1. Introduction
The clinical course of systemic autoimmune diseases is characterised by periods of relative quiescence punctuated by acute flares, episodes of intensified inflammation that drive cumulative organ damage and shape long-term prognosis [
1,
2]. Although the triggers for these flares remain incompletely understood, converging evidence implicates microbial exposures as key precipitants. Infections, viral reactivations, and perturbations in commensal microbiota have long been associated with disease onset and exacerbation in conditions ranging from systemic lupus erythematosus (SLE) to rheumatoid arthritis (RA), Sjogren’s syndrome, and inflammatory myopathies [
3,
4,
5,
6].
Three distinct categories of microbial input contribute to autoimmune flares, each operating through overlapping but mechanistically distinguishable pathways. First, latent herpesvirus reactivations, particularly Epstein–Barr virus (EBV), cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), and varicella-zoster virus (VZV), periodically emerge from dormancy to engage innate sensors and create inflammatory milieus permissive for autoreactive B-cell expansion [
7,
8]. Second, acute respiratory and gastrointestinal infections, including influenza and SARS-CoV-2, trigger important plasmablast responses that can include autoreactive clones [
9,
10]. Third, chronic microbiome dysbiosis at oral and gut barrier sites generates inflammatory signals that keep B cells poised near activation thresholds, lowering the stimulus required for full-blown plasmablast storms [
11,
12]. These three inputs, overt infection, subclinical reactivation, and dysbiosis, represent mechanistically distinct but convergent drivers of the plasmablast storm cycle.
It is important to acknowledge that plasmablast storms represent one of several interconnected mechanisms driving autoimmune flares, rather than the unique pathway. Neutrophil activation with release of neutrophil extracellular traps (NETosis), complement cascade amplification, and stromal cell inflammatory loops all contribute to flare pathophysiology and may operate independently of/or in concert with B-cell responses [
13,
14]. Nevertheless, the plasmablast storm model provides a particularly tractable framework for understanding how discrete microbial events translate into humoral autoimmune exacerbations.
A unifying theme is the central role of plasmablasts—rapidly proliferating, short-lived antibody-secreting cells—in bridging these diverse microbial encounters with autoimmune pathology [
15,
16]. Unlike long-lived plasma cells that maintain stable antibody titres over years, plasmablasts represent an acute-phase response: they expand explosively within days of antigenic challenge and can infiltrate inflamed tissues [
17,
18]. This review synthesises current understanding of how microbial signals orchestrate plasmablast responses that fuel autoimmune flares (
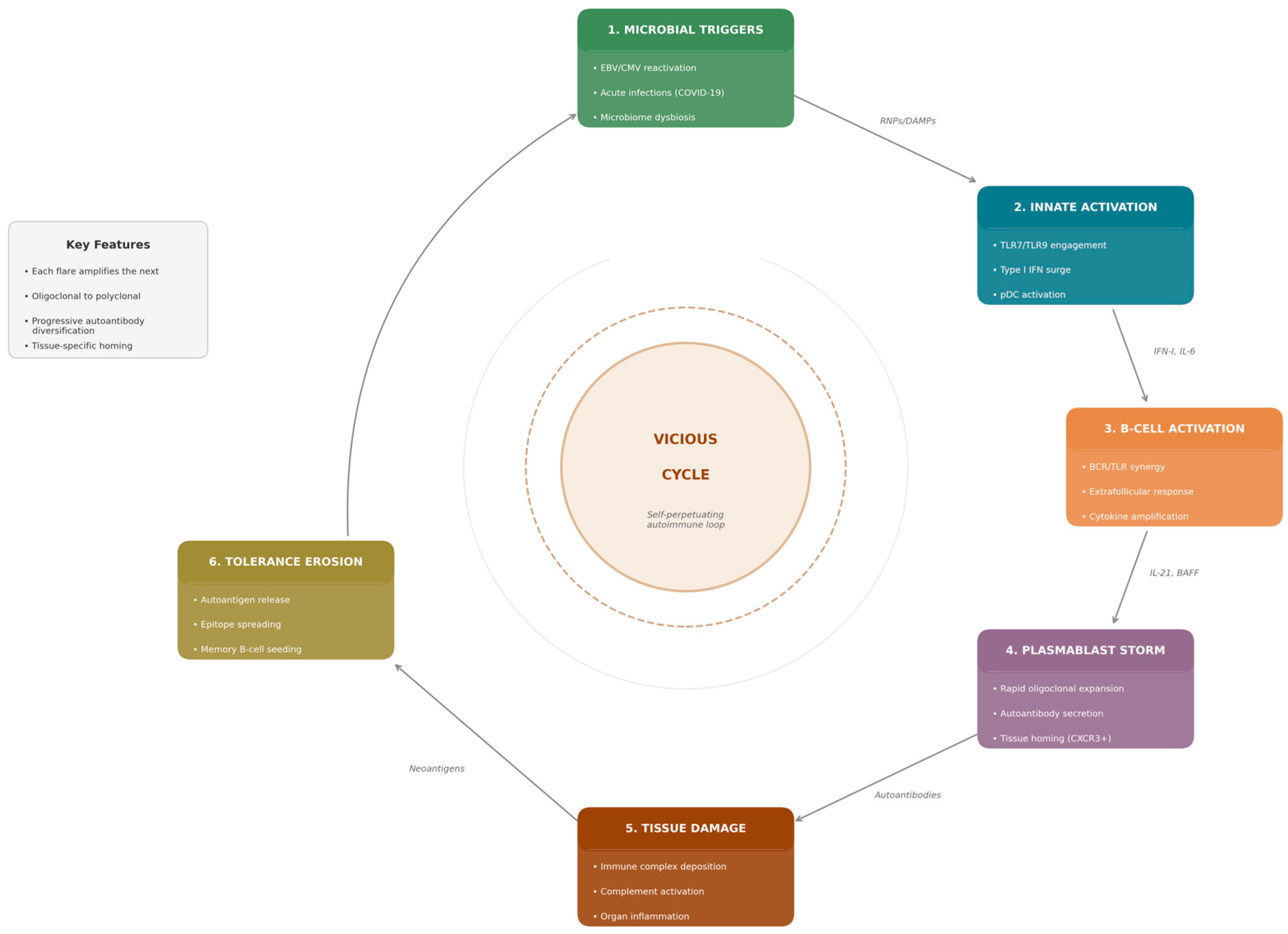
Figure 1).
