EPS—Then and Now
Abstract
:1. Too Good to Be True?
2. Slime
3. The Term EPS
4. The Structure of EPS
5. Functions of EPS
6. Extracellular DNA
7. Physical Properties of EPS
8. Self-Organization of the Matrix
9. Matrix Dispersal—An Organized Process
10. Good Enough to Be True
- -
- What is the distance over which the microbial cell is able to control its external microhabitat?
- -
- What is the nature of the matrix in relation to the genotype, phenotype and environmental cues? How is the dynamic of matrix expression controlled?
- -
- What influences EPS production and how can it be managed?
- -
- Which are the interactions of the various EPS components?
- -
- Which components contribute to matrix stability and how can we influence them?
- -
- Where and by which mechanisms are hydrophobic substances sorbed and retained in the matrix?
- -
- What are the mechanisms of water retention?
- -
- What is the role of EPS in light transmission to deeper layers of the biofilm?
- -
- What is the fate of released polymers in terms of lifetime, turnover and recycling?
Acknowledgments
Conflicts of Interest
References
- Haeckel, E. Beiträge zur Plastidentheorie; Gustav Fischer: Jena, Germany, 1870. [Google Scholar]
- Rehbock, P.F. Huxley, Haeckel and the oceanographers: The case of Bathybius haeckelii. Isis 1975, 66, 504–533. [Google Scholar] [CrossRef]
- Rice, A.L. Thomas Henry Huxley and the strange case of Bathybius haeckelii; a possible alternative explanation. Arch. Nat. Hist. 1983, 2, 169–180. [Google Scholar] [CrossRef]
- Beckwith, T.D. The bacteriology of pulp slime. J. Bacteriol. 1931, 22, 15–22. [Google Scholar] [PubMed]
- Flemming, H.-C.; Meier, M.; Schild, T. Mini-Review: Biofouling in paper production. Biofouling 2013, 29, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Cholodny, N. Über eine neue Methode zur Untersuchung der Bodenflora. Arch. Mikrobiol. 1930, 1, 620–652. [Google Scholar]
- ZoBell, C.E. The effect of solid surfaces upon bacterial activity. J. Bacteriol. 1943, 46, 39–56. (In German) [Google Scholar] [PubMed]
- Neu, T.R.; Marshall, K.C. Microbial “footprints”—A new approach to adhesive polymers. Biofouling 1991, 3, 101–112. [Google Scholar] [CrossRef]
- ZoBell, C.E.; Allen, E.C. Attachment of bacteria to submerged slides. Proc. Soc. Exp. Biol. Med. 1933, 30, 1409–1411. [Google Scholar] [CrossRef]
- ZoBell, C.E.; Allen, E.C. The significance of marine bacteria in the fouling of submerged surfaces. J. Bacteriol. 1935, 29, 239–251. [Google Scholar] [PubMed]
- Heukelekian, H.; Heller, A. Relation between food concentration and surface for bacterial growth. J. Bacteriol. 1940, 40, 547–558. [Google Scholar] [PubMed]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How bacteria stick. Sci. Am. 1978, 238, 85–95. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Repert, D.; Weiland, T.; Golladay, S.W.; Linkins, A.E. Exoenzyme accumulation in epilithic biofilms. Hydrobiologica 1991, 222, 29–37. [Google Scholar] [CrossRef]
- Flemming, H.-C. Biodeterioration of synthetic polymers—A brief review. Mat. Corross. 2010, 61, 986–992. [Google Scholar] [CrossRef]
- Decho, A.W. Microbial exopolymer secretions in ocean environments: Their role(s) in food webs and marine processes. Oceanogr. Mar. Biol. Annu. Rev. 1990, 28, 73–153. [Google Scholar]
