Combination of Posaconazole and Amphotericin B in the Treatment of Candida glabrata Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Organism and Growth Conditions
2.2. Antifungal Drugs
2.3. Minimum Inhibitory Concentrations (MICs)
2.4. Minimum Fungicidal Concentration (MFC)
2.5. Minimum Biofilm Eradication Concentration (MBEC)
2.6. XTT Reduction and Checkerboard Assay
2.7. Interpretation of Drug Combination Interaction
2.8. Biofilm Total Biomass Quantification—Crystal Violet Staining
2.9. Statistical Analysis
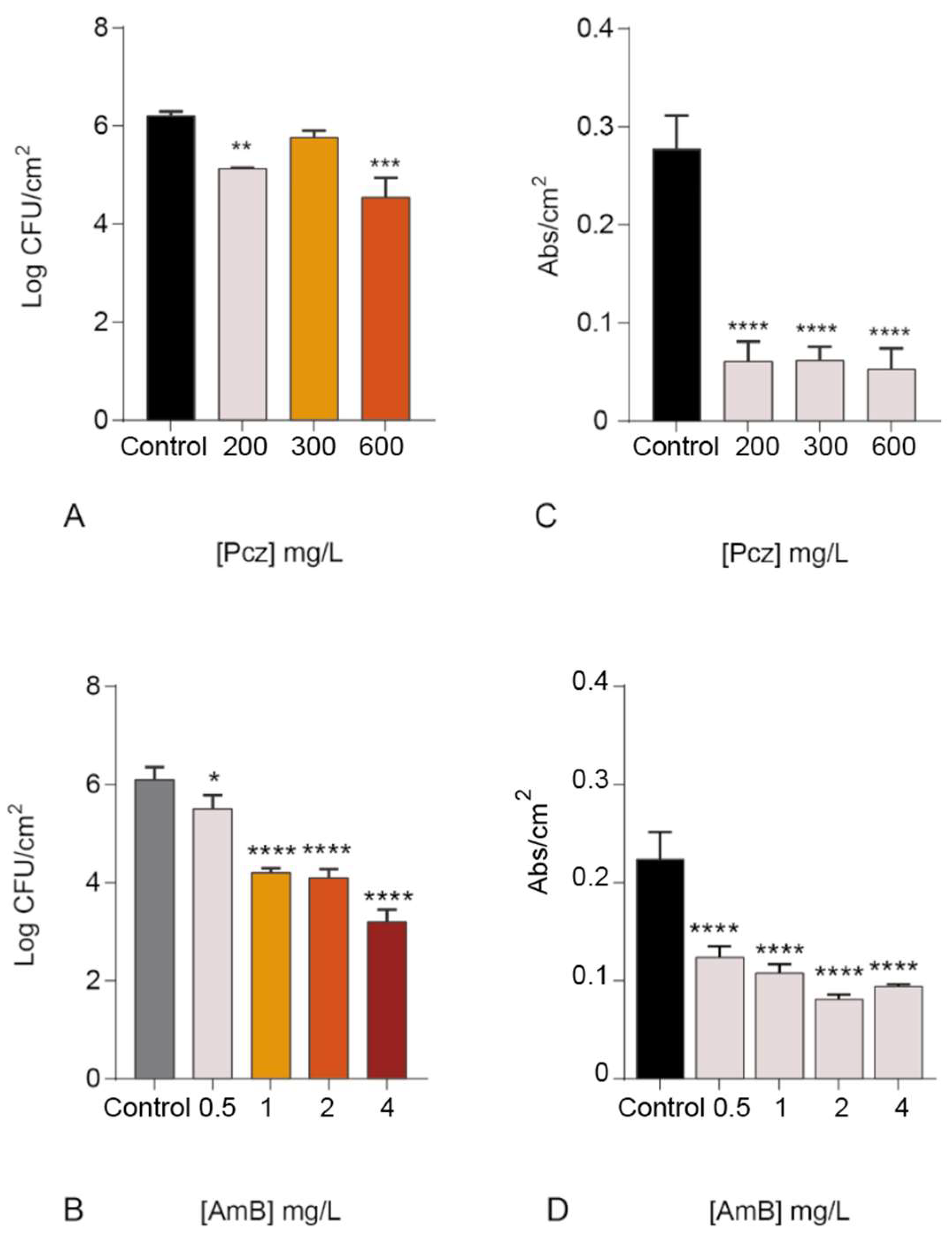
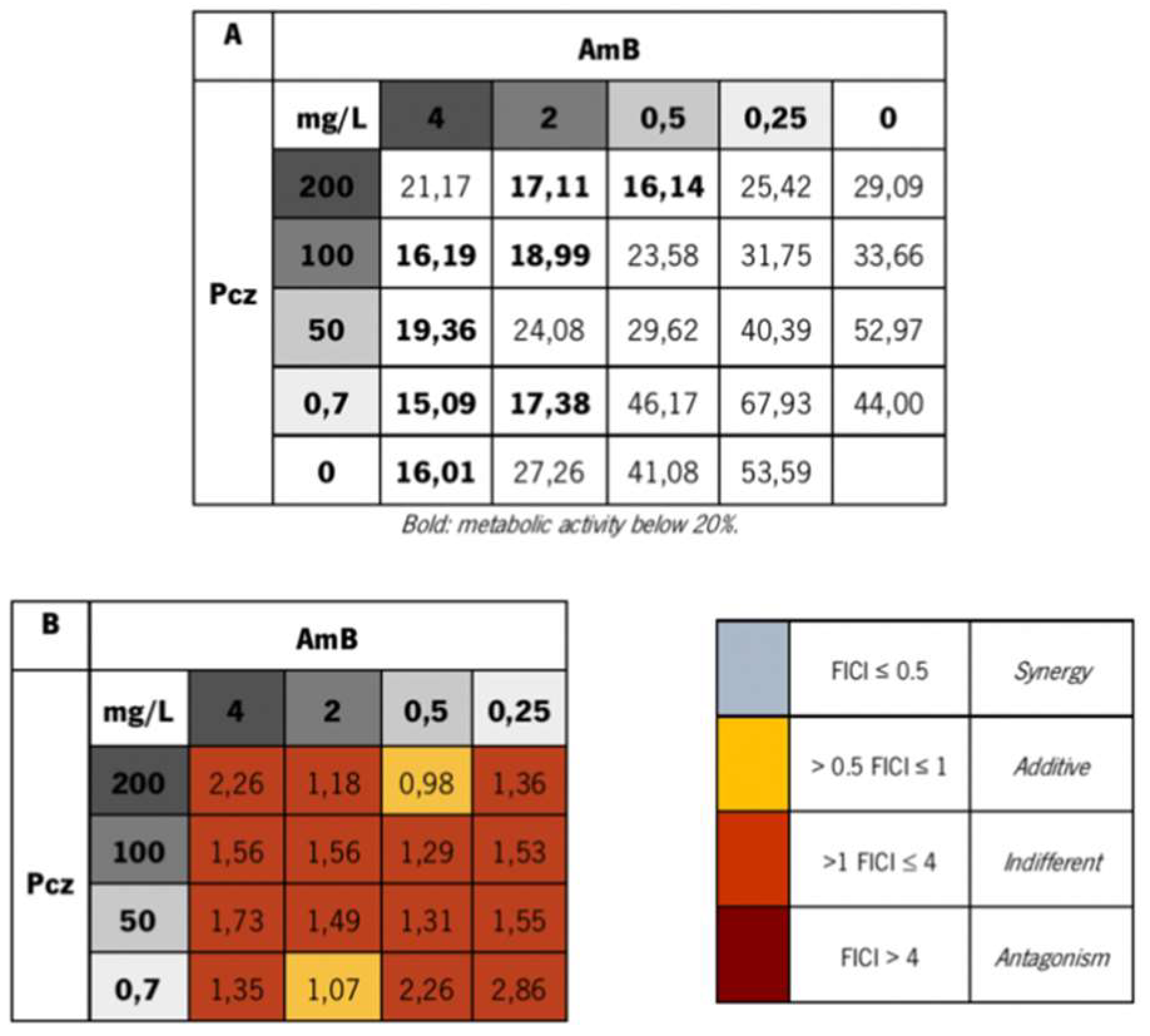
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodrigues, C.F.; Rodrigues, M.; Silva, S.; Henriques, M. Candida glabrata Biofilms: How Far Have We Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G. Invasive candidiasis. Infect. Dis. Clin. N. Am. 2006, 20, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Silva, S.; Henriques, M. Candida glabrata: A review of its features and resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Boas, D.V.; Haynes, K.; Henriques, M.; Rodrigues, C.F.; Vilas Boas, D.; Haynes, K.; Henriques, M. The MNN2 Gene Knockout Modulates the Antifungal Resistance of Biofilms of Candida glabrata. Biomolecules 2018, 8, 130. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Henriques, M. Liposomal and Deoxycholate Amphotericin B Formulations: Effectiveness against Biofilm Infections of Candida spp. Pathogens 2017, 6, 13. [Google Scholar] [CrossRef]
- Tscherner, M.; Schwarzmüller, T.; Kuchler, K. Pathogenesis and Antifungal Drug Resistance of the Human Fungal Pathogen Candida glabrata. Pharmaceuticals 2011, 4, 169–186. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M.C.R. Promising alternative therapeutics for oral candidiasis. Curr. Med. Chem. 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.; Nickels, J.; Edlind, T. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 2000, 44, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Susceptibility of Candida glabrata biofilms to echinocandins: Alterations in the matrix composition. Biofouling 2018, 892–7014. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Tronchin, G.; Larcher, G.; Ernoult, E.; Bergès, T.; Chabasse, D.; Bouchara, J.-P. A nonsense mutation in the ERG6 gene leads to reduced susceptibility to polyenes in a clinical isolate of Candida glabrata. Antimicrob. Agents Chemother. 2008, 52, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Tronchin, G.; Bergès, T.; Hennequin, C.; Chabasse, D.; Bouchara, J.-P. Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrob. Agents Chemother. 