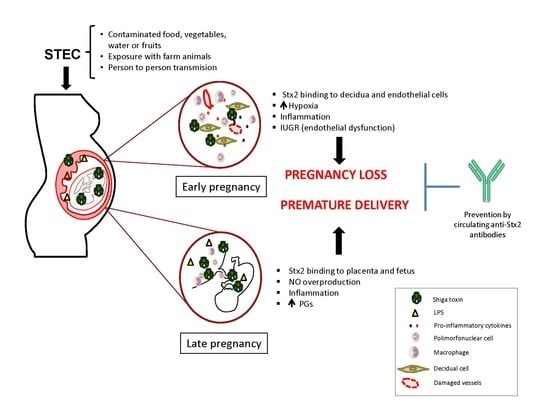
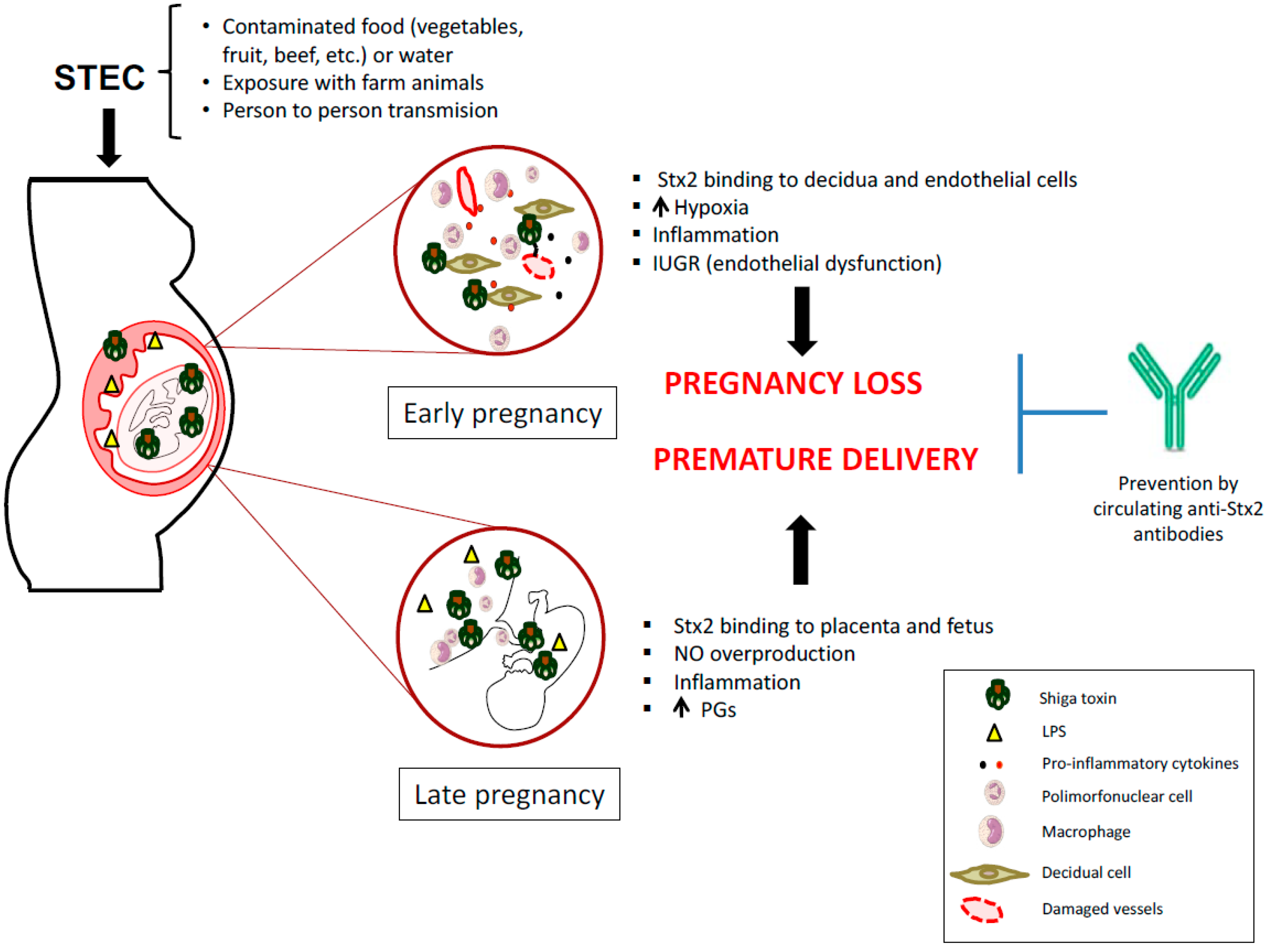
Shiga Toxin-Producing Escherichia coli Infections during Pregnancy
Abstract
1. Introduction
2. Foodborne Bacteria and Adverse Pregnancy Outcome
3. Pathophysiology of Adverse Pregnancy Outcomes Caused by Infections
4. STEC Infection May Be Responsible for Pregnancy Complications
5. Strategies to Prevent STEC Infection During Pregnancy
Funding
Conflicts of Interest
References
- Karmali, M.A.; Petric, M.; Lim, C.; Fleming, P.C.; Steele, B.T. Escherichia coli cytotoxin, haemolytic-uraemic syndrome, and haemorrhagic colitis. Lancet 1983, 2, 1299–1300. [Google Scholar] [CrossRef]
- Palermo, M.S.; Exeni, R.A.; Fernandez, G.C. Hemolytic uremic syndrome: Pathogenesis and update of interventions. Expert Rev. Anti-Infect. Ther. 2009, 7, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Raa, H.; Grimmer, S.; Schwudke, D.; Bergan, J.; Wälchli, S.; Skotland, T.; Shevchenko, A.; Sandvig, K. Glycosphingolipid requirements for endosome-to-Golgi transport of Shiga toxin. Traffic 2009, 10, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Repetto, H.A. Epidemic hemolytic-uremic syndrome in children. Kidney Int. 1997, 52, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Repetto, H.A. Long-term course and mechanisms of progression of renal disease in hemolytic uremic syndrome. Kidney Int. 2005, 68, S102–S106. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Chinen, I.; Miliwebsky, E.; Masana, M. Risk factors for Shiga toxin-producing Escherichia coli-associated human diseases. Microbiol. Spectr. 2014, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Werber, D.; Cramer, J.P.; Askar, M.; Faber, M.; an der Heiden, M.; Bernard, H.; Fruth, A.; Prager, R.; Spode, A. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 2011, 365, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Matovina, M.; Husnjak, K.; Milutin, N.; Ciglar, S.; Grce, M. Possible role of bacterial and viral infections in miscarriages. Fertil. Steril. 2004, 81, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Burdet, J.; Rubio, A.P.; Salazar, A.I.; Ribeiro, M.L.; Ibarra, C.; Franchi, A.M. Inflammation, infection and preterm birth. Curr. Pharm. Des. 2014, 20, 4741–4748. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Thompson, C. The infectious origins of stillbirth. Am. J. Obstet. Gynecol. 2003, 189, 861–873. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.J.; Theiler, R.N.; Rasmussen, S.A. Emerging infections and pregnancy. Emerg. Infect. Dis. 2006, 12, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Trevejo, R.T.; Barr, M.C.; Robinson, R.A. Important emerging bacterial zoonotic infections affecting the immunocompromised. Vet. Res. 2005, 36, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, N.F.; Tillett, J. Listeriosis and toxoplasmosis in pregnancy: Essentials for healthcare providers. J. Perinat. Neonatal Nurs. 2016, 30, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bazaco, M.C.; Albrecht, S.A.; Malek, A.M. Preventing foodborne infection in pregnant women and infants. Nurs. Womens Health 2008, 12, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Jia, H.; Kast, R.J.; Thomas, E.A. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav. Immun. 