- Decho, A.W. Microbial biofilms in intertidal systems: An overview. Cont. Shelf Res. 2000, 20, 1257–1273. [Google Scholar] [CrossRef]
- Geesey, G.G.; Mutch, R.; Costerton, J.W.; Green, R.B. Sessile bacteria: An important component of the microbial population in small mountain streams. Limnol. Oceanogr. 1978, 23, 1214–1223. [Google Scholar] [CrossRef]
- Geesey, G.G. Microbial exopolymers: Ecological and economic considerations. ASM News 1982, 48, 9–14. [Google Scholar]
- Flemming, H.-C.; Wingender, J.; Kjelleberg, S.; Steinberg, P.; Rice, S.; Szewzyk, U. Biofilms: An emergent form of microbial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Tielen, P.; Rosenau, F.; Wilhelm, S.; Jaeger, K.-C.; Flemming, H.-C.; Wingender, J. Extracellular enzymes affect biofilm formation in mucoid Pseudomonas aeruginosa. BMC Microbiol. 2013, 159, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 2007, 5, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Characklis, W.G.; Wilderer, P.A. Structure and Function of Biofilms; Wiley-Blackwell: Chichester, UK, 1989. [Google Scholar]
- Wingender, J.; Neu, T.; Flemming, H.-C. Microbial Extracellular Polymer Substances; Springer: Heidelberg, Germany, 1999. [Google Scholar]
- Nielsen, P.H.; Jahn, A. Extraction of EPS from biofilms. In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T.R., Flemming, H.C., Eds.; Springer: Heidelberg, Germany, 1999; pp. 49–72. [Google Scholar]
- Allison, D.G.; Sutherland, I.W.; Neu, T.R. EPS: What’s in an acronym? In Biofilm Communities: Order from Chaos; McBain, A., Ed.; BioLine: Cardiff, Wales, 2003; pp. 381–387. [Google Scholar]
- Sutherland, I.W. Bacterial exopolysaccharides. Adv. Microb. Physiol. 1972, 8, 143–213. [Google Scholar] [PubMed]
- Sutherland, I.W. EPS—A complex mixture. In The Perfect Slime—Microbial Extracellular Polymeric Substances; Flemming, H.-C., Wingender, J., Neu, T.R., Eds.; IWA Publishing: London, UK, 2016; pp. 15–24. [Google Scholar]
- Tsien, H.C.; Schmidt, E.L. Polarity in the exponential-phase Rhizobacterium japonicum cell. Can. J. Microbiol. 1977, 23, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Wingender, J.; Neu, T.; Flemming, H.-C. What are bacterial extracellular polymer substances? In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T., Flemming, H.-C., Eds.; Springer: Heidelberg/Berlin, Germany, 1999. [Google Scholar]
- Lawrene, J.R.; Swerhone, G.D.W.; Kuhlicke, U.; Neu, T.R. In situ evidence for microcomains in the polymer matrix of bacterial microcolonies. Can. J. Microbiol. 2007, 53, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Swerhone, G.D.W.; Kuhlicke, U.; Neu, T.R. In situ evidence for metabolic and chemical microdomains in the structured polymer matrix of bacterial microcolonies. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Swerhorne, G.D.W.; Lawrence, J.R. Assessment of lectin-binding analysis for in situ detection of glycoconjugates in biofilm systems. Microbiology 2001, 142, 288–313. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Neu, T.R.; Wingender, J. The Perfect Slime: Microbial Extracellular Polymeric Substances (EPS); IWA publishing: London, UK, 2016. [Google Scholar]
