A Two-Step Bioconversion Process for Canolol Production from Rapeseed Meal Combining an Aspergillus niger Feruloyl Esterase and the Fungus Neolentinus lepideus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Microorganisms and Culture Conditions
2.4. Accession Numbers of Protein Sequences
2.5. Enzyme Activity Assay
2.6. Screening of Resins for Aromatic Compound Adsorption
2.7. Enzymatic Hydrolysis of Rapeseed Meal
2.8. Batch Production of Sinapic Acid from Rapeseed Meal
2.9. Characterization of Rapeseed Meal
2.10. Extraction, Derivatization and Gas Chromatography–Mass Spectrometry (GC–MS) of the Monomeric Phenolics from N. lepideus Culture Broth
2.11. High Performance Liquid Chromatography (HPLC) Analysis of the Monomeric Phenolics from Fungal Culture Broth
3. Results
3.1. Characterization of RSM
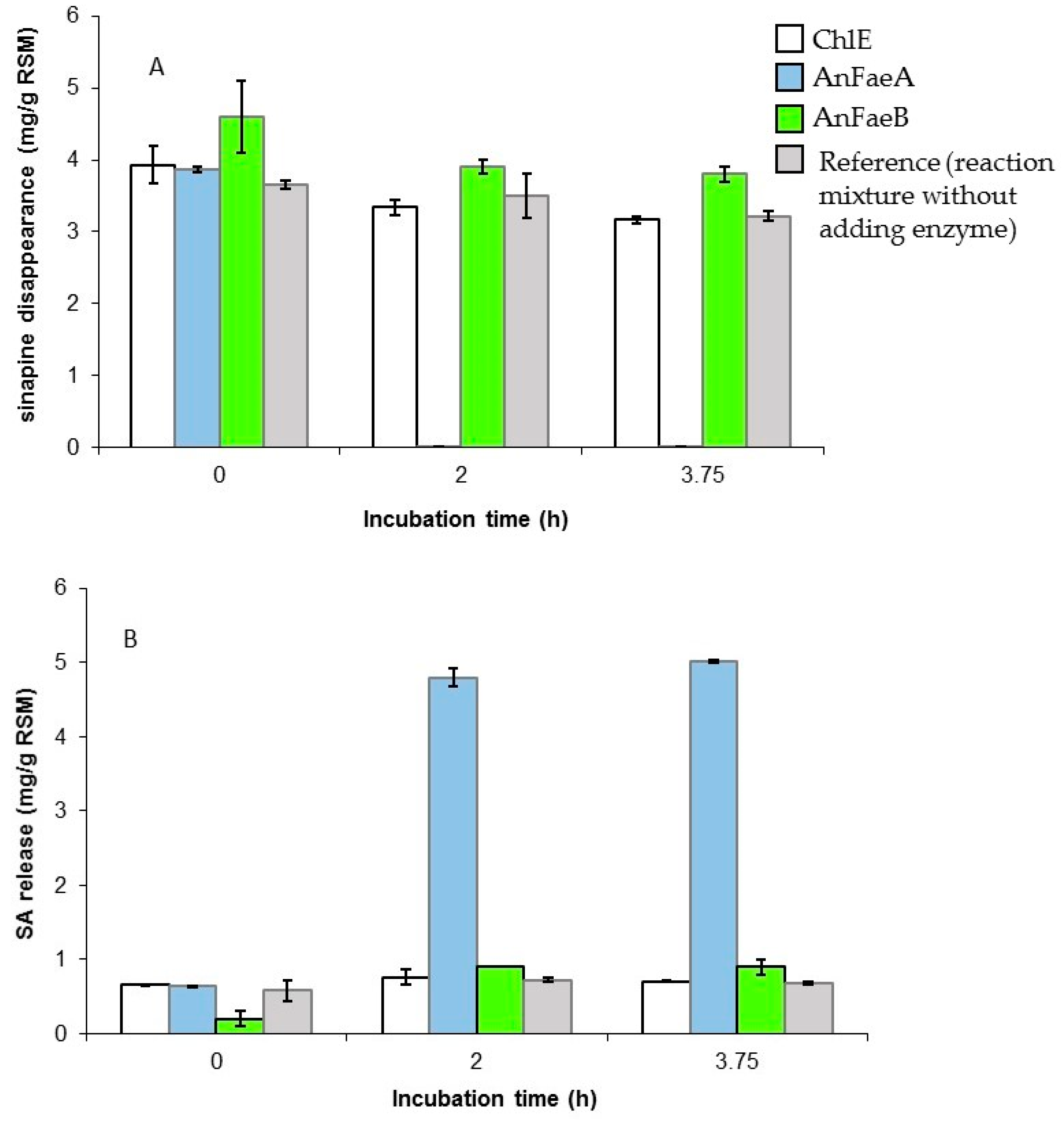
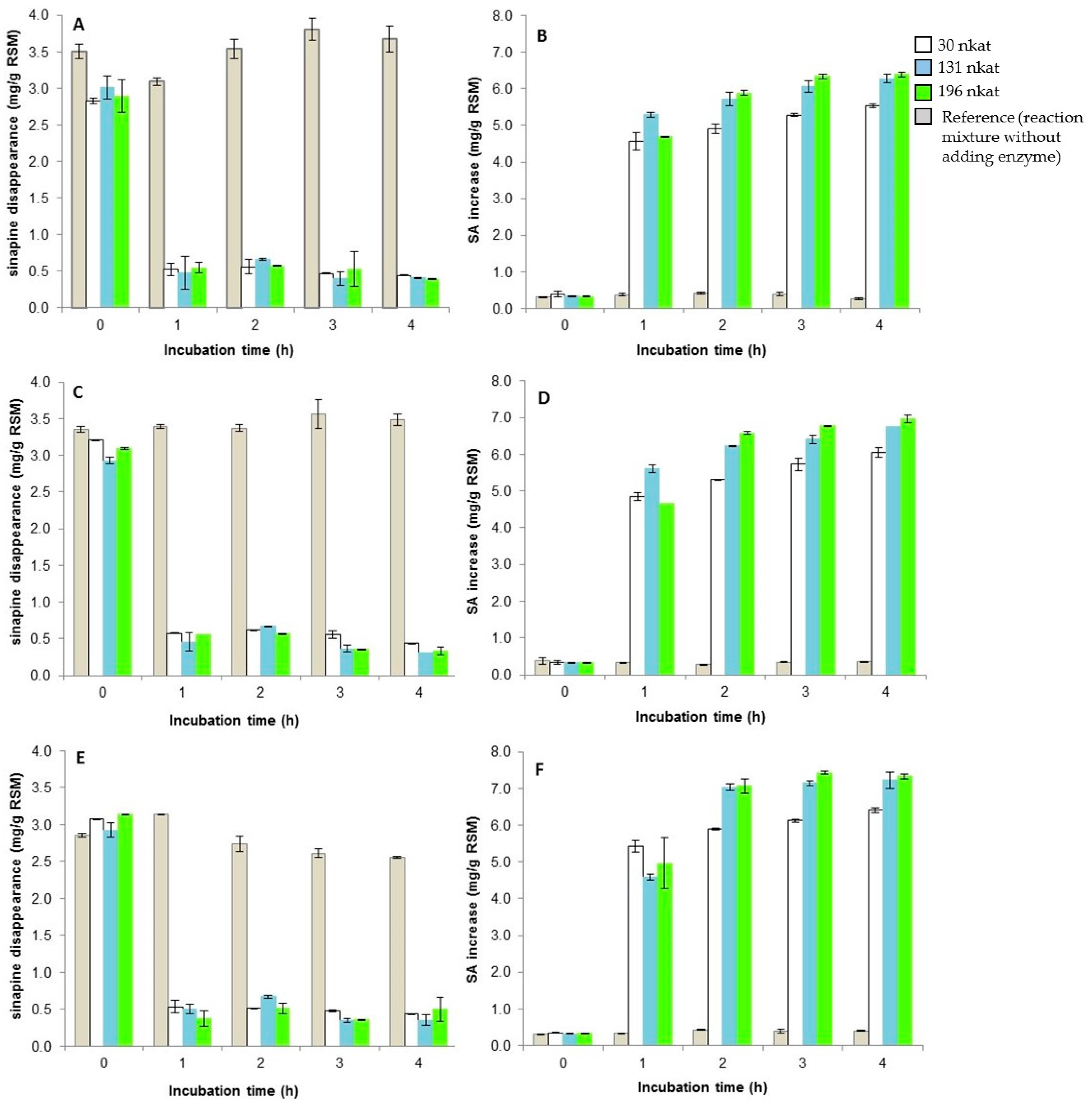
