Fluorescence Lectin Bar-Coding of Glycoconjugates in the Extracellular Matrix of Biofilm and Bioaggregate Forming Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Culture Conditions
2.2. Lectin-Screening
2.3. Epifluorescence and Laser Microscopy
2.4. Binary Bar-Coding and Heat-Mapping
2.5. Digital Image Analysis
3. Results
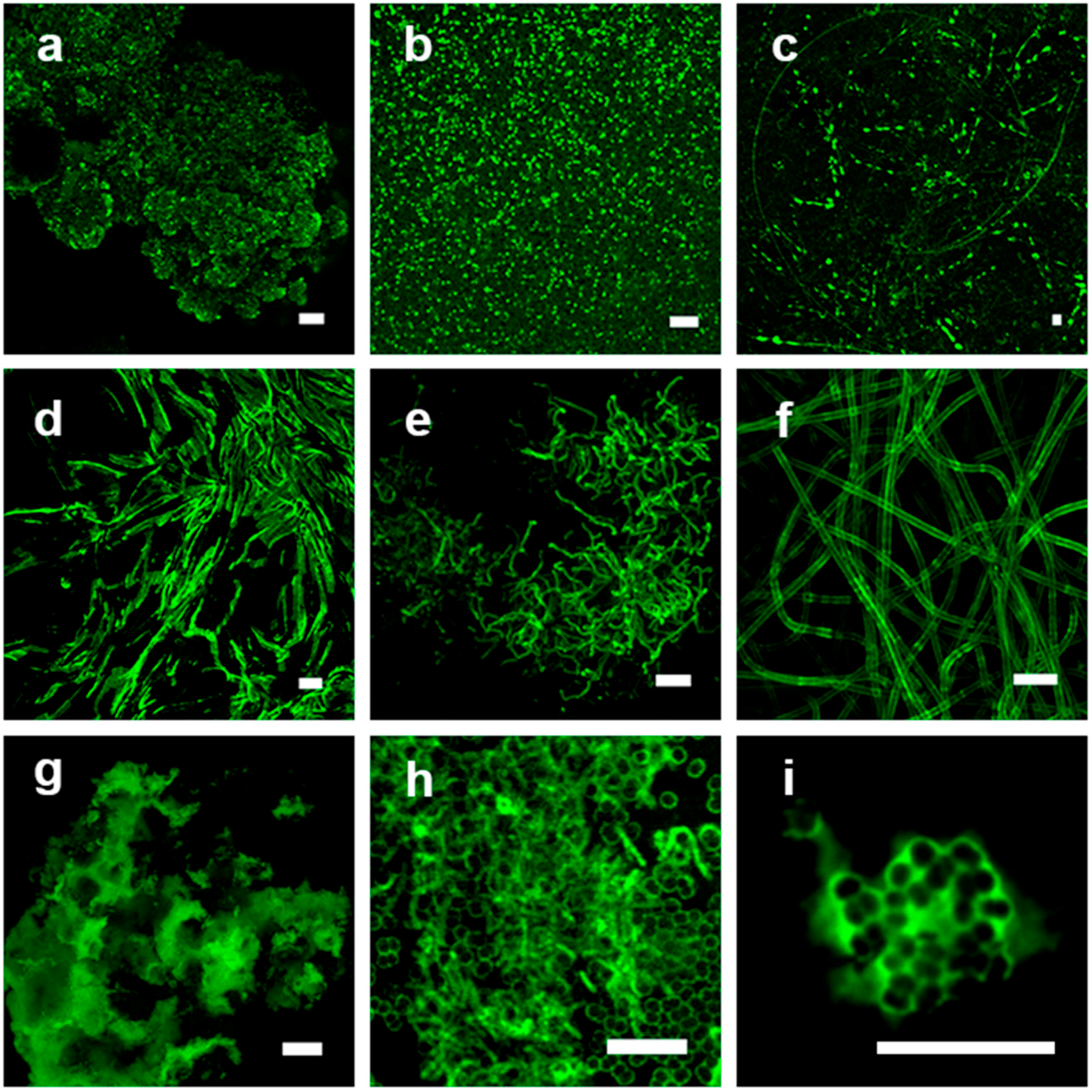
