The Species-Specific Responses of Freshwater Diatoms to Elevated Temperatures Are Affected by Interspecific Interactions
Abstract
1. Introduction
2. Material and Methods
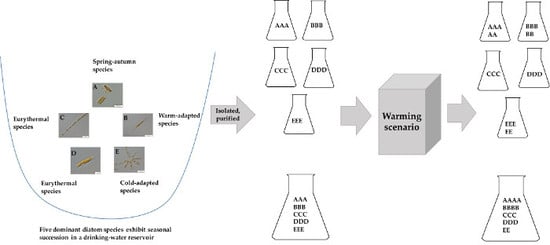
2.1. Diatom Isolation
2.2. Growth under Constant Temperature
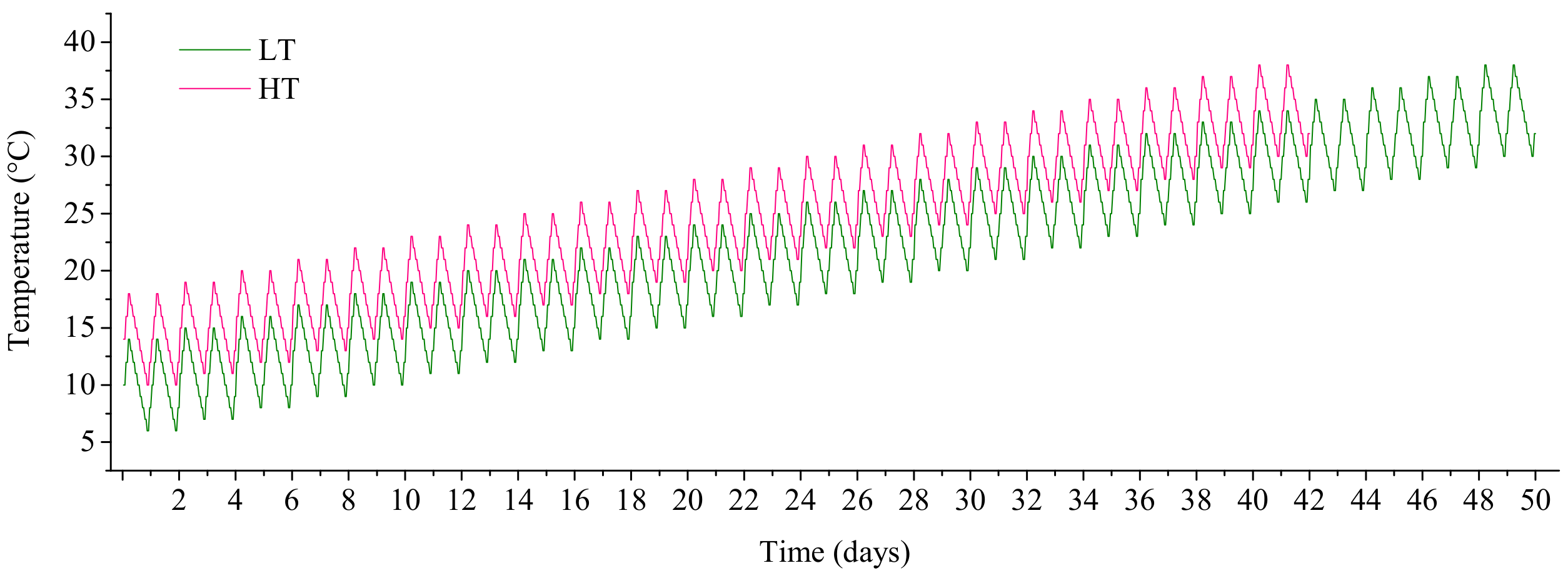
2.3. Experimental Design of the Simulated Warming Scenario from Early Spring to Summer
2.4. Growth Evaluation
2.5. Chlorophyll Fluorescence Measurement
2.6. Statistical Analysis
3. Results
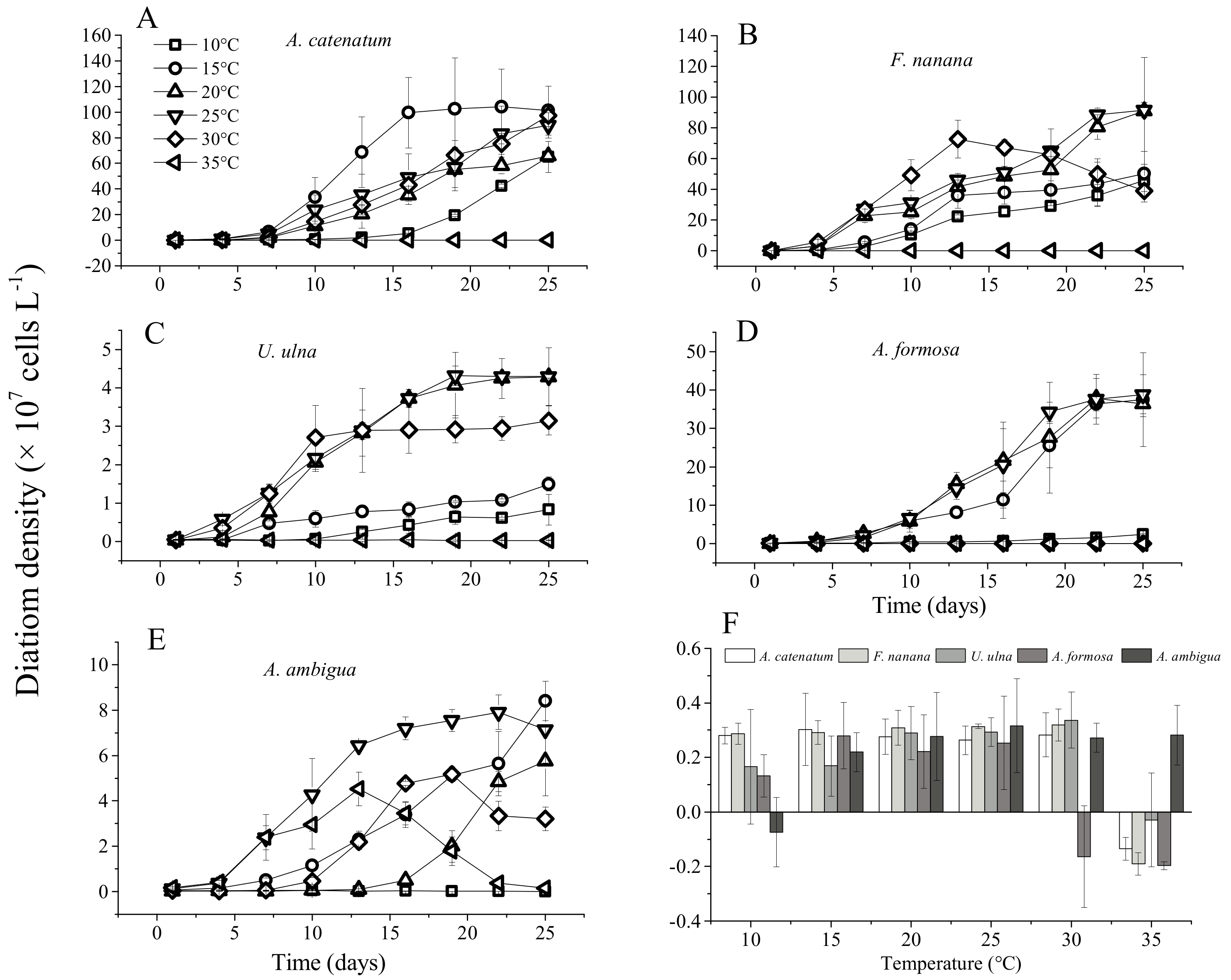
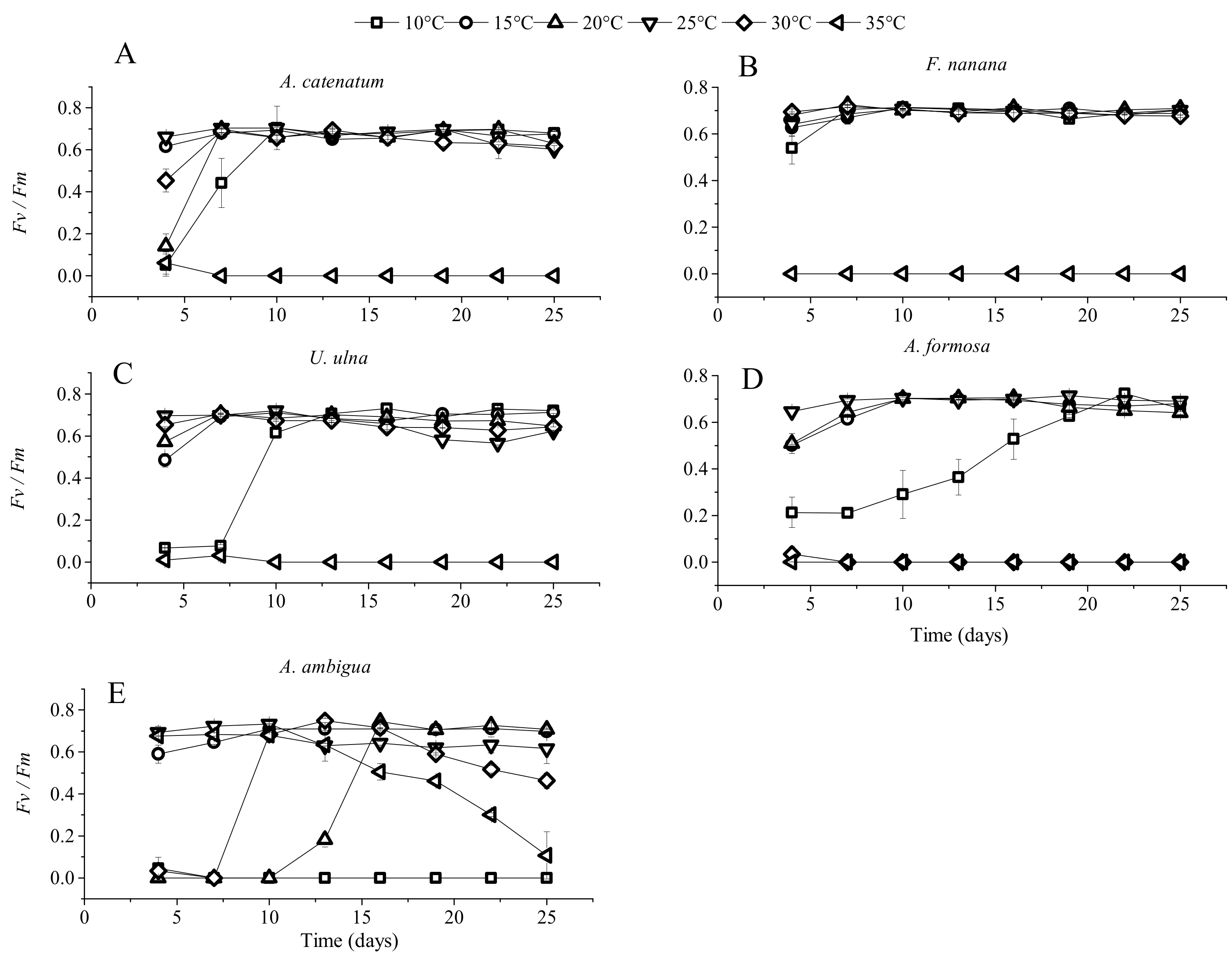
3.1. The Growth of Diatoms under Constant Temperature
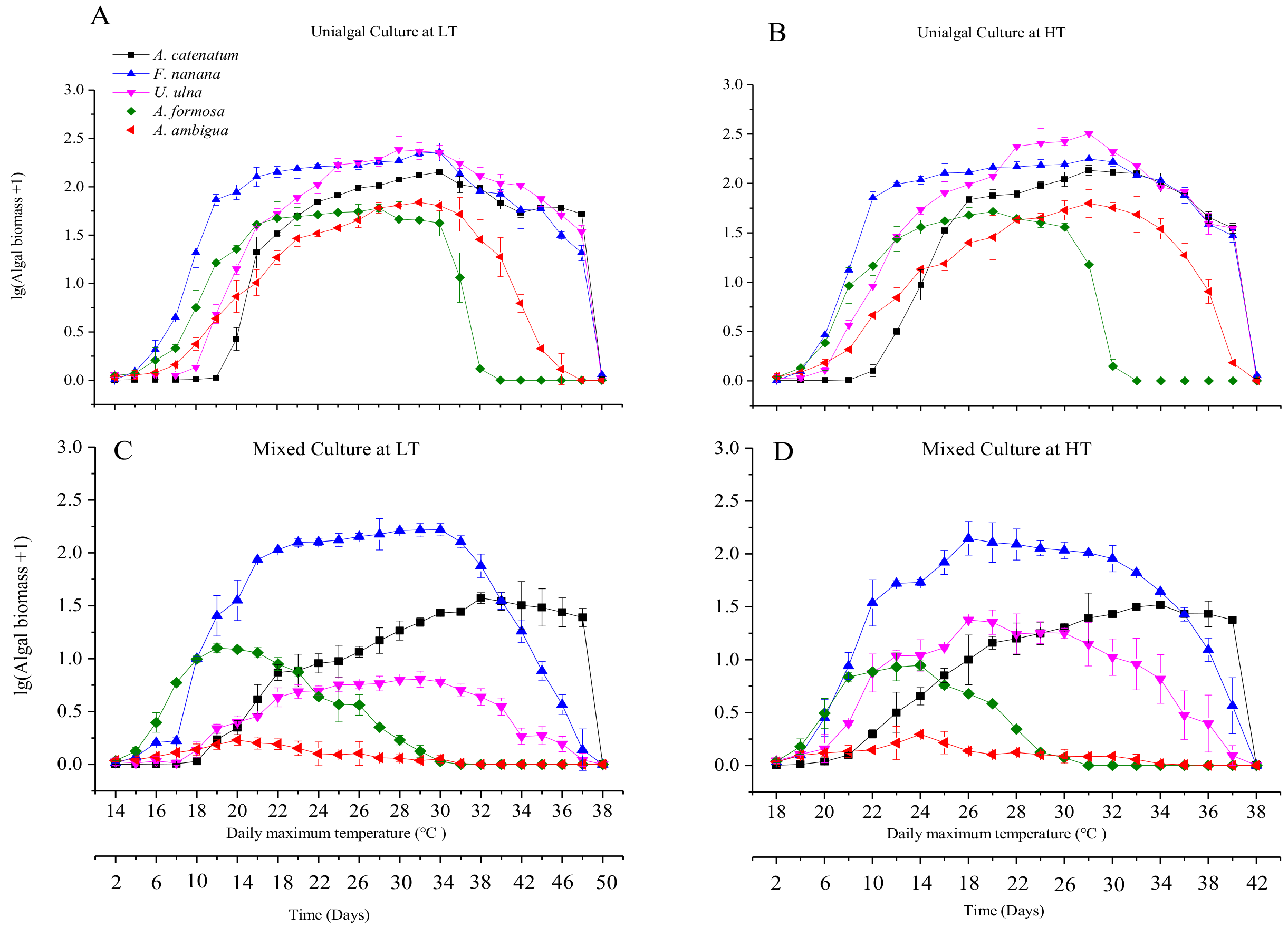
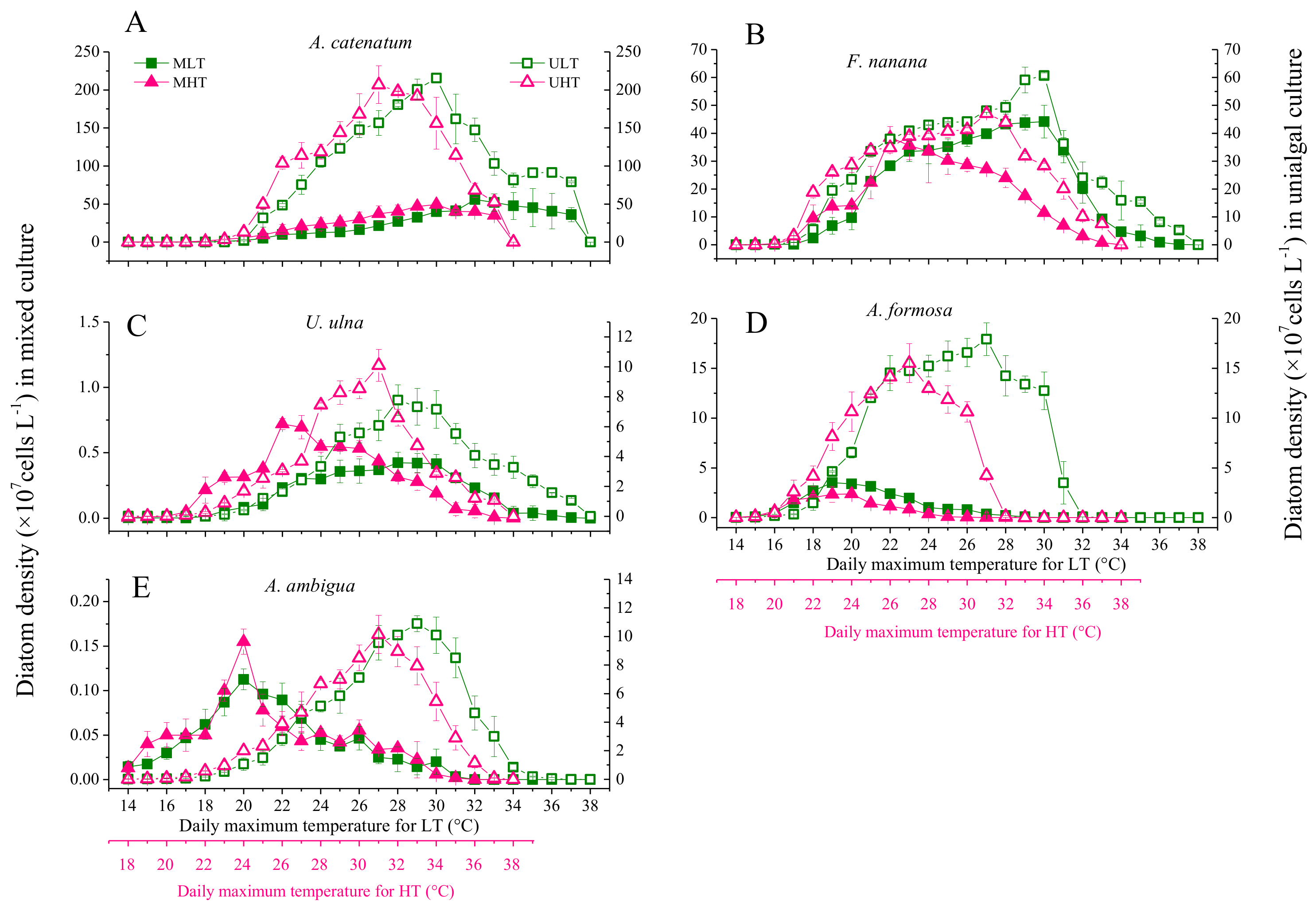
3.2. Diatom Growth under Fluctuant Temperatures in Unialgal and Mixed Cultures
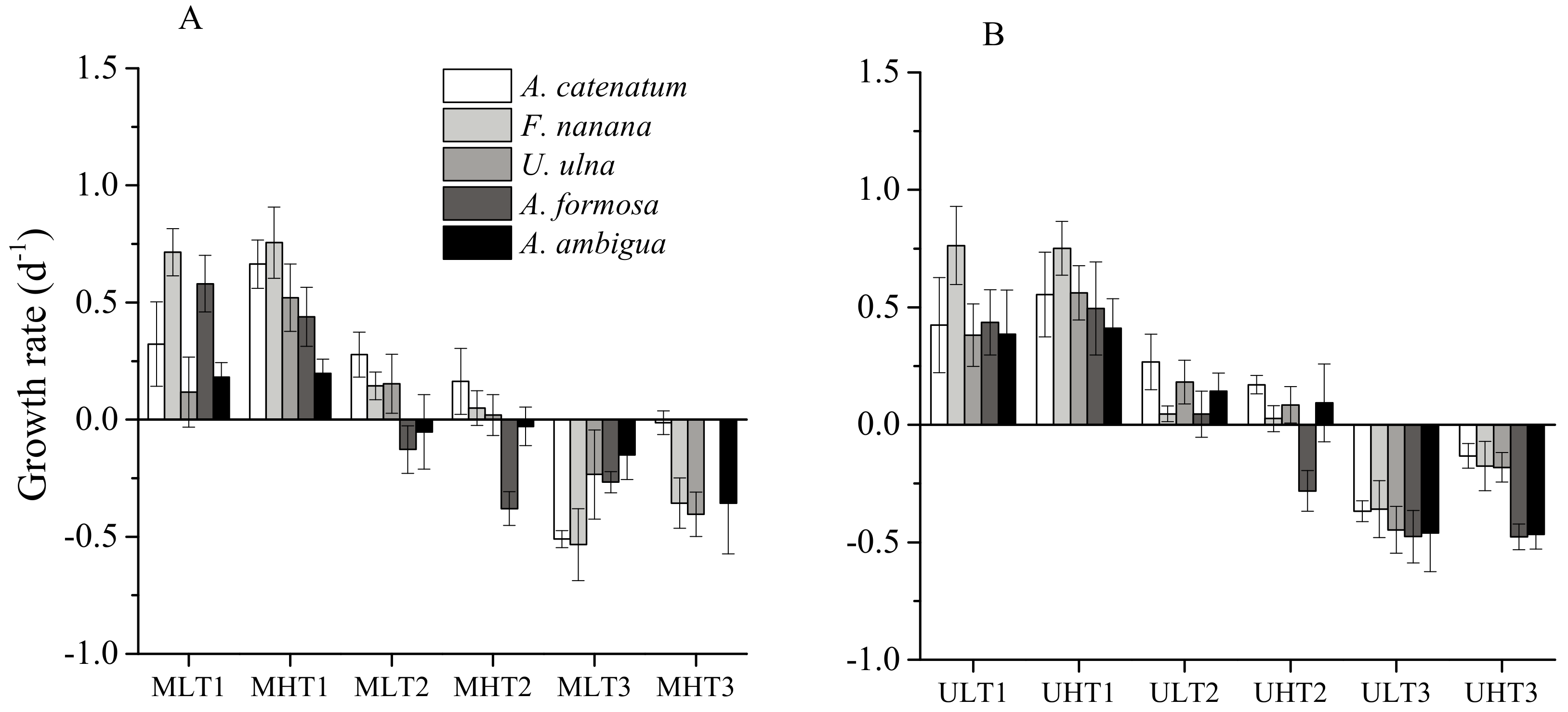
3.3. The Effect of Elevated Temperature (+4 °C) on Diatoms
3.4. The Differences in Diatom Responses to Warming Scenarios in Unialgal Versus Mixed Cultures
4. Discussion
4.1. Specific Responses of Diatoms to Warming
4.2. The Effect of Interspecific Interactions on the Response of Diatoms to Warming
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Rynearson, T.A.; Newton, J.A.; Armbrust, E.V. Spring bloom development, genetic variation, and population succession in the planktonic diatom Ditylum brightwellii. Limnol. Oceanogr. 2006, 51, 1249–1261. [Google Scholar] [CrossRef]
- B-Béres, V.; Török, P.; Kókai, Z.; Lukács, Á.; T-Krasznai, E.; Tóthmérész, B.; Bácsi, I. Ecological background of diatom functional groups: Comparability of classification systems. Ecol. Indic. 2017, 82, 183–188. [Google Scholar] [CrossRef]
