Physiological Peculiarities of Lignin-Modifying Enzyme Production by the White-Rot Basidiomycete Coriolopsis gallica Strain BCC 142
Abstract
1. Introduction
2. Materials and Methods
2.1. Organisms and Inocula Preparation
2.2. Lignocellulosic Materials
2.3. Shake-Flask Cultivation Conditions
2.4. Cultivation in Bioreactor
2.5. Enzyme Activity Assys
3. Results
3.1. Screening of White-Rot Basidiomycetes for Lignin-Modifying Enzyme Production
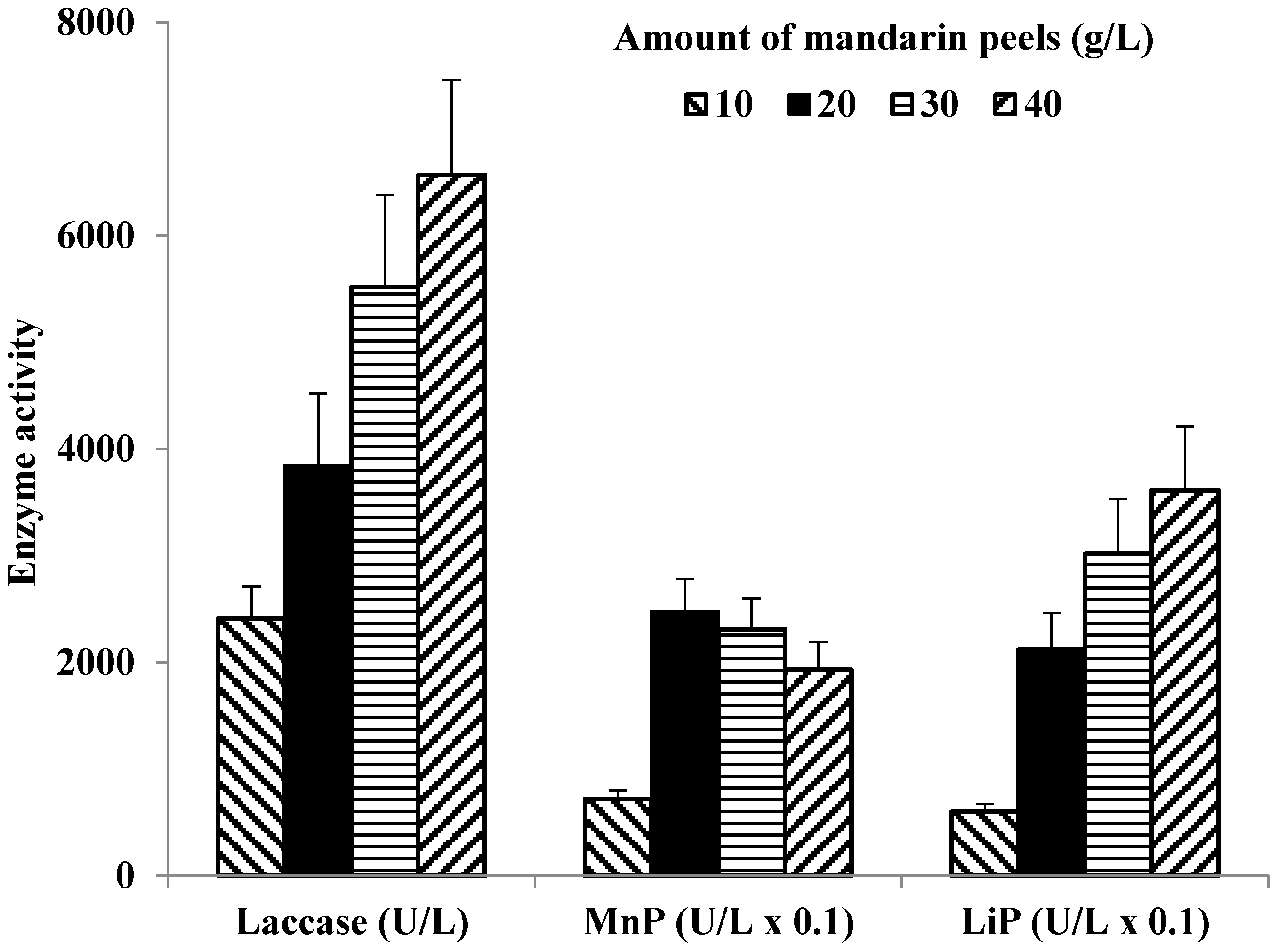
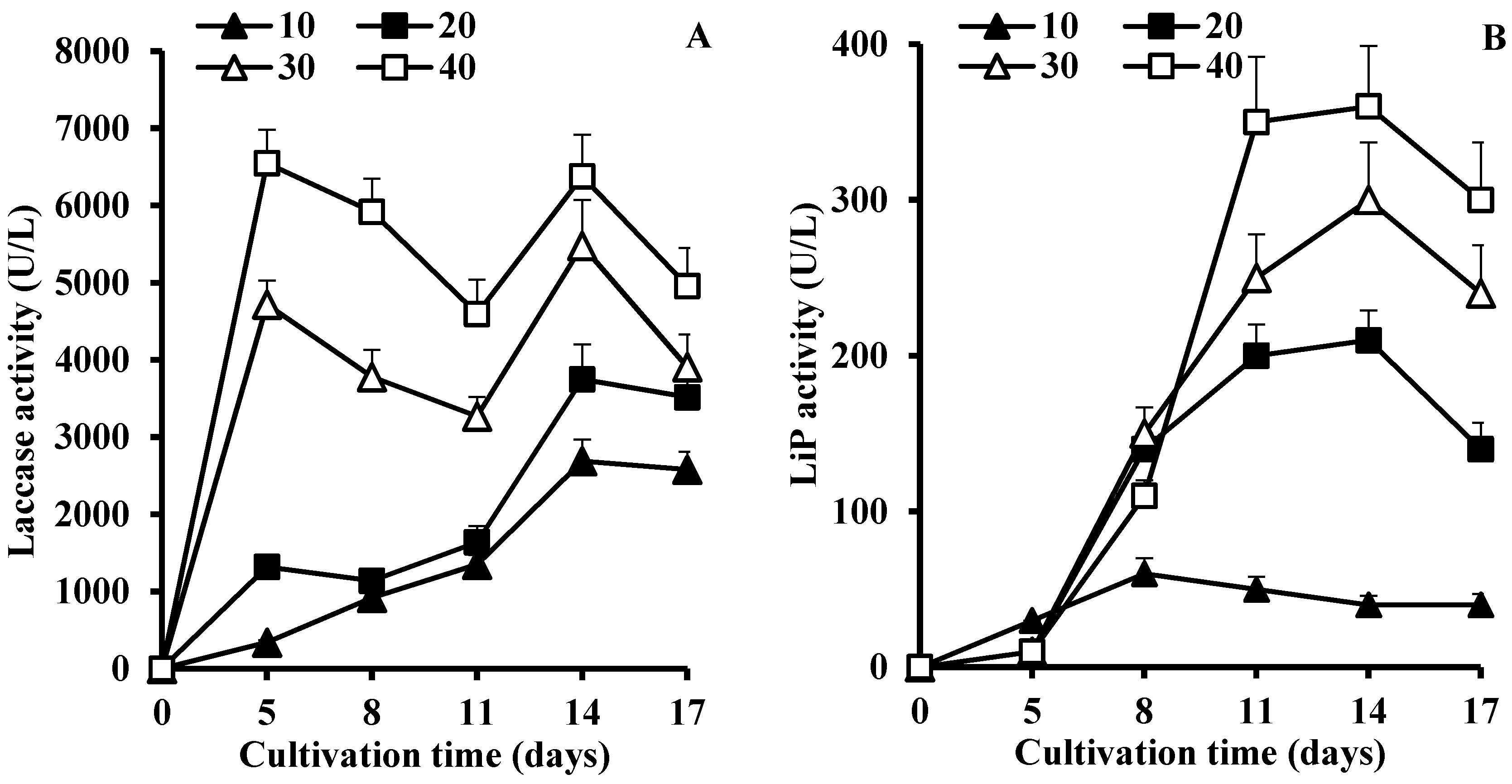
3.2. Effect of Lignocellulosic Growth Substrates on LME Production
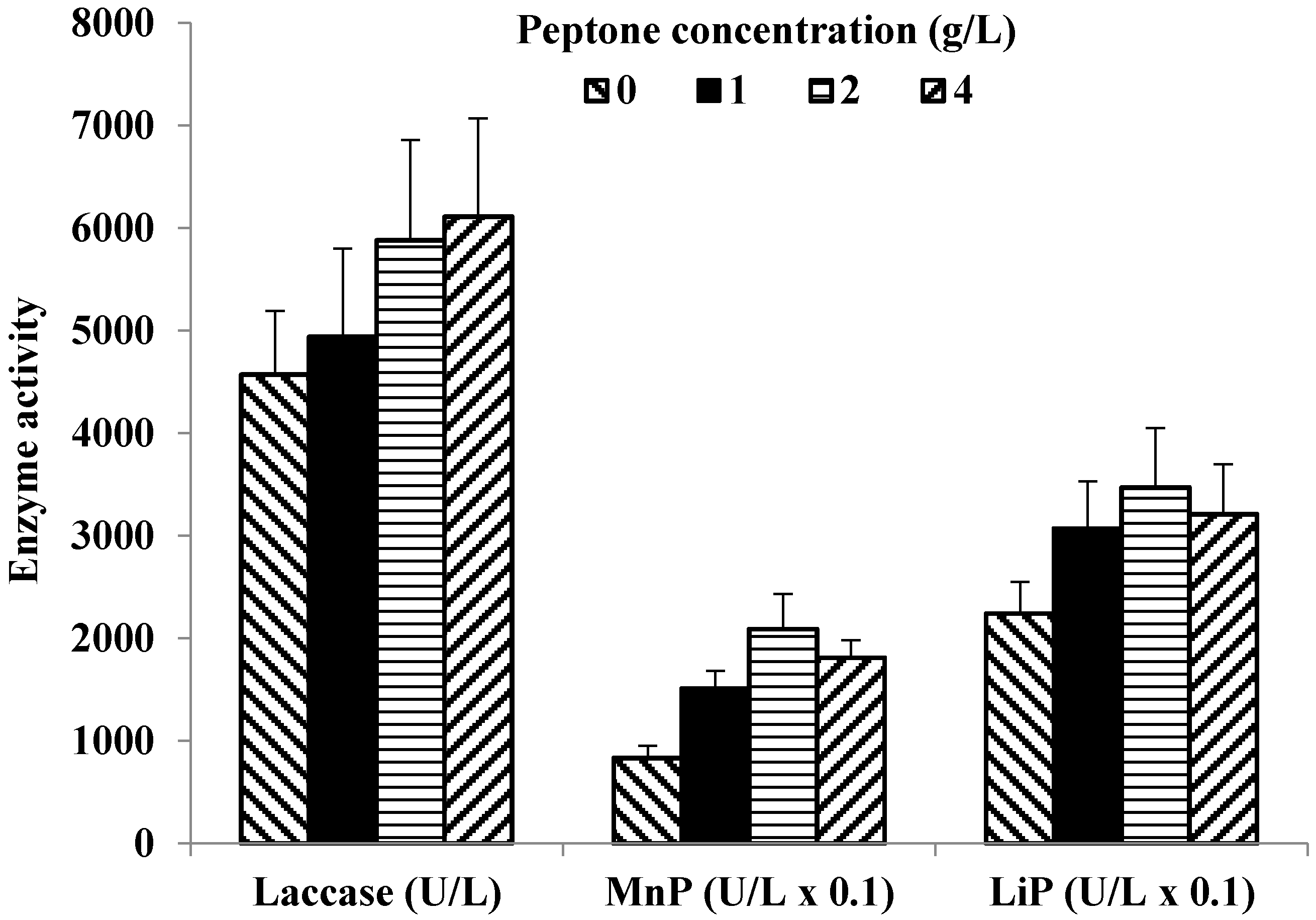
3.3. Effect of Nitrogen Source and Surfactant Concentration on LME Production
3.4. Effect of Aromatic Compounds
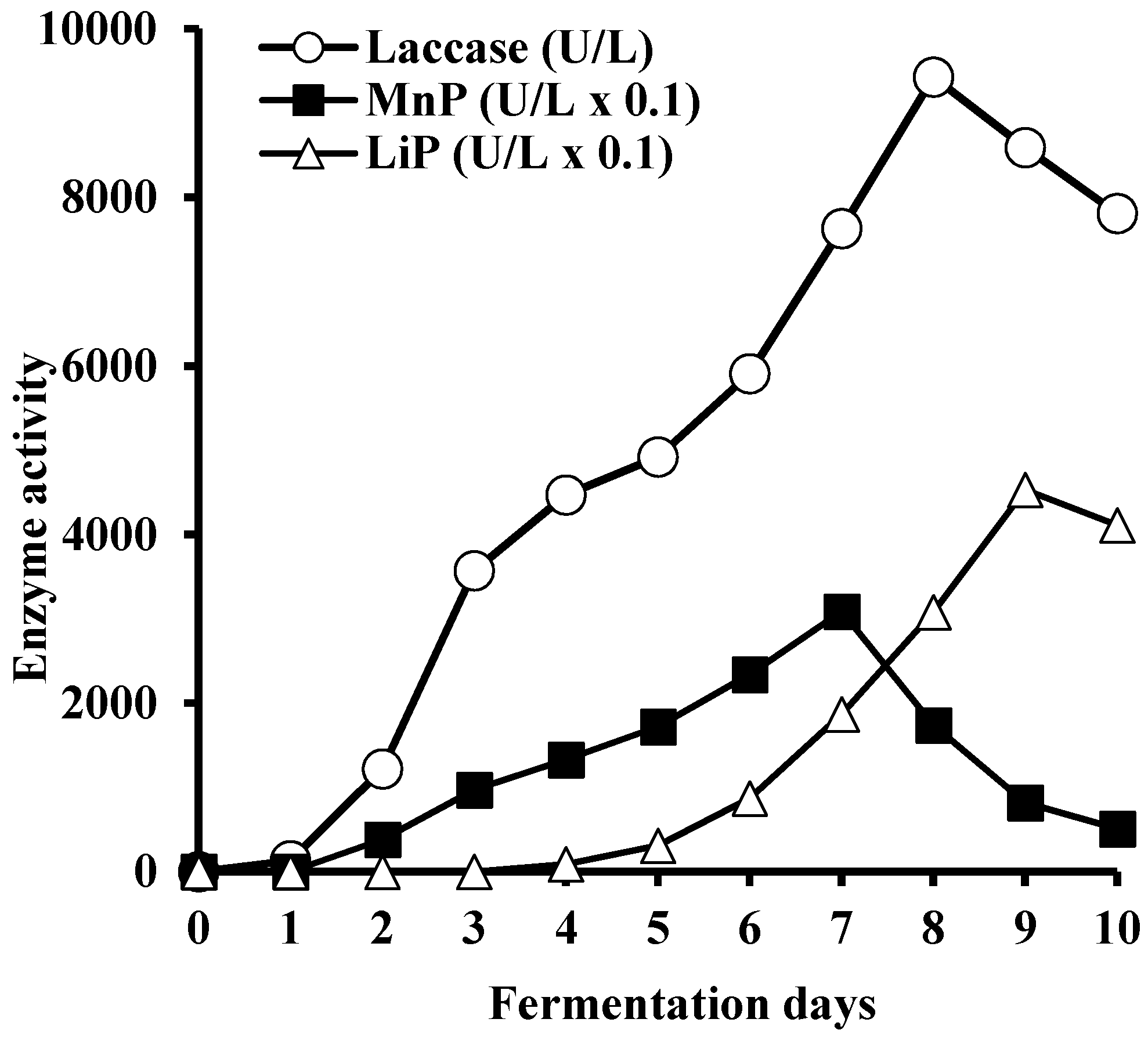
3.5. Enzyme Production in a Laboratory Bioreactor
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lundell, T.K.; Mäkelä, M.R.; de Vries, R.P.; Hildén, K.S. Genomics, lifestyles and future prospects of wood-decay and litter-decomposing Basidiomycota. In Advances in Botanical Research; Martin, F., Ed.; Academic Press: London, UK, 2014; Volume 70, pp. 329–370. [Google Scholar]
- Furukawa, T.; Bello, F.O.; Horsfall, L. Microbial enzyme systems for lignin degradation and their transcriptional regulation. Front. Biol. 2014, 9, 448–471. [Google Scholar] [CrossRef]