3.1. Visual Examination
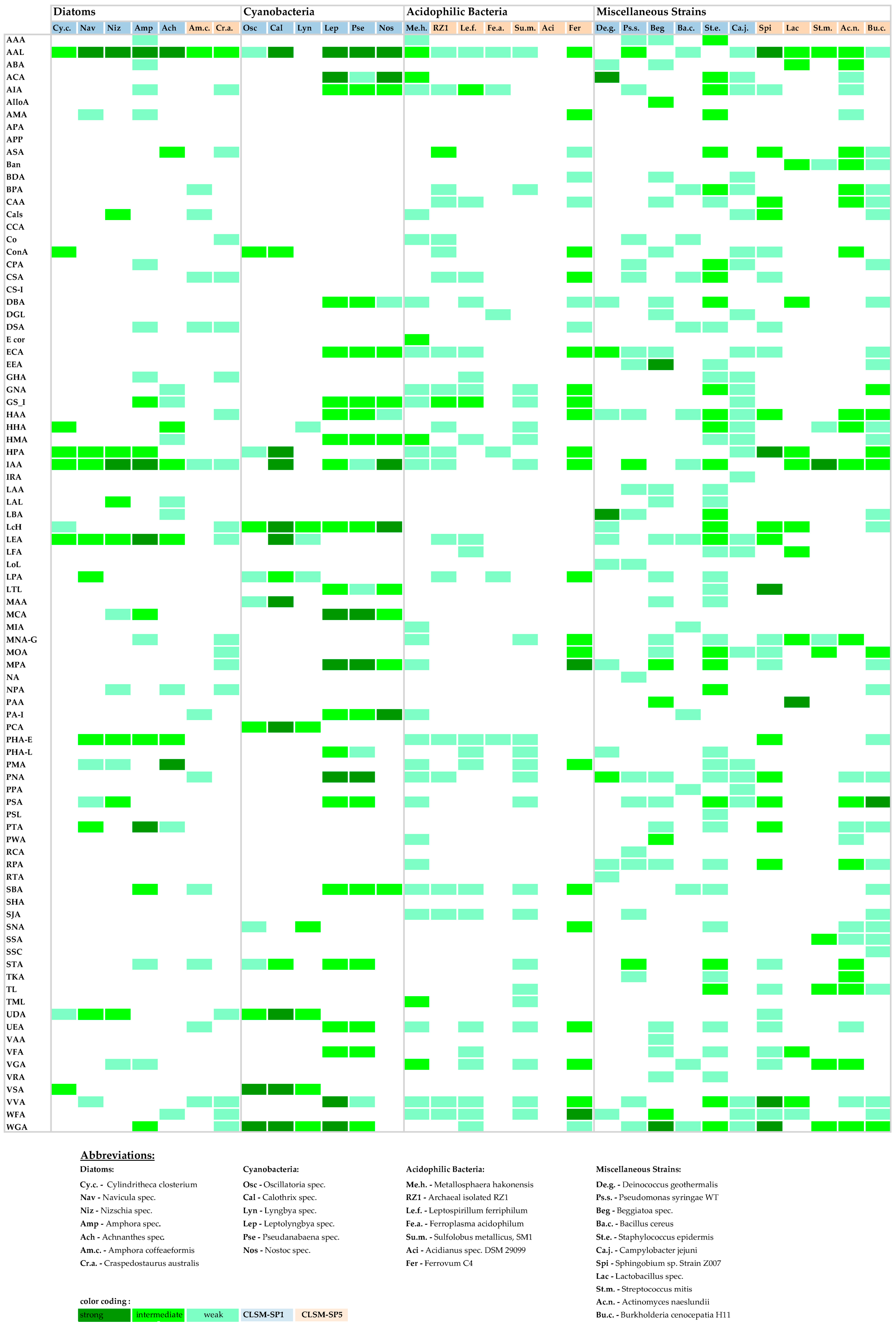
3.2. Binary Bar-Coding
3.3. Heat Map Bar-Coding
3.4. Lectin-Binding Information
4. Discussion
5. Conclusions and Perspective
- Fluorescence lectin bar-coding (FLBC) represents a powerful approach for selecting a panel of lectins useful for subsequent fluorescence lectin-binding analysis (FLBA).