- Bunse, C.; Pinhassi, J. Marine bacterioplankton seasonal succession dynamics. Trends Microbial. 2017, 25, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Padmakumar, K.B.; Thomas, L.C.; Vimalkumar, K.G.; Devi, C.A.; Maneesh, T.P.; Vijayan, A.K.; Gupta, G.; Sudhakar, M. Hydrobiological responses of the North Eastern Arabian Sea during late winter and early spring inter-monsoons and the repercussions on open ocean blooms. J. Mar. Biol. Assoc. UK 2017, 97, 1467–1478. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D.; Qian, S. Preliminary study on seasonal succession and development pathway of phytoplankton community in the Bohai Sea. Acta Oceanol. Sin. 2001, 20, 251–260. [Google Scholar]
- Wu, X.H.; Yin, D.C.; Chong, L.I.; Chen, L.; Yuan, L.I.; Zhao, Y. Analysis of factors influencing diatom blooms in the middle and lower Hanjiang River. J. Hydroecol. 2017, 38, 19–26. (In Chinese) [Google Scholar]
- Kolmakov, V.I.; Gaevskii, N.A.; Ivanova, E.A.; Dubovskaya, O.P.; Gribovskaya, I.V.; Kravchuk, E.S. Comparative analysis of ecophysiological characteristics of Stephanodiscus hantzschii Grun. in the periods of its bloom in recreational water bodies. Russ. J. Ecol. 2002, 33, 97–103. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U.; Aberle, N.; Engel, A.; Hansen, T.; Lengfellner, K.; Sandow, M.; Wohlers, J.; Zöllner, E.; Riebesell, U. An indoor mesocosm system to study the effect of climate change on the late winter and spring succession of Baltic Sea phyto-and zooplankton. Oecologia 2007, 150, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.I.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar]
- Shatwell, T.O.M.; Köhler, J.A.N.; Nicklisch, A. Warming promotes cold-adapted phytoplankton in temperate lakes and opens a loophole for Oscillatoriales in spring. Glob. Chang. Biol. 2008, 14, 2194–2200. [Google Scholar] [CrossRef]