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.A.; Scelza, R.; Acevedo, F.; Diez, M.C.; Gianfreda, L. Enzymes as useful tools for environmental purposes. Chemosphere 2014, 107, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V.; Kachlishvili, E. Physiological regulation of laccase and manganese peroxidase production by white-rot Basidiomycetes. J. Biotechnol. 2009, 144, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, A.; Giardina, P.; Lettera, V.; Pezzella, C.; Sannia, G.; Faraco, V. Induction and transcriptional regulation of laccases in fungi. Curr. Genom. 2011, 12, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Chen, S. The white-rot fungus Phanerochaete chrysosporium: Conditions for the production of lignin-degrading enzymes. Appl. Microbiol. Biotechnol. 2008, 81, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Galhaup, C.; Wagner, H.; Hinterstoisser, B.; Haltrich, D. Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enzyme Microb. Technol. 2002, 30, 529–536. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Metreveli, E.; Elisashvili, V. Modulation of Cerrena unicolor laccase and manganese peroxidase production. SpringerPlus 2014, 3, 463. [Google Scholar] [CrossRef] [PubMed]
- Revankar, M.S.; Lele, S.S. Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochem. 2006, 41, 581–588. [Google Scholar] [CrossRef]
- Songulashvili, G.; Jimenéz-Tobón, G.A.; Jaspers, C.; Penninckx, M.J. Immobilized laccase of Cerrena unicolor for elimination of endocrine disruptor micropollutants. Fungal Biol. 2012, 116, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Coconi-Linares, N.; Magaña-Ortíz, D.; Guzmán-Ortiz, D.A.; Fernández, F.; Loske, A.M.; Gómez-Lim, M.A. High-yield production of manganese peroxidase, lignin peroxidase, and versatile peroxidase in Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 2014, 98, 9283–9294. [Google Scholar] [CrossRef] [PubMed]
