The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists
Abstract
1. Introduction
2. Potential Intestinal Eco-Events That Affect the Microbiome
2.1. Nutrients, Food Additives, Bugs and Us
2.2. Microbial Metabolome as Mobilome
2.3. Post-Translational Modification of Naïve Proteins
2.4. Increased Intestinal Permeability: The Leaky Gut
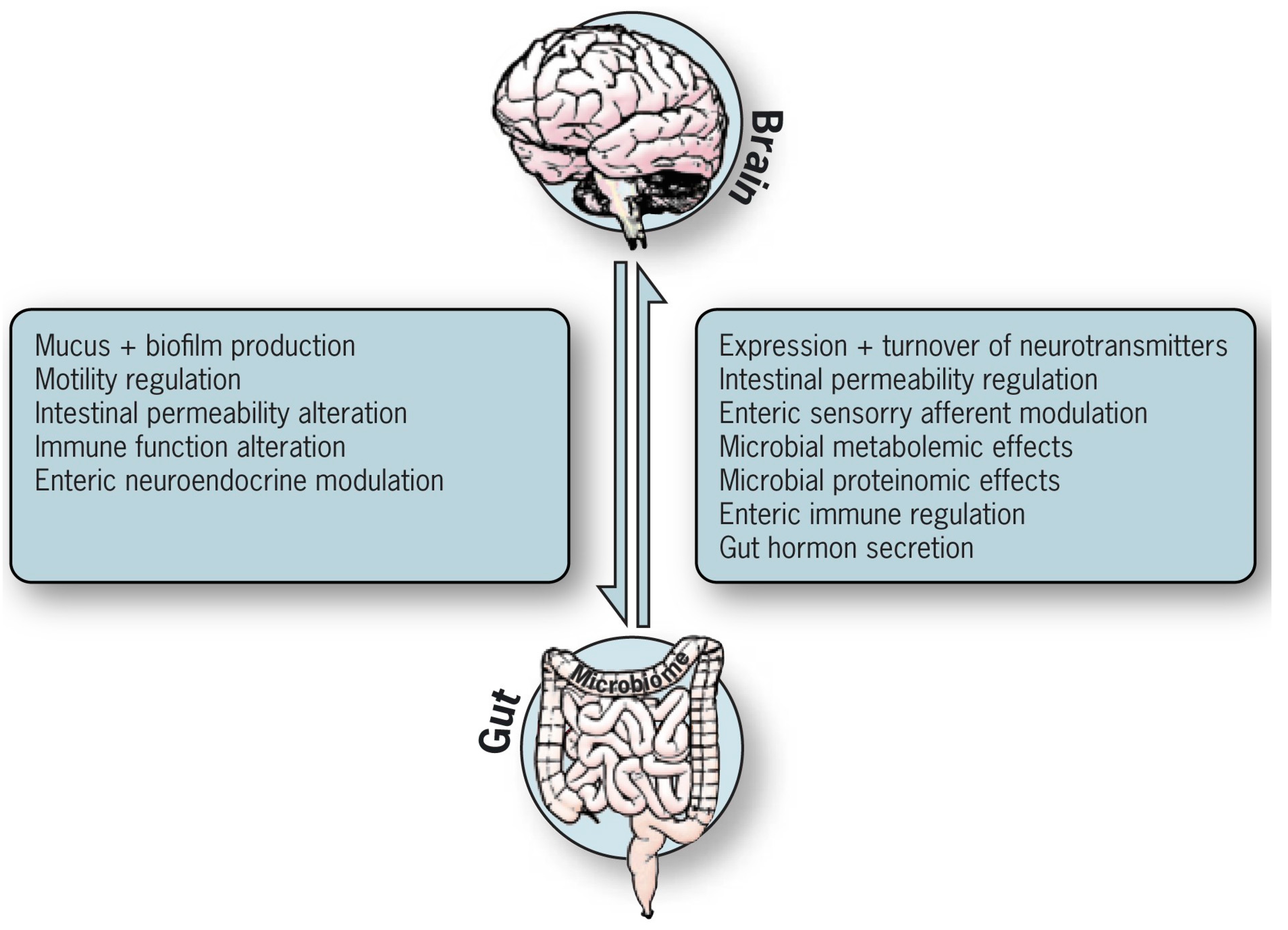
3. The Enteric Systems That Receive and Transmit Messages to the Brain
3.1. The Intestinal Glial Neuronal Bouncer (Microglial Network)
3.2. The Enteric Glial Roles
3.2.1. The Intestinal Local Roles
- Protect enteric functions by glial cell mediators or by the modulation of neurotransmission and secretion in the GI tract [87].
- By possessing receptors for various enteric neurotransmitters, they are activated by synaptic transmission e.g., ATP release from stimulated or damaged neurons or from the site of tissue trauma, infections, immune insult or inflammation [87].
- The glial cells respond to and produce cytokines and chemokines (IL-1 receptor, IL-1, IL-6, monocyte chemotactic protein1) that impact local events [87].
- Protect local tissue integrity [87].
- Active as progenitor cells [93].
- Constrain microbiota composition towards increased anti-inflammatory and decreased pro-inflammatory bacterial lineages [95].