- Kamjunke, N.; Herzsprung, P.; Neu, T.R. Quality of dissolved organic matter affects planktonic but not biofilm bacterial production in streams. Sci. Total Environ. 2015, 506–507, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Richter, A.M.; Hengge, R. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J. Bacteriol. 2013, 195, 5540–5554. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; van Heuvel, J.C. Gradients in biological systems. Anal. Chim. Acta 1988, 213, 259–265. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Jørgensen, B.B. Microelectrodes: Their use in microbial ecology. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer: New York, NY, USA, 1986; pp. 293–352. [Google Scholar]
- De Beer, D.; Stoodley, P.; Roe, F.; Lewandowski, Z. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 1994, 43, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Watnick, P.; Kolter, R. Biofilm, city of microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Neu, T.R.; Wozniak, D. The EPS matrix: The house of biofilm cells. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed]
- Corning, P.A. The re-emergence of “emergence”: A venerable concept in search of a theory. Complexity 2002, 7, 18–30. [Google Scholar] [CrossRef]
- Konopka, A. What is microbial community ecology? ISME J. 2009, 3, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPS)—Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [PubMed]
- Neu, T.R.; Lawrence, J.R. Extracellular polymeric substances in microbial biofilms. In Microbial Glycobiology: Structures, Relevance and Applications; Moran, A., Brenan, P., Holst, O., von Itzstein, M., Eds.; Elsevier: San Diego, CA, USA, 2009; pp. 735–758. [Google Scholar]
- Hobley, L.; Ostrowski, A.; Rao, F.V.; Bromley, K.M.; Porter, M.; Prescott, A.R.; MacPhee, C.E.; van Aalten, D.M.; Stanley-Wall, N.R. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. Proc. Natl. Acad. Sci. USA 2013, 110, 13600–13605. [Google Scholar] [CrossRef] [PubMed]
- Schooling, S.R.; Beveridge, T.J. Membrane vesicles: An overlooked component of the matrices of biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C. The perfect slime. Coll. Surf. B Biointerfaces 2011, 86, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Houry, A.; Gohar, M.; Deschamps, J.; Tischenko, E.; Aymerich, S.; Gruss, A.; Briandet, R. Bacterial swimmers that infiltrate and take over the biofilm matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 13088–13093. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, S.E.; Cooper, M.; Chhabra, S.R.; Glover, L.A.; Stewart, G.; Williams, P.; Prosser, J.I. Cell density-regulated recovery of starved biofilm populations of ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 1997, 63, 2281–2286. [Google Scholar] [PubMed]
- Morgan-Sastume, F.; Larsen, P.; Nielsen, J.L.; Nielsen, P.H. Characterization of the loosely attached fraction of activated sludge bacteria. Water Res. 2008, 42, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.; Kleerebezem, R.; Strous, M.; Chandran, K.; van Loosdrecht, M.C. Factors influencing the density of granular sludge. Appl. Microbiol. Biotechnol. 2013, 97, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Balzer, M.; Witt, N.; Flemming, H.-C.; Wingender, J. Accumulation of fecal indicator bacteria in river biofilms. Water Sci. Technol. 2010, 61, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Griebler, C.; Mindl, B.; Slezak, D. Combining DAPI and SYBR Green II for the enumeration of total bacterial numbers in aquatic sediments. Int. Rev. Hydrobiol. 2001, 86, 453–465. [Google Scholar] [CrossRef]