- Kapich, A.N.; Prior, B.A.; Botha, A.; Galkin, S.; Lundell, T.; Hatakka, A. Effect of lignocellulose-containing substrates on production of ligninolytic peroxidases in submerged cultures of Phanerochaete chrysosporium ME-446. Enzyme Microb. Technol. 2004, 34, 187–195. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E.; Penninckx, M. Effect of growth substrate, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J. Ind. Microbiol. Biotechnol. 2008, 35, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Kucharzyk, K.H.; Pawlik, A.; Staszczak, M.; Paszczynski, A.J. Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation. Enzyme Microb. Technol. 2013, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hu, X.; Cook, S.; Begonia, M.; Lee, K.S.; Hwang, H. Effect of culture conditions on the production of ligninolytic enzymes by white rot fungi Phanerochaete chrysosporium (ATCC 20696) and separation of its lignin peroxidase. World J. Microbiol. Biotechnol. 2008, 24, 2205–2212. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose in biological material. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Wariishi, H.; Valli, K.; Gold, M.H. Manganese (II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J. Biol. Chem. 1992, 267, 23688–23695. [Google Scholar] [PubMed]
- Glenn, J.K.; Gold, M.H. Purification and characterization of an extracellular Mn (II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch. Biochem. Biophys. 1985, 242, 329–341. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc. Natl. Acad. Sci. USA 1984, 81, 2280–2284. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Asatiani, M.; Kobakhidze, A.; Elisashvili, V. Trinitrotoluene and mandarin peels selectively affect lignin-modifying enzyme production in white-rot basidiomycetes. SpringerPlus 2016, 5, 252. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Michniewicz, A.; Ullrich, R.; Ledakowicz, S.; Hofrichter, M. The white-rot fungus Cerrena unicolor strain 137 produces two laccase isoforms with different physico-chemical and catalytic properties. Appl. Microbiol. Biotechnol. 2006, 69, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Hibi, M.; Hatahira, S.; Nakatani, M.; Yokozeki, K.; Shimizu, S.; Ogawa, J. Extracellular oxidases of Cerrena sp. complementarily functioning in artificial dye decolorization including laccase, manganese peroxidase, and novel versatile peroxidases. Biocatal. Agric. Biotechnol. 2012, 1, 220–225. [Google Scholar] [CrossRef]
- Kinnunen, A.; Maijala, P.; Järvinen, P.; Hatakka, A. Improved efficiency in screening for lignin-modifying peroxidases and laccases of basidiomycetes. Curr. Biotechnol. 2017, 6, 105–115. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E.; Khardziani, T.; Agathos, S.N. Effect of aromatic compounds on the production of laccase and manganese peroxidase by white-rot basidiomycetes. J. Ind. Microbiol. Biotechnol. 2010, 37, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
| Fungi | Final pH | Laccase (U/L) | MnP (U/L) (Mn2+) a | MnP (U/L) (Phenol Red) a | LiP (U/L) |
|---|---|---|---|---|---|