3.2.2. Role of Mucosal Glial Cells in Brain Disorders
3.2.3. Potential Enteric Neuron and Glial Cell Sensing Capacities
3.2.4. Potential Pathways of Neuronal/Glial Caudal–Cephalic Signal Trafficking to the Brain
Anatomical Pathways (Vagal and Spinal Afferent Neurons)
Neuroendocrine–Hypothalamic–Pituitary–Adrenal (HPA) Axis (Gut Hormones)
Immune Routes
Microbial Derived Signaling Neurotransmitters
The Enteric and Brain Barrier Dams
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jahng, J.; Kim, Y.S. Irritable Bowel Syndrome: Is It Really a Functional Disorder? A New Perspective on Alteration of Enteric Nervous System. J. Neurogastroenterol. Motil. 2016, 22, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. GUT-the Trojan horse in remote organs’ autoimmunity. J. Clin. Cell. Immunol. 2016, 7, 401. [Google Scholar]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Wang, Y.P. Gut Microbiota-brain Axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Microbiol. 2005, 3, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Harari, Y.N. Sapiens: A Brief History of Humankind; Vintage Books: New York, NY, USA; Harvill Secker: London, UK, 2011; pp. 1–466. [Google Scholar]
- Cresci, G.A.; Emmy Bawden, E. The Gut Microbiome: What we do and don’t know. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Jeremias, P.; Matthias, T. The world incidence and prevalence of autoimmune diseases is increasing: A review. Int. J. Celiac Dis. 2015, 3, 151–155. [Google Scholar] [CrossRef]
- Matthias, T.; Jeremias, P.; Neidhöfer, S.; Lerner, A. The industrial food additive microbial transglutaminase, mimics the tissue transglutaminase and is immunogenic in celiac disease patients. Autoimmun. Rev. 2016, 15, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. The jigsaw of breast feeding and celiac disease. Int. J. Celiac Dis. 2016, 4, 87–89. [Google Scholar] [CrossRef]
- Dahan, S.; Segal, Y.; Shoenfeld, Y. Dietary factors in rheumatic autoimmune diseases: A recipe for therapy? Nat. Rev. Rheumatol. 2017, 13, 248–358. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Jeremias, P.; Matthias, T. Nutrients, bugs and us: The short-chain fatty acids story in celiac disease. Int. J. Celiac Dis. 2016, 4, 92–94. [Google Scholar]
- Zimmer, J.; Lange, B.; Frick, J.S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A. Aluminum as an adjuvant in Crohn’s disease induction. Lupus 2012, 21, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. One size doesn’t fit all: IBD in Arabs and Jews in Israel, potential environmental and genetic impacts. J. Clin. Gastroenterol. Treat. 2017, 3, 40. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Extraintestinal manifestations of CD: Common pathways in the gut-remote organs’ axes. Int. J. Celiac Dis. 2017, 5, 24–27. [Google Scholar]
- Selmi, C.; Tsuneyama, K. Nutrition, geoepidemiology, and autoimmunity. Autoimmun. Rev. 2010, 9, A267–A270. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. Possible association between celiac disease and bacterial transglutaminase in food processing: A hypothesis. Nutr. Rev. 2015, 73, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Shoenfeld, Y.; Matthias, T. Gluten ingestion side effects and withdrawal advantages in non-celiac autoimmune diseases. Nutr. Rev. 2017, in press. [Google Scholar]
- Cosnes, J.; Cellier, C.; Viola, S.; Colombel, J.F.; Michaud, L.; Sarles, J.; Hugot, J.P.; Ginies, J.; Dabadie, A.; Mouterde, O.; et al. Incidence of autoimmune diseases in celiac disease: Protective effect of the gluten-free diet. Clin. Gastroenterol. Hepatol. 2008, 6, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Sategna Guidetti, C.; Solerio, E.; Scaglione, N.; Aimo, G.; Mengozzi, G. Duration of gluten exposure in adult coeliac disease does not correlate with the risk for autoimmune disorders. Gut 2001, 49, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Lowrie, M.; Garden, O.A.; Hadjivassiliou, M.; Harvey, R.J.; Sanders, D.S.; Powell, R.; Garosi, L. The Clinical and Serological Effect of a Gluten-Free Diet in Border Terriers with Epileptoid Cramping Syndrome. J. Vet. Intern. Med. 2015, 29, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Castaño, G.P.; Caro-Quintero, A.; Reyes, A.; Lizcano, F. Advances in Gut Microbiome Research, Opening New Strategies to Cope with a Western Lifestyle. Front. Genet. 2017, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Dumont-Driscoll, M. Foreword: We Will be What We Eat or What We Were Fed. Curr. Probl. Pediatr. Adolesc. Health Care 2015, 45, 133. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Aminov, R.; Matthias, T. Dysbiosis may trigger autoimmune diseases via inappropriate posttranslational modification of host proteins. Front. Microbiol. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Gilbert, J.A.; Nagler, C.R. The role of the commensal microbiota in the regulation of tolerance to dietary allergens. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Viladomiu, M.; Hon tecillas, R.; Yuan, L.; Lu, P.; Bassaganya-Riera, J. Nutritional protective mechanisms against gut inflammation. J. Nutr. Biochem. 2013, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell. 2016, 64, 982–992. [Google Scholar] [CrossRef] [PubMed]