- The time needed for lectin bar-coding is dependent on the difficulty in mounting the samples. It will also depend on the number of lectins bound, which is equal to the number of datasets to be recorded.
- Usually lectin bar-coding on one sample type with the current range of commercially available lectins (≈70) can be easily performed within two days.
- Subsequent fluorescence lectin-binding analysis (FLBA) with a selected panel of positive lectins is often combined with nucleic acid staining. This allows visualization of bacterial cell and glycoconjugate distribution as well as lectin-specific glycoconjugate typing see [16].
- The advantage of lectin bar-coding as compared to microarray techniques lies in the immediate visual information and identification of the lectin binding pattern.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DSM | Deutsche Sammlung für Mikroorganismen |
| DSMZ | Deutsche Sammlung für Mikroorganismen und Zellkulturen |
| ASW | artificial seawater |
| TSB | tryptic soy broth |
| THB | Todd Hewitt broth |
| MAC | Mackintosh basal salt solution |
| NYG | nutrient broth supplemented with yeast extract and glucose |
| YEB | complex medium with yeast extract, beef extract, peptone and sucrose |
| Fuc | Fucose |
| Gal | Galactose |
| GalNAc | N-Acetyl galactosamine |
| Glc | Glucose |
| GlcNAc | N-Acetyl glucosamine |
| Man | Manose |
References
- Neu, T.R.; Lawrence, J.R. Laser microscopy for the study of biofilms: Issues and options. In Aquatic Biofilms: Ecology, Water Quality and Wastewater Treatment; Romaní, A.M., Guasch, H., Balaguer, D., Eds.; Caister Academic Press: Norfolk, UK, 2016; pp. 29–45. [Google Scholar]
- Neu, T.R.; Lawrence, J.R. Advanced techniques for in situ analysis of the biofilm matrix (structure, composition, dynamics) by means of laser scanning microscopy. In Microbial Biofilms—Methods and Protocols; Donelli, G., Ed.; Springer: New York, NY, USA, 2014; Volume 1147, pp. 43–64. [Google Scholar]
- Gallaher, T.K.; Wu, S.; Webster, P.; Aguilera, R. Identification of biofilm proteins in non-typeable haemophilus influenzae. BMC Microbiol. 2006, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.D.; Atwood, J., III; Orlando, R.; Shimkets, L.J. Proteins associated with the myxococcus xanthus extracellular matrix. J. Bacteriol. 2007, 189, 7634–7642. [Google Scholar] [CrossRef] [PubMed]
- Dumas, E.; Meunier, B.; Berdague, J.-L.; Chambon, C.; Desvaux, M.; Hebraud, M. Comparative analysis of extracellular and intracellular proteomes of listeria monocytogenes strains reveals a correlation between protein expression and serovar. Appl. Environ. Microbiol. 2008, 74, 7399–7409. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Shi, L.; Brown, R.N.; Xiong, Y.; Fredrickson, J.K.; Romine, M.F.; Marshall, M.J.; Lipton, M.S.; Beyenal, H. Extracellular polymeric substances from shewanella sp. Hrcr-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environ. Microbiol. 2011, 13, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Paes Leme, A.F.; Bellato, C.M.; Bedi, G.; Del Bel Cury, A.A.; Koo, H.; Cury, J.A. Effects of sucrose on the extracellular matrix of plaque-like biofilm formed in vivo, studied by proteomic analysis. Caries Res. 2008, 42, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, M.; Stensballe, A.; Nielsen, K.L.; Nielsen, P.-H. Digging into the extracellular matrix of a complex microbial community using a combined metagenomic and metaproteomic approach. Water Sci. Technol. 