- Devlin, J.E.; Finkelstein, S.A. Local physiographic controls on the responses of Arctic lakes to climate warming in Sirmilik National Park, Nunavut, Canadian. J. Paleolimnol. 2011, 45, 23–39. [Google Scholar] [CrossRef]
- Smith, M.E.; Manoylov, K.M. Changes in diatom biodiversity in Lake Sinclair, Baldwin County, Georgia, USA. J. Water Resour. Prot. 2013, 5, 732–742. [Google Scholar] [CrossRef]
- Berthon, V.; Alric, B.; Rimet, F.; Perga, M.E. Sensitivity and responses of diatoms to climate warming in lakes heavily influenced by humans. Freshw. Biol. 2014, 59, 1755–1767. [Google Scholar] [CrossRef]
- Köhler, J.; Hilt, S.; Adrian, R.; Nicklisch, A.; Kozerski, H.P.; Walz, N. Long-term response of a shallow, moderately flushed lake to reduced external phosphorus and nitrogen loading. Freshw. Biol. 2005, 50, 1639–1650. [Google Scholar] [CrossRef]
- Peeters, F.; Straile, D.; Lorke, A.; Livingstone, D.M. Earlier onset of the spring phytoplankton bloom in lakes of the temperate zone in a warmer climate. Glob. Chang.Biol. 2007, 13, 1898–1909. [Google Scholar] [CrossRef]
- Deng, J.; Qin, B.; Paerl, H.W.; Zhang, Y.; Ma, J.; Chen, Y. Earlier and warmer springs increase cyanobacterial (Microcystis spp.) blooms in subtropical Lake Taihu, China. Freshw. Biol. 2014, 59, 1076–1085. [Google Scholar] [CrossRef]
- Saros, J.E.; Anderson, N.J. The ecology of the planktonic diatom Cyclotella and its implications for global environmental change studies. Biol. Rev. 2015, 90, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Han, B. Reservoir ecology and limnology in China: A retrospective comment. J. Lake Sci. 2010, 22, 151–160. (In Chinese) [Google Scholar]