- McOrist, A.L.; Miller, R.B.; Bird, A.R.; Keogh, J.B.; Noakes, M.; Topping, D.L.; Conlon, M.A. Fecal butyrate levels vary widely among individuals but are usually increased by a diet high in resistant starch. J. Nutr. 2011, 141, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; De Pasquale, I.; Ndagijimana, M.; Vernocchi, P.; Ricciuti, P.; Gagliardi, F.; Laghi, L.; Crecchio, C.; Guerzoni, M.E.; et al. Duodenal and faecal microbiota of celiac children: Molecular, phenotype and metabolome characterization. BMC Microbiol. 2011, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.E.; Dai, Y. Metabolome of human gut microbiome is predictive of host dysbiosis. Gigascience 2015, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front. Microbiol. 2016, 7, 1144. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Nighot, M.; Al-Sadi, R.; Alhmoud, T.; Nighot, P.; Ma, T.Y. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J. Immunol. 2015, 195, 4999–5010. [Google Scholar] [CrossRef] [PubMed]
- Villarraso, J.C.; Galvan, A.; Escribano, B.M.; Túnez, I. INTERRELATIONSHIPS BETWEEN GUT MICROBIOTA AND HOST: Paradigms, Role in Neurodegenerative Diseases and Future Prospects. CNS Neurol. Disord. Drug Targets 2017. [Google Scholar] [CrossRef]
- Van Spaendonk, H.; Ceuleers, H.; Witters, L.; Patteet, E.; Joossens, J.; Augustyns, K.; Lambeir, A.M.; De Meester, I.; De Man, J.G.; De Winter, B.Y. Regulation of intestinal permeability: The role of proteases. World J. Gastroenterol. 2017, 23, 2106–2123. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. Rheumatoid arthritis-celiac disease relationship: Joints get that gut feeling. Autoimmun. Rev. 2015, 14, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Neidhöfer, S.; Matthias, T. Beyond the joint: What’s happening in the gut. Int. J. Celiac Dis. 2016, 4, 127–129. [Google Scholar]
- Lerner, A. Serological Diagnosis of Celiac Disease—Moving Beyond the Tip of the Iceberg. Int. J. Celiac Dis. 2014, 2, 64–66. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Are Microbial Enzymes Used Safe in the Processed Food Industries? Food Bioprocess Technol. 2016, 9, 2125–2126. [Google Scholar] [CrossRef]
- Lerner, A.; Aminov, R.; Matthias, T. Intestinal dysbiotic transglutaminases are potential environmental drivers of systemic autoimmunogenesis. Front. Microbiol. 2017, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. Don’t forget the exogenous microbial transglutaminases: It is immunogenic and potentially pathogenic. AIMS Biophys. 2016, 3, 546–552. [Google Scholar] [CrossRef]
- Bolshette, N.B.; Thakur, K.K.; Bidkar, A.P.; Trandafir, C.; Kumar, P.; Gogoi, R. Protein folding and misfolding in the neurodegenerative disorders: A review. Rev. Neurol. 2014, 170, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Neidhöfer, S.; Matthias, T. Transglutaminase 2 and anti transglutaminase 2 autoantibodies in celiac disease and beyond: Part A: TG2 double-edged sword: Gut and extraintestinal involvement. Immunome Res. 2015, 11, 101–105. [Google Scholar] [CrossRef]
- Malandain, H. Transglutaminases: A meeting point for wheat allergy, celiac disease, and food safety. Eur. Ann. Allergy Clin. Immunol. 2005, 37, 397–403. [Google Scholar] [PubMed]
- Gruber, P.; Gadermaier, G.; Bauer, R.; Weiss, R.; Wagner, S.; Leonard, R.; Breiteneder, H.; Ebner, C.; Ferreira, F.; Egger, M. Role of the polypeptide backbone and post-translational modifications in cross-reactivity of Art v 1, the major mugwort pollen allergen. Biol. Chem. 2009, 390, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, G.; Banai, M.; Fishman, Y.; Bercovier, H. Delayed-type hypersensitivity activity of the Brucella L7/L12 ribosomal protein depends on posttranslational modification. Infect. Immun. 1997, 65, 267–271. [Google Scholar] [PubMed]