2. Plasmablast Biology and Extrafollicular Responses
2.1. Defining Plasmablasts: Phenotype and Function
Plasmablasts occupy a transitional position in B-cell differentiation. Phenotypically, human plasmablasts are typically defined as CD19
low/+CD20
−CD27
highCD38
high cells that retain proliferative capacity while actively secreting immunoglobulin [
19,
20]. The transient nature of plasmablasts, with circulating half-lives measured in days to weeks, belies their outsized impact on acute immune responses [
21].
Recent single-cell analyses have revealed substantial heterogeneity within plasmablast populations. Transcriptomic profiling identifies subsets with distinct functional properties, including cells with enhanced inflammatory cytokine production, differential chemokine receptor expression, and varying degrees of commitment to the plasma cell fate [
22,
23].
Emerging evidence suggests that different microbial triggers may preferentially induce distinct plasmablast subgroups. Viral triggers, particularly EBV and SARS-CoV-2, appear to preferentially expand ISG
high plasmablasts characterised by strong interferon-signature gene expression and CXCR3
+ tissue-homing phenotypes [
24]. In contrast, bacterial stimuli engaging TLR2/TLR4 pathways may favour IL-6-driven, ISG
low plasmablasts with distinct metabolic profiles. Chronic dysbiosis has been associated with ‘exhausted’ or ‘atypical’ plasmablast phenotypes showing altered survival signals and reduced antibody secretion capacity [
25]. Whether these microbe-specific signatures can be exploited diagnostically or therapeutically remains an active area of investigation requiring systematic single-cell comparisons across trigger types.
2.2. Extrafollicular Versus Germinal Centre Pathways
B-cell responses can proceed through two distinct pathways. The germinal centre (GC) reaction represents the classical route to high-affinity, class-switched, long-lived humoral immunity, unfolding over weeks [
26]. In contrast, extrafollicular (EF) responses generate plasmablasts rapidly, within 3–5 days of antigen encounter [
27,
28]. The EF pathway can operate with reduced T-cell help, relying instead on innate signals including TLR ligands and type I interferons [
29]. Critically, EF responses are subject to less stringent tolerance checkpoints, rendering them susceptible to autoreactive output. In SLE and other systemic autoimmune diseases, EF responses are pathologically expanded at the expense of GC reactions [
30,
31]. The dominance of EF responses in active autoimmune disease likely reflects the inflammatory cytokine milieu, which favours rapid plasmablast generation over the more deliberate GC pathway.
2.3. Why Plasmablasts, Not Germinal Centre B Cells, React to Microbial Triggers
The striking sensitivity of extrafollicular plasmablast responses to microbial triggers reflects evolutionary pressures that shaped humoral immunity. EF responses evolved as a rapid-reaction force against acute infections, providing antibody within days when waiting weeks for GC-derived high-affinity responses would prove fatal [
32]. This evolutionary imperative for speed necessitated relaxed tolerance checkpoints: EF B cells can differentiate into plasmablasts with minimal T-cell help, relying instead on innate signals—particularly TLR7 and TLR9 engagement by microbial nucleic acids—to license differentiation [
33].
Viruses uniquely favour EF over GC responses because the inflammatory milieu they induce—dominated by type I interferons and IL-6—actively promotes plasmablast differentiation while suppressing GC formation [
34]. IFN-I inhibits Tfh cell development and GC B-cell survival while simultaneously driving IRF4 and BLIMP-1 expression that commits B cells to the plasmablast fate. In autoimmune disease, viral reactivations create inflammatory contexts that repeatedly channel B-cell responses through tolerance-relaxed EF pathways.
2.4. Transcriptional Control of Plasmablast Differentiation
The differentiation of activated B cells into plasmablasts requires coordinate changes in transcription factor expression. Central to this process is BLIMP-1 (encoded by PRDM1), a transcriptional repressor that silences genes required for B-cell identity. IRF4 cooperates with BLIMP-1 to promote plasma cell differentiation [
35,
36]. The cytokine environment profoundly influences the balance between these transcription factors. Type I interferons, IL-6, and IL-21 all promote IRF4 and BLIMP-1 expression through STAT-dependent mechanisms [
37,
38]. Microbial triggers amplify these transcriptional programmes by generating the precise cytokine signatures that tilt this balance toward plasmablast commitment. Infection-associated cytokine signatures thus create transcriptional contexts that favour rapid plasmablast differentiation over alternative B-cell fates.
2.5. Operational Definition of Plasmablast Storms
To enhance the translational value of the plasmablast storm concept, we propose provisional operational criteria modelled on established definitions for cytokine storm syndromes [
39]. These criteria require prospective validation but provide a framework for clinical and research applications:
Quantitative threshold: Circulating plasmablasts exceeding 3–5% of total B cells (defined as CD19
lowCD27
highCD38
high by flow cytometry), based on studies correlating this threshold with active SLE nephritis and disease flares [
40].
Temporal dynamics: Rapid expansion (greater than 2-fold increase within 7–14 days) from patient baseline, distinguishing acute storms from chronically elevated plasmablast frequencies.
Clonality assessment: Oligoclonal dominance, operationally defined as the top 3 expanded clones representing greater than 30% of the plasmablast repertoire by BCR sequencing, indicating antigen-driven rather than polyclonal expansion.