2007, 51, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Sanguinetti, M.; De Bernardis, F.; Torelli, R.; Posteraro, B.; Vandeputte, P.; Sanglard, D. Loss of mitochondrial functions associated with azole resistance in Candida glabrata results in enhanced virulence in mice. Antimicrob. Agents Chemother. 2011, 55, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Prażyńska, M.; Gospodarek, E. In vitro effect of amphotericin B on Candida albicans, Candida glabrata and Candida parapsilosis biofilm formation. Mycopathologia 2014, 177, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Shin, J.H.; Jung, S.I.; Park, K.H.; Cho, D.; Kee, S.J.; Shin, M.G.; Suh, S.P.; Ryang, D.W. Species-specific differences in the susceptibilities of biofilms formed by Candida bloodstream isolates to echinocandin antifungals. Antimicrob. Agents Chemother. 2007, 51, 1520–1523. [Google Scholar] [CrossRef]
- Colombo, A.L.; Nucci, M.; Park, B.J.; Nouér, S.A.; Arthington-Skaggs, B.; da Matta, D.A.; Warnock, D.; Morgan, J. Epidemiology of candidemia in Brazil: A nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 2006, 44, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Zarei Mahmoudabadi, A.; Rezaei-Matehkolaei, A.; Ghanavati, F. The Susceptibility Patterns of Candida Species Isolated From Urine Samples to Posaconazole and Caspofungin. Jundishapur J. Microbiol. 2015, 8. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Prakash, A.; Meletiadis, J.; Sharma, C.; Chowdhary, A. Candida auris: Comparison of the EUCAST and CLSI reference microdilution MICs for eight antifungal compounds and associated tentative ECOFFs. Antimicrob. Agents Chemother. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vuichard, D.; Weisser, M.; Orasch, C.; Frei, R.; Heim, D.; Passweg, J.R.; Widmer, A.F. Weekly use of fluconazole as prophylaxis in haematological patients at risk for invasive candidiasis. BMC Infect. Dis. 2014, 14, 573. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Walsh, T.J. Antifungal efficacy and pharmacodynamics of posaconazole in experimental models of invasive fungal infections. Mycoses 2006, 49, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Skiest, D.J.; Vazquez, J.A.; Anstead, G.M.; Graybill, J.R.; Reynes, J.; Ward, D.; Hare, R.; Boparai, N.; Isaacs, R. Posaconazole for the Treatment of Azole-Refractory Oropharyngeal and Esophageal Candidiasis in Subjects with HIV Infection. Clin. Infect. Dis. 2007, 44, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Chau, A.S.; Mendrick, C.A.; Sabatelli, F.J.; Loebenberg, D.; McNicholas, P.M. Application of real-time quantitative PCR to molecular analysis of Candida albicans strains exhibiting reduced susceptibility to azoles. Antimicrob. Agents Chemother. 2004, 48, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.; Rawlings, B.; Caffrey, P. Streptomyces nodosus host strains optimized for polyene glycosylation engineering. Biosci. Biotechnol. Biochem. 2012, 76, 384–387. [Google Scholar] [CrossRef]