- Makarova, K.S.; Aravind, L.; Koonin, E.V. A superfamily of archaeal, bacterial and eukaryotic proteins homologous to animal transglutaminases. Protein Sci. 1999, 8, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Saska, I.; Craik, D.J. Protease-catalysed protein splicing: A new post-translational modification? Trends Biochem. Sci. 2008, 33, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Zaneveld, J.; Turnbaugh, P.; Lozupone, C.; Ley, R.E.; Hamady, M.; Gordon, J.I.; Knight, R. Host-bacterial coevolution and the search for new drug targets. Curr. Opin. Chem. Biol. 2008, 12, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017, 77, 1783–1812. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Shea-Donohue, T. Mechanisms of disease: The role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012, 42, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Ackland, L.; Suphioglu, C. Nuts ‘n’ guts: Transport of food allergens across the intestinal epithelium. Asia Pac. Allergy 2013, 3, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, J.M.; Wessells, R.J.; Lujan, H.L.; DiCarlo, S.E. My gut feeling says rest: Increased intestinal permeability contributes to chronic diseases in high-intensity exercisers. Med. Hypotheses 2015, 85, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.; Chen, Y.; Song, Z.; Jiang, X.; Hu, F.; Conlon, M.A.; Topping, D.L. Polyphenol-Rich Propolis Extracts Strengthen Intestinal Barrier Function by Activating AMPK and ERK Signaling. Nutrients 2016, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Scaldaferri, F.; Bruno, G.; Petito, V.; Franceschi, F.; Gasbarrini, A. The therapeutic management of gut barrier leaking: The emerging role for mucosal barrier protectors. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1068–1076. [Google Scholar] [PubMed]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P.; Kunze, W.A. Voices from within: Gut microbes and the CNS. Cell. Mol. Life Sci. 2013, 70, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, R.; Anzalone, M.; Calabrese, F.; Milazzo, M.; Capuana, M.; Italia, A.; Occhipinti, S.; Marotta, F. The gut microbiota and its correlations with the central nervous system disorders. Panminerva Med. 2015, 57, 127–143. [Google Scholar] [PubMed]
- Berer, K.; Krishnamoorthy, G. Microbial view of central nervous system autoimmunity. FEBS Lett. 2014, 588, 4207–4213. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Nakazato, M. Mechanistic relationship between the vagal afferent pathway, central nervous system and peripheral organs in appetite regulation. J. Diabetes Investig. 2016, 7, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Browning, K.N.; Verheijden, S.; Boeckxstaens, G.E. The Vagus Nerve in Appetite Regulation, Mood, and Intestinal Inflammation. Gastroenterology 2017, 152, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Chiu, I.M. Bacterial Signaling to the Nervous System through Toxins and Metabolites. J. Mol. Biol. 2017, 429, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Knauf, C. How gut microbes talk to organs: The role of endocrine and nervous routes. Mol. Metab. 2016, 5, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Sirisinha, S. The potential impact of gut microbiota on your health: Current status and future challenges. Asian Pac. J. Allergy Immunol. 2016, 34, 249–264. [Google Scholar] [PubMed]
- Sharkey, K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Investig. 2015, 125, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Li, Y.Q. Enteric glial cells and their role in the intestinal epithelial barrier. World J. Gastroenterol. 2014, 20, 11273–11280. [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D.; Sharkey, K.A. Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Neunlist, M.; Van Landeghem, L.; Mahé, M.M.; Derkinderen, P.; des Varannes, S.B.; Rolli-Derkinderen, M. The digestive neuronal-glial-epithelial unit: A new actor in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Charrier, B.; Pilon, N. Toward a better understanding of enteric gliogenesis. Neurogenesis 2017, 4, e1293958. [Google Scholar] [CrossRef] [PubMed]
- Kabouridis, P.S.; Lasrado, R.; McCallum, S.; Chng, S.H.; Snippert, H.J.; Clevers, H.; Pettersson, S.; Pachnis, V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 2015, 85, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gershon, M.D. Bugs, guts, and glia: How microbiota influence enteric gliogenesis and migration. Neuron 2015, 85, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Savidge, T.C. Role of enteric neurotransmission in host defense and protection of the gastrointestinal tract. Auton. Neurosci. 2014, 181, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Neunlist, M.; Rolli-Derkinderen, M.; Latorre, R.; Van Landeghem, L.; Coron, E.; Derkinderen, P.; De Giorgio, R. Enteric glial cells: Recent developments and future directions. Gastroenterology 2014, 147, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Bohórquez, D.V.; Samsa, L.A.; Roholt, A.; Medicetty, S.; Chandra, R.; Liddle, R.A. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS ONE 2014, 9, e89881. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H. Neurogastroenterology: A window on the ENS. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.I.; Heuckeroth, R.O. Enteric nervous system development: Migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G1–G24. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Aguiar Jde, M.; Bon-Frauches, A.C.; Gomes, A.L.; Veríssimo, C.P.; Aguiar, D.P.; Matias, D.; Thomasi, B.B.; Gomes, A.S.; Brito, G.A.; Moura-Neto, V. The enteric glia: Identity and functions. Glia 2015, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, R.; Giancola, F.; Boschetti, E.; Abdo, H.; Lardeux, B.; Neunlist, M. Enteric glia and neuroprotection: Basic and clinical aspects. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G887–G893. [Google Scholar] [CrossRef] [PubMed]