2013, 67, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Manz, B.; Volke, F.; Dynes, J.J.; Hitchcock, A.P.; Lawrence, J.R. Advanced imaging techniques for assessment of structure, composition and function in biofilm systems. FEMS Microbiol. Ecol. 2010, 72, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zippel, B.; Neu, T.R. Characterization of glycoconjugates of extracellular polymeric substances in tufa-associated biofilms by using fluorescence lectin-binding analysis. Appl. Environ. Microbiol. 2011, 77, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Lawrence, J.R. Extracellular polymeric substances in microbial biofilms. In Microbial Glycobiology: Structures, Relevance and Applications; Moran, A., Brenan, P., Holst, O., von Itzstein, M., Eds.; Elsevier: San Diego, CA, USA, 2009; pp. 735–758. [Google Scholar]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Micro. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Swerhone, G.D.W.; Lawrence, J.R. Assessment of lectin-binding analysis for in situ detection of glycoconjugates in biofilm systems. Microbiology 2001, 147, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Staudt, C.; Horn, H.; Hempel, D.C.; Neu, T.R. Screening of lectins for staining lectin-specific glycoconjugates in the eps of biofilms. In Biofilms in Medicine, Industry and Environmental Technology; Lens, P., Moran, A.P., Mahony, T., Stoodley, P., O’Flaherty, V., Eds.; IWA Publishing: London, UK, 2003; pp. 308–327. [Google Scholar]
- Laue, H.; Schenk, A.; Hongqiao, L.; Lambertsen, L.; Neu, T.R.; Molin, S.; Ullrich, M.S. Contribution of alginate and levan to biofilm formation by pseudomonas syringae. Microbiology 2006, 152, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Peltola, M.; Neu, T.R.; Kanto-Oqvist, L.; Raulio, M.; Kolari, M.; Salkinoja-Salonen, M.S. Architecture of deinococcus geothermalis biofilms on glass and steel: A lectin study. Environ. Microbiol. 2008, 10, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Luef, B.; Neu, T.R.; Zweimüller, I.; Peduzzi, P. Structure and composition of aggregates in two large european rivers, based on confocal laser scanning microscopy and image and statistical analysis. Appl. Environ. Microbiol. 2009, 75, 5952–5962. [Google Scholar] [CrossRef] [PubMed]
- Bennke, C.M.; Neu, T.R.; Fuchs, B.M.; Amann, R. Mapping glycoconjugate-mediated interactions of marine bacteroidetes with diatoms. Syst. Appl. Microbiol. 2013, 36, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Weissbrodt, D.G.; Neu, T.R.; Kuhlicke, U.; Rappaz, Y.; Holliger, C. Assessment of bacterial and structural dynamics in aerobic granular biofilms. Front. Microbiol. 2013, 4, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Neu, T.R.; Bellenberg, S.; Kuhlicke, U.; Sand, W.; Vera, M. Use of lectins to in situ visualize glycoconjugates of extracellular polymeric substances in acidophilic archaeal biofilms. Microb. Biotechnol. 2015, 8, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Buhmann, M.T.; Poulson, N.; Klemm, J.; Kennedy, M.R.; Sherrill, C.D.; Kröger, N. A tyrosine-rich cell surface protein in the diatom amphora coffeaeformis identified through transcriptome analysis and genetic transformation. PLoS ONE 2014, 9, e110369. [Google Scholar] [CrossRef] [PubMed]
- Hinck, S.; Neu, T.R.; Lavik, G.; Mußmann, M.; de Beer, D.; Jonkers, H.M. Physiological adaptation of nitrate storing beggiatoa to diel cycling in a phototrophic hypersaline mat. Appl. Environ. Microbiol. 2007, 73, 7013–7022. [Google Scholar] [CrossRef] [PubMed]