- Storm, G.; van Etten, E. Biopharmaceutical aspects of lipid formulations of amphotericin B. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 64–73. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Gonçalves, B.; Rodrigues, M.; Silva, S.; Azeredo, J.; Henriques, M. The Effectiveness of Voriconazole in Therapy of Candida glabrata’s Biofilms Oral Infections and Its Influence on the Matrix Composition and Gene Expression. Mycopathologia 2017, 182, 653–664. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Arikan, S.; Barchiesi, F.; Bille, J.; Dannaoui, E.; Denning, D.W.; Donnelly, J.P.; Fegeler, W.; Moore, C.; Richardson, M.; et al. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia—Forming moulds. ESCMID Tech. Notes 2008, 14, 982–984. [Google Scholar] [CrossRef]
- EUCAST Breakpoint Tables for Interpretation of MICs, Version 8.1, Valid from 2017-03-01; Version 8.1. Available online: http://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/ (accessed on 30 July 2017).
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Moody, J. Synergism testing. Broth microdilution checkerboard and broth microdilution methods. In Clinical Microbiology Procedures Handbook; Isenberg, H., Ed.; American Society for Microbiology: Washington, DC, USA, 1991; pp. 1–28. [Google Scholar]
- Sopirala, M.M.; Mangino, J.E.; Gebreyes, W.A.; Biller, B.; Bannerman, T.; Balada-Llasat, J.-M.; Pancholi, P. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 4678–4683. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, V.; Di Campli, E.; Fazii, P.; Traini, T.; Cellini, L.; Di Giulio, M. Candida species isolated from different body sites and their antifungal susceptibility pattern: Cross-analysis of Candida albicans and Candida glabrata biofilms. Med. Mycol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Castanheira, M. Nosocomial candidiasis: Antifungal stewardship and the importance of rapid diagnosis. Med. Mycol. 2016, 54. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Boyken, L.; Hollis, R.J.; Kroeger, J.; Messer, S.A.; Tendolkar, S.; Diekema, D.J. Wild-Type MIC Distributions and Epidemiological Cutoff Values for Posaconazole and Voriconazole and Candida spp. as Determined by 24-Hour CLSI Broth Microdilution. J. Clin. Microbiol. 2011, 49, 630–637. [Google Scholar] [CrossRef]
