Antifungal Microbial Agents for Food Biopreservation—A Review
Abstract
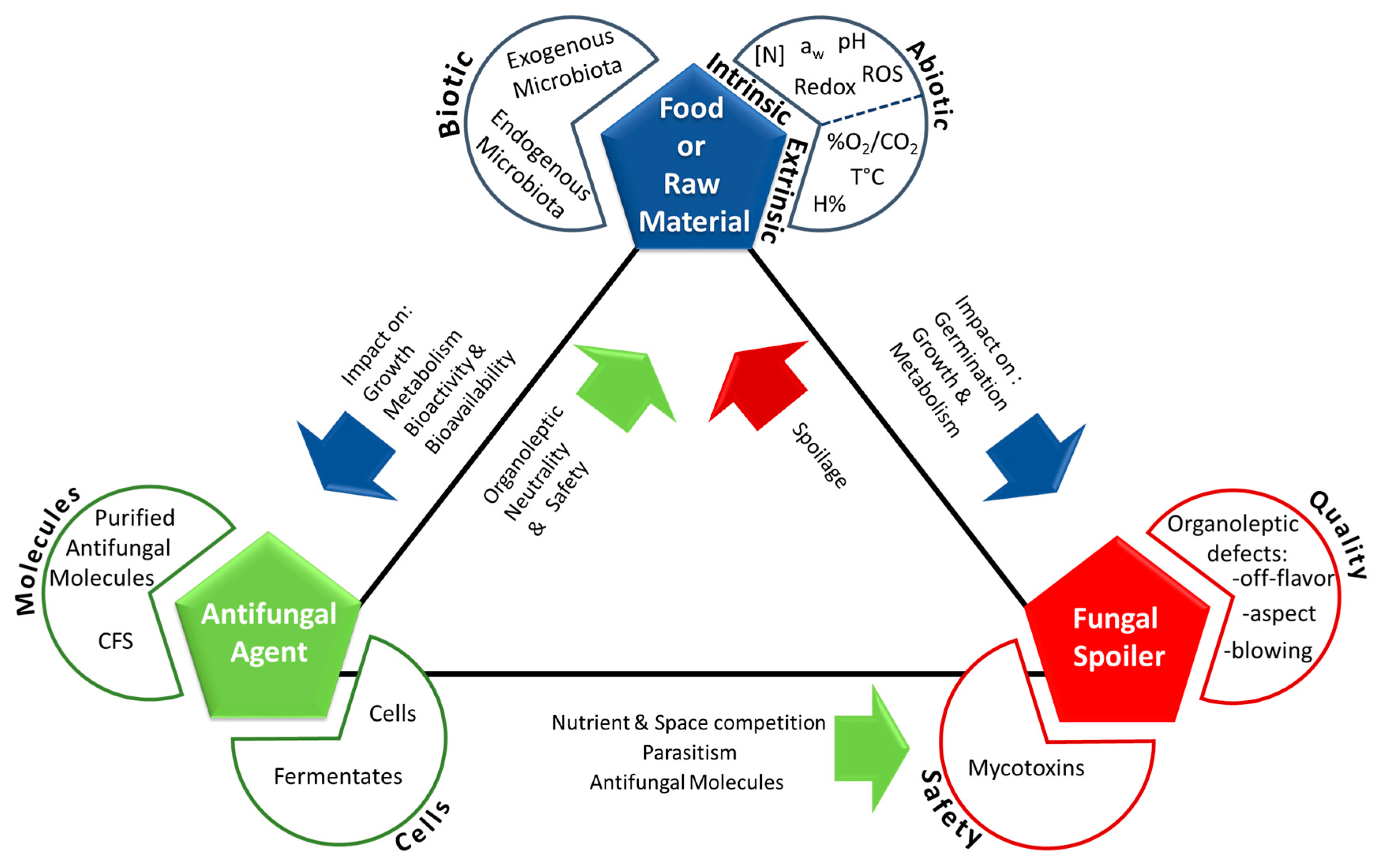
:1. Introduction
2. Fungal Spoilers
2.1. Quality and Safety Issues
2.2. Isolation and Identification of Fungal Spoilers
2.3. Fungal Spoilers and Food Chains
2.3.1. Fresh or Perishable Foods
2.3.2. Stored and Processed Foods
3. Antifungal Microorganisms in Food
3.1. Screening and Validation Methods
3.1.1. In Vitro Screening
3.1.2. Validation by Challenge-Test in the Food Products
3.2. Antifungal Microorganisms
3.2.1. Lactic Acid Bacteria
3.2.2. Propionibacteria
3.2.3. Bacillus and Other Bacteria
3.2.4. Yeasts
3.2.5. Filamentous Fungi
4. Action Mechanisms of Antifungal Microorganisms
4.1. Action Mechanisms of Antifungal Lactic Acid Bacteria and Propionibacteria in Fermented Foods
4.2. Action Mechanisms of Antifungal Yeasts and Molds in Fermented Foods
4.3. Action Mechanisms of Antifungal Yeasts and Bacillus spp. for Control of Postharvest Diseases
5. Implementation of Biopreservation Methods against Fungal Spoilage
5.1. Optimization and Application Modes
5.2. Constraints
6. Conclusions
Conflicts of Interest
References
- Gustavsson, J.; Cederberg, C.; Sonesson, U. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-107205-9. [Google Scholar]
- FAO. Save Food: Global Initiative on Food Loss and Waste Reduction—Key Findings. Available online: http://www.fao.org/save-food/resources/keyfindings/en/ (accessed on 2 May 2017).
- Kitinoja, L.; Saran, S.; Roy, S.K.; Kader, A.A. Postharvest technology for developing countries: Challenges and opportunities in research, outreach and advocacy. J. Sci. Food Agric. 2011, 91, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-92206-5. [Google Scholar]
- Sanzani, S.M.; Reverberi, M.; Geisen, R. Mycotoxins in harvested fruits and vegetables: Insights in producing fungi, biological role, conducive conditions, and tools to manage postharvest contamination. Postharvest Biol. Technol. 2016, 122, 95–105. [Google Scholar] [CrossRef]
- Krupinsky, J.M.; Bailey, K.L.; McMullen, M.P.; Gossen, B.D.; Turkington, T.K. Managing plant disease risk in diversified cropping systems. Agron. J. 2002, 94, 198–209. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. The use of packaging techniques to maintain freshness in fresh-cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2009, 44, 875–889. [Google Scholar] [CrossRef]
- Verma, L.R.; Joshi, D.V.K. Postharvest Technology of Fruits and Vegetables: General Concepts and Principles; Indus Publishing: Lahore, Pakistan, 2000; ISBN 978-81-7387-108-5. [Google Scholar]
- Dijksterhuis, J.; Houbraken, J.; Samson, R.A. 2 Fungal spoilage of crops and food. In Agricultural Applications; Kempken, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 35–56. ISBN 978-3-642-36821-9. [Google Scholar]
- Fellows, P.J. Food Processing Technology: Principles and Practice, 3rd ed.; Woodhead Publishing: Oxford, UK; Boca Raton, FL, USA, 2009; ISBN 978-1-84569-216-2. [Google Scholar]
- Silva, M.; Lidon, F. Food preservatives—An overview on applications and side effects. Emir. J. Food Agric. 2016, 1. [Google Scholar] [CrossRef]
- Stark, J.; Tan, H.S. Natamycin. In Food Preservatives; Springer: New York, NY, USA, 2003; pp. 179–195. [Google Scholar]
- Sharma, A.; Diwevidi, V.D.; Singh, S.; Pawar, K.K.; Jerman, M.; Singh, L.B.; Singh, S.; Srivastawav, D. Biological control and its important in agriculture. Int. J. Biotechnol. Bioeng. Res. 2013, 4, 175–180. [Google Scholar]
- Oliveira, P.M.; Zannini, E.; Arendt, E.K. Cereal fungal infection, mycotoxins, and lactic acid bacteria mediated bioprotection: From crop farming to cereal products. Food Microbiol. 2014, 37, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Kabaluk, J.T.; Svircev, A.M.; Goettel, M.S.; Woo, S.G. The Use and Regulation of Microbial Pesticides in Representative Jurisdictions Worldwide. Available online: http://www.iobc-global.org/download/Microbial_Regulation_Book_Kabaluk_et_al_2010.pdf (accessed on August 2010).