3.2. Release of Free SA from Meal by A. niger Esterases
3.3. Screening of Fungal Strains able to Biotransform Hydroxycinnamic Acids into the Corresponding Vinyl Compounds
3.4. Selection of a Specific Adsorbent for SA or Canolol Recovery
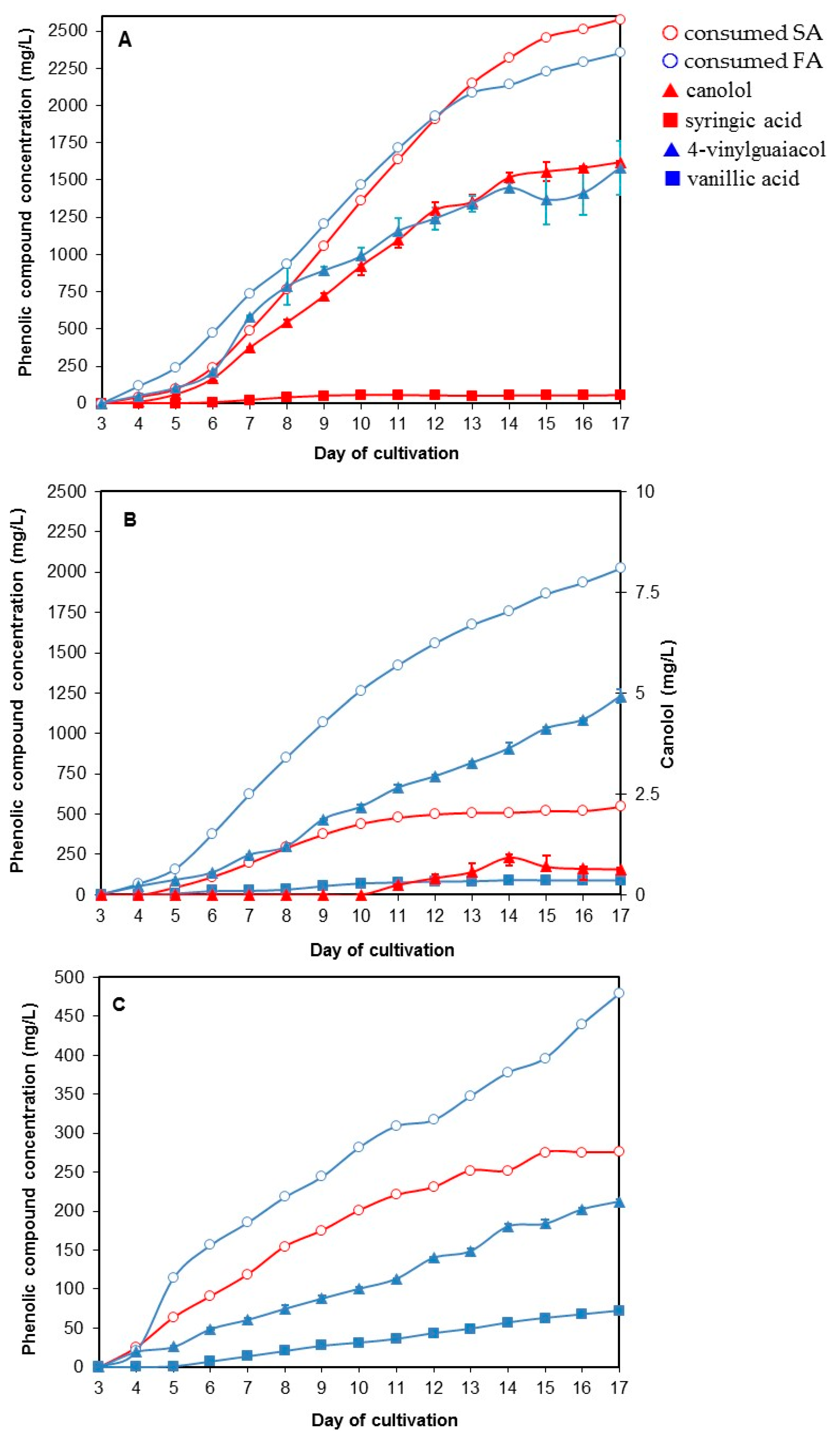
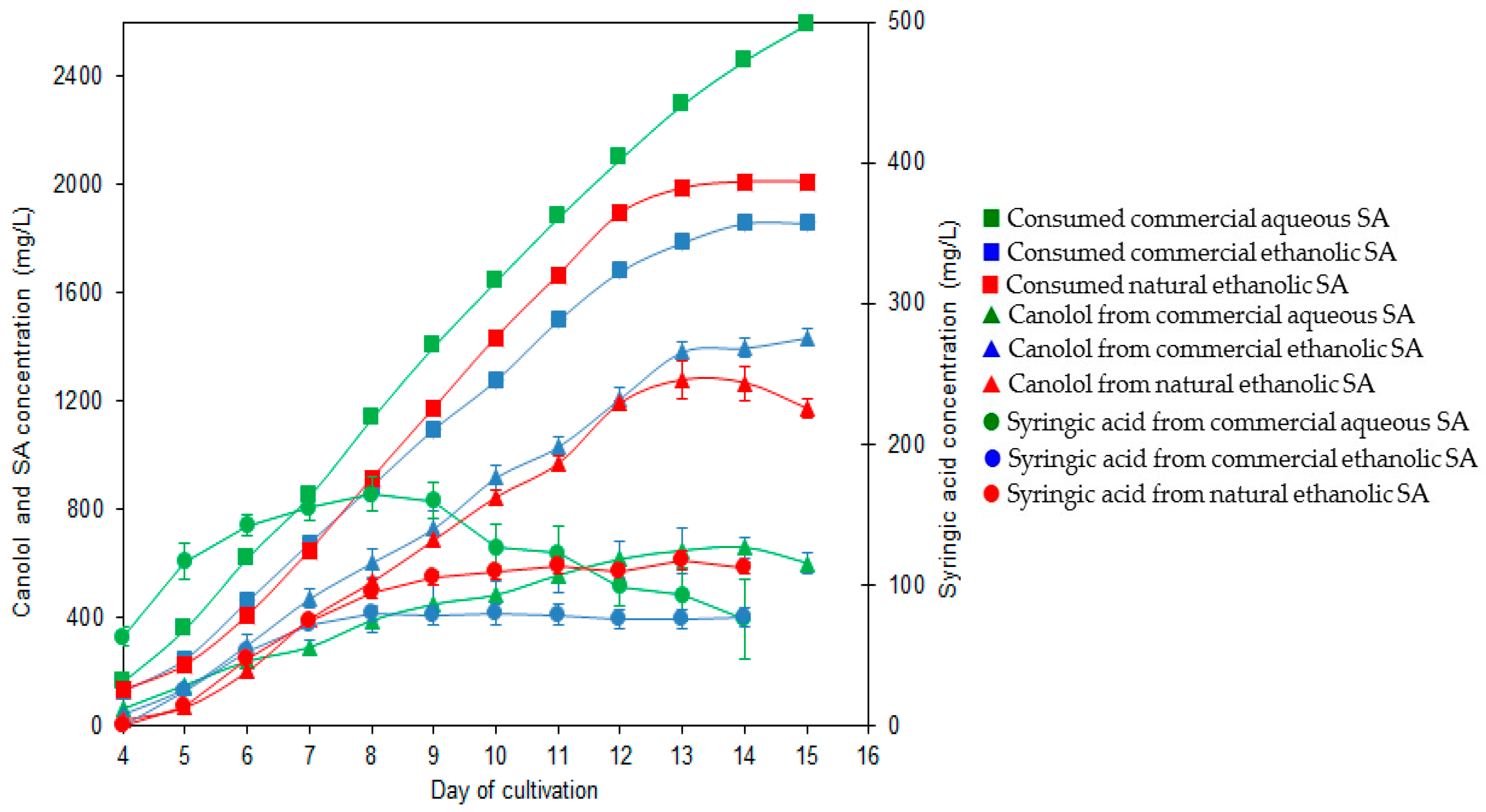
3.5. The Two-Step Bioconversion Process: Batch Production of Natural SA from Rapeseed Meal and Bioconversion into Canolol
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lomascolo, A.; Uzan-Boukris, E.; Sigoillot, J.-C.; Fine, F. Rapeseed and sunflower meal: A review on biotechnology status and challenge. Appl. Microbiol. Biotechnol. 2012, 95, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Agricultural Production Statistics by Country—IndexMundi. Available online: http://www.indexmundi.com/agriculture (accessed on 5 February 2017).