- Turonova, H.; Neu, T.R.; Ulbrich, P.; Pazlarova, J.; Tresse, O. The biofilm matrix of campylobacter jejuni determined by fluorescence lectin-binding analysis. Biofouling 2016, 32, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.; Neu, T.R.; Blom, J.F.; Pernthaler, J. Protistan predation interferes with bacterial long-term adaptation to substrate restriction by selecting for defence morphotypes. J. Evolut. Biol. 2016, 29, 2297–2310. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Lawrence, J.R. Lectin-binding-analysis in biofilm systems. Methods Enzymol. 1999, 310, 145–152. [Google Scholar] [PubMed]
- Neu, T.R.; Lawrence, J.R. In situ characterization of extracellular polymeric substances (EPS) in biofilm systems. In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T.R., Flemming, H.-C., Eds.; Springer: Heidelberg, Germany, 1999; pp. 21–47. [Google Scholar]
- Tao, S.-C.; Li, Y.; Zhou, J.; Qian, J.; Schnaar, R.L.; Zhang, Y.; Goldstein, I.J.; Zhu, H.; Schneck, J.P. Lectin microarrays identify cell-specific and functionally significant cell surface glycan markers. Glycobiology 2008, 18, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-L.; Gildersleeve, J.C.; Mahal, L.K. A simple strategy for the creation of a recombinant lectin microarray. Molecul. BioSyst. 2008, 4, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Swerhone, G.D.W.; Kuhlicke, U.; Neu, T.R. In situ evidence for microdomains in the polymer matrix of bacterial microcolonies. Can. J. Microbiol. 2007, 53, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Swerhone, G.D.W.; Kuhlicke, U.; Neu, T.R. In situ evidence for metabolic and chemical microdomains in the structured polymer matrix of bacterial microcolonies. FEMS Microbiol. Ecol. 2016, 92. Available online: femsec.oxfordjournals.org/content/92/11/fiw183.abstract (accessed on 24 August 2016). [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Swerhone, G.D.W.; Böckelmann, U.; Lawrence, J.R. Effect of CNP on composition and structure of lotic biofilms as detected with lectin-specific glycoconjugates. Aquat. Microb. Ecol. 2005, 38, 283–294. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Chenier, M.R.; Roy, R.; Beaumier, D.; Swerhone, G.D.W.; Neu, T.R.; Greer, C.W. Microscale and molecular assessment of the impacts of nickel, nutrients, and oxygen level on the structure and function of river biofilm communities. Appl. Environ. Microbiol. 2004, 70, 4326–4339. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Swerhone, G.D.W.; Wassenaar, L.I.; Neu, T.R. Effects of selected pharmaceuticals on riverine biofilm communities. Can. J. Microbiol. 2005, 51, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Zhu, B.; Swerhone, G.D.W.; Topp, E.; Roy, J.; Wassenaar, L.I.; Rema, T.; Korber, D.R. Community-level assessment of the effects of the broad-spectrum antimicrobial chlorhexidine on the outcome of river microbial biofilm development. Appl. Environ. Microbiol. 2008, 74, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Ardá, A.; Blasco, P.; Varón Silva, D.; Schubert, V.; André, S.; Bruix, M.; Cañada, F.J.; Gabius, H.-J.; Unverzagt, C.; Jiménez-Barbero, J. Molecular recognition of complex-type biantennary n-glycans by protein receptors: A three-dimensional view on epitope selection by nmr. J. Am. Chem. Soc. 2013, 135, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.L.; Gildersleeve, J.C.; Mahal, L.K. A simple strategy for the creation of a recombinant lectin microarray. Mol. BioSyst. 2008, 4, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, L.; Mahal, L.K. Glycomic analysis: An array of technologies. ACS Chem. Biol. 2009, 4, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Jingqing, G.; Lina, M.; Dianjun, L.; Zhenxin, W. Microarray-based technology for glycomics analysis. Comb. Chem. High Throughput Screen. 2012, 15, 90–99. [Google Scholar]