- Spreghini, E.; Maida, C.M.; Tomassetti, S.; Orlando, F.; Giannini, D.; Milici, M.E.; Scalise, G.; Barchiesi, F. Posaconazole against Candida glabrata isolates with various susceptibilities to fluconazole. Antimicrob. Agents Chemother. 2008, 52, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Henriques, M. Portrait of Matrix Gene Expression in Candida glabrata Biofilms with Stress Induced by Different Drugs. Genes 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.; Messer, S.; Boyken, L.; Hollis, R.; Rice, C.; Tendolkar, S.; Diekema, D. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 2004, 48, 201–205. [Google Scholar] [CrossRef]
- Lemke, A.; Kiderlen, A.F.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef]
- Baginski, M.; Czub, J. Amphotericin B and its new derivatives-mode of action. Curr. Drug Metab. 2009, 10, 459–469. [Google Scholar] [CrossRef]
- Botero, M.C.; Puentes-Herrera, M.; Cortés, J.A. Lipid formulations of amphotericin. Rev. Chilena Infectol. 2014, 31, 518–527. [Google Scholar] [CrossRef]
- Cacciapuoti, A.; Gurnani, M.; Halpern, J.; Norris, C.; Patel, R.; Loebenberg, D. Interaction between posaconazole and amphotericin B in concomitant treatment against Candida albicans in vivo. Antimicrob. Agents Chemother. 2005, 49, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Pappas, P.G.; Karchmer, A.W.; Sobel, J.; Edwards, J.E.; Hadley, S.; Brass, C.; Vazquez, J.A.; Chapman, S.W.; Horowitz, H.W.; et al. A Randomized and Blinded Multicenter Trial of High-Dose Fluconazole plus Placebo versus Fluconazole plus Amphotericin B as Therapy for Candidemia and Its Consequences in Nonneutropenic Subjects. Clin. Infect. Dis. 2003, 36, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lehman, V.N.; Averette, A.F.; Perfect, J.R.; Heitman, J. Posaconazole Exhibits In Vitro and In Vivo Synergistic Antifungal Activity with Caspofungin or FK506 against Candida albicans. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Sanati, H.; Ramos, C.F.; Bayer, A.S.; Ghannoum, M.A. Combination therapy with amphotericin B and fluconazole against invasive candidiasis in neutropenic-mouse and infective-endocarditis rabbit models. Antimicrob. Agents Chemother. 1997, 41, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.M.; Hitchcock, C.A.; Troke, P.F.; Picard, M. Combination Therapy of Murine Invasive Candidiasis with Fluconazole and Amphotericin B. Antimicrob. Agents Chemother. 1995, 39, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.M.; Salibian, M.; Goldani, L.Z. Saperconazole Therapy of Murine Disseminated Candidiasis: Efficacy and Interactions with Amphotericin B. Antimicrob. Agents Chemother. 1994, 38, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Canuto, M.M.; Rodero, F.G. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2002, 2, 550–563. [Google Scholar] [CrossRef]