- Rolig, A.S.; Mittge, E.K.; Ganz, J.; Troll, J.V.; Melancon, E.; Wiles, T.J.; Alligood, K.; Stephens, W.Z.; Eisen, J.S.; Guillemin, K. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol. 2017, 15, e2000689. [Google Scholar] [CrossRef] [PubMed]
- Bach-Ngohou, K.; Mahé, M.M.; Aubert, P.; Abdo, H.; Boni, S.; Bourreille, A.; Denis, M.G.; Lardeux, B.; Neunlist, M.; Masson, D. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12,14-prostaglandin J2. J. Physiol. 2010, 588, 2533–2544. [Google Scholar] [CrossRef] [PubMed]
- Van Landeghem, L.; Chevalier, J.; Mahé, M.M.; Wedel, T.; Urvil, P.; Derkinderen, P.; Savidge, T.; Neunlist, M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G976–G987. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, G. Enteric glial cells: New players in mucosal defence against bacteria? Gut 2011, 60, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Flamant, M.; Aubert, P.; Rolli-Derkinderen, M.; Bourreille, A.; Neunlist, M.R.; Mahé, M.M.; Meurette, G.; Marteyn, B.; Savidge, T.; Galmiche, J.P.; et al. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: A role for S-nitrosoglutathione. Gut 2011, 60, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, V.; Verkhratsky, A.; Zorec, R.; Parpura, V. Enteric glia regulate gut motility in health and disease. Brain Res. Bull. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Villanacci, V. Can “functional” constipation be considered as a form of enteric neuro-gliopathy? Glia 2011, 59, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Auber, F.; Danzer, E.; Noché-Monnery, M.E.; Sarnacki, S.; Trugnan, G.; Boudjemaa, S.; Audry, G. Enteric nervous system impairment in gastroschisis. Eur. J. Pediatr. Surg. 2013, 23, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Cortes, F.; Turco, F.; Linan-Rico, A.; Soghomonyan, S.; Whitaker, E.; Wehner, S.; Cuomo, R.; Christofi, F.L. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, L.; Cuomo, R. S100B protein in the gut: The evidence for enteroglial-sustained intestinal inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 2016, 7, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Pachnis, V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology 2016, 151, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Walker, M.M.; Talley, N.J. The mucosal immune system: Master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.K.; Gulbransen, B.D. Potential roles of enteric glia in bridging neuroimmune communication in the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G145–G152. [Google Scholar] [CrossRef] [PubMed]
- Kabouridis, P.S.; Lasrado, R.; McCallum, S.; Chng, S.H.; Snippert, H.J.; Clevers, H.; Pettersson, S.; Pachnis, V. The gut microbiota keeps enteric glial cells on the move; prospective roles of the gut epithelium and immune system. Gut Microbes 2015, 6, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Bohórquez, D.V.; Liddle, R.A. The gut connectome: Making sense of what you eat. J. Clin. Investig. 2015, 125, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.M.; Hofstra, R.M.; Burns, A.J. Building a brain in the gut: Development of the enteric nervous system. Clin. Genet. 2013, 83, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Benarroch, E.E. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol. Dis. 2012, 46, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Raina, G.B.; Pecci, C.; Pellene, A.; Calandra, C.R.; Gutiérrez, C.; Micheli, F.E.; Benarroch, E.E. Gastrointestinal manifestations in Parkinson’s disease: Prevalence and occurrence before motor symptoms. J. Neurol. 2013, 260, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef] [PubMed]
- Poirier, A.A.; Aubé, B.; Côté, M.; Morin, N.; Di Paolo, T.; Soulet, D. Gastrointestinal Dysfunctions in Parkinson’s Disease: Symptoms and Treatments. Parkinsons Dis. 2016, 2016, 6762528. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Rietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; van Wezel, R.J.; Kraneveld, A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Clairembault, T.; Leclair-Visonneau, L.; Neunlist, M.; Derkinderen, P. Enteric glial cells: New players in Parkinson’s disease? Mov. Disord. 2015, 30, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Clairembault, T.; Kamphuis, W.; Leclair-Visonneau, L.; Rolli-Derkinderen, M.; Coron, E.; Neunlist, M.; Hol, E.M.; Derkinderen, P. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem. 2014, 130, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pardo, P.; Kliest, T.; Dodiya, H.B.; Broersen, L.M.; Garssen, J.; Keshavarzian, A.; Kraneveld, A.D. The gut-brain axis in Parkinson’s disease: Possibilities for food-based therapies. Eur. J. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Magalingam, K.B.; Radhakrishnan, A.K.; Haleagrahara, N. Protective Mechanisms of Flavonoids in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 314560. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.K.; Shukla, S.; Seth, K.; Chauhan, S.; Sinha, C.; Shukla, Y.; Agrawal, A.K. Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Neurobiol. Dis. 2006, 22, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Sutalangka, C.; Wattanathorn, J. Neuroprotective and cognitive-enhancing effects of the combined extract of Cyperus rotundus and Zingiber officinale. BMC Complement. Altern. Med. 2017, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, M.; Wei, W.; Keller, J.N.; Adhikari, B.; King, J.F.; King, M.L.; Peng, N.; Laine, R.A. Dietary Plant Lectins Appear to Be Transported from the Gut to Gain Access to and Alter Dopaminergic Neurons of Caenorhabditis elegans, a Potential Etiology of Parkinson’s Disease. Front. Nutr. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Derkinderen, P.; Rouaud, T.; Lebouvier, T.; Bruley des Varannes, S.; Neunlist, M.; De Giorgio, R. Parkinson disease: The enteric nervous system spills its guts. Neurology 2011, 77, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Kraneveld, A.D.; Szklany, K.; de Theije, C.G.; Garssen, J. Gut-to-Brain Axis in Autism Spectrum Disorders: Central Role for the Microbiome. Int. Rev. Neurobiol. 2016, 131, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Savidge, T.C.; Williams, K.C. The Brain-Gut-Microbiome Axis: What Role Does It Play in Autism Spectrum Disorder? Curr. Dev. Disord. Rep. 2016, 3, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Oezguen, N.; Balderas, M.; Venkatachalam, A.; Runge, J.K.; Versalovic, J.; Veenstra-VanderWeele, J.; Anderson, G.M.; Savidge, T.; Williams, K.C. Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cell. Mol. Gastroenterol. Hepatol. 2016, 3, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, J.M. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 2016, 324, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, X.; Yang, S.; Meng, F.; Wang, X.; Wei, H.; Chen, T. Evaluation of the Microbial Diversity in Amyotrophic Lateral Sclerosis Using High-Throughput Sequencing. Front. Microbiol. 2016, 7, 1479. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yi, J.; Zhang, Y.G.; Zhou, J.; Sun, J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 2015, 3, e12356. [Google Scholar] [CrossRef] [PubMed]
- Luesma, M.J.; Cantarero, I.; Álvarez-Dotu, J.M.; Santander, S.; Junquera, C. New insights into c-Ret signaling pathway in the enteric nervous system and its relationship with ALS. Biomed. Res. Int. 2014, 2014, 328348. [Google Scholar] [CrossRef] [PubMed]
- Joachim, C.L.; Mori, H.; Selkoe, D.J. Amyloid beta-protein deposition in tissues other than brain in Alzheimer’s disease. Nature 1989, 341, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Shoemark, D.K.; Allen, S.J. The microbiome and disease: Reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimers Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.A.; Bryant, A.R.; Reynolds, J.D.; Jirik, F.R.; Sharkey, K.A. Prion diseases and the gastrointestinal tract. Can. J. Gastroenterol. 2006, 20, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Albanese, V.; Lawson, V.A.; Hill, A.F.; Cappai, R.; Di Guardo, G.; Staikopoulos, V.; Thacker, M.; Furness, J.B.; Chiocchetti, R. Evidence for prion protein expression in enteroglial cells of the myenteric plexus of mouse intestine. Auton. Neurosci. 2008, 140, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Shmakov, A.N.; McLennan, N.F.; McBride, P.; Farquhar, C.F.; Bode, J.; Rennison, K.A.; Ghosh, S. Cellular prion protein is expressed in the human enteric nervous system. Nat. Med. 2000, 6, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, D.S.; Sehgal, A.; Rios, D.; Williams, I.R.; Mabbott, N.A. Increased Abundance of M Cells in the Gut Epithelium Dramatically Enhances Oral Prion Disease Susceptibility. PLoS Pathog. 2016, 12, e1006075. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Dias, B.; Jovanovic, K.; Weiss, S.F. Alimentary prion infections: Touchdown in the intestine. Prion 2011, 5, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Beekes, M.; McBride, P.A. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J. 2007, 274, 588–605. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Ferrucci, M.; Lazzeri, G.; Paparelli, A.; Fornai, F. Transmission of prions within the gut and towards the central nervous system. Prion 2011, 5, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.M.; Tetlow, L.; Mabbott, N.A. Prion disease pathogenesis in the absence of the commensal microbiota. J. Gen. Virol. 