- Lacroix, C. Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage Biopreservation; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 0-85709-052-6. [Google Scholar]
- Robinson-Boyer, L.; Jeger, M.J.; Xu, X.-M.; Jeffries, P. Management of strawberry grey mould using mixtures of biocontrol agents with different mechanisms of action. Biocontrol Sci. Technol. 2009, 19, 1051–1065. [Google Scholar] [CrossRef]
- Spadaro, D.; Droby, S. Development of biocontrol products for postharvest diseases of fruit: The importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci. Technol. 2016, 47, 39–49. [Google Scholar] [CrossRef]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Piper, P.W. Resistance of yeasts to weak organic acid food Preservatives. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 77, pp. 97–113. ISBN 978-0-12-387044-5. [Google Scholar]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2016, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramirez-Corona, N.; Palou, E.; Lopez-Malo, A. Essential Oils: Antimicrobial Activities, Extraction Methods, and Their Modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Delgado, J.; Owens, R.A.; Doyle, S.; Asensio, M.A.; Núñez, F. Manuscript title: Antifungal proteins from moulds: Analytical tools and potential application to dry-ripened foods. Appl. Microbiol. Biotechnol. 2016, 100, 6991–7000. [Google Scholar] [CrossRef] [PubMed]
- Elsser-Gravesen, D.; Elsser-Gravesen, A. Biopreservatives. In Biotechnology of Food and Feed Additives; Zorn, H., Czermak, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 143, pp. 29–49. ISBN 978-3-662-43760-5. [Google Scholar]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Stiles, M.E. Biopreservation by lactic acid bacteria. Antonie Van Leeuwenhoek 1996, 70, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Punja, Z.K.; Utkhede, R.S. Using fungi and yeasts to manage vegetable crop diseases. Trends Biotechnol. 2003, 21, 400–407. [Google Scholar] [CrossRef]
- Gajbhiye, M.H.; Kapadnis, B.P. Antifungal-activity-producing lactic acid bacteria as biocontrol agents in plants. Biocontrol Sci. Technol. 2016, 26, 1451–1470. [Google Scholar] [CrossRef]
- Nguyen, P.-A.; Strub, C.; Fontana, A.; Schorr-Galindo, S. Crop molds and mycotoxins: Alternative management using biocontrol. Biol. Control 2017, 104, 10–27. [Google Scholar] [CrossRef]
- Buron, N.; Coton, M.; Legendre, P.; Ledauphin, J.; Kientz-Bouchart, V.; Guichard, H.; Barillier, D.; Coton, E. Implications of Lactobacillus collinoides and Brettanomyces/Dekkera anomala in phenolic off-flavour defects of ciders. Int. J. Food Microbiol. 2012, 153, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Perello, M.C.; de Revel, G.; Lonvaud-Funel, A. Growth and volatile compound production by Brettanomyces/Dekkera bruxellensis in red wine. J. Appl. Microbiol. 2008, 104, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- La Guerche, S.; Dauphin, B.; Pons, M.; Blancard, D.; Darriet, P. Characterization of Some Mushroom and Earthy Off-Odors Microbially Induced by the Development of Rot on Grapes. J. Agric. Food Chem. 2006, 54, 9193–9200. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, S.; Diguta, C.F.; Radoï-Matei, F.; Alexandre, H.; Guilloux-Bénatier, M. Non-Botrytis grape-rotting fungi responsible for earthy and moldy off-flavors and mycotoxins. Food Microbiol. 2014, 38, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, J.; Erl-Höning, C.; Wenning, M.; Böhnlein, C.; Gareis, M.; Pichner, R. Spoilage of vacuum-packed beef by the yeast Kazachstania psychrophila. Food Microbiol. 2016, 53, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Spanoghe, M.; Godoy Jara, M.; Rivière, J.; Lanterbecq, D.; Gadenne, M.; Marique, T. Development and application of a quantitative real-time PCR assay for rapid detection of the multifaceted yeast Kazachstania servazzii in food. Food Microbiol. 2017, 62, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wrent, P.; Rivas, E.-M.; de Prado, E.; Peinado, J.; de Silóniz, M.-I. Assessment of the Factors Contributing to the Growth or Spoilage of Meyerozyma guilliermondii in Organic Yogurt: Comparison of Methods for Strain Differentiation. Microorganisms 2015, 3, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Park, D.L.; Njapau, H.; Boutrif, E. Minimizing risks posed by mycotoxins utilizing the HACCP concept. Food Nutr. Agric. 1999, 23, 49–54. [Google Scholar]
- Sellamani, M.; Kalagatur, N.K.; Siddaiah, C.; Mudili, V.; Krishna, K.; Natarajan, G.; Putcha, V.L.R. Antifungal and Zearalenone Inhibitory Activity of Pediococcus pentosaceus Isolated from Dairy Products on Fusarium graminearum. Front. Microbiol. 2016, 7, 890. [Google Scholar] [CrossRef] [PubMed]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeasts in foods and beverages: Impact on product quality and safety. Curr. Opin. Biotechnol. 2007, 18, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Valence, F.; Pawtowski, A.; Auhustsinava-Galerne, L.; Frotté, N.; Baroncelli, R.; Deniel, F.; Coton, E.; Mounier, J. Diversity of spoilage fungi associated with various French dairy products. Int. J. Food Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Ojalvo, D.; Rodríguez, A.; Cordero, M.; Bernáldez, V.; Reyes-Prieto, M.; Córdoba, J.J. Characterisation and detection of spoilage mould responsible for black spot in dry-cured fermented sausages. Meat Sci. 2015, 100, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Kristiansson, E.; Ryberg, M.; Hallenberg, N.; Larsson, K.H. Intraspecific ITS variability in the kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol. Bioinform. 2008, 4, 193–201. [Google Scholar]
- Sholberg, P.L.; Conway, W.S. Postharvest pathology. In The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks, USDA-ARS Agriculture Handbook; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2004. [Google Scholar]
- Gill, C.O.; Lowry, P.D.; Di Menna, M.E. A Note on the Identities of Organisms Causing Black Spot Spoilage of Meat. J. Appl. Bacteriol. 1981, 51, 183–187. [Google Scholar] [CrossRef]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Stevenson, A.; Cray, J.A.; Williams, J.P.; Santos, R.; Sahay, R.; Neuenkirchen, N.; McClure, C.D.; Grant, I.R.; Houghton, J.D.; Quinn, J.P. Others is there a common water-activity limit for the three domains of life? ISME J. 2015, 9, 1333–1351. [Google Scholar] [CrossRef] [PubMed]
- Wolter, H.; Laing, E.; Viljoen, B.C. Isolation and identification of yeasts associated with intermediate moisture meats. Food Technol. Biotechnol. 2000, 38, 69–76. [Google Scholar]
- Dakal, T.C.; Solieri, L.; Giudici, P. Adaptive response and tolerance to sugar and salt stress in the food yeast Zygosaccharomyces rouxii. Int. J. Food Microbiol. 2014, 185, 140–157. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.L.; Pettersson, O.V.; Rice, T.; Hocking, A.D.; Schnürer, J. The extreme xerophilic mould Xeromyces bisporus—Growth and competition at various water activities. Int. J. Food Microbiol. 2011, 145, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.A.; Hocking, A.D.; Pitt, J.I.; Anggawati, A.M. Fungi associated with Indonesian dried fish. Food Microbiol. 1986, 3, 351–357. [Google Scholar] [CrossRef]
- Stevenson, A.; Hamill, P.G.; O’Kane, C.J.; Kminek, G.; Rummel, J.D.; Voytek, M.A.; Dijksterhuis, J.; Hallsworth, J.E. Aspergillus penicillioides differentiation and cell division at 0.585 water activity: Fungal cell division at 0.585 water activity. Environ. Microbiol. 2017, 19, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Miorelli, S.; Massaguer, P.R.; Aragão, G.M.F. Growth of Byssochlamys nivea in pineapple juice under the effect of water activity and ascospore age. Braz. J. Microbiol. 2011, 42, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.L.P.; Bernardi, A.O.; Pozza Morassi, L.L.; Silva, B.S.; Copetti, M.V.; Sant’Ana, A.S. Incidence, populations and diversity of fungi from raw materials, final products and air of processing environment of multigrain whole meal bread. Food Res. Int. 2016, 87, 103–108. [Google Scholar] [CrossRef]
- Le Lay, C.; Mounier, J.; Vasseur, V.; Weill, A.; Le Blay, G.; Barbier, G.; Coton, E. In vitro and in situ screening of lactic acid bacteria and propionibacteria antifungal activities against bakery product spoilage molds. Food Control 2016, 60, 247–255. [Google Scholar] [CrossRef]
- Hocking, A.D. Spoilage Problems: Problems caused by fungi. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 471–481. ISBN 978-0-12-384733-1. [Google Scholar]
- Gaggia, F.; Di Gioia, D.; Baffoni, L.; Biavati, B. The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends Food Sci. Technol. 2011, 22, S58–S66. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Xue, Y.-R.; Liu, C.-H. A Brief Review of Bioactive Metabolites Derived from Deep-Sea Fungi. Mar. Drugs 2015, 13, 4594–4616. [Google Scholar] [CrossRef] [PubMed]