2013, 30, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.K.; Velten, M. Maternal inflammation, growth retardation, and preterm birth: Insights into adult cardiovascular disease. Life Sci. 2011, 89, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Kundakovic, M.; Jaric, I. The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes 2017, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.; Gomez, L.; Montenegro, D.; de Avila, J.; Tamayo, M.; Lancheros, M.; Quiceno, J.; Trujillo, T.; Noriega, L.; Grueso, M.; et al. Periodontal condition is associated with adverse perinatal outcomes and premature rupture membranes in low income pregnant women in Bogota, Colombia: A case control study. J. Matern-Fetal Neonatal Med. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Chaiworapongsa, T. Preterm labor, intrauterine infection, and the fetal inflammatory response syndrome. NeoReviews 2002, 3, e73–e85. [Google Scholar] [CrossRef]
- Baud, D.; Greub, G. Intracellular bacteria and adverse pregnancy outcomes. Clin. Microbiol. Infect. 2011, 17, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Tichaczek-Dischinger, P.S.; Rau, J.; Sihto, H.-M.; Lehner, A.; Adam, M.; Stephan, R. Outbreak of Staphylococcal food poisoning due to SEA-producing Staphylococcus aureus. Foodborne Pathog. Dis. 2013, 10, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E. Infections in Obstetrics and Gynecology: Textbook and Atlas; Thieme: New York, NY, USA, 2006. [Google Scholar]
- Silver, R.M. Fetal death. Obstet. Gynecol. 2007, 109, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.M.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Update 2015, 22, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Rowe, D.C.; Golenbock, D.T. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004, 6, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, C.; Palermo, M. Host Responses to Pathogenic Escherichia coli. In Pathogenic Escherichia coli in Latin America; Torres, A.G., Ed.; Springer: Cham, Switzerland, 2016; pp. 122–141. [Google Scholar]
- Romero, R.; Espinoza, J.; Gonçalves, L.F.; Kusanovic, J.P.; Friel, L.; Hassan, S. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 2007, 25, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Ulinski, T.; Lervat, C.; Ranchin, B.; Gillet, Y.; Floret, D.; Cochat, P. Neonatal hemolytic uremic syndrome after mother-to-child transmission of Escherichia coli O157. Pediatr. Nephrol. 2005, 20, 1334–1335. [Google Scholar] [CrossRef] [PubMed]
- Stritt, A.; Stritt Tschumi, S.; Kottanattu, L.; Bucher, B.S.; Steinmann, M.; von Steiger, N.; Stephan, R.; Hächler, H.; Simonetti, G.D. Neonatal hemolytic uremic syndrome after mother-to-child transmission of a low-pathogenic stx2b harboring Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 2012, 56, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.T.; Goldie, J.; Alexopoulou, I.; Shimizu, A. Post-partum haemolytic-uremic syndrome and verotoxin-producing Escherichia coli. Lancet 1984, 1, 511. [Google Scholar] [CrossRef]
- Tanaka, H.; Toyoda, N.; Adachi, E.; Takeda, T. Immunologic evaluation of an Escherichia coli O157-infected pregnant woman. A case report. J. Reprod. Med. 2000, 45, 442–444. [Google Scholar] [PubMed]