| C. unicolor 300 | 6.1 | 8810 ± 1270 14,b | 1640 ± 300 4 | 1440 ± 210 4 | 0 |
| C. unicolor 301 | 5.8 | 3190 ± 360 10 | 810 ± 100 10 | 1350 ± 220 10 | Traces c |
| C. unicolor 302 | 6.0 | 7640 ± 1370 10 | 560 ± 150 7 | 670 ± 110 10 | 0 |
| C. unicolor 303 | 5.8 | 9410 ± 1420 10 | 1040 ± 210 7 | 880 ± 160 10 | Traces |
| C. gallica 142 | 5.7 | 1090 ± 150 14 | 140 ± 20 10 | 180 ± 20 7 | 70 ± 10 14 |
| F. trogii 146 | 5.9 | 640 ± 103 7 | 40 ± 10 10 | 0 | Traces |
| M. tremellosus 206 | 5.3 | 610 ± 71 7 | 0 | 0 | 0 |
| P. chrysosporium 1309 | 6.5 | 0 | 110 ± 20 7 | 40 ± 10 7 | Traces |
| P. chrysosporium 24725 | 6.1 | 0 | 0 | 0 | Traces |
| P. chrysosporium 34541 | 6.4 | 0 | 0 | 0 | 0 |
| P. radiata 64658 | 6.0 | 230 ± 40 7 | Traces c | 0 | Traces |
| T. hirsuta 119 | 5.5 | 810 ± 120 10 | Traces | 140 ± 30 4 | 0 |
| T. ochracea 1009 | 6.0 | 250 ± 30 7 | 280 ± 50 7 | 210 ± 30 4 | Traces |
| T. versicolor 113 | 6.4 | 1060 ± 170 10 | Traces | 0 | Traces |
| T. versicolor 159 | 6.4 | 2350 ± 380 4 | 100 ± 20 4 | 90 ± 20 4 | 0 |
| T. zonatus 540 | 6.5 | 4220 ± 690 4 | 160 ± 30 4 | 150 ± 20 4 | Traces |
| Fungal Strain | Final pH | Laccase (U/L) | MnP (U/L) (Mn2+) a | MnP (U/L) (Phenol Red) a | LiP (U/L) |
|---|---|---|---|---|---|
| C. unicolor 300 | 5.8 | 19,600 ± 3020 10,b | 1980 ± 370 4 | 820 ± 130 4 | 160 ± 20 10 |
| C. unicolor 301 | 5.0 | 16,600 ± 2810 7 | 2760 ± 420 7 | 1120 ± 180 7 | 110 ± 20 14 |
| C. unicolor 302 | 6.0 | 22,430 ± 2720 10 | 430 ± 40 7 | 330 ± 60 7 | 100 ± 30 10 |
| C. unicolor 303 | 5.2 | 38,290 ± 6080 7 | 1920 ± 280 4 | 1070 ± 220 7 | 60 ± 10 10 |