- Pierce, C.G.; Srinivasan, A.; Uppuluri, P.; Ramasubramanian, A.K.; López-Ribot, J.L. Antifungal therapy with an emphasis on biofilms. Curr. Opin. Pharmacol. 2013, 13. [Google Scholar] [CrossRef]
- Delattin, N.; Cammue, B.P.; Thevissen, K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med. Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Silva, S.; Azeredo, J.; Henriques, M. Candida glabrata’s recurrent infections: Biofilm formation during Amphotericin B treatment. Lett. Appl. Microbiol. 2016, 63, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Al-fattani, M.A.; Douglas, L.J. Penetration of Candida Biofilms by Antifungal Agents. Antimicrob. Agents Chemother 2004, 48, 3291–3297. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Guglielminetti, M.; Ferrario, A.; Calabrὸ, M.; Casari, E. Candidemia: Species involved, virulence factors and antimycotic susceptibility. New Microbiol. 2012, 35, 459–468. [Google Scholar] [PubMed]
- Grandesso, S.; Sapino, B.; Mazzuccato, S.; Solinas, M.; Bedin, M.; D’Angelo, M.; Gion, M. Study on in vitro susceptibility of Candida spp. isolated from blood culture. Infect. Med. 2012, 20, 25–30. [Google Scholar]
- Lewis, R.; Kontoyiannis, D.; Darouiche, R.; Raad, I.; Prince, R. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infection. Antimicrob. Agents Chemother. 2002, 46, 3499–3505. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.; Costerton, J. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Shigemura, K.; Yoshida, H.; Fujisawa, M.; Arakawa, S. Candida urinary tract infection and Candida species susceptibilities to antifungal agents. J. Antibiot. 2013, 66, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.M. Interactions of Amphotericin B and SCH 39304 in the Treatment of Experimental Murine Candidiasis: Lack of Antagonism of a Polyene-Azole Combination. Antimicrob. Agents Chemother. 1991, 35, 1669–1671. [Google Scholar] [CrossRef]
- Narita, A.; Muramatsu, H.; Sakaguchi, H.; Doisaki, S.; Tanaka, M.; Hama, A.; Shimada, A.; Takahashi, Y.; Yoshida, N.; Matsumoto, K.; et al. Correlation of CYP2C19 Phenotype With Voriconazole Plasma Concentration in Children. J. Pediatr. Hematol. Oncol. 2013, 35, e21. [Google Scholar] [CrossRef] [PubMed]
- Caira, M.; Alkhamis, K.; Obaidat, R. Preparation and crystal characterization of a polymorph, a monohydrate, and an ethyl acetate solvate of the antifungal fluconazole. J. Pharm. Sci. 2004, 93, 601–661. [Google Scholar] [CrossRef]