Functional correlate: Concurrent rise in disease-relevant autoantibodies (anti-dsDNA, ACPA, anti-Ro/La) or validated clinical flare markers (e.g., SLEDAI increase ≥4 points).
We acknowledge that these thresholds derive primarily from SLE studies and may require disease-specific calibration. Standardisation of flow cytometry panels and BCR sequencing protocols across centres represents a prerequisite for multi-site validation. Nevertheless, establishing quantitative criteria transforms ‘plasmablast storms’ from a descriptive metaphor into a measurable entity amenable to biomarker development and therapeutic targeting.
3. Microbial Triggers of Plasmablast Storms
3.1. Epstein–Barr Virus: The Prototypical Trigger
EBV stands as the most extensively studied viral trigger for systemic autoimmunity [
41,
42]. Prospective studies have shown that EBV infection precedes and predicts the development of multiple sclerosis, with a 32-fold increased risk following infectious mononucleosis [
43].
EBV occupies a unique position among plasmablast storm triggers because it is both a direct B-cell tropic virus, establishing latency specifically within memory B cells, and a potent amplifier of bystander innate sensing through release of immunostimulatory nucleic acids during reactivation [
44]. This dual capacity—to directly manipulate infected B cells while simultaneously creating systemic inflammatory contexts—explains why EBV is so central to plasmablast biology in autoimmune disease.
During periodic viral reactivations, EBV-infected B cells undergo lytic replication and release viral particles containing unmethylated CpG DNA and small RNAs that engage TLR9 and TLR7 in plasmacytoid dendritic cells and B cells [
45]. Studies in lupus patients demonstrate that EBV viral loads correlate with disease activity and that reactivation episodes precede clinical flares [
46,
47].
3.2. Cytomegalovirus and Other Herpesviruses
CMV contributes to autoimmune pathology through mechanisms distinct from EBV. Whereas EBV directly infects B cells, CMV’s primary latent reservoir is myeloid lineage cells. A distinctive feature of CMV infection is its profound impact on T-cell homeostasis, driving expansion of CD4
+CD28
null T cells with features of immunosenescence [
48].
CMV reactivation during immunosuppression or physiological stress releases viral particles containing immunostimulatory DNA that engages TLR9, potentially licensing bystander B-cell activation [
8]. Molecular mimicry between CMV antigens and self-proteins, including phospholipids, has been proposed as a mechanism linking CMV infection to antiphospholipid antibody production [
49]. Unlike EBV’s direct B-cell effects, CMV’s contribution to plasmablast storms operates primarily through T-cell-mediated pathways and innate immune activation.
3.2.1. Human Herpesvirus 6
HHV-6 (comprising HHV-6A and HHV-6B species) infects over 90% of humans by age two and establishes latency in multiple cell types, including T cells, monocytes, and neural cells [
50]. HHV-6 has been implicated in several autoimmune disorders, most notably multiple sclerosis, but also autoimmune connective tissue diseases and Hashimoto’s thyroiditis [
51].
HHV-6A demonstrates particular neurotropism and has been detected in active MS plaques, with elevated anti-HHV-6 antibody titres in MS patients compared to controls [
52]. The virus can trigger autoimmunity through several mechanisms: molecular mimicry between viral and myelin basic protein epitopes, bystander activation through lytic infection releasing sequestered autoantigens, and aberrant CD46 signalling that promotes IL-17-driven inflammation [
53]. HHV-6 reactivation has been associated with flares of autoimmune connective tissue diseases, although direct causality remains to be established [
54].
From a plasmablast storm perspective, HHV-6 reactivation provides type I interferon-inducing stimuli through TLR9 engagement by viral DNA, potentially lowering B-cell activation thresholds in genetically susceptible individuals.
3.2.2. Varicella-Zoster Virus
VZV occupies a unique position among herpesviruses as the only human virus definitively shown to productively infect arterial walls and cause vasculopathy [
55]. After primary varicella infection, VZV establishes latency in sensory ganglia along the entire neuraxis. Reactivation as herpes zoster can be followed by VZV vasculopathy, initially recognised in cerebral arteries but now known to affect extracranial vessels including temporal arteries.
The discovery of VZV antigen in temporal arteries of patients with giant cell arteritis (GCA) has generated intense interest in viral triggers for large-vessel vasculitis [
56]. VZV has been detected in 73% of GCA-positive temporal artery biopsies versus 22% of pathologically normal biopsies in some studies [
57]. The proposed mechanism involves transaxonal spread of reactivated virus from ganglionic neurons to arterial adventitia, followed by transmural infection that triggers granulomatous inflammation. However, inconsistent findings across studies and failure to reproduce positive results in some cohorts indicate that VZV is unlikely to explain all GCA cases [
56].
For plasmablast biology, VZV reactivation provides a model of how periodic herpesvirus emergence from latency can trigger localised inflammatory responses with potential for bystander autoimmune activation.
3.2.3. Herpesvirus Synergy and Co-Reactivation
Clinical and experimental evidence suggests that multiple herpesviruses may reactivate simultaneously during periods of immune stress, potentially amplifying inflammatory signals [
58]. EBV-CMV co-infection has been associated with more severe autoimmune manifestations than either virus alone. The concept of a ‘herpesvirus burden’—cumulative exposure and reactivation frequency across the herpesvirus family—may help explain individual variation in autoimmune susceptibility.
From the plasmablast storm perspective, herpesvirus co-reactivation provides multiple simultaneous innate sensing inputs through TLR7 (viral RNA) and TLR9 (viral DNA), creating particularly robust type I interferon responses that drive extrafollicular B-cell differentiation.
3.3. SARS-CoV-2 and Acute Respiratory Infections
SARS-CoV-2 infection induces profound plasmablast expansion, sometimes exceeding 30% of circulating B cells in severe cases, with evidence of extrafollicular pathway dominance [
59]. Single-cell analyses reveal oligoclonal plasmablast expansions with autoreactive specificities, including anti-interferon, anti-phospholipid, and anti-nuclear antibodies [
60].