- Briones, R.; Serrano, L.; Labidi, J. Valorisation of some lignocellulosic agro-industrial residues to obtain biopolyols. J. Chem. Technol. Biotechnol. 2011, 87, 244–249. [Google Scholar] [CrossRef]
- Yeoman, K.H.; Edwards, C. Protease production by Streptomyces thermovulgaris grown on rapemeal-derived media. J. Appl. Bacteriol. 1994, 77, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.I.; Huma, T.; Khalid, A.M.; Latif, F. Kinetics of enhanced substrate consumption and endo-β-xylanase production by a mutant derivative of Humicola lanuginosa in solid-state fermentation. World J. Microbiol. Biotechnol. 2005, 21, 869–876. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Ioannidou, O.; Skoulou, V. Rapeseed residues utilization for energy and 2nd generation biofuels. Fuel 2008, 87, 1492–1502. [Google Scholar] [CrossRef]
- Wang, R.; Shaarani, S.M.; Godoy, L.C.; Melikoglu, M.; Vergara, C.S.; Koutinas, A.; Webb, C. Bioconversion of rapeseed meal for the production of a generic microbial feedstock. Enzym. Microb. Technol. 2010, 47, 77–83. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H.; Miao, Y.; Wei, P.; Chen, J. Simultaneous saccharification and fermentation of acid-pretreated rapeseed meal for succinic acid production using Actinobacillus succinogenes. Enzym. Microb. Technol. 2011, 48, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Ji, Z.; Wang, C.; Qi, G.; Zhang, L.; Ma, X.; Chen, S. Co-producing iturin A and poly-γ-glutamic acid from rapeseed meal under solid state fermentation by the newly isolated Bacillus subtilis strain 3-10. World J. Microbiol. Biotechnol. 2012, 28, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Vuorela, S.; Meyer, A.; Heinonen, M. Impact of isolation method on the antioxidant activity of rapeseed meal phenolics. J. Agric. Food Chem. 2004, 52, 8202–8207. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.L.; Grosse, S.; Leisch, H.; Lau, P.C.K. Antioxidant canolol production from a renewable feedstock via an engineered decarboxylase. Green Chem. 2013, 15, 3312–3317. [Google Scholar] [CrossRef]
- Koski, A.; Pekkarinen, S.; Hopia, A.; Wähälä, K.; Heinonen, M. Processing of rapeseed oil: Effects on sinapic acid derivative content and oxidative stability. Eur. Food Res. Technol. 2003, 217, 110–114. [Google Scholar] [CrossRef]
- Wakamatsu, D.; Morimura, S.; Sawa, T.; Kida, K.; Nakai, C.; Maeda, H. Isolation, identification, and structure of a potent alkyl-peroxyl radical scavenger in crude canola oil, canolol. Biosci. Biotechnol. Biochem. 2005, 69, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, H.; Kanazawa, A.; Wakamatu, D.; Morimura, S.; Kida, K.; Akaike, T.; Maeda, H. Antioxidative and antimutagenic activities of 4-vinyl-2,6-dimethoxyphenol (canolol) isolated from canola oil. J. Agric. Food Chem. 2004, 52, 4380–4387. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, Z.; Wang, W.; Zhang, W.; Liu, S.; Zhang, X.; Fang, J.; Maeda, H.; Matsukura, M. Protective effect of canolol from oxidative stress-induced cell damage in ARPE-19 via an ERK mediated antioxidative pathway. Mol. Vision 2011, 17, 2040–2048. [Google Scholar]
- Cao, X.; Tsukamoto, T.; Seki, T.; Tanaka, H.; Morimura, S.; Cao, L.; Mizoshita, T.; Ban, H.; Toyoda, T.; Maeda, H.; Tatematsu, M. 4-Vinyl-2,6-dimethoxyphenol (canolol) suppresses oxidative stress and gastric carcinogenesis in Helicobacter pylori-infected carcinogen-treated Mongolian gerbils. Int. J. Cancer 2008, 122, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kumamoto, J.P.; Tsukamoto, T.; Tatematsu, M.; Nagoya-shi, J.P. Anti-Inflammatory Agent and Cancer-Preventive Agent Comprising Canolol or Prodrug Thereof and Pharmaceutical, Cosmetic and Food Comprising the Same. U.S. Patent 2012/0122995 A1, 2012. [Google Scholar]