- Herndl, G.J.; Peduzzi, P. The ecology of amorphous aggregations (Marine Snow) in the Northern Adriatic Sea. Mar. Ecol. 1988, 9, 79–90. [Google Scholar] [CrossRef]
- Catlin, B.W. Extracellular desoxyribonucleic acid of bacteria and a desoxyribonuclease inhibitor. Science 1956, 124, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Whitchurch, C.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Vilain, S.; Pretorius, J.M.; Theron, J.; Broezel, V.S. DNA as an adhesin: Bacillus cereus requires extracellular DNA to form biofilms. Appl. Environ. Microbiol. 2009, 75, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Schramm, A.; Neu, T.R.; Revsbech, N.P.; Meyer, R.L. Extracellular DNA in adhesion and biofilm formation of four environmental isolates: A quantitative study. FEMS Microbiol. Ecol. 2013, 86, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Huseby, M.J.; Kruse, A.C.; Digre, J.; Kohler, P.L.; Vocke, J.A.; Mann, E.E.; Bayles, K.W.; Bohach, G.A.; Schlievert, P.M.; Ohlendorf, D.H.; et al. Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms. Proc. Natl. Acad. Sci. USA 2010, 107, 14407–14412. [Google Scholar] [CrossRef] [PubMed]
- D’Alvise, P.; Siøholm, O.R.; Yankelevich, T.; Jin, Y.; Wuertz, S.; Smets, B.F. TOL plasmid carriage enhances biofilm formation and increases extracellular DNA content in Pseudomonas putida KT2440. FEMS Microbiol. Lett. 2010, 312, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Sharma, P.K.; Krom, B.P.; van der Mei, H.C.; Busscher, H.J. Role of eDNA on the adhesion forces between Streptococcus mutans and substratum surfaces: Influence of ionic strength and substratum hydrophobicity. Langmuir 2011, 27, 10113–10118. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Liu, H.; Zhang, L.; Guo, Y.; Yu, S.; Wozniak, D.J.; Ma, L.Z. The exopolysaccharide Psl-eDNA interaction enables the formation of a biofilm skeleton in Pseudomonas aeruginosa. Environ. Microbiol. Rep. 2015, 7, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.C.; Nilsson, M.; Jensen, P.Ø.; Høiby, N.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Extracellular DNA Shields against Aminoglycosides in Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2013, 57, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wloka, M.; Rehage, H.; Flemming, H.-C.; Wingender, J. Rheological properties of viscoelastic biofilm EPS and comparison to the behavior of calcium alginate gels. Colloid Polym. Sci. 2004, 282, 1067–1076. [Google Scholar] [CrossRef]
- Dominiak, D.M.; Nielsen, J.L.; Nielsen, P.H. Extracellular DNA is abundant and important for microcolony strength in mixed microbial biofilms. Environ. Microbiol. 2011, 13, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.O.; Hengge, R. Stress responses go three dimensional—The spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ. Microbiol. 2014, 16, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Jahn, C.E.; Selimi, D.A.; Barak, J.H.D.; Charkowski, A.O. The Dickeya dadantii biofilm matrix consists of cellulose nanofibres, and is an emergent property dependent upon the type II secretion system and the cellulose synthesis operon. Microbiology 2011, 157, 2733–2744. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.S.; Nielsen, P.H. Amyloids—A neglected child of the slime. In The Perfect Slime—Microbial Extracellular Substances; Flemming, H.-C., Wingender, J., Neu, T.R., Eds.; IWA Publishing: London, UK, 2016; pp. 113–134. [Google Scholar]