- Vero, S.; Garmendia, G.; González, M.B.; Bentancur, O.; Wisniewski, M. Evaluation of yeasts obtained from Antarctic soil samples as biocontrol agents for the management of postharvest diseases of apple (Malus × domestica). FEMS Yeast Res. 2013, 13, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; García, H.S. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pantelides, I.S.; Christou, O.; Tsolakidou, M.-D.; Tsaltas, D.; Ioannou, N. Isolation, identification and in vitro screening of grapevine yeasts for the control of black aspergilli on grapes. Biol. Control 2015, 88, 46–53. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Kaushik, N.; Sowemimo, A.; Chhipa, H.; Koekemoer, T.; van de Venter, M.; Odukoya, O.A. Antifungal and antiproliferative activities of endophytic fungi isolated from the leaves of Markhamia tomentosa. Pharm. Biol. 2017, 55, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Tokpah, D.P.; Li, H.; Wang, L.; Liu, X.; Mulbah, Q.S.; Liu, H. An assessment system for screening effective bacteria as biological control agents against Magnaporthe grisea on rice. Biol. Control 2016, 103, 21–29. [Google Scholar] [CrossRef]
- Inglin, R.C.; Stevens, M.J.A.; Meile, L.; Lacroix, C.; Meile, L. High-throughput screening assays for antibacterial and antifungal activities of Lactobacillus species. J. Microbiol. Methods 2015, 114, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Delavenne, E.; Mounier, J.; Déniel, F.; Barbier, G.; Le Blay, G. Biodiversity of antifungal lactic acid bacteria isolated from raw milk samples from cow, ewe and goat over one-year period. Int. J. Food Microbiol. 2012, 155, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Penkar, S.; Plocková, M.; Chumchalová, J.; Bullerman, L.B. Antifungal Activity of Sodium Acetate and Lactobacillus rhamnosus. J. Food Prot. 2002, 65, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Lind, H.; Sjögren, J.; Gohil, S.; Kenne, L.; Schnürer, J.; Broberg, A. Antifungal compounds from cultures of dairy propionibacteria type strains. FEMS Microbiol. Lett. 2007, 271, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Delavenne, E.; Ismail, R.; Pawtowski, A.; Mounier, J.; Barbier, G.; Le Blay, G. Assessment of lactobacilli strains as yogurt bioprotective cultures. Food Control 2013, 30, 206–213. [Google Scholar] [CrossRef]
- Garnier, L.; Leyva Salas, M.; Pinon, N.; Wiernasz, N.; Patowski, A.; Coton, E.; Mounier, J.; Valence, F. High-throughput method for antifungal activity screening in a cheese-mimicking model. 2017; manuscript in preparation. [Google Scholar]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Muhammad, Z.; Evivie, S.E.; Luo, G.-W.; Xu, M.; Huo, G.-C. Screening of antifungal potentials of Lactobacillus helveticus KLDS 1.8701 against spoilage microorganism and their effects on physicochemical properties and shelf life of fermented soybean milk during preservation. Food Control 2016, 66, 183–189. [Google Scholar] [CrossRef]
- Nagaraja, H.; Chennappa, G.; Rakesh, S.; Naik, M.K.; Amaresh, Y.S.; Sreenivasa, M.Y. Antifungal activity of Azotobacter nigricans against trichothecene-producing Fusarium species associated with cereals. Food Sci. Biotechnol. 2016, 25, 1197–1204. [Google Scholar] [CrossRef]
- Axel, C.; Brosnan, B.; Zannini, E.; Peyer, L.C.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal activities of three different Lactobacillus species and their production of antifungal carboxylic acids in wheat sourdough. Appl. Microbiol. Biotechnol. 2016, 100, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Kilani-Feki, O.; Khedher, S.B.; Dammak, M.; Kamoun, A.; Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Tounsi, S. Improvement of antifungal metabolites production by Bacillus subtilis V26 for biocontrol of tomato postharvest disease. Biol. Control 2016, 95, 73–82. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Li, P.; Apaliya, M.T.; Yang, Q.; Peng, Y.; Zhang, X. Biocontrol of postharvest green mold of oranges by Hanseniaspora uvarum Y3 in combination with phosphatidylcholine. Biol. Control 2016, 103, 30–38. [Google Scholar] [CrossRef]
- Ma, H.; Sun, X.; Wang, M.; Gai, Y.; Chung, K.-R.; Li, H. The citrus postharvest pathogen Penicillium digitatum depends on the PdMpkB kinase for developmental and virulence functions. Int. J. Food Microbiol. 2016, 236, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Delavenne, E.; Cliquet, S.; Trunet, C.; Barbier, G.; Mounier, J.; Le Blay, G. Characterization of the antifungal activity of Lactobacillus harbinensis K.V9.3.1Np and Lactobacillus rhamnosus K.C8.3.1I in yogurt. Food Microbiol. 2015, 45, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Zhang, S.; Uluko, H.; Cui, W.; Lv, J. Potential use of Lactobacillus casei AST18 as a bioprotective culture in yogurt. Food Control 2013, 34, 675–680. [Google Scholar] [CrossRef]
- Lynch, K.M.; Pawlowska, A.M.; Brosnan, B.; Coffey, A.; Zannini, E.; Furey, A.; McSweeney, P.L.H.; Waters, D.M.; Arendt, E.K. Application of Lactobacillus amylovorus as an antifungal adjunct to extend the shelf-life of Cheddar cheese. Int. Dairy J. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Axel, C.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal sourdough lactic acid bacteria as biopreservation tool in quinoa and rice bread. Int. J. Food Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bullerman, L.B.; Giesova, M.; Hassan, Y.; Deibert, D.; Ryu, D. Antifungal activity of sourdough bread cultures. In Advances in Food Mycology; Hocking, A.D., Pitt, J.I., Samson, R.A., Thrane, U., Eds.; Springer: Boston, MA, USA, 2006; Volume 571, pp. 307–316. ISBN 978-0-387-28385-2. [Google Scholar]
- Coda, R.; Cassone, A.; Rizzello, C.G.; Nionelli, L.; Cardinali, G.; Gobbetti, M. Antifungal Activity of Wickerhamomyces anomalus and Lactobacillus plantarum during Sourdough Fermentation: Identification of Novel Compounds and Long-Term Effect during Storage of Wheat Bread. Appl. Environ. Microbiol. 2011, 77, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, A.; Gobbetti, M.; Rossi, J.; Damiani, P. Antimould activity of sourdough lactic acid bacteria: identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Appl. Microbiol. Biotechnol. 1998, 50, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Brosnan, B.; Jacob, F.; Furey, A.; Coffey, A.; Zannini, E.; Arendt, E.K. Lactic acid bacteria bioprotection applied to the malting process. Part II: Substrate impact and mycotoxin reduction. Food Control 2015, 51, 444–452. [Google Scholar] [CrossRef]
- Sangmanee, P.; Hongpattarakere, T. Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control 2014, 40, 224–233. [Google Scholar] [CrossRef]
- Cray, J.A.; Bell, A.N.W.; Bhaganna, P.; Mswaka, A.Y.; Timson, D.J.; Hallsworth, J.E. The biology of habitat dominance; can microbes behave as weeds? The biology of habitat dominance. Microb. Biotechnol. 2013, 6, 453–492. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, M.; Lavermicocca, P.; Lonigro, S.L.; Valerio, F. Lactobacillus brevis-based bioingredient inhibits Aspergillus niger growth on pan bread. Ital. J. Agron. 2014, 9, 146. [Google Scholar] [CrossRef]
- Ryu, E.H.; Yang, E.J.; Woo, E.R.; Chang, H.C. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiol. 2014, 41, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Gerez, C.L.; Fornaguera, M.J.; Obregozo, M.D.; Font de Valdez, G.; Torino, M.I. Antifungal starter culture for packed bread: Influence of two storage conditions. Rev. Argent. Microbiol. 2015, 47, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Saladino, F.; Luz, C.; Manyes, L.; Fernández-Franzón, M.; Meca, G. In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Aunsbjerg, S.D.; Honoré, A.H.; Marcussen, J.; Ebrahimi, P.; Vogensen, F.K.; Benfeldt, C.; Skov, T.; Knøchel, S. Contribution of volatiles to the antifungal effect of Lactobacillus paracasei in defined medium and yogurt. Int. J. Food Microbiol. 2015, 194, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Torres, N.; Ávila, M.; Delgado, D.; Garde, S. Effect of reuterin-producing Lactobacillus reuteri coupled with glycerol on the volatile fraction, odour and aroma of semi-hard ewe milk cheese. Int. J. Food Microbiol. 2016, 232, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Cheong, E.Y.L.; Sandhu, A.; Jayabalan, J.; Kieu Le, T.T.; Nhiep, N.T.; My Ho, H.T.; Zwielehner, J.; Bansal, N.; Turner, M.S. Isolation of lactic acid bacteria with antifungal activity against the common cheese spoilage mould Penicillium commune and their potential as biopreservatives in cheese. Food Control 2014, 46, 91–97. [Google Scholar] [CrossRef]
- Fernandez, B.; Vimont, A.; Desfossés-Foucault, É.; Daga, M.; Arora, G.; Fliss, I. Antifungal activity of lactic and propionic acid bacteria and their potential as protective culture in cottage cheese. Food Control 2017, 78, 350–356. [Google Scholar] [CrossRef]
- Axel, C.; Zannini, E.; Arendt, E.K.; Waters, D.M.; Czerny, M. Quantification of cyclic dipeptides from cultures of Lactobacillus brevis R2Δ by HRGC/MS using stable isotope dilution assay. Anal. Bioanal. Chem. 2014, 406, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic acid bacteria as sensory biomodulators for fermented cereal-based beverages. Trends Food Sci. Technol. 2016, 54, 17–25. [Google Scholar] [CrossRef]