- Chart, H.; Perry, N.T.; Cheasty, T.; Wright, P.A. The kinetics of antibody production to antigens of Escherichia coli O157 in a pregnant woman with haemolytic uraemic syndrome. J. Med. Microbiol. 2002, 51, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Tanimoto, A.; Abe, T.; Ogawa, M.; Yutsudo, T.; Kashimura, M.; Yoshida, S. Shiga toxin 1 and 2 induce apoptosis in the amniotic cell line WISH. J. Soc. Gynecol. Investig. 2002, 9, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.L.; Sacerdoti, F.; Ibarra, C. Shiga toxin type 2 impairs trophoblast migration and invasion. Insight into the role of inducible nitric oxide synthase in damages. In Proceedings of the 10th International Symposium on Shiga Toxin (Verocytotoxin)-Producing Escherichia coli Infections, Florence, Italy, 6–9 May 2018. [Google Scholar]
- Burdet, J.; Zotta, E.; Franchi, A.M.; Ibarra, C. Intraperitoneal administration of shiga toxin type 2 in rats in the late stage of pregnancy produces premature delivery of dead fetuses. Placenta 2009, 30, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Burdet, J.; Zotta, E.; Cella, M.; Franchi, A.M.; Ibarra, C. Role of nitric oxide in Shiga toxin-2-induced premature delivery of dead fetuses in rats. PLoS ONE 2010, 5, e15127. [Google Scholar] [CrossRef] [PubMed]
- Ogando, D.G.; Paz, D.; Cella, M.; Franchi, A.M. The fundamental role of increased production of nitric oxide in lipopolysaccharide-induced embryonic resorption in mice. Reproduction 2003, 125, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Aisemberg, J.; Vercelli, C.; Billi, S.; Ribeiro, M.L.; Ogando, D.; Meiss, R.; McCann, S.M.; Rettori, V.; Franchi, A.M. Nitric oxide mediates prostaglandins’ deleterious effect on lipopolysaccharide-triggered murine fetal resorption. Proc. Natl. Acad. Sci. USA 2007, 104, 7534–7539. [Google Scholar] [CrossRef] [PubMed]
- Swaisgood, C.M.; Zu, H.X.; Perkins, D.J.; Wu, S.; Garver, C.L.; Zimmerman, P.D.; Iams, J.D.; Kniss, D.A. Coordinate expression of inducible nitric oxide synthase and cyclooxygenase-2 genes in uterine tissues of endotoxin-treated pregnant mice. Am. J. Obstet. Gynecol. 1997, 177, 1253–1262. [Google Scholar] [CrossRef]
- Burdet, J.; Sacerdoti, F.; Cella, M.; Franchi, A.M.; Ibarra, C. Role of TNF-α in the mechanisms responsible for preterm delivery induced by Stx2 in rats. Br. J. Pharmacol. 2013, 168, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Peltier, M.R. Immunology of term and preterm labor. Reprod. Biol. Endocrinol. 2003, 1, 122. [Google Scholar] [CrossRef] [PubMed]
- Gravett, M.G.; Adams, K.M.; Sadowsky, D.W.; Grosvenor, A.R.; Witkin, S.S.; Axthelm, M.K.; Novy, M.J. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am. J. Obstet. Gynecol. 2007, 197, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Azizieh, F.Y.; Raghupathy, R.G. Tumor necrosis factor-alpha and pregnancy complications: A prospective study. Med. Princ. Pract. 2015, 24, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Fujii, J.; Tanimoto, A.; Yutsudo, T.; Kashimura, M.; Yoshida, S. Effects of Shiga toxin 2 on lethality, fetuses, delivery, and puerperal behavior in pregnant mice. Infect. Immun. 2000, 68, 2254–2258. [Google Scholar] [CrossRef] [PubMed]
- Sacerdoti, F.; Amaral, M.M.; Zotta, E.; Franchi, A.M.; Ibarra, C. Effects of Shiga toxin type 2 on maternal and fetal status in rats in the early stage of pregnancy. BioMed Res. Int. 2014, 2014, 384645. [Google Scholar] [CrossRef] [PubMed]