- Chew, S.; Kundukad, B.; Seviour, T.; van der Maarel, J.R.; Yang, L.; Rice, S.A.; Doyle, P.; Kjelleberg, S. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. mBio 2014, 5, e01536-14. [Google Scholar] [CrossRef] [PubMed]
- Körstgens, V.; Wingender, J.; Flemming, H.-C.; Borchard, W. Influence of calcium-ion concentration on the mechanical properties of a model biofilm of Pseudomonas aeruginosa. Water Sci. Technol. 2001, 43, 49–57. [Google Scholar] [PubMed]
- Mayer, C.; Moritz, R.; Kirschner, C.; Borchard, W.; Maibaum, R.; Wingender, J.; Flemming, H.-C. The role of intermolecular interactions: Studies on model systems for bacterial biofilms. Int. J. Biol. Macromol. 1999, 26, 3–16. [Google Scholar] [CrossRef]
- Laspidou, C.S.; Rittmann, B.E. Modeling the development of biofilm density including active bacteria, inert biomass, and extracellular polymeric substances. Water Res. 2004, 38, 3349–3361. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.W.; He, Y.; Ren, Y.; Zerdoum, A.; Libera, M.R.; Sharma, P.K.; van Winkelhoff, A.J.; Neut, D.; Stoodley, P.; van der Mei, H.C.; et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 2015, 39, 234–245. [Google Scholar] [CrossRef] [PubMed]
- De Dier, R.; Fauvart, M.; Michiels, J.; Veermant, J. The role of biosurfactants in bacterial systems. In The Physical Basis of Quorum Communication; Hagen, S.J., Ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Rochex, A.; Godon, J.J.; Bernet, N.; Escudié, R. Role of shear stress on composition, diversity and dynamics of biofilm bacterial communities. Water Res. 2008, 42, 4915–4922. [Google Scholar] [CrossRef] [PubMed]
- Persat, A.; Nadell, C.D.; Kim, M.K.; Ingremeau, F.; Siryaporn, A.; Drescher, K.; Wingreen, N.S.; Bassler, B.L.; Gitai, Z.; Stone, H.A. The mechanical world of bacteria. Cell 2015, 161, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskov, M.; Tolker-Nielsen, T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2006, 59, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Birjiniuk, A.; Samad, T.S.; Doyle, P.S.; Ribbeck, K. Material properties of biofilms—A review of methods for understanding permeability and mechanics. Rep. Prog. Phys. 2015, 78, 036601. [Google Scholar] [CrossRef] [PubMed]
- Kunin, V.; Raes, J.; Harris, J.K.; Spear, J.R.; Walker, J.J.; Ivanova, N.; von Mering, C.; Bebout, B.M.; Pace, N.R.; Bork, P.; et al. Millimeter-scale genetic gradients and community-level molecular convergence in a hypersaline microbial mat. Mol. Syst. Biol. 2008, 4, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gestel, J.; Weissing, F.J.; Kuipers, O.P.; Tovàcs, A.T. Density of founder cells affects spatial pattern formation and cooperation in Bacillus subtilis biofilms. ISME J. 2014, 8, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Kearns, D.B. A field guide to swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Ninawe, A.S.; Lipton, A.N.; Pandian, V.; Selvin, J. Rhamnolipid biosurfactants: Evolutionary implications, applications and future prospects from untapped marine resource. Crit. Rev. Biotechnol. 