- Peyer, L.C.; De Kruijf, M.; O’Mahony, J.; De Colli, L.; Danaher, M.; Zarnkow, M.; Jacob, F.; Arendt, E.K. Lactobacillus brevis R2Δ as starter culture to improve biological and technological qualities of barley malt. Eur. Food Res. Technol. 2017. [Google Scholar] [CrossRef]
- Ngang, J.-J.E.; Yadang, G.; Kamdem, S.L.S.; Kouebou, C.P.; Fanche, S.A.Y.; Kougan, D.L.T.; Tsoungui, A.; Etoa, F.-X. Antifungal properties of selected lactic acid bacteria and application in the biological control of ochratoxin A producing fungi during cocoa fermentation. Biocontrol Sci. Technol. 2015, 25, 245–259. [Google Scholar] [CrossRef]
- Adedokun, E.O.; Rather, I.A.; Bajpai, V.K.; Park, Y.-H. Biocontrol efficacy of Lactobacillus fermentum YML014 against food spoilage moulds using the tomato puree model. Front. Life Sci. 2016, 9, 64–68. [Google Scholar] [CrossRef]
- Varsha, K.K.; Devendra, L.; Shilpa, G.; Priya, S.; Pandey, A.; Nampoothiri, K.M. 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int. J. Food Microbiol. 2015, 211, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Srivastava, S. Antifungal effect of antimicrobial peptides (AMPs LR14) derived from Lactobacillus plantarum strain LR/14 and their applications in prevention of grain spoilage. Food Microbiol. 2014, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Rather, I.; Seo, B.J.; Rejish Kumar, V.J.; Choi, U.-H.; Choi, K.-H.; Lim, J.H.; Park, Y.-H. Isolation and characterization of a proteinaceous antifungal compound from Lactobacillus plantarum YML007 and its application as a food preservative. Lett. Appl. Microbiol. 2013, 57, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gamba, R.R.; Caro, C.A.; Martínez, O.L.; Moretti, A.F.; Giannuzzi, L.; De Antoni, G.L.; León Peláez, A. Antifungal effect of kefir fermented milk and shelf life improvement of corn arepas. Int. J. Food Microbiol. 2016, 235, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Barman, S.; Mukhopadhyay, A.; Mandal, N.C. Biological control of fruit-rot of jackfruit by rhizobacteria and food grade lactic acid bacteria. Biol. Control 2015, 83, 29–36. [Google Scholar] [CrossRef]
- Lipinska, L.; Klewicki, R.; Klewicka, E.; Kolodziejczyk, K.; Sojka, M.; Nowak, A. Antifungal Activity of Lactobacillus sp Bacteria in the Presence of Xylitol and Galactosyl-Xylitol. Biomed. Res. Int. 2016, 5897486. [Google Scholar] [CrossRef]
- Matei, G.M.; Matei, S.; Matei, A.; Cornea, C.P.; Draghici, E.M.; Jerca, I.O. Bioprotection of fresh food products against blue mold using lactic acid bacteria with antifungal properties. Romanian Biotechnol. Lett. 2016, 21, 11201–11208. [Google Scholar]
- Zhang, N.; Liu, J.; Li, J.; Chen, C.; Zhang, H.; Wang, H.-K.; Lu, F.-P. Characteristics and Application in Food Preservatives of Lactobacillus plantarum TK9 Isolated from Naturally Fermented Congee. Int. J. Food Eng. 2016, 12, 377–384. [Google Scholar] [CrossRef]
- Kumar, S.N.; Sreekala, S.R.; Chandrasekaran, D.; Nambisan, B.; Anto, R.J. Biocontrol of Aspergillus Species on Peanut Kernels by Antifungal Diketopiperazine Producing Bacillus cereus Associated with Entomopathogenic Nematode. PLoS ONE 2014, 9, e106041. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, M.H.; Sathe, S.J.; Marathe, R.J.; Deshmukh, R.B. Antifungal Bacillus subtilis AFB22 from pomegranate with potential to control fruit rot. Res. J. Biotechnol. 2013, 8, 26–35. [Google Scholar]
- Zhang, C.H.; Li, Y.; Liu, P.; Liu, M.J. Identification of two Bacillus amyloliquefaciens strains with high suppression to the key fruit pathogens of Chinese jujube. Biocontrol Sci. Technol. 2015, 25, 573–582. [Google Scholar] [CrossRef]
- Kim, Y.S.; Balaraju, K.; Jeon, Y. Effects of rhizobacteria Paenibacillus polymyxa APEC136 and Bacillus subtilis APEC170 on biocontrol of postharvest pathogens of apple fruits. J. Zhejiang Univ. Sci. B 2016, 17, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Calvo, H.; Marco, P.; Blanco, D.; Oria, R.; Venturini, M.E. Potential of a new strain of Bacillus amyloliquefaciens BUZ-14 as a biocontrol agent of postharvest fruit diseases. Food Microbiol. 2017, 63, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, J.; Li, B.; He, C.; Chen, Y.; Tian, S. Influence of Oxidative Stress on Biocontrol Activity of Cryptococcus laurentii against Blue Mold on Peach Fruit. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Gotor-Vila, A.; Teixidó, N.; Di Francesco, A.; Usall, J.; Ugolini, L.; Torres, R.; Mari, M. Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol. 2017, 64, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Passera, A.; Venturini, G.; Battelli, G.; Casati, P.; Penaca, F.; Quaglino, F.; Bianco, P.A. Competition assays revealed Paenibacillus pasadenensis strain R16 as a novel antifungal agent. Microbiol. Res. 2017, 198, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Mao, S.; Tu, K. Effect of preharvest spraying Cryptococcus laurentii on postharvest decay and quality of strawberry. Biol. Control 2014, 73, 68–74. [Google Scholar] [CrossRef]
- Qin, X.; Xiao, H.; Xue, C.; Yu, Z.; Yang, R.; Cai, Z.; Si, L. Biocontrol of gray mold in grapes with the yeast Hanseniaspora uvarum alone and in combination with salicylic acid or sodium bicarbonate. Postharvest Biol. Technol. 2015, 100, 160–167. [Google Scholar] [CrossRef]
- Campos-Martínez, A.; Velázquez-del Valle, M.G.; Flores-Moctezuma, H.E.; Suárez-Rodríguez, R.; Ramírez-Trujillo, J.A.; Hernández-Lauzardo, A.N. Antagonistic yeasts with potential to control Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. and Colletotrichum acutatum J.H. Simmonds on avocado fruits. Crop Prot. 2016, 89, 101–104. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Zeng, K. Efficacy of Pichia membranaefaciens combined with chitosan against Colletotrichum gloeosporioides in citrus fruits and possible modes of action. Biol. Control 2016, 96, 39–47. [Google Scholar] [CrossRef]
- Ferraz, L.P.; da Cunha, T.; da Silva, A.C.; Kupper, K.C. Biocontrol ability and putative mode of action of yeasts against Geotrichum citri-aurantii in citrus fruit. Microbiol. Res. 2016, 188–189, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.F.; Contreras, L.; Garnica, N.M.; Fernández-Zenoff, M.V.; Farías, M.E.; Sepulveda, M.; Ramallo, J.; Dib, J.R. Native Killer Yeasts as Biocontrol Agents of Postharvest Fungal Diseases in Lemons. PLoS ONE 2016, 11, e0165590. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, M.; Żarowska, B.; Restuccia, C.; Cirvilleri, G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017, 61, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhimo, V.Y.; Dilip, D.; Sten, J.; Ravat, V.K.; Bhutia, D.D.; Panja, B.; Saha, J. Antagonistic Yeasts for Biocontrol of the Banana Postharvest Anthracnose Pathogen Colletotrichum musae. J. Phytopathol. 2017, 165, 35–43. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Zhang, H.; Zhang, X.; Apaliya, M.T.; Zheng, X.; Mahunu, G.K. Effect of Yarrowia lipolytica on postharvest decay of grapes caused by Talaromyces rugulosus and the protein expression profile of T. rugulosus. Postharvest Biol. Technol. 2017, 126, 15–22. [Google Scholar] [CrossRef]
- Andrade, M.J.; Thorsen, L.; Rodríguez, A.; Córdoba, J.J.; Jespersen, L. Inhibition of ochratoxigenic moulds by Debaryomyces hansenii strains for biopreservation of dry-cured meat products. Int. J. Food Microbiol. 2014, 170, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Núñez, F.; Lara, M.S.; Peromingo, B.; Delgado, J.; Sánchez-Montero, L.; Andrade, M.J. Selection and evaluation of Debaryomyces hansenii isolates as potential bioprotective agents against toxigenic penicillia in dry-fermented sausages. Food Microbiol. 2015, 46, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Bernáldez, V.; Córdoba, J.J.; Rodríguez, M.; Cordero, M.; Polo, L.; Rodríguez, A. Effect of Penicillium nalgiovense as protective culture in processing of dry-fermented sausage “salchichón”. Food Control 2013, 32, 69–76. [Google Scholar] [CrossRef]
- Alía, A.; Andrade, M.J.; Rodríguez, A.; Reyes-Prieto, M.; Bernáldez, V.; Córdoba, J.J. Identification and control of moulds responsible for black spot spoilage in dry-cured ham. Meat Sci. 2016, 122, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Liu, Y.; Liu, S.; Cheng, M.; Zhang, Y.; Wang, R.; Chen, H.; Li, J.; Chen, X.; Wang, A. Analysis of Clonostachys rosea-induced resistance to grey mould disease and identification of the key proteins induced in tomato fruit. Postharvest Biol. Technol. 2017, 123, 83–93. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Scholz, C.F.P.; Kilian, M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4422–4432. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.; Mahony, J.; van Sinderen, D. Comparative analysis of two antifungal Lactobacillus plantarum isolates and their application as bioprotectants in refrigerated foods. J. Appl. Microbiol. 2012, 113, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Shi, Y.; Shen, F.; Wang, H. A new high phenyl lactic acid-yielding Lactobacillus plantarum IMAU10124 and a comparative analysis of lactate dehydrogenase gene. FEMS Microbiol. Lett. 2014, 356, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Muhialdin, B.J.; Hassan, Z.; Bakar, F.A.; Saari, N. Identification of antifungal peptides produced by Lactobacillus plantarum IS10 grown in the MRS broth. Food Control 2016, 59, 27–30. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Yapar, N.; Yetim, H. Diversity and probiotic potentials of lactic acid bacteria isolated from gilaburu, a traditional Turkish fermented European cranberrybush (Viburnum opulus L.) fruit drink. Food Res. Int. 2014, 64, 537–545. [Google Scholar] [CrossRef]