The COVID-19 pandemic served as a large-scale natural experiment validating the plasmablast storm model beyond classical rheumatic disease. The observations that severe infection drives extrafollicular B-cell responses with autoreactive output parallel findings in SLE [
61]. Recent studies of long COVID have demonstrated persistence of autoreactive plasmablast clones and autoantibodies months after acute infection, suggesting that intense innate activation can establish durable autoimmune memory in susceptible individuals [
62,
63].
3.4. Mucosal Infections and the Mouth-to-Flare Connection
Periodontal pathogens, notably Porphyromonas gingivalis, have been mechanistically linked to RA through their capacity to citrullinate host proteins via PAD enzymes, generating neoepitopes recognised by ACPA [
64,
65]. The ‘mouth-to-flare’ model positions periodontal infection as an active, ongoing source of antigenic stimulation that can repeatedly trigger systemic plasmablast responses [
66].
4. Innate Sensing Pathways Licensing Plasmablast Responses
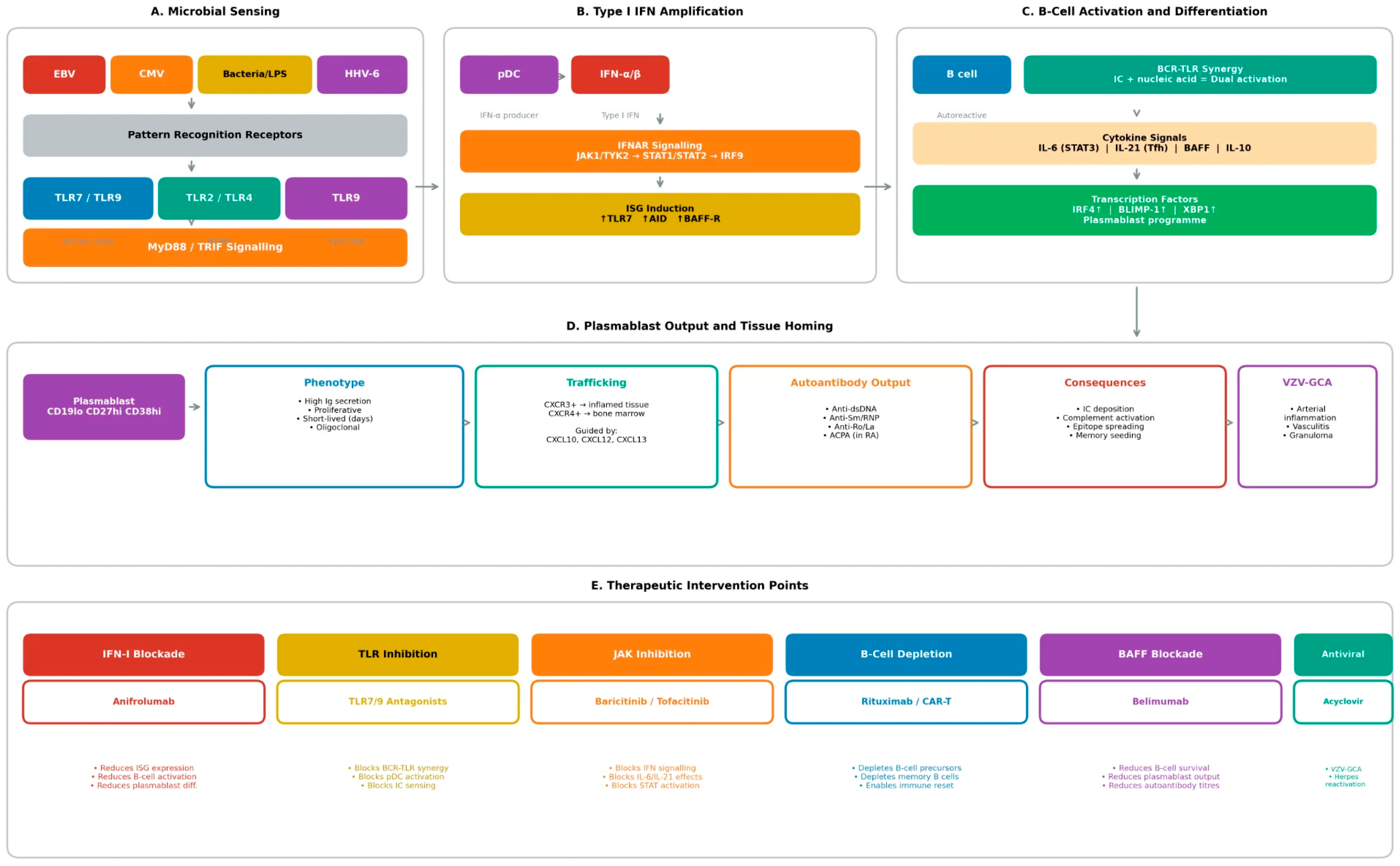
The molecular mechanisms through which microbial signals license plasmablast differentiation centre on innate sensing pathways, particularly Toll-like receptor signalling and type I interferon production (
Figure 2). These pathways integrate detection of specific pathogen-associated molecular patterns (PAMPs) with B-cell activation, creating permissive contexts for extrafollicular responses that bypass normal tolerance checkpoints.
4.1. Toll-like Receptor Signalling and Pathogen-Associated Molecular Patterns
TLRs represent the frontline sensors through which B cells detect microbial presence. Each TLR recognises specific PAMPs: TLR9 detects unmethylated CpG DNA motifs; TLR7 recognises single-stranded RNA; TLR4 responds to lipopolysaccharide; and TLR2 senses bacterial lipopeptides and lipoglycans [
67,
68]. Dual engagement of the BCR and TLRs dramatically lowers activation thresholds—a phenomenon termed BCR-TLR synergy [
69].
