.38 | 190.90 ± 12.42 | 59.05 ± 2.93 | |
| dD64 | + | - | 0.67 ± 0.05 | 1.78 ± 0.34 | 257.68 ± 14.04 | n.d. | n.d. | |
| dD74 | - | - | 1.63 ± 0.08 | n.d. | 2318.05 ± 93.43 | n.d. | n.d. | |
| Excretory system diseases | ||||||||
| Renal system diseases | cD7 | - | - | 3.05 ± 0.11 | 2.71 ± 0.39 | n.d. | n.d. | n.d. |
| cD9 | + | + | 1.94 ± 0.07 | 7.86 ± 0.39 | n.d. | n.d. | n.d. | |
| cD17 | - | - | 4.79 ± 0.20 | 3.92 ± 1.09 | n.d. | n.d. | n.d. | |
| cD28 | - | - | 6.57 ± 0.23 | 9.05 ± 1.76 | n.d. | n.d. | n.d. | |
| dD38 | + | + | 1.99 ± 0.17 | n.d. | n.d. | n.d. | 42.40 ± 4.08 | |
| Kidney diseases | cD5 | - | + | 2.26 ± 0.12 | 4.32 ± 0.37 | 53.53 ± 3.82 | n.d. | 21.50 ± 3.28 |
| cD8 | + | + | 2.03 ± 0.07 | 19.99 ± 1.42 | 116.67 ± 5.49 | n.d. | 26.29 ± 5.44 | |
| cD19 | - | + | 2.95 ± 0.09 | 6.34 ± 0.47 | 79.69 ± 7.12 | n.d. | 70.02 ± 3.92 | |
| cD27 | + | + | 0.80 ± 0.01 | 2.25 ± 0.26 | 188.62 ± 13.41 | 37.25 ± 4.55 | 74.83 ± 5.02 | |
| dD44 | + | - | 3.15 ± 0.17 | n.d. | n.d. | 39.82 ± 1.35 | 61.94 ± 5.54 | |
| dD61 | - | + | 0.29 ± 0.02 | 1.95 ± 0.20 | 448.35 ± 16.78 | 86.23 ± 4.30 | n.d. | |
| dD73 | - | - | 0.99 ± 0.05 | 1.51 ± 0.26 | 93.93 ± 6.66 | n.d. | n.d. | |
| Digestive system diseases | ||||||||
| Intestinal tract diseases | cD18 | - | + | 4.02 ± 0.09 | 1.77 ± 0.24 | n.d. | n.d. | 41.22 ± 4.21 |
| dD63 | + | - | 0.43 ± 0.03 | n.d. | 191.75 ± 9.93 | n.d. | 14.50 ± 2.89 | |
| dD66 | - | - | 0.58 ± 0.03 | 1.90 ± 0.41 | 27.76 ± 2.68 | 106.29 ± 10.43 | n.d. | |
| dD72 | - | + | 1.44 ± 0.14 | 3.16 ± 0.27 | 296.13 ± 7.37 | 53.98 ± 2.89 | n.d. | |
| dD75 | - | - | 0.99 ± 0.04 | n.d. | 94.85 ± 10.19 | n.d. | n.d. | |
| Liver diseases | cD6 | - | + | 2.62 ± 0.16 | 8.96 ± 1.13 | 41.53 ± 4.66 | n.d. | 41.64 ± 5.39 |
| cD23 | - | + | 4.59 ± 0.22 | 7.14 ± 0.34 | n.d. | 28.28 ± 3.32 | 23.46 ± 3.82 | |
| dD37 | - | - | 0.31 ± 0.02 | n.d. | n.d. | n.d. | n.d. | |
| dD70 | - | - | 0.29 ± 0.03 | n.d. | n.d. | n.d. | n.d. | |
| dD71 | - | - | 4.88 ± 0.15 | 3.37 ± 0.58 | n.d. | 20.06 ± 2.58 | n.d. | |
| Skeletal system diseases | ||||||||
| Bone disorders | dD41 | - | + | 0.60 ± 0.05 | n.d. | 140.63 ± 16,63 | n.d. | n.d. |
| dD67 | - | - | 1.07 ± 0.07 | n.d. | 37.23 ± 4.55 | n.d. | 16.66 ± 1.42 | |
| dD76 | - | - | 0.53 ± 0.03 | 40.07 ± 3.06 | 394.86 ± 8.55 | n.d. | n.d. | |
| Teeth | dD65 | - | - | 0.60 ± 0.03 | n.d. | 42.23 ± 4.62 | n.d. | n.d. |
| Specification of Diet | ERG [μg/g] | ||
|---|---|---|---|
| Minimum | Maximum | Mean ± s.d. | |
| Allergies | 0.05 | 2.68 | 0.74 ± 0.79 c |