- Garofalo, C.; Zannini, E.; Aquilanti, L.; Silvestri, G.; Fierro, O.; Picariello, G.; Clementi, F. Selection of Sourdough Lactobacilli with Antifungal Activity for Use as Biopreservatives in Bakery Products. J. Agric. Food Chem. 2012, 60, 7719–7728. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.K.; Dal Bello, F.; Ryan, L. Increasing the Shelf Life of Bakery and Patisserie Products by Using the Antifungal Lactobacillus Amylovorus DSM 19280. US Patent WO200,9141,427 A2, 26 November 2009. [Google Scholar]
- Hemme, D.; Foucaud-Scheunemann, C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int. Dairy J. 2004, 14, 467–494. [Google Scholar] [CrossRef]
- Kalschne, D.L.; Womer, R.; Mattana, A.; Sarmento, C.M.P.; Colla, L.M.; Colla, E. Characterization of the spoilage lactic acid bacteria in in “sliced vacuum-packed cooked ham”. Braz. J. Microbiol. 2015, 46, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Raeisi, M.; Ebrahimi, M.; Sadeghi, B. Antifungal Activity of Pediococcus pentosaceus Isolated from Whole Barley Sourdough. J. Food Qual. Hazards Control 2016, 3, 30–36. [Google Scholar]
- Oliveira, P.M.; Brosnan, B.; Furey, A.; Coffey, A.; Zannini, E.; Arendt, E.K. Lactic acid bacteria bioprotection applied to the malting process. Part I: Strain characterization and identification of antifungal compounds. Food Control 2015, 51, 433–443. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Cummins, C.S.; Johnson, J.L. Family Propionibacteriaceae: The genus Propionibacterium. In The Prokaryotes; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 400–418. ISBN 978-0-387-25493-7. [Google Scholar]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and Mechanistic Insights into the Direct and Indirect Antimicrobial Properties of Bacillus subtilis Lipopeptides on Plant Pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, D.; van Rooyen, J.; Clarke, K.G. Enhanced production of antifungal lipopeptides by Bacillus amyloliquefaciens for biocontrol of postharvest disease. New Biotechnol. 2015, 32, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Wei, Z.; Guan, Z.; Cai, Y.; Liao, X. Antifungal Activity of Isolated Bacillus amyloliquefaciens SYBC H47 for the Biocontrol of Peach Gummosis. PLoS ONE 2016, 11, e0162125. [Google Scholar] [CrossRef] [PubMed]
- Muccilli, S.; Restuccia, C. Bioprotective Role of Yeasts. Microorganisms 2015, 3, 588–611. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Iimura, Y.; Otsuka, K. Breeding of Useful Killer Wine Yeasts. Am. J. Enol. Vitic. 1980, 31, 28–33. [Google Scholar]
- Janisiewicz, W.J.; Korsten, L. Biological cotrol of postharvest diseases of fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, R.; Labrie, S.; Fliss, I. Antimicrobial and Probiotic Properties of Yeasts: From Fundamental to Novel Applications. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Chanchaichaovivat, A.; Panijpan, B.; Ruenwongsa, P. Putative modes of action of Pichia guilliermondii strain R13 in controlling chilli anthracnose after harvest. Biol. Control 2008, 47, 207–215. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhang, Y.; Xu, X.-Y.; Qi, S.-H. Diverse Deep-Sea Fungi from the South China Sea and Their Antimicrobial Activity. Curr. Microbiol. 2013, 67, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, T.; Li, Y.; Cai, D.; Liu, X.; Lu, H.; Zheng, X. Postharvest biocontrol of Alternaria alternata in Chinese winter jujube by Rhodosporidium paludigenum. J. Appl. Microbiol. 2009, 107, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Banjara, N.; Suhr, M.J.; Hallen-Adams, H.E. Diversity of Yeast and Mold Species from a Variety of Cheese Types. Curr. Microbiol. 2015, 70, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Hammes, W.P.; Knauf, H.J. Starters in the processing of meat products. Meat Sci. 1994, 36, 155–168. [Google Scholar] [CrossRef]
- Delgado, J.; Acosta, R.; Rodríguez-Martín, A.; Bermúdez, E.; Núñez, F.; Asensio, M.A. Growth inhibition and stability of PgAFP from Penicillium chrysogenum against fungi common on dry-ripened meat products. Int. J. Food Microbiol. 2015, 205, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Owens, R.A.; Doyle, S.; Asensio, M.A.; Núñez, F. Impact of the antifungal protein PgAFP from Penicillium chrysogenum on the protein profile in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2015, 99, 8701–8715. [Google Scholar] [CrossRef] [PubMed]
- Cray, J.A.; Bhaganna, P.; Singhal, R.S.; Patil, S.V.; Saha, D.; Chakraborty, R; Timson, D.J.; Hallsworth, J.E. Modern fungicides and antifungal compounds VII. In Proceedings of the 17th International Reinhardsbrunn Symposium, April 21–25, 2013, Friedrichroda, Germany; DPG Spectrum Phytomedizin; DPG-Verl: Braunschweig, Germany, 2014; ISBN 978-3-941261-13-6. [Google Scholar]
- Thierry, A.; Deutsch, S.-M.; Falentin, H.; Dalmasso, M.; Cousin, F.J.; Jan, G. New insights into physiology and metabolism of Propionibacterium freudenreichii. Int. J. Food Microbiol. 2011, 149, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Dagnas, S.; Gauvry, E.; Onno, B.; Membré, J.-M. Quantifying Effect of Lactic, Acetic, and Propionic Acids on Growth of Molds Isolated from Spoiled Bakery Products. J. Food Prot. 2015, 78, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, L.T.; Chung, T.C.; Dobrogosz, W.J.; Lindgren, S.E. Production of a Broad Spectrum Antimicrobial Substance by Lactobacillus reuteri. Microb. Ecol. Health Dis. 1989, 2, 131–136. [Google Scholar] [CrossRef]
- Black, B.A.; Zannini, E.; Curtis, J.M.; Ganzle, M.G. Antifungal Hydroxy Fatty Acids Produced during Sourdough Fermentation: Microbial and Enzymatic Pathways, and Antifungal Activity in Bread. Appl. Environ. Microbiol. 2013, 79, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Broberg, A.; Jacobsson, K.; Strom, K.; Schnurer, J. Metabolite Profiles of Lactic Acid Bacteria in Grass Silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Zhang, S.; Cui, W.; Lv, J. Identification of Antifungal Compounds Produced by Lactobacillus casei AST18. Curr. Microbiol. 2012, 65, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Mieszkin, S.; Hymery, N.; Debaets, S.; Coton, E.; Le Blay, G.; Valence, F.; Mounier, J. Action mechanisms involved in the bioprotective effect of Lactobacillus harbinensis K.V9.3.1.Np against Yarrowia lipolytica in fermented milk. Int. J. Food Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Strom, K.; Sjogren, J.; Broberg, A.; Schnurer, J. Lactobacillus plantarum MiLAB 393 Produces the Antifungal Cyclic Dipeptides Cyclo(L-Phe-L-Pro) and Cyclo(L-Phe-trans-4-OH-L-Pro) and 3-Phenyllactic Acid. Appl. Environ. Microbiol. 2002, 68, 4322–4327. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; Di Biase, M.; Lattanzio, V.M.T.; Lavermicocca, P. Improvement of the antifungal activity of lactic acid bacteria by addition to the growth medium of phenylpyruvic acid, a precursor of phenyllactic acid. Int. J. Food Microbiol. 2016, 222, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Chang, H.C. Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. Int. J. Food Microbiol. 2010, 139, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, C.; Coton, E.; Le Blay, G.; Chobert, J.-M.; Haertlé, T.; Choiset, Y.; Van Long, N.N.; Meslet-Cladière, L.; Mounier, J. Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int. J. Food Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Paik, H.D.; Glatz, B.A. Purification and partial amino acid sequence of propionicin PLG-1, a bacteriocin produced by Propionibacterium thoenii P127. Le Lait 1995, 75, 367–377. [Google Scholar] [CrossRef]
- Schwenninger, S.M.; Lacroix, C.; Truttmann, S.; Jans, C.; Spörndli, C.; Bigler, L.; Meile, L. Characterization of low-molecular-weight antiyeast metabolites produced by a food-protective Lactobacillus-Propionibacterium coculture. J. Food Prot. 2008, 71, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Theron, M.M.; Lues, J.F.R. Organic Acids and Food Preservation; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011; ISBN 978-1-4200-7842-8. [Google Scholar]