TLR7 gain-of-function variants cause monogenic lupus, with a Y99H variant identified as sufficient to drive SLE in both humans and mice [
70]. TLR7 gene duplication increases lupus risk, potentially contributing to the female predominance through gene dosage effects [
71].
4.2. Type I Interferon: The Master Amplifier
Type I interferons emerge as master amplifiers of plasmablast responses. pDCs serve as the principal IFN-I producers, secreting 1000-fold more IFN-alpha than other cell types upon TLR7 or TLR9 engagement [
72]. IFN-I drives plasmablast differentiation through induction of IRF4 and BLIMP-1, and upregulates TLR7 expression on B cells, creating positive feedback [
73]. The IFN signature is present in 50–80% of active SLE patients. Therapeutic targeting with anifrolumab has demonstrated efficacy, validating this pathway [
74].
Similar signatures have been identified in subsets of patients with Sjogren syndrome, dermatomyositis, and systemic sclerosis. From the plasmablast perspective, IFN-I functions as the inflammatory context that licenses extrafollicular B-cell responses to proceed unchecked.
4.3. Cytokine Networks Supporting Plasmablast Differentiation
IL-6 is indispensable for plasma cell differentiation, acting through STAT3 to induce BLIMP-1 [
75]. IL-21, produced by Tfh cells, potently drives B-cell proliferation and plasmablast differentiation [
76]. Microbial triggers that activate multiple arms of this cytokine network simultaneously generate particularly robust plasmablast responses [
77].
5. Microbiome Dysbiosis and B-Cell Activation
5.1. The Gut as a Chronic, Low-Grade Microbial Stimulus
The intestinal microbiota, comprising trillions of bacteria, fungi, and viruses, profoundly shapes immune development and homeostasis. Dysbiosis, pathological alterations in microbiome composition, has been documented across autoimmune conditions including SLE, RA, Sjogren syndrome, and inflammatory myopathies. While establishing causality remains challenging, experimental models demonstrate that microbiome manipulation can modulate autoimmune phenotypes, and emerging human data support mechanistic connections.
From the plasmablast storm perspective, the gut microbiome functions as a chronic, low-grade source of microbial stimulation that keeps B cells poised near activation thresholds, thereby lowering the stimulus required for discrete triggers to ignite full-blown plasmablast responses. Unlike acute infections that provide transient, high-intensity signals, dysbiosis creates a persistent state of immune priming [
78]. This tonic stimulation may not be sufficient to trigger overt plasmablast storms independently but sensitises the B-cell compartment such that subsequent viral reactivations, acute infections, or other triggers more readily precipitate autoreactive responses. The gut-immune axis influences B-cell biology through multiple mechanisms. Intestinal epithelial cells and dendritic cells sense microbial products through pattern recognition receptors, producing cytokines including BAFF, APRIL, and IL-6 that can act systemically to modulate B-cell survival and differentiation. Gut-associated lymphoid tissue (GALT), including Peyer patches and isolated lymphoid follicles, serves as a major site of B-cell activation, class switching, and IgA production. Perturbations in intestinal homeostasis can alter the output of these responses, potentially seeding systemic autoimmunity.
Specific bacterial taxa have been associated with autoimmune disease. Ruminococcus gnavus, expanded in active SLE, produces lipoglycans that stimulate dendritic cells via TLR2 (demonstrated in human cell culture and murine models) [
79]. Enterococcus gallinarum translocates across the gut barrier in lupus-prone mice and has been detected in liver tissue of SLE patients, inducing autoantibody production through type I interferon pathways [
80].
5.2. Metabolite-Mediated B-Cell Modulation
Microbiota-derived metabolites represent a key mechanism through which dysbiosis influences systemic immunity and B-cell function. SCFAs, including acetate, propionate, and butyrate, are produced by bacterial fermentation of dietary fibre and exert dose-dependent effects on immune cells. Butyrate inhibits histone deacetylases (HDACs), enabling epigenetic modulation of gene expression, and can suppress B-cell class switching and antibody production through effects on AID expression (established in murine models and human B-cell cultures) [
81]. Reduced SCFA-producing bacteria, commonly observed in autoimmune dysbiosis, may thus release B cells from metabolic restraint.
The mechanistic chain from dysbiosis to plasmablast permissiveness operates through defined molecular steps. Loss of SCFA-producing taxa removes butyrate-mediated HDAC inhibition. Reduced butyrate releases epigenetic brakes on plasmablast differentiation genes, lowering the threshold for IRF4 and BLIMP-1 induction [
82].
While experimental models strongly support a role for microbial metabolites in shaping B-cell differentiation thresholds, direct causal evidence in humans remains limited. Most human studies demonstrate associative links between dysbiosis, altered metabolite profiles (e.g., reduced short-chain fatty acids), and autoimmune disease activity.
The effects of SCFAs on B-cell differentiation have been demonstrated in vitro and in murine models, but direct causal evidence in human autoimmune disease remains limited to correlational observations (reduced SCFA-producing bacteria in patient cohorts, inverse correlations between faecal butyrate and disease activity). Similarly, while tryptophan metabolite shifts have been documented in SLE and RA patients, whether these represent drivers or consequences of inflammation requires interventional studies. Ongoing clinical trials of dietary fibre supplementation and defined bacterial consortia will help establish which microbiome-plasmablast connections are therapeutically tractable in humans.
At present, it remains unresolved whether metabolite alterations act as primary drivers of plasmablast differentiation in humans or function predominantly as permissive modulators that lower activation thresholds in genetically predisposed hosts. Accordingly, microbiome-derived metabolite effects should be interpreted as context-setting rather than deterministic, pending interventional validation in human studies.