- Aouf, C.; Zago, E.; Lecomte, J.; Villeneuve, P.; Fulcrand, H.; Fine, F.; Rous, J.-F. Polyaromatic Dimers, Method for Preparing Same and Use of Same. Patent WO2016/097657, 1 September 2016. [Google Scholar]
- Zago, E.; Dubreucq, E.; Lecomte, J.; Villeneuve, P.; Fine, F.; Fulcrand, H.; Aouf, C. Synthesis of bio-based epoxy monomers from natural ally- and vinyl phenols and the estimation of their affinity to the oestrogen receptot α by molecular docking. New J. Chem. 2016, 40, 7701–7710. [Google Scholar] [CrossRef]
- Pudel, F.; Habicht, V.; Piofczyk, T.; Matthäus, B.; Quirin, K.W.; Cawelius, A. Fluidized bed treatment of rapeseed meal and cake as possibility for the production of canolol. Oilseeds Fats Crop. Lipids 2014, 21, D103. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Liu, C.; Li, W.; Huang, F. Influence of microwaves treatment of rapeseed on phenolic compounds and canolol content. J. Agric. Food. Chem. 2014, 62, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Zago, E.; Lecomte, J.; Barouh, N.; Aouf, C.; Carré, P.; Fine, F.; Villeneuve, P. Influence of rapeseed meal treatments on its total phenolic content and composition in sinapine, sinapic acid and canolol. Ind. Crop. Prod. 2015, 76, 1061–1070. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Concurrent extraction and transformation of bioactive phenolic compounds from rapeseed meal using pressurized solvent extraction system. Ind. Crop. Prod. 2016, 94, 152–159. [Google Scholar] [CrossRef]
- Steinke, R.D.; Paulson, M.C. The production of steam-volatile phenols during the cooking and alcoholic fermentation of grain. J. Agric. Food Chem. 1964, 12, 381–387. [Google Scholar] [CrossRef]
- Godoy, L.; Martinez, C.; Carrasco, N.; Ganga, M.A. Purification and characterization of p-coumarate decarboxylase and a vinylphenol reductase from Brettanomyces bruxellensis. Int. J. Food Microbiol. 2008, 127, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Matte, A.; Grosse, F.; Bergeron, H.; Abokitse, K.; Lau, P.C.K. Structural analysis of Bacillus pumilus acid decarboxylase, a lipocalin-fold enzyme. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-K.; Tokashiki, M.; Maeno, S.; Onaga, S.; Taira, T.; Ito, S. Purification and properties of phenolic acid decarboxylase from Candida guilliermondii. J. Ind. Microbiol. Biotechnol. 2012, 39, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Record, E.; Asther, Mi.; Sigoillot, C.; Pages, S.; Punt, P.J.; Delattre, M.; Haon, M.; Van den Hondel, C.A.; Sigoillot, J.-C.; Lesage-Meessen, L.; Asther, M. Overproduction of Aspergillus niger feruloyl esterase for pulp bleaching application. Appl. Microbiol. Biotechnol. 2003, 62, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, A.; Benoit, I.; Asther, Mi.; Asther, M.; Record, E. Homologous expression of the feruloyl esterase B gene from Aspergillus niger and characterization of the recombinant enzyme. Protein Expr. Purif. 2004, 37, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Benoit, I.; Asther, Mi.; Bourne, Y.; Navarro, D.; Canaan, S.; Lesage-Meessen, L.; Herweijer, M.; Coutinho, P.M.; Asther, M.; Record, E. Gene overexpression and biochemical characterization of the biotechnologically relevant chlorogenic acid hydrolase from Aspergillus niger. Appl. Environ. Microbiol. 2007, 73, 5624–5632. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/protein/ (accessed on 1 July 2017).