| C. gallica 142 | 5.2 | 5300 ± 780 10 | 170 ± 30 7 | 220 ± 30 14 | 210 ± 30 14 |
| F. trogii 146 | 6.0 | 1200 ± 130 4 | 90 ± 10 10 | 270 ± 50 10 | 60 ± 10 14 |
| M. tremellosus 206 | 4.9 | 3900 ± 470 14 | 70 ± 10 10 | 60 ± 10 10 | 0 |
| P. chrysosporium 1309 | 6.7 | 0 | 160 ± 20 4 | 0 | 150 ± 30 10 |
| P. chrysosporium 24725 | 6.2 | 0 | 150 ± 304 | 0 | Traces c |
| P. chrysosporium 34541 | 6.2 | 0 | Traces c | 0 | Traces |
| P. radiata 64658 | 5.1 | 4200 ± 640 7 | 240 ± 30 4 | 50 ± 10 4 | 40 ± 10 10 |
| T. hirsuta 119 | 4.8 | 1300 ± 190 4 | 30 ± 0 4 | 0 | 30 ± 10 4 |
| T. ochracea 1009 | 5.6 | 5530 ± 1070 4 | 380 ± 70 4 | 170 ± 30 10 | 80 ± 10 14 |
| T. versicolor 113 | 5.7 | 3180 ± 390 10 | 50 ± 10 10 | 80 ± 10 10 | 110 ± 20 4 |
| T. versicolor 159 | 6.0 | 3920 ± 480 10 | Traces | 0 | 40 ± 10 10 |
| T. zonatus 540 | 5.7 | 8430 ± 1390 4 | 220 ± 407 | 130 ± 20 4 | 60 ± 10 14 |
| Growth Substrate | Final pH | Laccase (U/L) | MnP (U/L) (Mn2+) a | MnP (U/L) (Phenol Red) a | LiP (U/L) | CMCase (U/mL) |
|---|---|---|---|---|---|---|
| Banana peels | 5.0 | 7120 ± 1470 7,b | 240 ± 30 4 | 200 ± 20 7 | 70 ± 20 10 | 1.6 ± 0.2 7 |
| EPR | 5.4 | 6130 ± 990 7 | 110 ± 20 7 | 260 ± 30 7 | 40 ± 10 10 | 2.7 ± 0.2 10 |
| Mandarin peels | 5.0 | 6490 ± 820 14 | 160 ± 20 7 | 240 ± 30 10 | 230 ± 30 10 | 5.2 ± 0.8 10 |
| Sunflower oil cake | 6.0 | 27,280 ± 4970 10 | Traces c | 170 ± 50 7 | 80 ± 10 7 | 3.5 ± 0.5 10 |
| Walnut pericarp | 5.8 | 4680 ± 610 7 | Traces | 180 ± 30 7 | 60 ± 10 10 | 2.1 ± 0.3 7 |
| Wheat bran | 5.0 | 19,720 ± 2790 7 | 170 ± 30 7 | 260 ± 40 7 | 60 ± 10 10 | 4.1 ± 0.5 7 |