- Li, H.; Wei, L.; Fang, P.; Yang, P. Recent advances in the fabrication and detection of lectin microarrays and their application in glycobiology analysis. Analyt. Methods 2014, 6, 2003–2014. [Google Scholar] [CrossRef]
- Wang, L.; Cummings, R.D.; Smith, D.F.; Huflejt, M.; Campbell, C.T.; Gildersleeve, J.C.; Gerlach, J.Q.; Kilcoyne, M.; Joshi, L.; Serna, S.; et al. Cross-platform comparison of glycan microarray formats. Glycobiology 2014, 24, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.L.; Pilobello, K.T.; Mahal, L.K. Analyzing the dynamic bacterial glycome with a lectin microarray approach. Nat. Chem. Biol. 2006, 2, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-L.; Mahal, L.K. A lectin microarray approach for the rapid analysis of bacterial glycans. Nat. Protoc. 2006, 1, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.C.; Blixt, O.; Collins, B.E. Sweet spots in functional glycomics. Nat. Chem. Biol. 2006, 2, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Arthur, C.M.; McBride, R.; Berger, O.; Razi, N.; Heimburg-Molinaro, J.; Rodrigues, L.C.; Gourdine, J.-P.; Noll, A.J.; von Gunten, S.; et al. Microbial glycan microarrays define key features of host-microbial interactions. Nat. Chem. Biol. 2014, 10, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Shoseyov, O.; Shani, Z.; Levy, I. Carbohydrate binding modules: Biochemical properties and novel applications. Microbiol. Mol. Biol. Rev. 2006, 70, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Ojima, Y.; Sakka, M.; Sakka, K.; Taya, M. Probing of exopolysaccharides with green fluorescence protein-labeled carbohydrate-binding module in escherichia coli biofilms and flocs induced by bcsb overexpression. J. Biosci. Bioeng. 2014, 118, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Suparman, A.; Nguyen, M.H.; Sakka, M.; Sakka, K.; Taya, M. Exopolysaccharide assay in escherichia coli microcolonies using a cleavable fusion protein of gfp-labeled carbohydrate-binding module. J. Microbiol. Methods 2015, 114, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Angelov, A.; Loderer, C.; Pompei, S.; Liebl, W. Novel family of carbohydrate-binding modules revealed by the genome sequence of spirochaeta thermophila DSM 6192. Appl. Environ. Microbiol. 2011, 77, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Cuskin, F.; Flint, J.E.; Gloster, T.M.; Morland, C.; Baslé, A.; Henrissat, B.; Coutinho, P.M.; Strazzulli, A.; Solovyova, A.S.; Davies, G.J.; et al. How nature can exploit nonspecific catalytic and carbohydrate binding modules to create enzymatic specificity. Proc. Natl. Acad. Sci. USA 2012, 109, 20889–20894. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.H.; Gilbert, P.; High, N.J.; Kolenbrander, P.E.; Handley, P.S. Bacterial coaggregation: An integral process in the development of multi-species biofilms. Trends Microbiol. 2003, 11, 94–100. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell−cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Min, K.R.; Rickard, A.H. Coaggregation by the freshwater bacterium sphingomonas natatoria alters dual-species biofilm formation. Appl. Environ. Microbiol. 2009, 75, 3987–3997. [Google Scholar] [CrossRef] [PubMed]