2016, 36, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, O.; Ghigo, J.M. Multi-species biofilms: How to avoid unfriendly neighbours. FEMS Microbiol. Rev. 2012, 36, 972–989. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Karig, D.; Kumar, A.; Ardekani, A.M. Interplay of physical mechanisms and biofilm processes: Review of microfluidic methods. Lab Chip 2015, 15, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Wilking, J.N.; Zaburdaev, V.; De Volder, M.; Losick, R.; Brenner, M.P.; Weitz, D.A. Liquid transport facilitated by channels in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2013, 110, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. Biofilms and antimicrobial resistance. Clin. Orthop. Relat. Res. 2005, 437, 41–47. [Google Scholar] [CrossRef]
- Fabbri, S.; Stoodley, P. Mechanical properties of biofilms. In The Perfect Slime—Microbial Extracellular Polymeric Substances; Flemming, H.-C., Wingender, J., Neu, T., Eds.; IWA Publishing: London, UK, 2016; pp. 153–178. [Google Scholar]
- Decho, A.W.; Kawaguchi, T.; Allen, M.A.; Louchard, E.M.; Reid, R.P.; Stephens, F.C.; Voss, K.J.; Wheatcroft, R.A.; Taylor, B.B. Sediment properties influencing upwelling spectral reflectance signatures: The “biofilm gel effect”. Limnol. Oceanogr. 2003, 48, 431–443. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, J.; Ding, W.; Lin, J.; Tian, R.; Lu, L.; Liu, X.; Shen, X.; Qian, P.-Y. Extracellular matrix-associated proteins form an integral and dynamic system during Pseudomonas aeruginosa biofilm development. Front. Microbiol. 2015, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.-G.; O’Toole, G.A. c-di-GMP and its effects on biofilm formation and dispersion: A Pseudomonas aeruginosa review. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2011, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.K. Biofilm dispersal: Deciding when it is better to travel. Mol. Microbiol. 2014, 94, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Gjermansen, M.; Niellson, M.; Yang, L.; Tolker-Nielsen, T. Characzerization of starvation-induced dispersion in Pseudomonas putida biofilms. Mol. Microbiol. 2010, 75, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Manuel, S.G.A.; Ragunath, C.; Sait, H.B.R.; Izano, E.A.; Kaplan, J.B.; Ramasubbu, N. Role of active-site residues of dispersin B, a biofilm-releasing beta-hexosaminidase from a periodontal pathogen, in substrate hydrolysis. FEBS J. 2007, 274, 5987–5999. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Rice, K.C.; Boles, B.R.; Endres, J.L.; Ranjit, D.; Chandramohan, L.; Tsang, L.H.; Smeltzer, M.S.; Horswill, A.R.; Bayles, K.W. Modulation of edna release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE 2009, 4, e5822. [Google Scholar] [CrossRef] [PubMed]