- Ralet, M.-C.; Faulds, C.B.; Williamson, G.; Thibaut, J.-F. Degradation of feruloylated oligosaccharides from sugar-beet pulp and wheat bran by ferulic acid esterases from Aspergillus niger. Carbohydr. Res. 1994, 263, 257–269. [Google Scholar] [CrossRef]
- Bonnin, E.; Brunel, M.; Gouy, Y.; Lesage-Meessen, L.; Asther, M.; Thibault, J.-F. Aspergillus niger I-1472 and Pycnoporus cinnabarinus MUCL39533, selected for the biotransformation of ferulic acid to vanillin, are also able to produce cell wall polysaccharide-degrading enzymes and feruloyl esterases. Enzym. Microb. Technol. 2001, 28, 70–80. [Google Scholar] [CrossRef]
- Falconnier, B.; Lapierre, C.; Lesage-Meesen, L.; Yonnet, G.; Brunerie, P.; Colonna-Ceccaldi, B.; Corrieu, G.; Asther, M. Vanillin as a product of ferulic acid biotransformation by the white-rot fungus Pyncoporus cinnabarinus I-937: Identification of metabolic pathways. J. Biotechnol. 1994, 37, 123–132. [Google Scholar] [CrossRef]
- Vuorela, S.; Meyer, A.; Heinonen, M. Quantitative analysis of the main phenolics in rapeseed meal and oils processed differently using enzymatic hydrolysis and HPLC. Eur. Food Res. Technol. 2003, 217, 517–523. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Lomascolo, A.; Bonnin, E.; Thibault, J.-F.; Buleon, A.; Roller, M.; Asther, M.; Record, E.; Colonna Ceccaldi, B.; Asther, M. A biotechnological process involving filamentous fungi to produce natural crystalline vanillin from maize bran. Appl. Biochem. Biotechnol. 2002, 102–103, 141–153. [Google Scholar] [CrossRef]
- Faulds, C.B. What can feruloyl esterases do for us? Phytochem. Rev. 2010, 9, 121–132. [Google Scholar] [CrossRef]
- Faulds, C.B.; Williamson, G. Purification and characterization of a ferulic acid esterase (FAE-III) from Aspergillus niger: Specificity for the phenolic moiety and binding to microcrystalline cellulose. Microbiology 1994, 140, 779–787. [Google Scholar] [CrossRef]
- Brezillon, C.; Kroon, P.A.; Faulds, C.B.; Brett, G.M.; Williamson, G. Novel ferulic acid esterases are induced by growth of Aspergillus niger on sugar beet pulp. Appl. Microbiol. Biotechnol. 1996, 45, 371–376. [Google Scholar] [CrossRef]
- Bonnin, E.; Lesage-Meessen, L.; Asther, M.; Thibault, J.-F. A new process using Aspergillus niger and its enzymes for the production of vanillin and related compounds from agro-industrial by-products. Afinidad LVII 2000, 489, 357–364. [Google Scholar]
- Shimazono, H. Investigations on lignins and lignification. Identification of a phenolic ester in the culture medium of Lentinus lepideus and the O-methylation of methyl p-coumarate to methyl p-methoxycinnamate in vivo. Arch. Biochem. Biophys. 1959, 83, 206–215. [Google Scholar] [CrossRef]
- Jin, M.; Kim, S.; Kim, B. Induction of B-cell proliferation and NF-B activation by water-soluble glycan from Neolentinus lepideus. Int. J. Immunopharmacol. 1996, 18, 439–448. [Google Scholar] [CrossRef]
- Jin, M.; Jeon, H.; Jung, J.-H.; Kim, C.; Shin, S.; Choi, J.; Lee, K.; Kang, Y.; Kim, Y. Activation of selective transcription factor and cytokines by a water-soluble exctract from Neolentinus lepideus. Exp. Biol. Med. 2003, 228, 749–758. [Google Scholar] [CrossRef]
- Yoon, K.N.; Alam, N.; Lee, K.R.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; Lee, T.S. Antioxidant and antityrosinase activities of various extracts from the fruiting bodies of Lentinus lepideus. Molecules 2011, 16, 2334–2347. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kanawaku, R.; Masumoto, M.; Yanase, H. Efficient xylose fermentation by the brown rot fungus Neolentinus lepideus. Enzym. Microb. Technol. 2012, 50, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Krings, U.; Pilawa, S.; Theobald, C.; Berger, R.G. Phenyl propenoic side chain degradation of ferulic acid by Pycnoporus cinnabarinus—Elucidation of metabolic pathways using [5-2H]-ferulic acid. J. Biotechnol. 2001, 85, 305–314. [Google Scholar] [CrossRef]