- Kocer, M.A.T.; Şen, B.L. The seasonal succession of diatoms in phytoplankton of a soda lake (Lake Hazar, Turkey). Turk. J. Bot. 2012, 36, 738–746. [Google Scholar]
- Cullen, J.J.; MacIntyre, H.L. On the use of the serial dilution culture method to enumerate viable phytoplankton in natural communities of plankton subjected to ballast water treatment. J. Appl. Phycol. 2016, 28, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Tatters, A.O.; Roleda, M.Y.; Schnetzer, A.; Fu, F.; Hurd, C.L.; Boyd, P.W.; Caron, D.A.; Lie, A.A.Y.; Hoffmann, L.J.; Hutchins, D.A. Short-and long-term conditioning of a temperate marine diatom community to acidification and warming. Phil. Trans. R. Soc. B 2013, 368, 20120437. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, J.; Chen, J.; Chen, Q.; Yan, X.; Xuan, J.; Zeng, J. Responses of summer phytoplankton community to drastic environmental changes in the Changjiang (Yangtze River) estuary during the past 50 years. Water Res. 2014, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.; Mckee, D.; Atkinson, D.; Collings, S.E.; Eaton, J.W.; Gill, A.B.; Wilson, D. How important is climate? Effects of warming, nutrient addition and fish on phytoplankton in shallow lake microcosms. J. Appl. Ecol. 2003, 40, 782–792. [Google Scholar] [CrossRef]
- Lassen, M.K.; Nielsen, K.D.; Richardson, K.; Garde, K.; Schlüter, L. The effects of temperature increases on a temperate phytoplankton community—A mesocosm climate change scenario. J. Exp. Mar. Biol. Ecol. 2010, 383, 79–88. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Allen, A.P.; Cellamare, M.; Dossena, M.; Gaston, K.J.; Leitao, M.; Montoya, J.M.; Reuman, D.C.; Woodward, G.; Trimmer, M. Five Years of Experimental Warming Increases the Biodiversity and Productivity of Phytoplankton. PLoS Biol. 2015, 13, e1002324. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, B.M.; Mehner, T.; Adrian, R. Reconciling the opposing effects of warming on phytoplankton biomass in 188 large lakes. Sci. Rep. 2017, 7, 10762. [Google Scholar] [CrossRef] [PubMed]
- Staehr, P.A.; Sand-Jensen, K. Seasonal changes in temperature and nutrient control of photosynthesis, respiration and growth of natural phytoplankton communities. Freshw. Biol. 2006, 51, 249–262. [Google Scholar] [CrossRef]
- Hillebrand, H.; Borer, E.T.; Bracken, M.E.S.; Cardinale, B.J.; Cebrian, J.; Cleland, E.E.; Cleland, E.E.; Elser, J.J.; Gruner, D.; Harpole, W.S.; et al. Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol. Lett. 2009, 12, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Striebel, M.; Schabhüttl, S.; Hodapp, D.; Hingsamer, P.; Hillebrand, H. Phytoplankton responses to temperature increases are constrained by abiotic conditions and community composition. Oecologia 2016, 182, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.R. Predicting the response of populations to environmental change. Ecology 1995, 76, 926–941. [Google Scholar] [CrossRef]
- Fox, J.W.; Morin, P.J. Effects of intra- and interspecific interactions on species responses to environmental change. J. Anim. Ecol. 2001, 70, 80–90. [Google Scholar]
- Wacker, A.; Marzetz, V.; Spijkerman, E. Interspecific competition in phytoplankton drives the availability of essential mineral and biochemical nutrients. Ecology 2015, 96, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Dunker, S.; Jakob, T.; Wilhelm, C. Contrasting effects of the cyanobacterium Microcystis aeruginosa on the growth and physiology of two green algae, Oocystis marsonii and Scenedesmus obliquus, revealed by flow cytometry. Freshw. Biol. 2013, 58, 1573–1587. [Google Scholar] [CrossRef]