- Stratford, M.; Nebe-von-Caron, G.; Steels, H.; Novodvorska, M.; Ueckert, J.; Archer, D.B. Weak-acid preservatives: pH and proton movements in the yeast Saccharomyces cerevisiae. Int. J. Food Microbiol. 2013, 161, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Cray, J.A.; Russell, J.T.; Timson, D.J.; Singhal, R.S.; Hallsworth, J.E. A universal measure of chaotropicity and kosmotropicity: A universal measure of chao- and kosmotropicity. Environ. Microbiol. 2013, 15, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.; Ezeji, T.C.; Singhal, R.S.; Thevelein, J.M.; Timson, D.J.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. Rapid identification, by use of the LTQ Orbitrap hybrid FT mass spectrometer, of antifungal compounds produced by lactic acid bacteria. Anal. Bioanal. Chem. 2012, 403, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. The QuEChERS approach in a novel application for the identification of antifungal compounds produced by lactic acid bacteria cultures. Talanta 2014, 129, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, B.; Coffey, A.; Arendt, E.K.; Furey, A. A comprehensive investigation into sample extraction and method validation for the identification of antifungal compounds produced by lactic acid bacteria using HPLC-UV/DAD. Anal. Methods 2014, 6, 5331–5344. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; Morrissey, J.P.; van Sinderen, D. Transcriptomic and morphological profiling of Aspergillus fumigatus Af293 in response to antifungal activity produced by Lactobacillus plantarum 16. Microbiology 2013, 159, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Rizzello, C.G.; Di Cagno, R.; Trani, A.; Cardinali, G.; Gobbetti, M. Antifungal activity of Meyerozyma guilliermondii: Identification of active compounds synthesized during dough fermentation and their effect on long-term storage of wheat bread. Food Microbiol. 2013, 33, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.; Peromingo, B.; Núñez, F.; Asensio, M.A. Use of molds and their antifungal proteins for biocontrol of toxigenic molds on dry-ripened cheese and meats. Curr. Opin. Food Sci. 2016, 11, 40–45. [Google Scholar] [CrossRef]
- Haidar, R.; Fermaud, M.; Calvo-Garrido, C.; Roudet, J.; Deschamps, A. Modes of action for biological control of Botrytis cinerea by antagonistic bacteria. Phytopathol. Mediterr. 2016, 55, 301–322. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal peptides: To be or not to be membrane active. Biochimie 2016, 130, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Selitrennikoff, C.P. Antifungal Proteins. Appl. Environ. Microbiol. 2001, 67, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Sumi, C.D.; Yang, B.W.; Yeo, I.-C.; Hahm, Y.T. Antimicrobial peptides of the genus Bacillus: A new era for antibiotics. Can. J. Microbiol. 2015, 61, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Lin, B.; Zhang, R. A novel antifungal protein of Bacillus subtilis B25. SpringerPlus 2013, 2, 543. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Martini, C.; Mari, M. Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action? Eur. J. Plant Pathol. 2016, 145, 711–717. [Google Scholar] [CrossRef]
- Rouissi, W.; Ugolini, L.; Martini, C.; Lazzeri, L.; Mari, M. Control of Postharvest Fungal Pathogens by Antifungal Compounds from Penicillium expansum. J. Food Prot. 2013, 76, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, S.; Lu, J.; Liu, L.; Uluko, H.; Pang, X.; Sun, Y.; Xue, H.; Zhao, L.; Kong, F.; et al. Antifungal activities and effect of Lactobacillus casei AST18 on the mycelia morphology and ultrastructure of Penicillium chrysogenum. Food Control 2014, 43, 57–64. [Google Scholar] [CrossRef]
- Nally, M.C.; Pesce, V.M.; Maturano, Y.P.; Rodriguez Assaf, L.A.; Toro, M.E.; Castellanos de Figueroa, L.I.; Vazquez, F. Antifungal modes of action of Saccharomyces and other biocontrol yeasts against fungi isolated from sour and grey rots. Int. J. Food Microbiol. 2015, 204, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.S.T.; Beck, J.J.; Sarreal, S.B.L.; Gee, W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014, 30, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. Bacillus spp.: A promising biocontrol agent of root, foliar, and postharvest diseases of plants. In Bacilli and Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Jha, C.K., Aeron, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 113–141. ISBN 978-3-319-44408-6. [Google Scholar]
- Li, C.; Wang, J.; Luo, C.; Ding, W.; Cox, D.G. A new cyclopeptide with antifungal activity from the co-culture broth of two marine mangrove fungi. Nat. Prod. Res. 2014, 28, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Klewicka, E. Antifungal activity of lactic acid bacteria of genus Lactobacillus sp in the presence of polyols. Acta Aliment. 2007, 36, 495–499. [Google Scholar] [CrossRef]
- Serrazanetti, D.I.; Guerzoni, M.E.; Corsetti, A.; Vogel, R. Metabolic impact and potential exploitation of the stress reactions in lactobacilli. Food Microbiol. 2009, 26, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.Z.; Tian, S.P.; Xu, Y.; Wan, Y.K. Enhancement of biocontrol efficacy of antagonistic yeasts by salicylic acid in sweet cherry fruit. Physiol. Mol. Plant Pathol. 2003, 62, 147–154. [Google Scholar] [CrossRef]
- Geremew, T.; Kebede, A.; Andualem, B. The role of spices and lactic acid bacteria as antimicrobial agent to extend the shelf life of metata ayib (traditional Ethiopian spiced fermented cottage cheese). J. Food Sci. Technol. 2015, 52, 5661–5670. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 4: Suitability of taxonomic units notified to EFSA until March 2016. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef] [PubMed]
- FDA Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ (accessed on 9 April 2017).
- Coton, M.; Lebreton, M.; Leyva Salas, M.; Navarro, M.; Patowsky, A.; Le Blay, G.; Valence, F.; Coton, E.; Mounier, J. Safety risk assessment of potential antifungal lactic acid bacteria and propionibacteria. 2017; in preparation. [Google Scholar]
- Gasser, F. Safety of lactic acid bacteria and their occurrence in human clinical infections. Bull. Inst. Pasteur 1994, 92, 45–67. [Google Scholar]
- Mogensen, G.; Salminen, S.; O’brien, J.; Ouwehand, A.; Holzapfel, W.; Shortt, C.; Fonden, R.; Miller, G.; Donohue, D.; Playne, M. Food microorganisms: Health benefits, safety evaluation and strains with documented history of use in foods. Bull. Int. Dairy Fed. 2002, 377, 4–9. [Google Scholar]
- Vankerckhoven, V.; Huys, G.; Vancanneyt, M.; Vael, C.; Klare, I.; Romond, M.-B.; Entenza, J.M.; Moreillon, P.; Wind, R.D.; Knol, J.; et al. Biosafety assessment of probiotics used for human consumption: Recommendations from the EU-PROSAFE project. Trends Food Sci. Technol. 2008, 19, 102–114. [Google Scholar] [CrossRef]
- Ladero, V.; Fernández, M.; Calles-Enríquez, M.; Sánchez-Llana, E.; Cañedo, E.; Martín, M.C.; Alvarez, M.A. Is the production of the biogenic amines tyramine and putrescine a species-level trait in enterococci? Food Microbiol. 2012, 30, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Coton, M. Blue cheese. In Fermented Foods in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 275–303. ISBN 978-0-12-802309-9. [Google Scholar]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef] [PubMed]
- Coton, E.; Coton, M. Evidence of horizontal transfer as origin of strain to strain variation of the tyramine production trait in Lactobacillus brevis. Food Microbiol. 2009, 26, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Marcobal, Á.; Martín-Álvarez, P.J.; Polo, M.C.; Muñoz, R.; Moreno-Arribas, M.V. Formation of biogenic amines throughout the industrial manufacture of red wine. J. Food Prot. 2006, 69, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ladero, V.; Calles-Enríquez, M.; Fernández, M.; Alvarez, A.M. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Sillasantos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Melin, P.; Hakansson, S.; Eberhard, T.H.; Schnurer, J. Survival of the biocontrol yeast Pichia anomala after long-term storage in liquid formulations at different temperatures, assessed by flow cytometry. J. Appl. Microbiol. 2006, 100, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Melin, P.; Sundh, I.; Hakansson, S.; Schnurer, J. Biological preservation of plant derived animal feed with antifungal microorganisms: Safety and formulation aspects. Biotechnol. Lett. 2007, 29, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Tian, S.P. Effects of trehalose on stress tolerance and biocontrol efficacy of Cryptococcus laurentii. J. Appl. Microbiol. 2006, 100, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, E.A.; Klaenhammer, T.R. The genomics of lactic acid bacteria. Trends Microbiol. 2007, 15, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Rouse, S.; Harnett, D.; Vaughan, A.; Van Sinderen, D. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 2008, 104, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, U.; Villarreal, J.V. Inhibition of Penicillium nordicum in MRS medium by lactic acid bacteria isolated from foods. Food Control 2010, 21, 107–111. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Gamarra, S.; Garcia-Effron, G.; Park, S.; Perlin, D.S.; Rao, R. Requirement for Ergosterol in V-ATPase Function Underlies Antifungal Activity of Azole Drugs. PLoS Pathog. 2010, 6, e1000939. [Google Scholar] [CrossRef] [PubMed]