- Trevors, J.T. Hypothesized origin of microbial life in a prebiotic gel and the transition to a living biofilm and microbial mats. Comptes Rendues Biol. 2011, 334, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Nutman, A.P.; Bennett, V.C.; Friend, C.R.L.; van Kranendonk, M.J.; Chivas, A.R. Rapid emergence of life shown by discovery of 3700-million-year-old microbial structures. Nature 2016, 537, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W.; Packer, B.M. Early Archaean (3.3 billion to 3.5 billion-year old) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C. The perfect slime—And the “dark matter” of biofilms. In The Perfect Slime: Microbial Extracellular Polymeric Substances; Flemming, H.-C., Neu, T.R., Wingender, J., Eds.; Springer: Heidelberg, Germany; New York, NY, USA, 2016; pp. 1–14. [Google Scholar]
- Neu, T.R.; Lawrence, J.R. The extracellular matrix: An intractable part of biofilm systems. In The Perfect Slime—Microbial Extracellular Substances; Flemming, H.-C., Wingender, J., Neu, T.R., Eds.; IWA Publishing: London, UK, 2016; pp. 25–60. [Google Scholar]
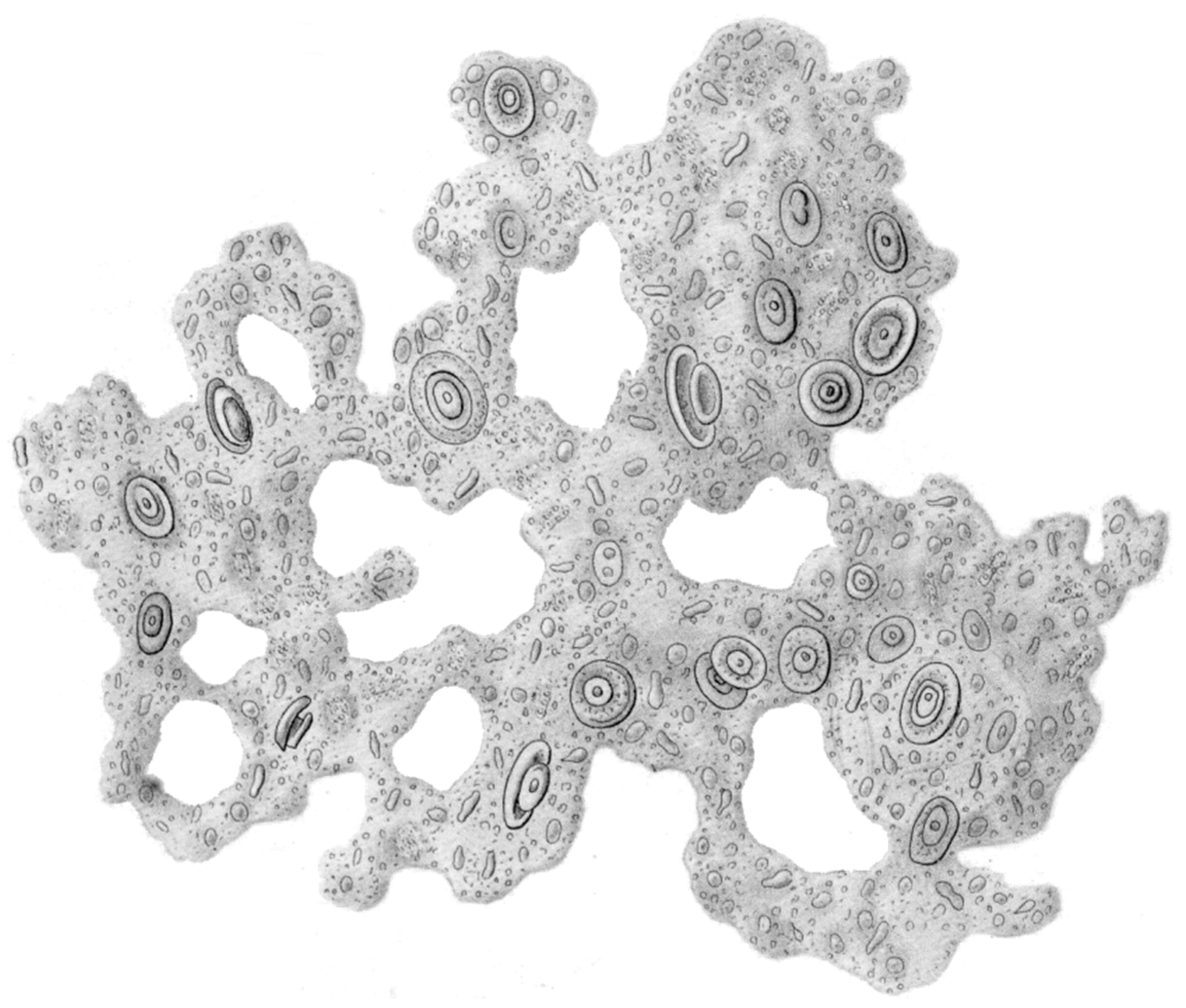
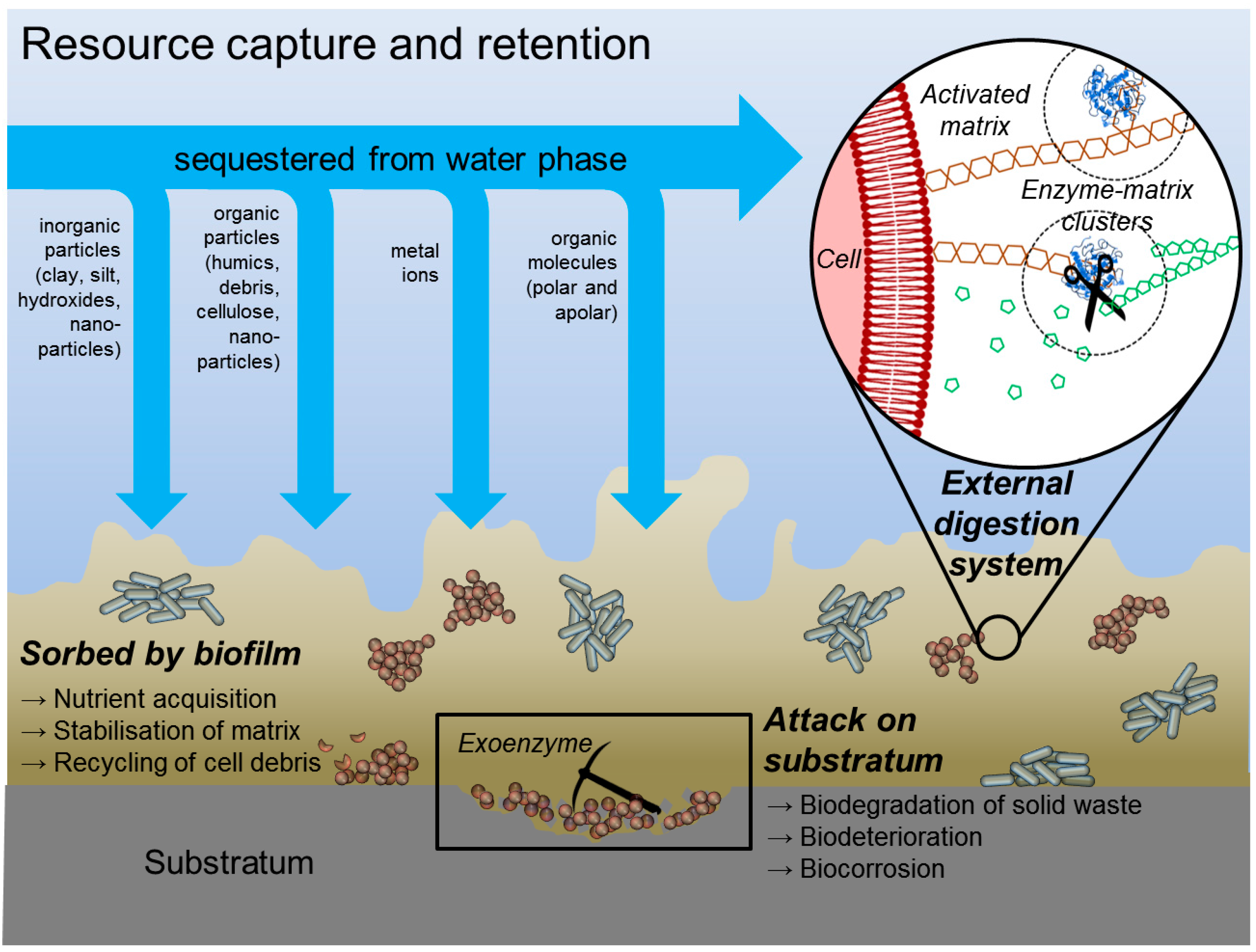
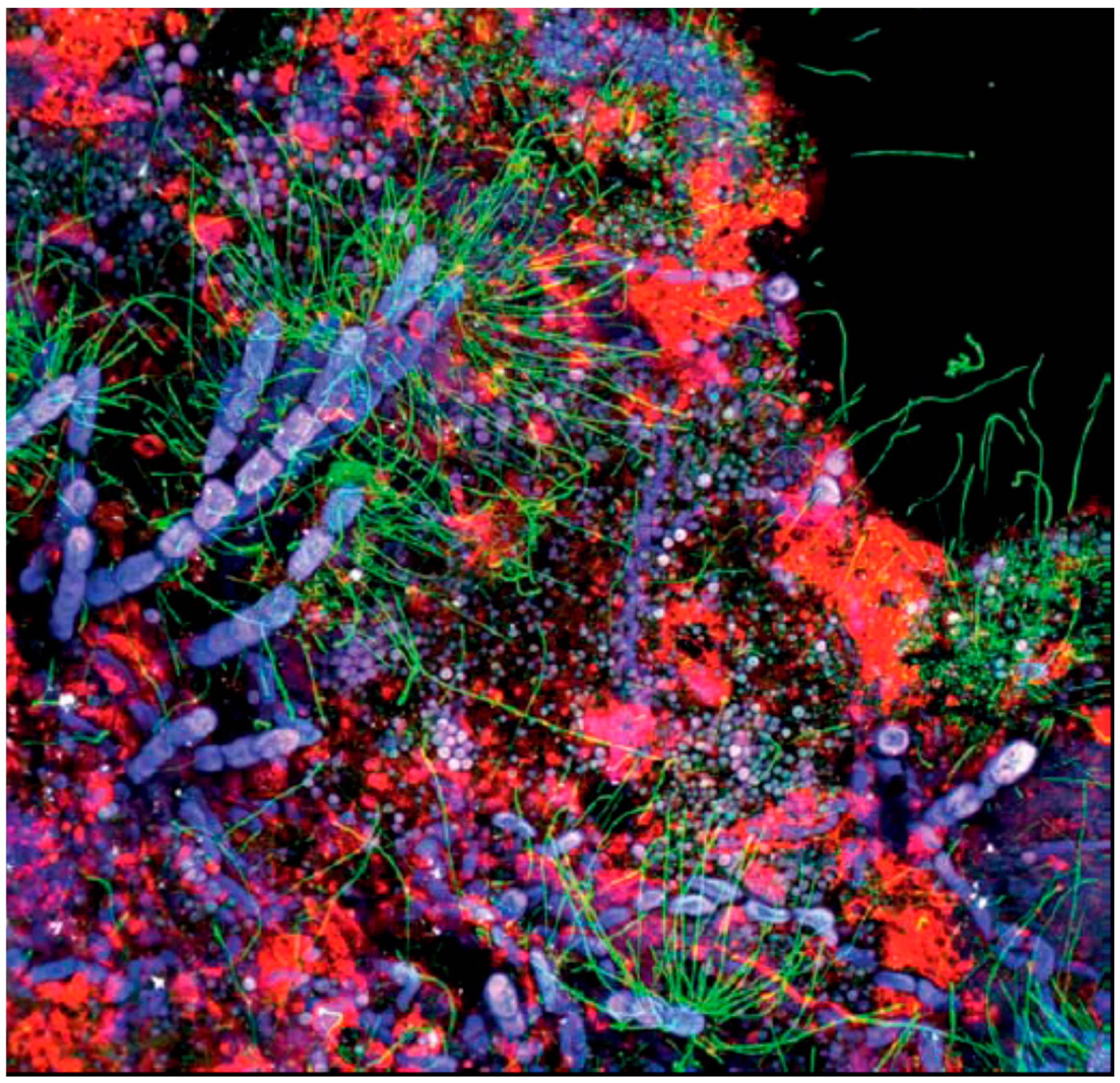
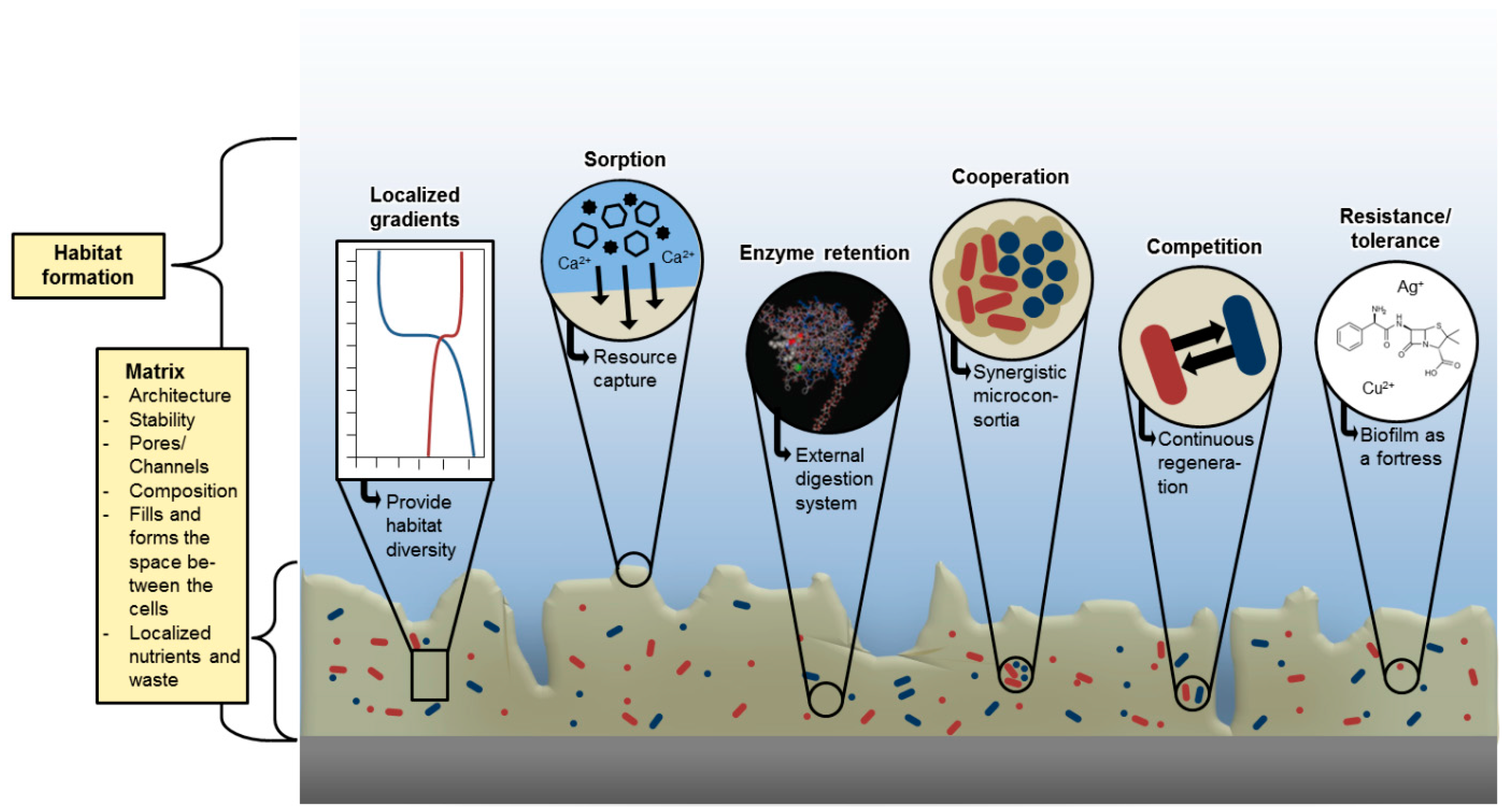

| Function of EPS Component | Relevance for Biofilm Organism | EPS Components Involved |
|---|---|---|
| Adhesion | Initial steps in colonization of abiotic and biotic surfaces by planktonic cells, long-term attachment of whole biofilms to surfaces | Polysaccharides, proteins (e.g., fimbriae), eDNA |
| Aggregation of bacterial cells | Bridging between cells, (temporary) immobilization of bacterial populations, basis for development of high cell densities, cell-cell recognition | Polysaccharides, proteins, DNA |
| Cohesion of biofilms | Structural elements forming a hydrated polymer network (biofilm matrix), mediation of mechanical stability of biofilms (frequently in conjunction with multivalent cations or hydrophobic interactions), determination of EPS structure (capsule, slime, sheath) and biofilm architecture, generation of matrix | Neutral and charged polysaccharides, proteins (e.g., amyloids, lectins), DNA |
| Retention of water | Maintenance of highly hydrated microenvironment around biofilm organisms, dessication tolerance in water-deficient environments | Hydrophilic polysaccharides and Proteins; skin-forming hydrophobic proteins (BslA [46]) |
| Protective barrier against antimicrobials | Resistance to nonspecific and specific host defenses during infection, tolerance to various antimicrobial agents (e.g., disinfectants, antibiotics), protection of cyanobacterial nitrogenase from harmful effects of oxygen; protection against some (but not all!) grazers | Polysaccharides, proteins |
| Sorption of polar organic compounds | Accumulation of nutrients from the environment, sorption of xenobiotics (detoxification) | Charged or hydrophobic polysaccharides and proteins |
| Sorption of inorganic ions | Promotion of polysaccharide gel formation, ion exchange, mineral formation, accumulation of toxic metal ions (detoxification) | Charged polysaccharides and proteins, including inorganic substituents such as phosphate and sulphate |
| Sorption of apolar organic substances | Resource capture | Proteins, not yet defined hydrophobic pockets in matrix |
| Sorption of particles | Resource capture | Sticky matrix components |
| Enhanced access to resources captured in the matrix | Providing additional enzymatic competence and capacity in the matrix; killer-vesicles as a weapon in competition (Schooling and Beveridge [47] | Membrane vesicles (contain nucleic acids, enzymes, proteins, LPS etc.) |