- Lin, J.; Morin, P.J. Temperature-dependent interactions explain unexpected responses to environmental warming in communities of competitors. J. Anim. Ecol. 2004, 73, 569–576. [Google Scholar]
- Zhu, Z.; Qu, P.; Fu, F.; Tennenbaum, N.; Tatters, A.O.; Hutchins, D.A. Understanding the blob bloom: Warming increases toxicity and abundance of the harmful bloom diatom pseudo-nitzschia in california coastal waters. Harmful Algae 2017, 67, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bertness, M.D.; Ewanchuk, P.J. Latitudinal and climate-driven variation in the strength and nature of biological interactions in New England marshes. Oecologia 2002, 132, 392–401. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2007: Synthesis Report Geneva; IPCC: Geneva, Switzerland, 2007. [Google Scholar]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, C.R.; Wang, J.; Huang, S.; Hu, Y.; Zhang, J.L.; Li, D.H. Temperature and silicate are significant driving factors for the seasonal shift of dominant diatoms in a drinking water reservoir. J. Oceanol. Limnol. 2018, in press. [Google Scholar] [CrossRef]
- Olenina, I.; Hajdu, S.; Andersson, A.; Edler, L.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. Balt. Sea Environ. Proc. 2006, 106, 144. [Google Scholar]
- HELCOM. Manual for Marine Monitoring in the COMBINE Programme of HELCOM. 2014. Available online: http://www.helcom.fi/Lists/Publications/Manual%20for%20Marine%20Monitoring%20in%20the%20COMBINE%20Programme%20of%20HELCOM.pdf (accessed on 1 June 2018).