| Food Field | Group | Antifungal Microorganisms (Active In Situ/Tested Strains) | In Situ Test | Source of Microorganism | Application Method | Activity Spectrum (Inhibited/Tested) | Reference |
|---|---|---|---|---|---|---|---|
| bakery | LAB | Lactobacillus brevis ITM18 | yeast-leavened bread | sourdough | CFS as ingredient | Aspergillus niger | [97] |
| bakery | LAB | Lactobacillus plantarum HD1 | Korean draft rice wine | kimchi | CFS | Aspergillus fumigatus and Pichia kudriavzevii | [98] |
| bakery | LAB | Lactobacillus amylovorus DSM19280 (1/1) | sourdough quinoa bread | cereal isolate | cells in sourdough | environmental molds | [80] |
| bakery | LAB | Lactobacillus plantarum CRL778 | wheat bread | homemade wheat dough | SL778: fermentate as ingredient | environmental molds | [99] |
| bakery | LAB | Lactobacillus plantarum UFG 121 (only 1 in situ from best 2/88 in vitro) | oat-based product | food | cells in sourdough | Fusarium culmorum (only 1 tested in situ), Penicillium chrysogenum, Penicillium expansum, Penicillium roqueforti, and Aspergillus flavus (5/7 in vitro) | [69] |
| bakery | LAB | Lactobacillus amylovorus DSM19280 (1/3) | sourdough wheat bread | cereal isolate | cells as starter | Fusarium culmorum | [83] |
| bakery | LAB | Lactobacillus reuteri R29, Lactobacillus brevis R2, Lactobacillus amylovorus DSM19280 | sourdough of quinoa and rice bread | human, pork, and cereal | cells in sourdough | environmental molds | [90] |
| bakery | LAB | Lactobacillus bulgaricus CECT 4005, Lactobacillus plantarum CECT 749 (active in situ 2/6), Lactobacillus johnsonii CECT 289, Lactobacillus rhamnosus CECT 288, Lactobacillis ruminis CECT 1324, and Bifidobacterium bifidum CECT 870T (6 active in vitro/16) | loaf bread | not detailed | cells in sourdough | Aspergillus parasiticus (only one tested in situ) and Penicillium expansum | [100] |
| bakery | LAB & PAB | Leuconostoc citreum (5 strains), Lactobacillus sakei, Lactobacillus plantarum, Lactobacillus spicheri O15, Lactobacillus reuteri 5529, Lactobacillus brevis Lu35, Propionibacterium acidipropionici and Propionibacterium freudenreichii LSaci68 (by surface-spraying 12 LAB/69) | pound cake and milk bread roll | milk, milk roll sourdough, and others not detailed | whole culture as sourdough ingredient for milk bread roll and sprayed | Cladosporium sphaerospermum and Wallemia sebi on pound cake; and Eurotium repens, Aspergillus niger, and Penicillium corylophilum on milk bread roll | [63] |
| dairy | LAB | Lactobacillus harbinensis K.V9.3.1Np, Lactobacillus. rhamnosus K.C8.3.1I, and Lactobacillus paracasei K.C8.3.1Hc1 (3/11) | yogurt | cow and goat milk | cells as adjunct culture | Debaryomyces hansenii, Kluyveromyces lactis, Kluyveromyces marxianus, Penicillium brevicompactum, Rhodotorula mucilaginosa, and Yarrowia lipolytica (6/6) | [78] |
| dairy | LAB | Lactobacillus casei AST18 (1/1) | yogurt | Chinese dairy products | cells as adjunct culture | Penicillium sp. (1/1) | [88] |
| dairy | LAB | Lactobacillus paracasei DCS302 | yogurt | not detailed | cells as adjunct culture | Penicillium sp. nov. DCS 1541, Penicillium solitum (2/8) | [101] |
| dairy | LAB | Lactobacillus harbinensis K.V9.3.1Np (1/2) | yogurt | cow milk | cells as adjunct culture | Yarrowia lipolytica (1/1) | [87] |
| dairy | LAB | Lactobacillus reuteri INIA P57 | semi-hard ewe milk cheese | pig feces (isolated by Langa 2003) | cells as adjunct culture supplemented with glycerol | Not evaluated | [102] |
| dairy | LAB | Lactobacillus amylovorus DSM 19280 (1/1) | cheddar cheese | cereal environment | cells as adjunct culture | Penicillium expansum (1/1) and environmental molds | [89] |
| dairy | LAB | 12 strains of Lactobacillus plantarum (12/897) | cottage cheese | fresh herbs, fruits, and vegetables | cells as added to the finished product | Penicillium commune | [103] |
| dairy | LAB | L. rhamnosus A238, L. rhamnosus A119 (2/5) The association of L. rhamnosus A238 with B. animalis subsp. lactis A026, and L. rhamnosus A119 with B. animalis subsp. lactis A026 | cottage cheese | not detailed | cells added to the finished product | Penicillium chrysogenum (1/1) | [104] |
| malting | LAB | Lactobacillus brevis R2Δ (1/1) | barley malt extract fermentation | porcine isolate | cells as starter | Not evaluated | [105] |
| malting | LAB | Lactobacillus brevis R2Δ and Lactobacillus plantarum FST1.7 (2/2) | barley malt extract (wort) fermentation | porcine and barley isolate | cells as starter | Fusarium culmorum | [106] |
| malting | LAB | Lactobacillus brevis R2Δ (1/1) | barley in malting process | porcine isolate | cells as starters and CFS | Fusarium culmorum and Fusarium graminearum | [107] |
| malting | LAB | Lactobacillus reuteri R29 and Lactobacillus amylovorus DSM19280 | malting process (steeping and germination) | human and cereal isolates | CFS (wort as growth media) as the steeping liquor | Fusarium culmorum | [94] |
| fermented vegetables | LAB | Pediococcus spp. A19 (tested in situ) Pediococcus spp. A21, Lactobacillus plantarum B4496, Lactobacillus brevis 207, and Lactobacillus sanfranciscensis BB12 (5/13) | cocoa | fermenting cocoa | cells as starter | Aspergillus carbonarius, Aspergillus niger, and Aspergillus ochraceus | [108] |
| fermented vegetables | LAB | Lactobacillus fermentum YML014 | tomato puree | gari, fermented cassava (starchy root) | cells | Penicillium expansum (only one tested in situ), Aspergillus flavus, Aspergillus niger, Candida albicans, and Zygosaccharomyces rouxii (low inhibition of yeasts) | [109] |
| fermented vegetables | LAB | Lactobacillus helveticus KLDS 1.8701 (1/4 also L. helveticus) | fermented soybean milk | dairy products | cells as adjunct culture | Penicillium sp. (1/1) | [81] |
| grain/seed | LAB | Lactococcus sp. BSN307 | wheat grains | rotten jackfruit, guava, and animals fecal samples | submerged in purified volatile organic compound 2,4-di-tert-butylphenol | A. niger, F. moniliforme, F. graminearum, F. chlamydosporum, and F. oxysporum | [110] |
| grain/seed | LAB | Lactobacillus plantarum LR/14 | wheat seeds (Triticum aestivum var. HD 2824) | not detailed | AMP LR14 solution | Aspergillus niger, Rhizopus stolonifer, Mucor racemosus, and Penicillium chrysogenum. | [111] |
| grain/seed | LAB | Lactobacillus plantarum YML007 (1/1400) | soybean | kimchi | CFS | Aspergillus niger | [112] |
| grain/seed | LAB & Fungi | kefir grains contain a symbiotic consortium of LAB and yeasts (Lactobacillus plantarum, L. kefir, Lactococcus lactis subsp. lactis, Saccharomyces and Acetobacter) | arepa (corn cakes) | kefir grains | CFS | Aspergillus flavus | [113] |
| fruit | LAB & other bacteria | Lactobacillus lactis subsp. lactis LABW1, LABW3, LABW4, Burkholderia cenocepacia VBC7 and Pseudomonas poae VBK1 | jackfruit | rotten jackfruit | cells sprayed over the fruit | Rhizopus stolonifer | [114] |
| fruit | LAB | Lactobacillus paracasei ŁOCK0921 (1 tested in situ) (1/(9 in vitro/60)) | wild cherries | plant and human | CFS cultivated with xylitol or galactosyl-xylitol directly on wild cherries | Alternaria brassicicola (1 tested in situ), Alternaria alternata, Aspergillus niger, Fusarium lateritium, Geotrichum candidum, and Mucor hiemalis (6/8) | [115] |
| fruit | LAB | Lactic acid bacteria strains LCM5, LAB 58, LAB 13, and LAB 43 (4/6) | apple | plants, fermented wheat bran, pickles, and sauerkraut | cells sprayed over the fruit (wounded and non-wounded) | Penicillium expansum | [116] |
| fruit/dairy | LAB | Lactobacillus plantarum TK9 | citrus, apples and yogurt | Chinese naturally fermented congee | cells | Penicillium roqueforti, Penicillium citrinum, Penicillium oxalicum, Aspergillus fumigatus, Aspergillus flavus, and Rhizopus nigricans (6/7) | [117] |
| grain/seed | Other bacteria | Acetobacter nigricans AZT 54 (0/1) | maize, sorghum and wheat grains | paddy field soil samples | cereals submerged in suspension | Fusarium sporotrichioides, Fusarium graminearum, Fusarium poae, and Fusarium equiseti (4/10 in vitro) | [82] |
| grain/seed | Other bacteria | Bacillus cereus | peanut kernels | entomopathogenic nematode | cells and purified cyclo(4-hydroxy-l-Pro-l-Trp) | Aspergillus flavus | [118] |
| fruit | Other bacteria | Bacillus subtilis AFB22 [1/(50/200 in vitro)] | pomegranate | pomegranate leaves and fruits | cells and CFS by spraying on wounded fruits | P. varsoniana (only in situ), A. flavus, A. clavatus, B. humicola, F. graminearum, and R. stolonifer (in vitro) | [119] |
| fruit | Other bacteria | Bacillus amyloliquefaciens ZJ01 and ZJ02 | jujube fruit | phyllosphere of Chinese jujube | whole culture in created wounds-prevention treatment | Phoma destructiva (2 strains), Alternaria alterna (2 strains), and Fusicoccum spp. (5/5) | [120] |
| fruit | Other bacteria | Bacilllus subtilis V26 | tomato fruit | rhizosphere of almond trees | whole culture, endospores, and CFS in created wounds | Botrytis cinerea | [84] |
| fruit | Other bacteria | Paenibacillus polymyxa APEC136 and Bacillus subtilis APEC170 | apple | soil from several apple orchards | cells over created wounds | Colletotrichum gloeosporioides, Colletotrichum acutatum, and Botryosphaeria dothidea | [121] |