- Kline, K.A.; Fälker, S.; Dahlberg, S.; Normark, S.; Henriques-Normark, B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 2009, 5, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Ielasi, F.S.; Alioscha-Perez, M.; Donohue, D.; Claes, S.; Sahli, H.; Schols, D.; Willaert, R.G. Lectin-glycan interaction network-based identification of host receptors of microbial pathogenic adhesins. mBio 2016, 7, e00584-16. [Google Scholar] [CrossRef] [PubMed]
- Böckelmann, U.; Manz, W.; Neu, T.R.; Szewzyk, U. A new combined technique of fluorescent in situ hybridization and lectin-binding-analysis (fish-lba) for the investigation of lotic microbial aggregates. J. Microbiol. Methods 2002, 49, 75–87. [Google Scholar] [CrossRef]
| Strain | Medium/Growth Conditions/Reference |
|---|---|
| Various diatoms | ASW plus inorganic nutrients, liquid culture |
| Amphora caffeaeformis | [21] |
| Craspedostauros australis | [21] |
| Various cyanobacteria | BG 11, liquid culture |
| Metallosphaera hakonensis | DSMZ 88 medium, 65 °C, pH 2.5, grown on 10 µm chalcopyrite grains in Erlenmeyer flasks |
| Leptospirillum ferriphilum | MAC, 0.02 % yeast extract, pH 1.8, pyrite grains in Erlenmeyer flasks |
| Acidianus sp. DSM 29099 | [20] |
| Ferroplasma acidiphilum DSM 29986 | [20] |
| Sulfolobus metallicus DSM 6482 | [20] |
| Ferrovum C4 | iFeo medium, pH 2.5, liquid culture |
| Deinococcus geothermalis | [16] |
| Pseudomonas syringae | [15] |
| Beggiatoa | [22] |
| Bacillus cereus 10987 | TSB + 0.5% yeast extract, IBIDI 96 well microplates |
| Streptococcus epidermidis 1457 | TSB, IBIDI 96 well microplates |
| Campylobacter jejuni | [23] |
| Sphingobium sp. Z007 | [24] |
| Lactobacillus sp. | THB, liquid culture, 35 °C |
| Streptococcus mitis | THB, liquid culture, 35 °C |
| Acinetobacter naesludii | THB, liquid culture, 35 °C |
| Burkholderia cenocepacia H111 | NYG, YEB, membrane filters on agar plates |
| Lectin | Specificity |
|---|---|
| AAL | α-Fuc |
| IAA | n. d. |
| WGA | β-GlcNAc |
| VVA | α-Man, α-GalNAc |
| LEA | β-GlcNAc |
| PNA | β-Gal |
| AIA | n.d. |
| ECA | α-Gal, β-Gal, α-GalNAc, β-GalNAc |
| HAA | α-GlcNAc, α-GalNAc |
| HPA | α-GalNAc |
| PSA | α-Man, α-Glc, α-GlcNAc |
| SBA | α-GalNAc, β-GalNAc |
| WFA | α-GalNAc, β-GalNAc |
| DBA | α-GalNAc |
| LcH | α-Man, α-Glc, α-GlcNAc |
| MNA | n.d. |
| PHA-E | n.d. |
| STA | β-GlcNAc |
| UEA | β-GlcNAc, α-Fuc |
| GS-I | α-Gal, α-GalNAc |
| HHA | α-Man |
| MPA | n.d. |
| PMA | n.d. |
| VGA | n.d. |
| ConA | α-Man, α-Glc, α-GlcNAc |
| GNA | α-Man |
| HMA | α-Fuc, α-GalNAc |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neu, T.R.; Kuhlicke, U. Fluorescence Lectin Bar-Coding of Glycoconjugates in the Extracellular Matrix of Biofilm and Bioaggregate Forming Microorganisms. Microorganisms 2017, 5, 5. https://doi.org/10.3390/microorganisms5010005
Neu TR, Kuhlicke U. Fluorescence Lectin Bar-Coding of Glycoconjugates in the Extracellular Matrix of Biofilm and Bioaggregate Forming Microorganisms. Microorganisms. 2017; 5(1):5. https://doi.org/10.3390/microorganisms5010005
Chicago/Turabian StyleNeu, Thomas R., and Ute Kuhlicke. 2017. "Fluorescence Lectin Bar-Coding of Glycoconjugates in the Extracellular Matrix of Biofilm and Bioaggregate Forming Microorganisms" Microorganisms 5, no. 1: 5. https://doi.org/10.3390/microorganisms5010005
APA StyleNeu, T. R., & Kuhlicke, U. (2017). Fluorescence Lectin Bar-Coding of Glycoconjugates in the Extracellular Matrix of Biofilm and Bioaggregate Forming Microorganisms. Microorganisms, 5(1), 5. https://doi.org/10.3390/microorganisms5010005