- Cox, E.J. Freshwater diatom ecology: Developing an experimental approach as an aid to interpreting field data. Hydrobiologia 1993, 269–270, 447–452. [Google Scholar] [CrossRef]
- Vinson, D.K.; Rushforth, S.R. Diatom species composition along a thermal gradient in the Portneuf River, Idaho, USA. Hydrobiologia 1989, 185, 41–54. [Google Scholar] [CrossRef]
- Hlúbiková, D.; Ector, L.; Hoffmann, L. Examination of the type material of some diatom species related to Achnanthidium minutissimum (Kütz.) Czarn. (Bacillariophyceae). Algol. Stud. 2011, 136, 19–43. [Google Scholar] [CrossRef]
- Coste, M.; Ector, L. Diatomées invasives exotiques ou rares em France: Principales observations effectuées au cours dês dernières décennies. Syst. Geogr. Plants 2000, 70, 373–400. [Google Scholar] [CrossRef]
- Straub, F. Apparition envahissante de la diatomée Achnanthes catenata Bily and Marvan (Heterokontophyta, Bacillariophyceae) dans le Lac de Neuchâtel (Suisse). Bull. Soc. Neuchatel. Sci. Nat. 2002, 125, 59–65. [Google Scholar]
- Zhang, Y.; Ma, X.F.; Guo, F.F.; Li, J.Z.; Xiong, B.X. Community structures of phytoplankton and their relationships with environmental factors in the Jinshahe Reservoir, Hubei Province. J. Lake Sci. 2015, 27, 902–910. (In Chinese) [Google Scholar]
- Lv, G.J.; Xiong, B.X.; Chen, P. Community structure and diversity of phytoplankton of four reservoirs in middle China. J. Fish. Sci. China 2012, 19, 690–699. (In Chinese) [Google Scholar]
- Hayakawa, T.; Kudoh, S.; Suzuki, Y.; Takahashi, M. Temperature-dependent changes in colony size of the freshwater pennate diatom Asterionella formosa (Bacillariophyceae) and their possible ecological implications. J. Phycol. 1994, 30, 955–964. [Google Scholar] [CrossRef]
- Solovieva, N.; Jones, V.; Birks, J.H.; Appleby, P.; Nazarova, L. Diatom responses to 20th century climate warming in lakes from the northern Urals, Russia. Palaeogeogr. Palaeocl. 2008, 259, 96–106. [Google Scholar] [CrossRef]
- Cremer, H.; Wagner, B. Planktonic diatom communities in High Arctic lakes (Store Koldewey, Northeast Greenland). Can. J. Bot. 2004, 82, 1744–1757. [Google Scholar] [CrossRef]
- Michelutti, N.; Cooke, C.A.; Hobbs, W.O.; Smol, J.P. Climate-driven changes in lakes from the Peruvian Andes. J. Paleolimnol. 2015, 54, 153–160. [Google Scholar] [CrossRef]
- Lewis, R.J.; Jensen, S.I.; DeNicola, D.M.; Miller, V.I.; Hoagland, K.D.; Ernst, S.G. Genetic variation in the diatom Fragilaria capucina (Fragilariaceae) along a latitudinal gradient across North America. Plant Syst. Evol. 1997, 204, 99–108. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Mishra, S.; Kuhaneck, R.M.; Heckathorn, S.A.; Bridgeman, T.B. Environmental controls on growth and lipid content for the freshwater diatom, Fragilaria capucina: A candidate for biofuel production. J. Appl. Phycol. 2012, 24, 1045–1051. [Google Scholar] [CrossRef]
- Snitko, L.V.; Rogozin, A.G.; Timoshkin, O.A. Thermoindicator properties of phytoplankton species (by the example of waterbodies of the Southern Urals). Inland Water Biol. 2015, 8, 147–156. [Google Scholar] [CrossRef]
- Rühland, K.M.; Paterson, A.M.; Smol, J.P. Lake diatom responses to warming: Reviewing the evidence. J. Paleolimnol. 2015, 54, 1–35. [Google Scholar] [CrossRef]
- Bradbury, J.P. Limnologic history of Lago de Patzcuaro, Michoacan, Mexico for the past 48,000 years: Impacts of climate and man. Palaeogeogr. Palaeocl. 2000, 163, 69–95. [Google Scholar] [CrossRef]
- Schabhttl, S.; Hingsamer, P.; Weigelhofer, G.; Hein, T.; Weigert, A.; Striebel, M. Temperature and species richness effects in phytoplankton communities. Oecologia 2013, 171, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Gerten, D.; Adrian, R. Climate-driven changes in spring plankton dynamics and the sensitivity of shallow polymictic lakes to the North Atlantic Oscillation. Limnol. Oceanogr. 2000, 45, 1058–1066. [Google Scholar] [CrossRef]