- Lomascolo, A.; Asther, M.; Navarro, D.; Antona, C.; Delattre, M.; Lesage-Meessen, L. Shifting the biotransformation pathways of L-phenylalanine into benzaldehyde by Trametes suaveolens CBS334.85 using HP20 resin. Lett. Appl. Microbiol. 2001, 32, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.G.; Huang, C.Y.; Fu, W.Q.; Dai, C.C. Potential of endophytic fungus Phomopsis liquidambari for transformation and degradation of recalcitrant pollutant sinapic acid. Fungal Biol. 2016, 120, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.P.; Liu, Z.Q.; Xu, M.; Wanga, Y.J.; Zhenga, Y.G.; Shen, Y.C. Enhanced biotransformation of (R,S)-mandelonitrile to (R)-(−)-mandelic acid with in situ production removal by addition of resin. Biochem. Eng. J. 2010, 53, 143–149. [Google Scholar] [CrossRef]
- Wang, P.; He, J.Y.; Yin, J.F. Enhanced biocatalytic production of L-cysteine by Pseudomonas sp. B-3 with in situ product removal using ion-exchange resin. Bioprocess Biosyst. Eng. 2015, 38, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Cavin, J.F.; Dartois, V.; Divies, C. Gene cloning, transcriptional analysis, purification, and characterization of phenolic acid decarboxylase from Bacillus subtilis. Appl. Environ. Microbiol. 1998, 64, 1466–1471. [Google Scholar] [PubMed]
- Landete, J.M.; Rodríguez, H.; Curiel, J.A.; de las Rivas, B.; Mancheño, J.M.; Muñoz, R. Gene cloning, expression, and characterization of phenolic acid decarboxylase from Lactobacillus brevis RM84. J. Ind. Microbiol. Biotechnol. 2012, 37, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, L.; Ding, S. An organic solvent-tolerant phenolic acid decarboxylase from Bacillus licheniformis for the efficient bioconversion of hydroxycinnamic acids to vinyl phenol derivatives. Appl. Microbiol. Biotechnol. 2015, 99, 5071–5081. [Google Scholar] [CrossRef] [PubMed]

| Adsorption Ratio a (%) | ||||
|---|---|---|---|---|
| pH 4 | pH 7 | |||
| Type of adsorbent | SA | canolol | SA | canolol |
| XAD1180 | 94.2 ± 0.1 | 98.1 ± 0 | 37.7 ± 0.3 | 98.1 ± 0 |
| XAD16 | 98.8 ± 0 | 99.1 ± 0 | 56.3 ± 1.0 | 99.2 ± 0 |
| XAD2 | 84.9 ± 0.1 | 96.9 ± 0.2 | 30.8 ± 0.2 | 97.3 ± 0 |
| SP207 | 99.3 ± 0 | 100 ± 0 | 75.2 ± 1.0 | 100 ± 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odinot, E.; Fine, F.; Sigoillot, J.-C.; Navarro, D.; Laguna, O.; Bisotto, A.; Peyronnet, C.; Ginies, C.; Lecomte, J.; Faulds, C.B.; et al. A Two-Step Bioconversion Process for Canolol Production from Rapeseed Meal Combining an Aspergillus niger Feruloyl Esterase and the Fungus Neolentinus lepideus. Microorganisms 2017, 5, 67. https://doi.org/10.3390/microorganisms5040067
Odinot E, Fine F, Sigoillot J-C, Navarro D, Laguna O, Bisotto A, Peyronnet C, Ginies C, Lecomte J, Faulds CB, et al. A Two-Step Bioconversion Process for Canolol Production from Rapeseed Meal Combining an Aspergillus niger Feruloyl Esterase and the Fungus Neolentinus lepideus. Microorganisms. 2017; 5(4):67. https://doi.org/10.3390/microorganisms5040067
Chicago/Turabian StyleOdinot, Elise, Frédéric Fine, Jean-Claude Sigoillot, David Navarro, Oscar Laguna, Alexandra Bisotto, Corinne Peyronnet, Christian Ginies, Jérôme Lecomte, Craig B. Faulds, and et al. 2017. "A Two-Step Bioconversion Process for Canolol Production from Rapeseed Meal Combining an Aspergillus niger Feruloyl Esterase and the Fungus Neolentinus lepideus" Microorganisms 5, no. 4: 67. https://doi.org/10.3390/microorganisms5040067
APA StyleOdinot, E., Fine, F., Sigoillot, J.-C., Navarro, D., Laguna, O., Bisotto, A., Peyronnet, C., Ginies, C., Lecomte, J., Faulds, C. B., & Lomascolo, A. (2017). A Two-Step Bioconversion Process for Canolol Production from Rapeseed Meal Combining an Aspergillus niger Feruloyl Esterase and the Fungus Neolentinus lepideus. Microorganisms, 5(4), 67. https://doi.org/10.3390/microorganisms5040067