| Wheat straw | 6.4 | 11,210 ± 1580 7 | 240 ± 10 7 | 330 ± 50 7 | 90 ± 10 7 | 3.5 ± 0.4 7 |
| Surfactant Concentration (g/L) | Final pH | Laccase (U/L) | MnP (U/L) (Phenol Red) | LiP (U/L) | CMCase (U/mL) |
|---|---|---|---|---|---|
| Control | 5.8 | 6680 ± 820 14 | 230 ± 30 14 | 340 ± 50 14 | 6.0 ± 0.8 14 |
| +1.0 Tween 80 | 5.8 | 6830 ± 1060 14 | 310 ± 40 14 | 370 ± 40 14 | 6.5 ± 1.0 14 |
| +2.0 Tween 80 | 5.8 | 6630 ± 1150 14 | 350 ± 50 14 | 360 ± 50 14 | 6.9 ± 1.0 14 |
| +4.0 Tween 80 | 5.8 | 7120 ± 1070 14 | 450 ± 70 14 | 370 ± 60 14 | 6.6 ± 1.1 14 |
| +2.0 PEG | 5.8 | 6390 ± 1180 14 | 220 ± 30 14 | 340 ± 40 14 | 6.6 ± 0.9 14 |
| +4.0 PEG | 5.8 | 6720 ± 930 14 | 230 ± 30 14 | 370 ± 40 14 | 6.7 ± 1.1 14 |
| +6.0 PEG | 5.8 | 6900 ± 760 14 | 220 ± 40 14 | 370 ± 50 14 | 6.9 ± 1.2 14 |
| +1.0 Triton X-100 | 5.1 | 6310 ± 980 14 | 40 ± 10 14 | 400 ± 60 14 | 4.0 ± 0.5 14 |
| +2.0 Triton X-100 | 5.0 | 6370 ± 1190 14 | 50 ± 10 14 | 400 ± 70 14 | 4.3 ± 0.2 14 |
| +4.0 Triton X-100 | 4.9 | 6630 ± 1410 14 | 40 ± 10 14 | 390 ± 70 14 | 3.5 ± 0.2 14 |
| Compound Concentration (mol/L) | Final pH | Laccase (U/L) | MnP (U/L) (Phenol Red) | LiP (U/L) |
|---|---|---|---|---|
| Control | 5.8 | 6500 ± 90 9 | 260 ± 30 12 | 330 ± 40 12 |
| +0.3 mM hydroquinone | 5.2 | 4920 ± 110 12 | 240 ± 30 12 | 140 ± 30 12 |
| +0.3 mM trinitrotoluene | 5.8 | 5320 ± 100 6 | 210 ± 30 12 | 290 ± 60 12 |
| +0.5 mM 2.6 dimethoxyphenol | 5.6 | 6190 ± 70 6 | 220 ± 20 12 | 370 ± 60 12 |
| +0.5 mM ferulic acid | 5.5 | 9730 ± 1680 12 | 240 ± 20 12 | 300 ± 40 12 |
| +0.5 mM guaiacol | 5.5 | 6780 ± 970 12 | 270 ± 30 12 | 370 ± 40 12 |