- Sacerdoti, F.; Amaral, M.M.; Aisemberg, J.; Cymeryng, C.B.; Franchi, A.M.; Ibarra, C. Involvement of hypoxia and inflammation in early pregnancy loss mediated by Shiga toxin type 2. Placenta 2015, 36, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, C.; Amaral, M.M.; Palermo, M.S. Advances in pathogenesis and therapy of hemolytic uremic syndrome caused by Shiga toxin-2. IUBMB Life 2013, 65, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F.; Petraglia, F. Inflammation and pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Giaccia, A.J.; Simon, M.C.; Johnson, R. The biology of hypoxia: The role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004, 18, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.G.; Parashar, U.D.; Lye, M.S.; Ong, F.G.; Zaki, S.R.; Alexander, J.P.; Ho, K.K.; Han, L.L.; Pallansch, M.A.; Suleiman, A.B.; et al. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: Clinical and pathological characteristics of the disease. Clin. Infect. Dis. 2000, 31, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Current paradigms in cellular oxygen sensing. Adv. Exp. Med. Biol. 2003, 543, 57–71. [Google Scholar] [PubMed]
- Radom-Aizik, S.; Zaldivar, F.P.; Nance, D.M.; Haddad, F.; Cooper, D.M.; Adams, G.R. Growth inhibition and compensation in response to neonatal hypoxia in rats. Pediatr. Res. 2013, 74, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002, 16, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Winer, N.; Resche-Rigon, M.; Morin, C.; Ville, Y.; Rozenberg, P. Is induced abortion with misoprostol a risk factor for late abortion or preterm delivery in subsequent pregnancies? Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 145, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Osol, G.; Mandala, M. Maternal uterine vascular remodeling during pregnancy. Physiology 2009, 24, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, P.; Tan, A.; Romagosa, C.; Radford, J.; Mwapasa, V.; Molyneux, M.E.; Meshnick, S.R.; Hunt, N.H.; Rogerson, S.J. Placental hypoxia during placental malaria. J. Infect. Dis. 2008, 197, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.A.; Mascarenhas, M.; Petric, M.; Dutil, L.; Rahn, K.; Ludwig, K.; Arbus, G.S.; Michel, P.; Sherman, P.M.; Wilson, J.; et al. Age-specific frequencies of antibodies to Escherichia coli verocytotoxins (Shiga toxins) 1 and 2 among urban and rural populations in southern Ontario. J. Infect. Dis. 2003, 188, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.A.; Petric, M.; Winkler, M.; Bielaszewska, M.; Brunton, J.; van de Kar, N.; Morooka, T.; Nair, G.B.; Richardson, S.E.; Arbus, G.S. Enzyme-linked immunosorbent assay for detection of immunoglobulin G antibodies to Escherichia coli Vero cytotoxin 1. J. Clin. Microbiol. 1994, 32, 1457–1463. [Google Scholar] [PubMed]
- Fernández-Brando, R.J.; Bentancor, L.V.; Mejías, M.P.; Ramos, M.V.; Exeni, A.; Exeni, C.; Laso Mdel, C.; Exeni, R.; Isturiz, M.A.; Palermo, M.S. Antibody response to Shiga toxins in Argentinean children with enteropathic hemolytic uremic syndrome at acute and long-term follow-up periods. PLoS ONE 2011, 6, e19136. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Brando, R.J.; Amaral, M.M.; Ciocchini, A.E.; Bentancor, L.V.; Trelles, J.A.; Da Rocha, M.; Landriel, M.; Ugarte, M.; Briones, G.; Ibarra, C.; et al. Microbiological and serological control of Escherichia coli O157:H7 in kindergarten staff in Buenos Aires city and suburban areas. Medicina 2017, 77, 185–190. [Google Scholar] [PubMed]