2017, 98, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zeng, B.; Wang, H.; Li, B.; Huo, R.; Zheng, P.; Zhang, X.; Du, X.; Liu, M.; Fang, Z.; et al. Microbiota Modulates Behavior and Protein Kinase C mediated cAMP response element-binding protein Signaling. Sci. Rep. 2016, 6, 29998. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Mahony, S.M. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.; Thiennimitr, P.; Chattipakorn, N.; Chattipakorn, S.C. Diet, gut microbiota and cognition. Metab. Brain Dis. 2017, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Sarnelli, G.; Capoccia, E.; Cirillo, C.; Pesce, M.; Lu, J.; Cali, G.; Cuomo, R.; Steardo, L. Autologous transplantation of intestine-isolated glia cells improves neuropathology and restores cognitive deficits in β amyloid-induced neurodegeneration. Sci. Rep. 2016, 6, 22605. [Google Scholar] [CrossRef] [PubMed]
- MacFabe, D.F.; Cain, N.E.; Boon, F.; Ossenkopp, K.P.; Cain, D.P. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav. Brain Res. 2011, 217, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Mood by microbe: Towards clinical translation. Genome Med. 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, G.; Surette, M.; Moayyedi, P. The gut microbiota and psychiatric illness. J. Psychiatry Neurosci. 2017, 42, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.C.; Huus, K.E.; Finlay, B.B. Microbes and the mind: Emerging hallmarks of the gut microbiota-brain axis. Cell. Microbiol. 2016, 18, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; Desbonnet, L.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The microbiome: Stress, health and disease. Mamm. Genome. 2014, 25, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, Y.; Tominaga, K.; Tanaka, F.; Tanigawa, T.; Watanabe, T.; Fujiwara, Y.; Arakawa, T. Enteric glial cells are associated with stress-induced colonic hyper-contraction in maternally separated rats. Neurogastroenterol. Motil. 2015, 27, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Szakal, D.; Williams, B.L.; Mishra, N.; Che, X.; Lee, B.; Bateman, L.; Klimas, N.G.; Komaroff, A.L.; Levine, S.; Montoya, J.G.; et al. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2017, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A. Gut inflammation in chronic fatigue syndrome. Nutr. Metab. 2010, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, S.; Ercan, E.; Breedveld, P.; Brakenhoff, L.; Ghariq, E.; Schmid, S.; van Osch, M.; van Buchem, M.; Emmer, B.; van der Grond, J.; et al. Cerebral magnetic resonance imaging in quiescent Crohn’s disease patients with fatigue. World J. Gastroenterol. 2017, 23, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W. (Dis) Trust your gut: The gut microbiome in age-related inflammation, health, and disease. Microbiome 2017, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Saffrey, M.J. Cellular changes in the enteric nervous system during ageing. Dev. Biol. 2013, 382, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Cryan, J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Di Giancamillo, A.; Vitari, F.; Bosi, G.; Savoini, G.; Domeneghini, C. The chemical code of porcine enteric neurons and the number of enteric glial cells are altered by dietary probiotics. Neurogastroenterol. Motil. 2010, 22, e271–e278. [Google Scholar] [CrossRef] [PubMed]
- El Aidy, S.; Stilling, R.; Dinan, T.G.; Cryan, J.F. Microbiome to Brain: Unravelling the Multidirectional Axes of Communication. Adv. Exp. Med. Biol. 2016, 874, 301–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Stribinskis, V.; Rane, M.J.; Demuth, D.R.; Gozal, E.; Roberts, A.M.; Jagadapillai, R.; Liu, R.; Choe, K.; Shivakumar, B.; et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci. Rep. 2016, 6, 34477. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Neuropeptides, Microbiota, and Behavior. Int. Rev. Neurobiol. 2016, 131, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.M.; Scott, L.V.; Dinan, T.G. Cytokines: Abnormalities in major depression and implications for pharmacological treatment. Hum. Psychopharmacol. 2004, 19, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, A.; Kashyap, P.C.; Nelson, T.A.; Aronov, P.A.; Donia, M.S.; Spormann, A.; Fischbach, M.A.; Sonnenburg, J.L. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013, 7, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. The gut microbiome and the brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
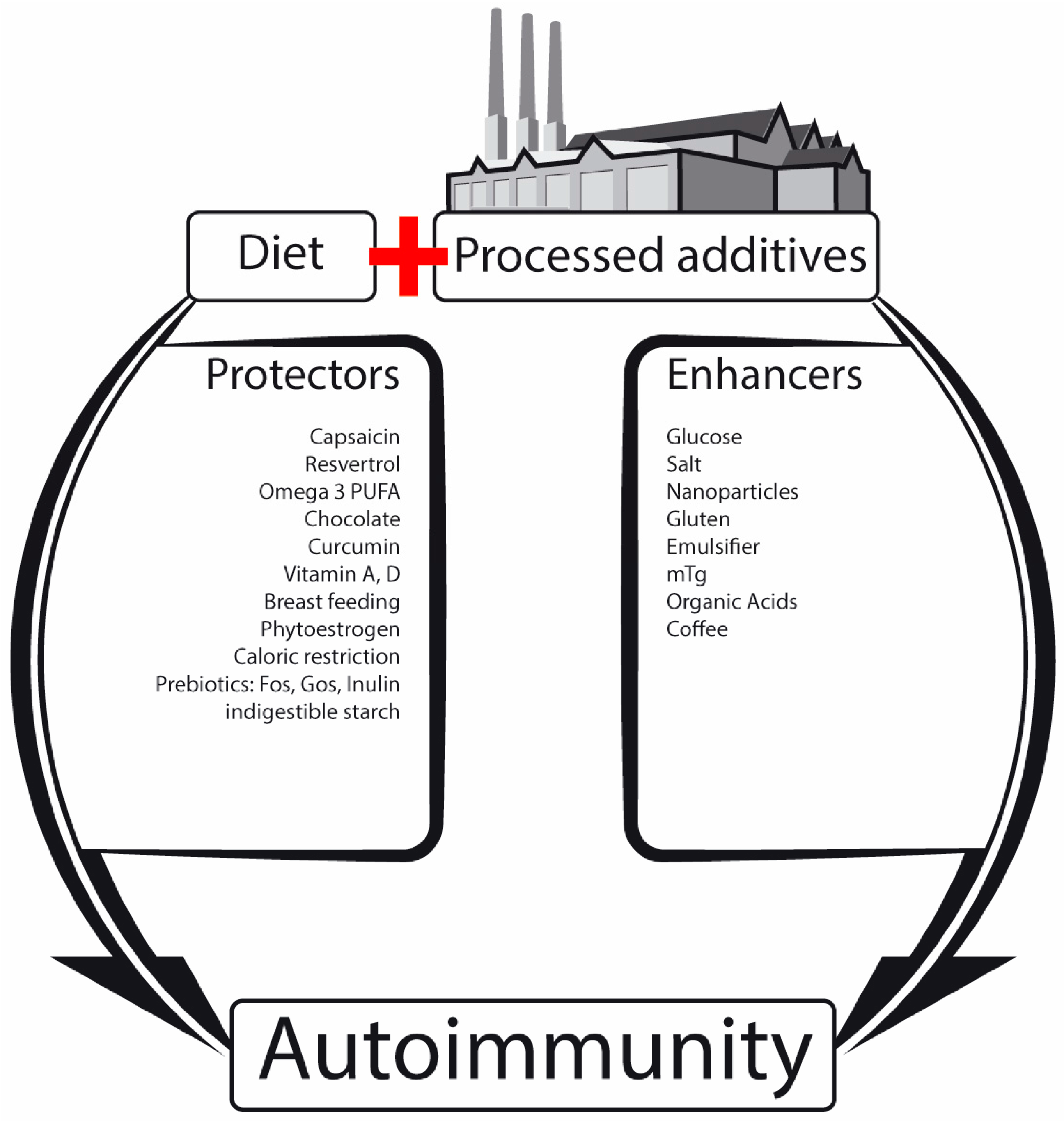
| Beneficial Microbial Metabolites or Constituents | Advantages | Harmful Microbial Metabolites | Disadvantages |
|---|---|---|---|
| SCFAs | Nutrient, energy providing | Lipopolysaccharide supply | Obesity, metabolic syndrome, leaky gut |
| Propionate production | Gluconeogenesis, cholesterol lowering | Toxin production | Cancer promotion |