5.3. Oral-Gut-Joint Axis in Rheumatoid Arthritis
Prevotella copri expansion in new-onset RA represents one of the most replicated microbiome findings in autoimmunity [
83]. Prevotella-derived metabolites may promote Th17 differentiation and joint inflammation [
84].
5.4. Barrier Dysfunction and Microbial Translocation
Intestinal barrier dysfunction, or ‘leaky gut,’ permits translocation of bacterial products into the systemic circulation [
85]. SLE patients show elevated serum lipopolysaccharide (LPS) and correlations between microbial translocation markers and autoantibody levels have been reported [
86].
6. T-Cell Cooperation and Tissue-Level Inflammation
While extrafollicular plasmablast responses can proceed with reduced T-cell dependence relative to germinal centre reactions, T-cell help remains important for sustaining and amplifying these responses. Peripheral helper T cells (Tph), distinct from classical Tfh cells, have been identified in inflamed tissues of patients with RA and other autoimmune conditions [
87]. Tph cells express high levels of PD-1 and produce IL-21, providing B-cell help in ectopic lymphoid structures [
88].
Microbial triggers can expand these T-cell populations through bystander activation and cytokine-driven proliferation, further amplifying the capacity for plasmablast storms.
7. Plasmablast Trafficking and Tissue Homing
Chemokine Guidance of Plasmablast Migration
Plasmablasts express distinct chemokine receptor profiles that determine their migratory behaviour and tissue localisation. CXCR4, the receptor for CXCL12 (SDF-1), guides plasmablasts toward bone marrow niches where they may mature into long-lived plasma cells [
89]. In contrast, CXCR3, which binds CXCL9, CXCL10, and CXCL11, directs plasmablasts toward inflamed tissues expressing these interferon-induced chemokines. Microbial triggers shape this chemokine landscape through induction of IFN-I and inflammatory cytokines, determining whether circulating plasmablasts seed bone marrow long-term reservoirs or home to inflamed organs where they mediate local antibody-dependent damage. The balance between CXCR3 and CXCR4 expression on individual plasmablasts thus influences the anatomical distribution and persistence of autoantibody production. In active SLE, plasmablasts demonstrate enhanced CXCR3 expression, correlating with renal and cutaneous manifestations [
90]. Inflamed kidneys in lupus nephritis upregulate CXCL10, creating chemotactic gradients that recruit CXCR3
+ plasmablasts to sites of tissue injury. This tissue-directed homing amplifies local autoantibody deposition and immune complex formation.
The chemokine receptor repertoire of plasmablasts is dynamically regulated during differentiation. Early plasmablasts express high levels of CXCR3, facilitating rapid tissue infiltration, while maturing cells progressively upregulate CXCR4, enabling bone marrow homing [
91]. Inflammatory cytokines, particularly IFN-I and IL-6, can arrest this developmental programme, maintaining CXCR3 expression and prolonging tissue residence.
8. Autoantibody Diversification and Epitope Spreading
8.1. Plasmablast-Driven Repertoire Evolution
A critical consequence of repeated plasmablast storms is progressive diversification of the autoantibody repertoire [
92]. While individual plasmablast bursts are often oligoclonal, dominated by expanded clones responding to acute triggers, cumulative episodes drive epitope spreading and affinity maturation that entrench autoimmunity. Analysis of longitudinal patient samples reveals that autoantibody specificities broaden over disease duration, with new epitope reactivities emerging during flares and often persisting into remission [
93].
Somatic hypermutation (SHM) within extrafollicular responses, though less extensive than in germinal centres, nonetheless contributes to antibody diversification [
94]. IFN-I and TLR signalling can induce activation-induced cytidine deaminase (AID), the enzyme responsible for SHM and class-switch recombination, in extrafollicular B cells. This enables ongoing antibody diversification outside classical GC structures, potentially generating higher-affinity autoreactive variants during successive plasmablast storms. BCR sequencing studies in SLE patients demonstrate clonal evolution with accumulating mutations over time [
95].
8.2. Epitope Spreading and Tolerance Erosion
Epitope spreading, the extension of immune responses from initial target epitopes to additional epitopes on the same or related antigens, is a hallmark of progressive autoimmunity [
96]. In SLE, responses may begin against a single component of ribonucleoprotein complexes (e.g., SmB) and spread to additional components (SmD, RNP-A, RNP-C) over time. Studies of pre-clinical lupus demonstrate sequential appearance of autoantibody specificities, with anti-Ro often preceding anti-La and anti-DNA responses [
97]. ACPA responses in RA similarly diversify from initial citrullinated targets to encompass multiple citrullinated proteins.
Plasmablast storms contribute to epitope spreading through several mechanisms. First, inflammatory tissue damage releases intracellular and sequestered antigens, exposing new epitopes to the immune system [
98]. Second, SHM introduces mutations that may broaden BCR cross-reactivity. Third, the inflammatory cytokine milieu during flares disrupts peripheral tolerance mechanisms that normally restrain autoreactive B cells.
Each microbial trigger thus creates not only an immediate plasmablast storm but also an opportunity for repertoire diversification that progressively erodes tolerance and stabilises pathogenic B-cell clones [
99].
8.3. Memory B Cells and Long-Lived Plasma Cells
While plasmablasts themselves are short-lived, the B-cell responses they emerge from generate memory populations that enable rapid recall during subsequent triggers [
100]. Memory B cells arising from extrafollicular responses may carry autoreactive specificities that permit immediate plasmablast differentiation upon re-encounter with self-antigens or during subsequent microbial triggers that create permissive inflammatory contexts. Longitudinal studies of SLE patients reveal persistence of autoreactive B-cell clones across years, with clone frequencies expanding during flares and contracting during remission [
101].