- Horn, H.; Paul, L.; Horn, W.; Petzoldt, T. Long-term trends in the diatom composition of the spring bloom of a German reservoir: Is Aulacoseira subarctica favoured by warm winters? Freshw. Biol. 2011, 56, 2483–2499. [Google Scholar] [CrossRef]
- Adrian, R.; Wilhelm, S.; Gerten, D. Life-history traits of lake plankton species may govern their phenological response to climate warming. Glob. Chang. Biol. 2006, 12, 652–661. [Google Scholar] [CrossRef]
- Hancock, F.D. Diatom associations in the aufwuchs of inundated trees and underwater leaves of Salvinia, drowned Mwenda River, Lake Kariba, Zimbabwe. Hydrobiologia 1985, 121, 65–76. [Google Scholar] [CrossRef]
- Post, E.; Peterson, R.O.; Stenseth, N.C.; McLaren, B.E. Ecosystem consequences of wolf behavioural responses to climate. Nature 1999, 401, 905–907. [Google Scholar] [CrossRef]
- Abrams, P.A. Will small population sizes warn us of impending extinctions? Am. Nat. 2002, 160, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.; Travis, J.M.J.; Johst, K. Interspecific interactions affect species and community responses to climate shifts. Oikos 2013, 122, 358–366. [Google Scholar] [CrossRef]
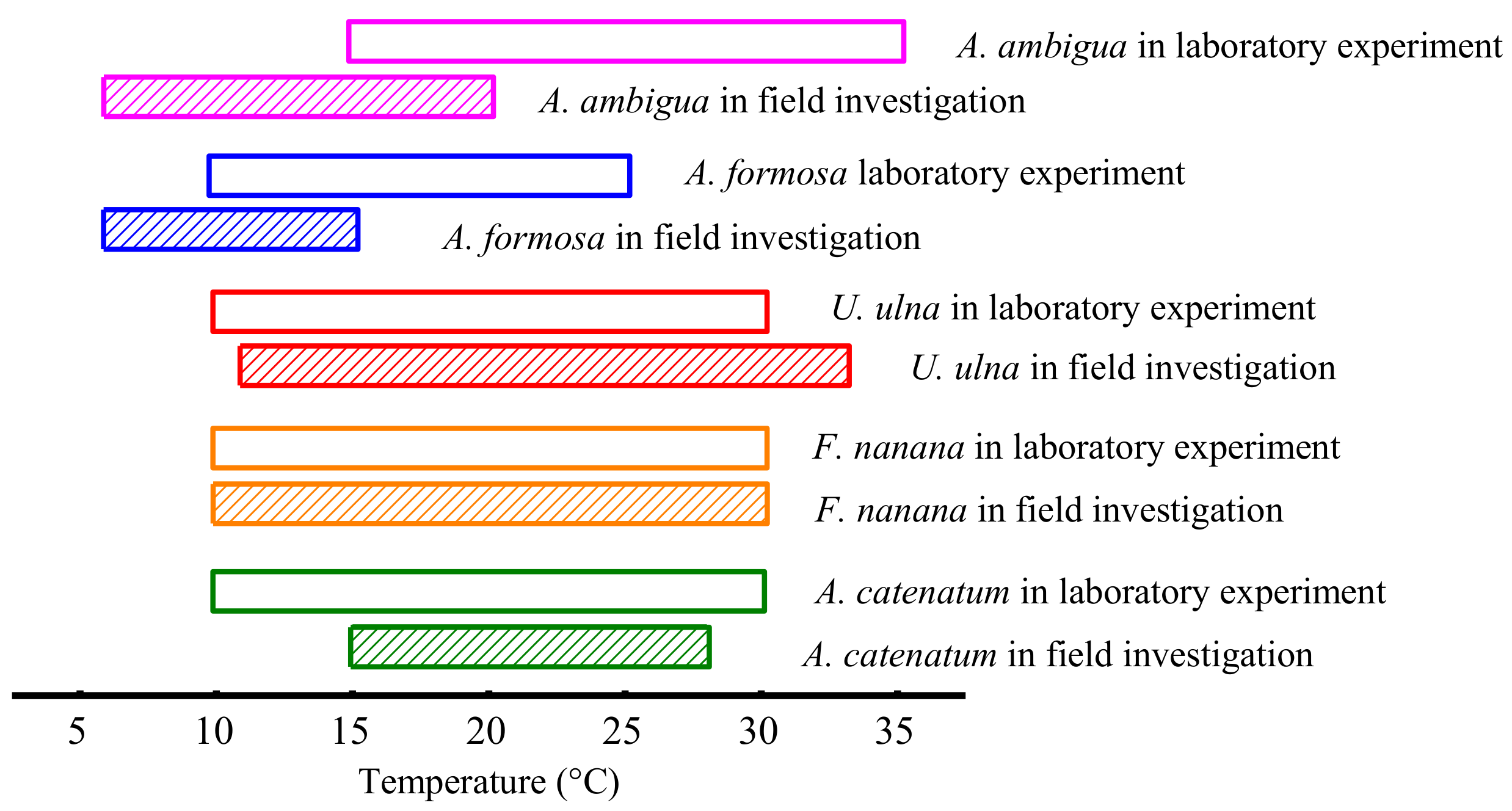
| Diatom Species | Micrographs | Volume (μm3·cell−1) | Isolation Season | Range of Growth Temperature in the Field |
|---|---|---|---|---|
| Achnanthidium catenatum |  | 65.2 | Autumn | 15–28 °C |
| Fragilaria nanana |  | 374.5 | Autumn | 10–30 °C |
| Ulnaria ulna |  | 3169.0 | Summer | 11–33 °C |
| Asterionella formosa |  | 625.5 | Winter | 6–15 °C |
| Aulacoseira ambigua |  | 331.0 | Autumn | 6–20 °C |
| O’clock | Day 1 | Day 42 | Day 43 | Day 50 | ||||
|---|---|---|---|---|---|---|---|---|
| LT (°C) | HT (°C) | LT (°C) | HT (°C) | LT (°C) | HT (°C) | LT (°C) | HT (°C) | |
| 9:00 | 10 | 14 | 30 | 34 | 31 | - | 34 | - |
| 11:00 | 12 | 16 | 32 | 36 | 33 | - | 36 | - |
| 13:00 | 14 | 18 | 34 | 38 | 35 | - | 38 | - |
| 15:00 | 13 | 17 | 33 | 37 | 34 | - | 37 | - |
| 17:00 | 12 | 16 | 32 | 36 | 33 | - | 36 | - |
| 19:00 | 11 | 15 | 31 | 35 | 32 | - | 35 | - |
| 21:00 | 10 | 14 | 30 | 34 | 31 | - | 34 | - |
| 23:00 | 9 | 13 | 29 | 33 | 30 | - | 33 | - |
| 1:00 | 8 | 12 | 28 | 32 | 29 | - | 32 | - |
| 3:00 | 7 | 11 | 27 | 31 | 28 | - | 31 | - |
| 5:00 | 6 | 10 | 26 | 30 | 27 | - | 30 | - |
| 7:00 | 8 | 12 | 28 | 32 | 29 | - | 32 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Peng, C.; Wang, Z.; Zhang, J.; Li, L.; Huang, S.; Li, D. The Species-Specific Responses of Freshwater Diatoms to Elevated Temperatures Are Affected by Interspecific Interactions. Microorganisms 2018, 6, 82. https://doi.org/10.3390/microorganisms6030082
Zhang Y, Peng C, Wang Z, Zhang J, Li L, Huang S, Li D. The Species-Specific Responses of Freshwater Diatoms to Elevated Temperatures Are Affected by Interspecific Interactions. Microorganisms. 2018; 6(3):82. https://doi.org/10.3390/microorganisms6030082
Chicago/Turabian StyleZhang, Yun, Chengrong Peng, Zhicong Wang, Jinli Zhang, Lijie Li, Shun Huang, and Dunhai Li. 2018. "The Species-Specific Responses of Freshwater Diatoms to Elevated Temperatures Are Affected by Interspecific Interactions" Microorganisms 6, no. 3: 82. https://doi.org/10.3390/microorganisms6030082
APA StyleZhang, Y., Peng, C., Wang, Z., Zhang, J., Li, L., Huang, S., & Li, D. (2018). The Species-Specific Responses of Freshwater Diatoms to Elevated Temperatures Are Affected by Interspecific Interactions. Microorganisms, 6(3), 82. https://doi.org/10.3390/microorganisms6030082