- Louie, A.; Liu, W.; Miller, D.A.; Sucke, A.C.; Liu, Q.-F.; Drusano, G.L.; Mayers, M.; Miller, M.H. Efficacies of High-Dose Fluconazole plus Amphotericin B and High-Dose Fluconazole plus 5-Fluorocytosine versus Amphotericin B, Fluconazole, and 5-Fluorocytosine Monotherapies in Treatment of Experimental Endocarditis, Endophthalmitis, and Pyelonephritis D. Antimicrob. Agents Chemoter. 1999, 43, 2831–2840. [Google Scholar] [CrossRef]
- Lewis, R.E.; Lund, B.C.; Klepser, M.E.; Ernst, E.J.; Pfaller, M.A. Assessment of antifungal activities of fluconazole and amphotericin B administered alone and in combination against Candida albicans by using a dynamic in vitro mycotic infection model. Antimicrob. Agents Chemother. 1998, 42, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Sugar, A.M.; Liu, X.P. Interactions of itraconazole with amphotericin B in the treatment of murine invasive candidiasis. J. Infect. Dis. 1998, 177, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; De Mello, T.; De Souza Ramos, L.; Branquinha, M. Biofilm: A Robust and Efficient Barrier to Antifungal Chemotherapy. J. Antimicrob. Agents 2015. [Google Scholar] [CrossRef]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Shor, E.; Zhao, Y. Update on Antifungal Drug Resistance. Curr. Clin. Microbiol. Rep. 2015, 2, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, J.; d’Enfert, C. Candida albicans biofilms: Building a heterogeneous, drug-tolerant environment. Curr. Opin. Microbiol. 2013, 16, 398–403. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghan-noum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef]

| Drug | MIC (mg/L) | MFC (mg/L) | MBEC (mg/L) |
|---|---|---|---|
| Posaconazole | 0.7 | 0.8–0.9 | >300 |
| Amphotericin B | 0.25 | 1–2 | 2–4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, C.F.; Alves, D.F.; Henriques, M. Combination of Posaconazole and Amphotericin B in the Treatment of Candida glabrata Biofilms. Microorganisms 2018, 6, 123. https://doi.org/10.3390/microorganisms6040123
Rodrigues CF, Alves DF, Henriques M. Combination of Posaconazole and Amphotericin B in the Treatment of Candida glabrata Biofilms. Microorganisms. 2018; 6(4):123. https://doi.org/10.3390/microorganisms6040123
Chicago/Turabian StyleRodrigues, Célia F., Diana F. Alves, and Mariana Henriques. 2018. "Combination of Posaconazole and Amphotericin B in the Treatment of Candida glabrata Biofilms" Microorganisms 6, no. 4: 123. https://doi.org/10.3390/microorganisms6040123
APA StyleRodrigues, C. F., Alves, D. F., & Henriques, M. (2018). Combination of Posaconazole and Amphotericin B in the Treatment of Candida glabrata Biofilms. Microorganisms, 6(4), 123. https://doi.org/10.3390/microorganisms6040123