A proportion of plasmablasts also mature into long-lived plasma cells (LLPCs) that seed bone marrow niches, establishing durable autoantibody production independent of ongoing B-cell activation [
102]. LLPCs can persist for decades and are resistant to conventional immunosuppression and B-cell depletion therapies. The interplay between acute plasmablast storms and these long-term memory and plasma cell populations creates the complex serological dynamics characteristic of chronic autoimmunity. Targeting LLPCs may be necessary for achieving true immunological remission [
103].
9. Clinical Implications and Therapeutic Opportunities
9.1. Plasmablasts as Disease Biomarkers
Circulating plasmablast frequencies correlate with disease activity across multiple autoimmune conditions, positioning them as potential biomarkers for monitoring and predicting flares [
40]. In SLE, plasmablast percentages above 2–3% of peripheral B cells strongly associate with active disease, particularly nephritis. Flow cytometric enumeration of CD19
lowCD27
highCD38
high cells provides a readily implementable assay for clinical practice [
104]. Rising plasmablast counts may herald impending flares before clinical symptoms manifest, enabling pre-emptive therapeutic intensification.
Beyond enumeration, molecular characterisation of plasmablasts offers additional insights. Single-cell RNA sequencing and BCR repertoire analysis can reveal clonal dynamics, identify disease-relevant specificities, and track responses to therapy [
105]. The oligoclonal nature of many plasmablast expansions suggests that specific clones drive individual flares, potentially enabling targeted intervention against pathogenic specificities. Plasmablast-derived autoantibody profiles may also inform prognosis and guide therapeutic selection.
9.2. Viral Monitoring and Antiviral Strategies
Given the strong associations between herpesvirus reactivation and autoimmune flares, monitoring EBV and CMV viral loads or serological markers may identify patients at risk for reactivation-associated plasmablast surges [
47]. Serial measurement of EBV DNA by quantitative PCR could potentially predict impending flares in patients with established SLE or Sjogren syndrome, enabling pre-emptive immunosuppressive adjustment. Similarly, CMV antigenaemia or DNA monitoring, already standard practice in transplant medicine, might have applications in heavily immunosuppressed autoimmune patients at high risk for CMV-triggered disease exacerbations.
Antiviral prophylaxis or pre-emptive treatment represents a theoretical but largely unexplored strategy to blunt microbe-licensed plasmablast storms in high-risk patients. Acyclovir and valacyclovir suppress herpesvirus replication and could, in principle, reduce the frequency of reactivation-triggered flares, particularly in heavily immunosuppressed patients receiving rituximab, cyclophosphamide, or other lymphocyte-depleting therapies who are at increased risk for herpesvirus reactivation [
106]. However, clinical trial data supporting this approach in autoimmune disease are lacking, and the cost-effectiveness of universal prophylaxis is uncertain. A more targeted approach might involve viral monitoring to identify patients with rising EBV or CMV loads, followed by pre-emptive antiviral therapy before clinical flare develops. This strategy remains investigational and requires prospective validation. Integrated biomarker panels combining plasmablast enumeration with viral load monitoring may enhance flare prediction. Preliminary data from lupus cohorts suggest that rising EBV DNA titres preceding plasmablast expansion could identify a ‘pre-storm’ window amenable to pre-emptive intervention. A proposed monitoring panel includes: (1) plasmablast frequency by flow cytometry (CD19
lowCD27
highCD38
high); (2) EBV/CMV viral loads by quantitative PCR; (3) IFN-signature score (gene expression or serum protein panel); and (4) disease-relevant autoantibody titres. Prospective studies evaluating whether this combined approach outperforms single biomarkers for flare prediction are warranted.
9.3. Targeting Innate Sensing Pathways
Therapeutic blockade of innate sensing pathways offers a strategy for preventing the inflammatory contexts that license plasmablast storms (
Table 1). Anifrolumab, a monoclonal antibody blocking the type I IFN receptor (IFNAR1), has demonstrated efficacy in moderate-to-severe SLE and represents proof of concept for this approach [
107,
108]. By neutralising IFN-I signalling regardless of whether it originates from viral infection, immune complex stimulation, or other sources, anifrolumab reduces B-cell activation signals, diminishes plasmablast differentiation, and lowers autoantibody titres. The selective benefit in IFN-high patients highlights the importance of stratifying patients by pathway activation.
TLR7 and TLR9 inhibitors are in clinical development for autoimmune indications. Enpatoran (M5049), a dual TLR7/8 inhibitor, has shown promising results in early lupus trials [
109]. Given the central role of these receptors in sensing nucleic acid-containing immune complexes and microbial PAMPs, their inhibition may directly restrain plasmablast responses to both endogenous triggers and exogenous microbial stimuli. JAK inhibitors, which block signalling downstream of IFN receptors and multiple cytokine receptors, have shown efficacy in RA and SLE and may exert part of their benefit through dampening plasmablast-permissive inflammation [
110].
9.4. B-Cell Directed Therapies
Direct targeting of B-cell lineage cells remains a cornerstone of autoimmune therapy. Rituximab, depleting CD20+ B cells, effectively eliminates precursors capable of generating plasmablasts while sparing mature plasma cells that lack CD20 (
Table 2) [
111]. The clinical observation that rituximab responses are often delayed and may require multiple cycles is consistent with gradual depletion of autoreactive memory populations that seed plasmablast storms. Variable responses across patients and conditions may reflect differences in the contribution of B-cell-dependent versus plasma cell-dependent autoantibody production.
Belimumab, blocking BAFF, reduces B-cell survival signals and modestly diminishes plasmablast frequencies over time [
111,
112]. Combination of belimumab with rituximab has shown enhanced efficacy in lupus nephritis, suggesting synergy between survival signal blockade and B-cell depletion. Obinutuzumab, a glycoengineered anti-CD20 antibody with enhanced antibody-dependent cellular cytotoxicity, has demonstrated superiority to rituximab in lupus nephritis trials [
113].