| Enzymatic activity | Digestion of exogenous macromolecules for nutrient acquisition, degradation of structural EPS allowing release of cells from biofilms, utilization of substratum as substrate | Proteins |
| Nutrient source | Source of C, N and P compounds for utilization by biofilm community | Potentially all EPS components |
| Genetic information | Horizontal gene transfer between biofilm cells | DNA |
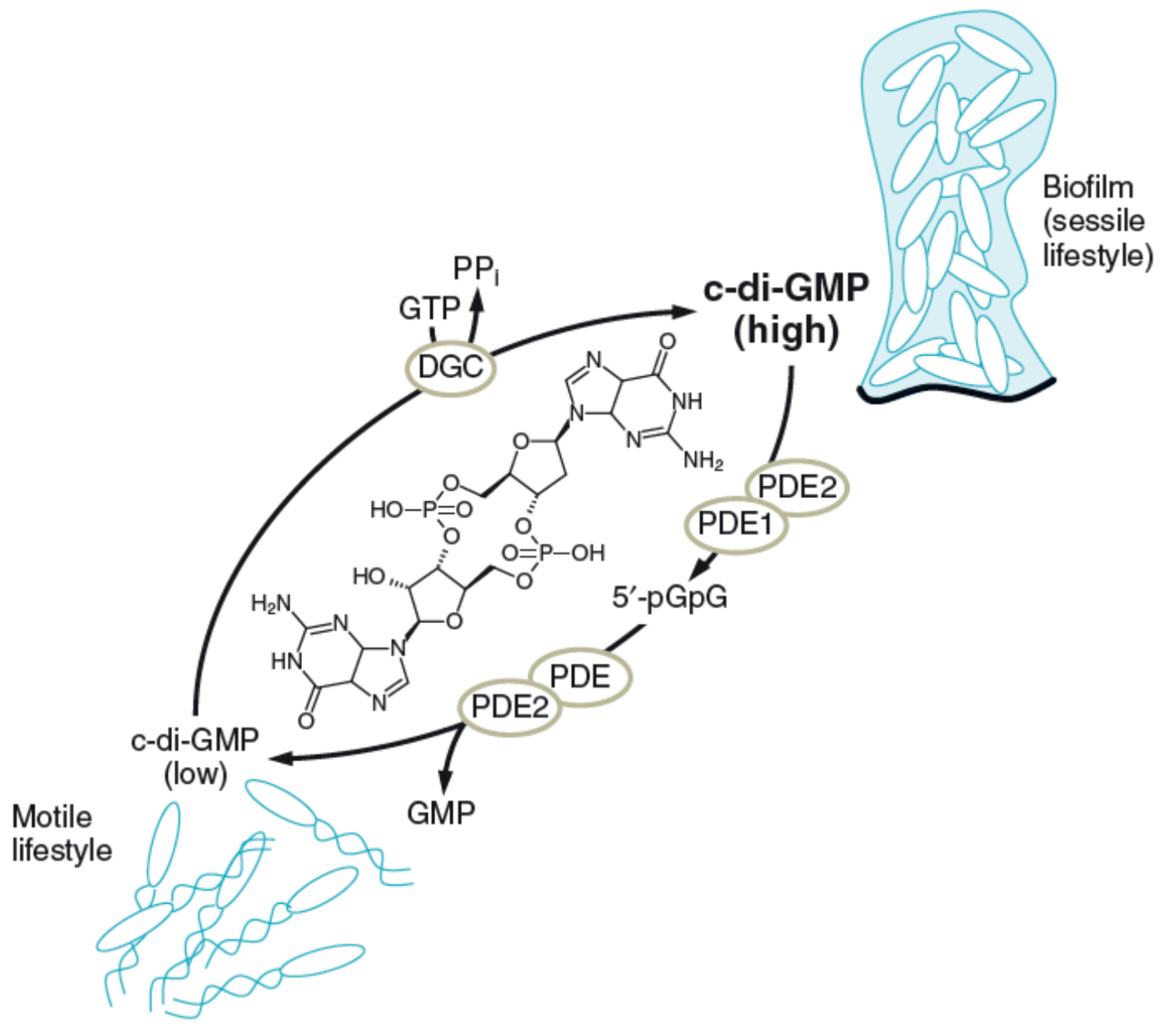
| Intercellular information | Regulation of biofilm dynamics and responses, regulating c-di-GMP concentration | Polysaccharides |
| Electron donor or acceptor | Redox activity in biofilm matrix, electron transport mediation to surfaces | Proteins (e.g., pili, nanowires), humic substances |
| Resource capture by export of enzymes into matrix | Providing additional enzymatic competence and capacity in the matrix | Outer membrane vesicles (contain nucleic acids, enzyme proteins, lipopolysaccharides, phospholipids) |
| Sink for excess energy | Sink for excess carbon under unbalanced C:N metabolic conditions | Polysaccharides |
| Binding of enzymes | Accumulation, retention and stabilization of enzymes through their interaction with polysaccharides | Polysaccharides, enzyme proteins |
| Microbial Modification | Effect on EPS |
|---|---|
| Degradation of EPS components by hydrolases, esterases, lipases, proteases and other lytic enzymes | Shortening of chain length, degradation of EPS, change of matrix structure and stability, formation of pores and channels Destabilization of matrix, dispersion, release of biofilm organisms |
| Variation of EPS composition in mixed biofilms during development | EPS of different properties, resistance to EPS-lysing enzymes |
| Post-excretional addition of substituents to polysaccharides | Influence on shape, charge, hydrophobicity of polymer, surface activity |
| Molecular structure suitable for protein-polysaccharide interaction | Retention, possible protection and activation of extracellular enzymes |
| Excretion of rhamnolipids | Increase of porosity, favouring of cell motility, influencing mass transport |
| Movement of “stealth swimmers” Houry et al. [49] | Formation of channels, improvement of convective mass transport |
| Environmental influence | Effect |
| Shear forces | Washout of well soluble EPS, accumulation of less soluble EPS, increase of stability of remaining matrix, sloughing off, erosion |
| Grazing by higher organisms (protozoa, larvae, snails etc.) | Selective removal of EPS and EPS producing organisms, formation of channels, destabilization of matrix |
© 2016 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flemming, H.-C. EPS—Then and Now. Microorganisms 2016, 4, 41. https://doi.org/10.3390/microorganisms4040041
Flemming H-C. EPS—Then and Now. Microorganisms. 2016; 4(4):41. https://doi.org/10.3390/microorganisms4040041
Chicago/Turabian StyleFlemming, Hans-Curt. 2016. "EPS—Then and Now" Microorganisms 4, no. 4: 41. https://doi.org/10.3390/microorganisms4040041
APA StyleFlemming, H.-C. (2016). EPS—Then and Now. Microorganisms, 4(4), 41. https://doi.org/10.3390/microorganisms4040041