| +0.5 mM pyrogallol | 5.5 | 12,610 ± 1670 9 | 270 ± 30 9 | 430 ± 60 12 |
| +0.5 mM vanillic acid | 5.5 | 8330 ± 980 12 | 240 ± 20 12 | 330 ± 50 12 |
| +0.5 mM veratryl alcohol | 5.3 | 11,790 ± 1370 12 | 250 ± 20 12 | 330 ± 40 12 |
| +0.5 mM xylidine | 5.7 | 7100 ± 1280 6 | 280 ± 40 12 | 270 ± 60 12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elisashvili, V.; Kachlishvili, E.; Asatiani, M.D.; Darlington, R.; Kucharzyk, K.H. Physiological Peculiarities of Lignin-Modifying Enzyme Production by the White-Rot Basidiomycete Coriolopsis gallica Strain BCC 142. Microorganisms 2017, 5, 73. https://doi.org/10.3390/microorganisms5040073
Elisashvili V, Kachlishvili E, Asatiani MD, Darlington R, Kucharzyk KH. Physiological Peculiarities of Lignin-Modifying Enzyme Production by the White-Rot Basidiomycete Coriolopsis gallica Strain BCC 142. Microorganisms. 2017; 5(4):73. https://doi.org/10.3390/microorganisms5040073
Chicago/Turabian StyleElisashvili, Vladimir, Eva Kachlishvili, Mikheil D. Asatiani, Ramona Darlington, and Katarzyna H. Kucharzyk. 2017. "Physiological Peculiarities of Lignin-Modifying Enzyme Production by the White-Rot Basidiomycete Coriolopsis gallica Strain BCC 142" Microorganisms 5, no. 4: 73. https://doi.org/10.3390/microorganisms5040073
APA StyleElisashvili, V., Kachlishvili, E., Asatiani, M. D., Darlington, R., & Kucharzyk, K. H. (2017). Physiological Peculiarities of Lignin-Modifying Enzyme Production by the White-Rot Basidiomycete Coriolopsis gallica Strain BCC 142. Microorganisms, 5(4), 73. https://doi.org/10.3390/microorganisms5040073