- Thomas, D.E.; Elliott, E.J. Interventions for preventing diarrhea-associated hemolytic uremic syndrome: Systematic review. BMC Public Health 2013, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Mejias, M.P.; Ghersi, G.; Craig, P.O.; Panek, C.A.; Bentancor, L.V.; Baschkier, A.; Goldbaum, F.A.; Zylberman, V.; Palermo, M.S. Immunization with a chimera consisting of the B subunit of Shiga toxin type 2 and brucella lumazine synthase confers total protection against Shiga toxins in mice. J. Immunol. 2013, 191, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Mejias, M.P.; Fernandez-Brando, R.J.; Ramos, M.V.; Abrey-Recalde, M.J.; Zotta, E.; Meiss, R.; Palermo, M.S. Development of a mouse model of shiga toxin 2 (Stx2) intoxication for testing therapeutic agents against hemolytic uremic syndrome (HUS). Curr. Pharm. Des. 2016, 22, 5294–5299. [Google Scholar] [CrossRef] [PubMed]
- Adachi, E.; Tanaka, H.; Toyoda, N.; Takeda, T. Detection of bactericidal antibody in the breast milk of a mother infected with enterohemorrhagic Escherichia coli O157:H7. Kansenshogaku Zasshi 1999, 73, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitz, B.C.; Gerhardt, E.; Tironi Farinati, C.; Abdala, A.; Galarza, R.; Vilte, D.A.; Ibarra, C.; Cataldi, A.; Mercado, E.C. Vaccination of pregnant cows with EspA, EspB, γ-intimin, and Shiga toxin 2 proteins from Escherichia coli O157:H7 induces high levels of specific colostral antibodies that are transferred to newborn calves. J. Dairy Sci. 2012, 95, 3318–3326. [Google Scholar] [CrossRef] [PubMed]
- Mejias, M.P.; Cabrera, G.; Fernández-Brando, R.J.; Baschkier, A.; Ghersi, G.; Abrey-Recalde, M.J.; Miliwebsky, E.; Meiss, R.; Goldbaum, F.; Zylberman, V. Protection of mice against Shiga toxin 2 (Stx2)-associated damage by maternal immunization with a Brucella lumazine synthase-Stx2 B subunit chimera. Infect. Immun. 2014, 82, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Sacerdoti, F.; Mejías, M.P.; Bruballa, A.C.; Alvarez, R.S.; Amaral, M.M.; Palermo, M.S.; Ibarra, C. Immunization with BLS-Stx2B chimera totally protects dams from early pregnancy loss induced by Shiga toxin type 2 (Stx2) and confers anti-Stx2 immunity to the offspring. Vaccine 2016, 34, 4732–4737. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacerdoti, F.; Scalise, M.L.; Burdet, J.; Amaral, M.M.; Franchi, A.M.; Ibarra, C. Shiga Toxin-Producing Escherichia coli Infections during Pregnancy. Microorganisms 2018, 6, 111. https://doi.org/10.3390/microorganisms6040111
Sacerdoti F, Scalise ML, Burdet J, Amaral MM, Franchi AM, Ibarra C. Shiga Toxin-Producing Escherichia coli Infections during Pregnancy. Microorganisms. 2018; 6(4):111. https://doi.org/10.3390/microorganisms6040111
Chicago/Turabian StyleSacerdoti, Flavia, María Luján Scalise, Juliana Burdet, María Marta Amaral, Ana María Franchi, and Cristina Ibarra. 2018. "Shiga Toxin-Producing Escherichia coli Infections during Pregnancy" Microorganisms 6, no. 4: 111. https://doi.org/10.3390/microorganisms6040111
APA StyleSacerdoti, F., Scalise, M. L., Burdet, J., Amaral, M. M., Franchi, A. M., & Ibarra, C. (2018). Shiga Toxin-Producing Escherichia coli Infections during Pregnancy. Microorganisms, 6(4), 111. https://doi.org/10.3390/microorganisms6040111