| Obesity/overweight | 0.35 | 3.16 | 1.30 ± 0.84 bc |
| Excretory system diseases | 0.27 | 6.82 | 2.57 ± 1.70 a |
| Digestive system diseases | 0.25 | 5.04 | 2.01 ± 1.79 ab |
| Skeletal system diseases | 0.51 | 1.13 | 0.70 ± 0.23 c |
| LSD0.05 | 0.90 | ||
| Specification of Diet | ZON [ng/g] | FB1 [ng/g] | DON [ng/g] | NIV [ng/g] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± s.d. | Min | Max | Mean ± s.d. | Min | Max | Mean ± s.d. | Min | Max | Mean ± s.d. | |
| Allergies | 1.22 | 51.7 | 15.38 ± 19.07 b | n.d. | n.d. | n.d. | 59.61 | 202.58 | 123.65 ± 68.7 b | 30.18 | 47.6 | 38.92 ± 8.68 b |
| Obesity/overweight | 1.49 | 30.16 | 9.14 ± 7.05 c | 4.89 | 61.29 | 26.64 ± 24.90 b | 26.43 | 2415.03 | 611.89 ± 891.47 a | 17.71 | 200.4 | 106.12 ± 93.24 a |
| Excretory system diseases | 1.25 | 21.38 | 5.99 ± 5.40 c | 18.84 | 80.13 | 49.50 ± 21.73 a | 49.81 | 463.22 | 163.46 ± 138.25 b | 32.23 | 90.9 | 53.68 ± 20.94 b |
| Digestive system diseases | 1.54 | 10.15 | 4.38 ± 2.83 c | 11.27 | 47.29 | 30.21 ± 12.70 b | 24.87 | 303.47 | 130.40 ± 104.6 b | 17.43 | 118.3 | 52.15 ± 35.51 b |
| Skeletal system diseases | 36.55 | 42.14 | 40.07 ± 3.00 a | 15.45 | 18.22 | 16.66 ± 1.42 b | 33.18 | 404.52 | 153.73 ± 151.8 b | n.d. | n.d. | n.d. |
| LSD0.05 | 5.69 | LSD0.05 | 13.3 | LSD0.05 | 294.2 | LSD0.05 | 31.6 | |||||
| ERG | ZON | DON | NIV | FB1 | |
|---|---|---|---|---|---|
| ERG | 1 | −0.115 | 0.096 | −0.408 * | 0.099 |
| ZON | 1 | 0.281 | −0.022 | −0.183 | |
| DON | 1 | −0.203 | −0.271 | ||
| NIV | 1 | 0.343 | |||
| FB1 | 1 | ||||
| * p < 0.05 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witaszak, N.; Stępień, Ł.; Bocianowski, J.; Waśkiewicz, A. Fusarium Species and Mycotoxins Contaminating Veterinary Diets for Dogs and Cats. Microorganisms 2019, 7, 26. https://doi.org/10.3390/microorganisms7010026
Witaszak N, Stępień Ł, Bocianowski J, Waśkiewicz A. Fusarium Species and Mycotoxins Contaminating Veterinary Diets for Dogs and Cats. Microorganisms. 2019; 7(1):26. https://doi.org/10.3390/microorganisms7010026
Chicago/Turabian StyleWitaszak, Natalia, Łukasz Stępień, Jan Bocianowski, and Agnieszka Waśkiewicz. 2019. "Fusarium Species and Mycotoxins Contaminating Veterinary Diets for Dogs and Cats" Microorganisms 7, no. 1: 26. https://doi.org/10.3390/microorganisms7010026
APA StyleWitaszak, N., Stępień, Ł., Bocianowski, J., & Waśkiewicz, A. (2019). Fusarium Species and Mycotoxins Contaminating Veterinary Diets for Dogs and Cats. Microorganisms, 7(1), 26. https://doi.org/10.3390/microorganisms7010026