| Butyrate production | Cancer prevention, colonocyte energy | Tissue invasion of metabolites | Infections, leaky gut |
| Vitamin productions: B:1,2,5,6,7,8,9,11,12. Vitamin K | Various metabolic cellular effects | Leaky gut induced by metabolites | Autoimmune disease, Inflammatory bowel disease, immune disorders |
| Anti-inflammatory signals | Normal gut immune function | Microbial enzyme’s PTMP | Autoimmune and allergic disease |
| Antimicrobial production | Pathogen fighting | Pro-inflammatory signals3 | Inflammatory bowel disease, immune disorders |
| Non-digestible carbohydrates-bulk effect | Improved intestinal motility | Acetate production | Hypercholesterolemia, cardiovascular diseases |
| Bile acids | Improved fat/vitamin absorption, gut barrier, regulate serum lipids and glucose | Secondary bile acids | Colon cancer |
| Microbial proteases | Protective of intestinal permeability [45] | Microbial proteases | Harmful for intestinal permeability [45] |
| Red meat rich L-carnitine metabolism | Atherosclerosis | ||
| Organic acids | Hypertension, obesity, colonic cancer, autism | ||
| Metabolic imbalance | Irritable bowel syndrome, metabolic syndrome | ||
| Amino acids: tyrosine to phenols | Colonic cancer, autism | ||
| Trimethylamine production | coronary vascular disease |
| Categories | Names | Categories | Names |
|---|---|---|---|
| Pathogens | H. pylori | drugs | Proton pump inhibitors |
| Enteropathogenic E. coli | Non-steroidal anti-inflammatory drugs | ||
| Enterohemorrhagic E. coli | Selected bile salts | ||
| V. parahemolyticus | |||
| Salmonella enterica/typhimurium | toxins | Clostridium toxin | |
| Clostridium difficile | Ochratoxin A | ||
| Clostridium perfringens | Marine toxins | ||
| Bacteroides fragilis | EDTA | ||
| Vibrio cholerae | |||
| Shigella flexneri | Lifestyle factors | Western diet | |
| Campylobacter jejuni | |||
| Reovirus | Obesity | ||
| Rotavirus | Gut perfusion | Hypoperfusion | |
| Nutrients | High fat diet | Microbial enzymes | Proteases [45] |
| High carbohydrate diet | Allergens | Peanuts, soybean, wheat, milk proteins, nuts, sesame | |
| Vitamin A deprivation | Carcinogens | Arsenic, phenols, mercury, azoxymethane | |
| Vitamin D deprivation | Stress | Stress related psychiatric disorders | |
| Fructose | High-intensity exercise | ||
| Gluten | |||
| Processed food additives: sugar, salt, organic acids, microbial transglutaminase, emulsifiers, nanoparticles | |||
| Medium chain fatty acids | |||
| Acyl carnitines |
| Categories | Names |
|---|---|
| Prebiotic Nutrients | Galactooligosaccharides |
| Fructooligosaccharides | |
| Short chain fatty acids | Butyrate |
| Polyunsaturated fatty acids | PUFA |
| Nutrients | Glutamine |
| Zinc | |
| Plant-derived flavonoids | Quercetin and its metabolites |
| Propolis | |
| Green tea, coffee, berries, grapes, and other fruits/vegetables | |
| Vitamins | A, D |
| Probiotics | E. coli nissle 1917 |
| VSL#3 | Lactobacillus plantarum MB452 |
| VSL#3 | Bifidobacterium infantis Y1 |
| Lactobacillus salivarius UCC118 | |
| Lactobacillus salivarius CCUG38008 | |
| Lactobacillus rhamnosus GG | |
| Lactobacillus casei DN-114 001 | |
| Lactobacillus casei Shirota | |
| Microbial enzymes | Proteases [45] |
| Chemical compounds | Gelatin tannate [71] |
| Diseases | Reference |
|---|---|
| Parkinson’s disease | [84,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128] |
| Autism spectrum disorder | [84,129,130,131,132,133] |
| Amyotrophic lateral sclerosis | [84,134,135,136] |
| Alzheimer diseases | [84,137,138,139] |
| Prion diseases | [81,84,94,139,140,141,142,143,144,145,146,147] |
| Creutzfeldt-Jakob disease | [81,143,145] |
| Transmissible spongiform encephalopathies | [84,139,143,145,146] |
| Additional conditions | |
| Depression | [148,149,150,151,152] |
| Anxiety | [150,151,153] |
| Behavior | [154,155,156] |
| Cognition | [157,158,159] |
| Mood | [67,160,161] |
| Stress | [151,162,163,164] |
| Fatigue | [165,166,167,168] |
| Aging | [108,138,169] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerner, A.; Neidhöfer, S.; Matthias, T. The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists. Microorganisms 2017, 5, 66. https://doi.org/10.3390/microorganisms5040066
Lerner A, Neidhöfer S, Matthias T. The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists. Microorganisms. 2017; 5(4):66. https://doi.org/10.3390/microorganisms5040066
Chicago/Turabian StyleLerner, Aaron, Sandra Neidhöfer, and Torsten Matthias. 2017. "The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists" Microorganisms 5, no. 4: 66. https://doi.org/10.3390/microorganisms5040066
APA StyleLerner, A., Neidhöfer, S., & Matthias, T. (2017). The Gut Microbiome Feelings of the Brain: A Perspective for Non-Microbiologists. Microorganisms, 5(4), 66. https://doi.org/10.3390/microorganisms5040066