| fruit | Other bacteria | Bacillus amyloliquefaciens BUZ-14 | apple, orange, grape, and cherries | surface of peach fruit from an orchard | cells, endospores and CFS | Botrytis cinerea, Monilinia fructicola, Monilinia laxa, Penicillium digitatum, Penicillium expansum, and Penicillium italicum | [122] |
| fruit | Other bacteria | Cryptococcus laurentii | peach fruit | surfaces of apple fruits | cells in created wounds | Penicillium expansum | [123] |
| fruit | Other bacteria | Bacillus amyloliquefaciens CPA-8 | cherries | nectarine surface | wounded fruits packaged with in situ produced volatile organic compounds | Monilia fructicola (1/3) | [124] |
| fruit | Other bacteria | Paenibacillus pasadenensis R16 | grape berries | leaf of grapevine plant | wounded fruit submerged in cell suspension | Botrytis cinerea | [125] |
| fruit | Yeast | Cryptococcus laurentii 2.3803 | strawberries | not detailed | cells sprayed over fruits prior to harvest | Botrytis cinerea | [126] |
| fruit | Yeast | Hanseniaspora uvarum | grape berries | strawberries surface | cells in wounds and fruit submerged in salicylic acid or sodium bicarbonate | Botrytis cinerea | [127] |
| fruit | Yeast | Wickerhamomyces anomalus BS91, Metschnikowia pulcherrima MPR3, Aureobasidium pullulans PI1, and Saccharomyces cerevisiae BCA62 | grape berries | fermented olive brine and pomegranate | cells in created wounds | Botrytis cinerea | [71] |
| fruit | Yeast | Aureobasidium pullulan (25 strains), Cryptococcus magnus (2 strains), Candida sake 2AM3 [(28/33 in situ) (33/55 in vitro)] | grape berries | surface of grape berries | wounded fruits submerged in cells suspension | Aspergillus tubingensis | [70] |
| fruit | Yeast | Candida intermedia and Wickerhamomyces anomalus | avocado | fruits, leaves, and the soil of the avocado orchards | cells in created wounds | Colletotrichum gloeosporioides and Colletotrichum acutatum | [128] |
| fruit | Yeast | Hanseniaspora uvarum Y3 | orange | surfaces of grapes in vineyard | cells in created wounds | Penicillium digitatum | [85] |
| fruit | Yeast | Pichia membranaefaciens | citrus fruits Citrus sinensis | not detailed | cells in created wounds | Colletotrichum gloerosporioides | [129] |
| fruit | Yeast | Rhodotorula minuta ACBL-23, Candida azyma ACBL-44, S. cerevisiae ACBL-52, Rhodotorula mucilaginosa ACBL-68, and Aureobasidium pullulans ACBL-77 | ‘Pera’ orange fruits | citrus leaves, flowers, fruits, and citrus-growing soils | cells in created wounds | Geotrichum citri-aurantii | [130] |
| fruit | Yeast | Pichia fermentans (2 strains), Wickerhamomyces anomalus, Kazachstania exigua, and Saccharomyces cerevisiae | lemons | surface of leaves and fruits of different citrus and wash-water from lemon shells | wounded fruits submerged cells suspension | Penicillium digitatum and Penicillium italicum | [131] |
| fruit | Yeast | Debaryomyces hansenii KI2a, D. hansenii MI1a, and Wickerhamomyces anomalus BS91 | peach and plum fruits | blue-veined Rokpol cheese and fermented olive brine | cells in created wounds | Monilinia fructigena and Monilinia fructicola | [132] |
| fruit | Yeast | Candida tropicalis YZ27 | banana | from bitter gourd | cells in created wounds | Colletotrichum musae | [133] |
| fruit | Yeast | Yarrowia lipolytica | grape berries | surface of grapes | cells in created wounds | Talaromyces rugulosus | [134] |
| meat | Yeast | Debaromyces hansenii FHSCC 253H | dry-cured ham slices | dry-cured meat products | cells over slices (aw controlled) | Penicillium nordicum | [135] |
| meat | Yeast | Debaromyces hansenii 253H and 226G G | dry-fermented sausage | dry-cured meat products | cells over slices after fermentation | Penicillium verrucosum | [136] |
| meat | Molds | Penicillium nalgoviense | dry-fermented sausages | TEXEL PN1 from Danisco (Niebüll, Germany) | immersion of sausages in cells suspension | Penicillium verrucosum | [137] |
| meat | Molds | Penicillium chrysogenum CECT 20922 | dry-cured ham slices | not detailed | cells | Cladosporium cladosporioides, C. herbarum, and C. oxysporum | [138] |
| fruit | Molds | Clonostachys rosea | tomato fruit | not detailed | cells sprayed over the fruit | Botrytis cinerea | [139] |
| Product Name | Application Field | Properties | Composition | Manufacturer |
|---|---|---|---|---|
| Holdbac YM-B or YM-C | fermented food and white cheeses | protection against yeasts and molds | Lactobacillus rhamnosus and Propionibacterium freudenreichii subsp. shermanii | DuPont Danisco |
| Holdbac YM-XPM | fermented dairy and mild acidic yogurt | protection against yeasts and molds | Lactobacillus plantarum | DuPont Danisco |
| Holdbac YM-XPK | all types of cheeses | protection against yeasts and molds | Lactobacillus plantarum | DuPont Danisco |
| FreshQ 1 and FreshQ 4 | cottage cheese | protection against yeasts and molds | Lactobacillus rhamnosus and Lactobacillus paracasei | CHR Hansen |
| FreshQ 2 | cottage cheese | protection against yeasts and molds | Lactobacillus rhamnosus | CHR Hansen |
| FreshQ 5 | cottage cheese | protection against yeasts and molds | Lactobacillus paracasei | CHR Hansen |
| Natamax | fruit juices, wine, surface of dry-ripened food, dairy, and bakery products | protection against yeasts and molds | Natamycin produced by Streptomyces natalensis | DuPont Danisco |
| MicroGard | sauces, salad dressings, prepared meals, cured meat, pastas, bakery and dairy products, hash brown potatoes | protection against Gram-positive bacteria, Gram-negative bacteria, yeasts, and molds | Fermentate (skim milk or dextrose) of Propionibacterium freudenreichii subsp. shermani | DuPont Danisco |
| Hi Shield P | bakery products, salad dressings, and general used in food industry | protection against molds, yeasts (Pichia anomala), and bacteria (Bacillus subtilis); increase sour taste; and reduce salt content (flavor improver and enhancer) | Fermentate (corn) of lactic acid bacteria and yeasts | HI-FOOD S.p.A. |
| Inhibit FOG, Inhibit 2800, Inhibit 1900CW, Inhibit 3600 and Inhibit 2100NF | bakery products, cheeses, meats, salad dressings, condiments, dips, spreads, and meats | protection against molds, yeasts, and Gram-negative bacteria | Fermentates (dextrose, wheat, wheat flour, whey, brown rice) of Propionibacterium freudenreichii | Mezzoni Foods |
| Biosafe 10LP | cherries, pome fruits, citrus, and potatoes | protection against Penicillium expansum, Botrytis cinerea, Mucor piriformis, Fusarium sambucinum, Helminthosporium solani, and Rhizopus stolonifer | Pseudomonas syringae | Nu Farm Inc. USA |
| Aspire | citrus and pome fruit | protection against molds (P. expansum and Botrytis cinerea) | Candida oleophila | Ecogen Inc. USA |
| Befresh | fresh fermented milk products | control the growth of yeast and molds | Lactobacillus paracasei and Propionibacterium freudenreichii subsp. shermanii, | Handary |
| Candifruit | pome fruit | protection against Botrytis cinerea, Penicillium expansum, and Rhizopus stolonifer | Candida sake | Sipcam-Inaagri, SA (Valencia, Spain) |
| Boni-Protect | pome fruit | protection against Botrytis cinerea, Monilinia fructigena, Penicillium expansum, and Pezicula malicortici | Aureobasidium pullulan | BioFerm GMbH, Germany |
| Shemer | citrus fruit, stone fruits, and berries | protection against Aspergillus niger, Botrytis cinerea, Penicillium expansum, Penicillium digitatum, Penicilllium italicum, and Rhizopus stolonifer | Metschnikowia fructicola | Bayer Cropscience, Israel |
| Pantovital | citrus and pome fruit | protection against Botrytis cinerea, Penicillium expansum, Penicillium digitatum, Penicillium italicum, and Rhizopus stolonifer | Pantoea agglomerans | BioDURCAL S.L. |
| YieldPlus | citrus, apple, and pear fruit | not detailed | Cryptococcus albidus | Anchor Bio-Technologies, Cape Town, South Africa |
| Nexy | pome fruit | protection against Botrytis cinerea and Penicillium expansum | Candida oleophila | BioNext sprl, France |
| Serenade | grapes, legumes, pome fruits, and peanuts | protection against fungi causing powdery mildew, late blight brown rot, fireblight | Bacillus subtilis | Agra Quees Inc. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal Microbial Agents for Food Biopreservation—A Review. Microorganisms 2017, 5, 37. https://doi.org/10.3390/microorganisms5030037
Leyva Salas M, Mounier J, Valence F, Coton M, Thierry A, Coton E. Antifungal Microbial Agents for Food Biopreservation—A Review. Microorganisms. 2017; 5(3):37. https://doi.org/10.3390/microorganisms5030037
Chicago/Turabian StyleLeyva Salas, Marcia, Jérôme Mounier, Florence Valence, Monika Coton, Anne Thierry, and Emmanuel Coton. 2017. "Antifungal Microbial Agents for Food Biopreservation—A Review" Microorganisms 5, no. 3: 37. https://doi.org/10.3390/microorganisms5030037
APA StyleLeyva Salas, M., Mounier, J., Valence, F., Coton, M., Thierry, A., & Coton, E. (2017). Antifungal Microbial Agents for Food Biopreservation—A Review. Microorganisms, 5(3), 37. https://doi.org/10.3390/microorganisms5030037