More recently, CD19-directed therapies, including the bispecific T-cell engager blinatumomab and CD19 CAR-T cells, have shown remarkable efficacy in refractory autoimmunity, achieving deep B-cell depletion that encompasses both CD20
+ B cells and CD20
− plasmablasts [
114,
115]. Early reports suggest potential for drug-free remission following B-cell reconstitution, consistent with elimination of pathogenic clones and immune reset. The durability of these responses and long-term safety require further investigation, but they provide proof of concept that elimination of autoreactive B-cell populations can fundamentally alter disease trajectory.
CD19-directed chimeric antigen receptor T-cell (CAR-T) therapy has emerged as a transformative approach for refractory autoimmune disease. The landmark study by Mackensen et al. (2022) demonstrated drug-free remissions in five patients with refractory SLE following CD19 CAR-T infusion [
116]. Subsequent reports have extended these findings to antisynthetase syndrome, systemic sclerosis, and other refractory conditions [
116,
117,
118,
119,
120]. The mechanism involves deep B-cell depletion encompassing both CD20+ B cells and CD20− plasmablasts/plasma cells, achieving a degree of B-lineage elimination not possible with rituximab. Updated follow-up data (2024) demonstrate sustained remissions exceeding 24 months in most patients, with B-cell reconstitution occurring from naive precursors apparently purged of autoreactive memory. Ongoing trials (NCT05765006, NCT05030779) are evaluating CAR-T in broader autoimmune populations and optimising conditioning regimens.
Bispecific antibodies engaging T cells to eliminate B-lineage cells represent an ‘off-the-shelf’ alternative to CAR-T. CD19 × CD3 bispecifics (blinatumomab) and CD20 × CD3 bispecifics (mosunetuzumab, glofitamab) are approved for B-cell malignancies and under investigation in autoimmunity. Potential advantages include immediate availability, titratable dosing, and avoidance of lymphodepletion conditioning. BCMA-targeting approaches, originally developed for myeloma, may enable selective depletion of long-lived plasma cells responsible for persistent autoantibody production [
116,
119].
9.5. Microbiome-Targeted Interventions
The recognition of microbiome contributions to autoimmune pathogenesis has generated interest in microbiome-targeted therapeutics [
121]. Probiotics, prebiotics, faecal microbiota transplantation (FMT), and defined bacterial consortia are under investigation, though clinical evidence in autoimmune disease remains preliminary. From the plasmablast perspective, successful microbiome modulation could reduce circulating microbial products, restore SCFA production, and dampen the tonic inflammation that primes B cells for extrafollicular responses.
The mechanistic rationale for microbiome-targeted therapy in plasmablast-driven disease centres on reducing tonic microbial TLR signalling and restoring metabolic restraints on B-cell differentiation. Dietary interventions, particularly those increasing fibre intake to support SCFA-producing bacteria, may restore butyrate-mediated HDAC inhibition and thereby raise the threshold for B-cell activation and plasmablast differentiation. FMT or defined consortia enriched in SCFA-producers could achieve similar effects. Strategies to repair barrier integrity, including glutamine supplementation, zinc, and probiotics with demonstrated effects on tight junction proteins, could reduce translocation of LPS, bacterial DNA, and other PAMPs that provide ongoing TLR stimulation to circulating B cells [
121]. While diet and microbiome modulation alone are unlikely to induce remission in established autoimmunity, they may reduce flare frequency by keeping B cells further from activation thresholds, such that stronger triggers are required to ignite plasmablast storms [
122,
123]. Microbiome engineering approaches under investigation include: (1) Live biotherapeutic products (LBPs) comprising defined bacterial consortia designed to restore eubiosis (e.g., VE303, SER-287); (2) Precision prebiotics selectively promoting beneficial taxa; (3) Phage therapy targeting specific pathobionts such as Ruminococcus gnavus or Enterococcus gallinarum; and (4) Barrier repair strategies using glutamine, zinc, or probiotics with demonstrated effects on tight junction integrity.
9.6. Plasmablast Storms Across Disease Contexts
While the plasmablast storm model was initially developed from SLE research, emerging evidence reveals both shared features and disease-specific patterns across autoimmune conditions:
Systemic lupus erythematosus: Characterized by dominant extrafollicular pathway activation, ISG
high plasmablast signatures, and autoantibodies targeting nuclear antigens (dsDNA, Sm, RNP). Plasmablast frequencies correlate strongly with nephritis activity and predict renal flares. EBV reactivation is a well-documented trigger [
122].
Rheumatoid arthritis: ACPA-secreting plasmablasts arise through the ‘mouth-to-flare’ pathway involving periodontal pathogens. Synovial ectopic lymphoid structures harbour local plasmablast generation. Unlike SLE, IFN-signature is less prominent; IL-6-driven pathways may predominate [
124].
Sjögren syndrome: Salivary gland-resident plasmablasts producing anti-Ro/La antibodies are characteristic. Strong EBV association with elevated viral loads in salivary tissue. Risk of B-cell lymphoma transformation reflects chronic B-cell stimulation [
125].
ANCA-associated vasculitis: Anti-PR3 and anti-MPO plasmablasts drive disease. Excellent response to rituximab supports central role of B-cell lineage. Early CAR-T data show promise in refractory cases [
126].
Inflammatory myopathies: Anti-synthetase and anti-MDA5 antibody-secreting plasmablasts expanded during active disease. Strong IFN-signature parallels SLE. Viral triggers (including SARS-CoV-2) documented as precipitants [
127,
128].
10. Future Directions and Research Priorities
Several key questions warrant investigation to translate the plasmablast storm model into improved patient outcomes. First, prospective studies with intensive longitudinal sampling are needed to define the temporal dynamics of plasmablast responses relative to microbial triggers, clinical symptoms, and serological changes [
128]. Integration of viral monitoring (EBV, CMV loads), microbiome profiling, and plasmablast enumeration could identify the relative contributions of different microbial inputs to individual flares and enable biomarker-guided pre-emptive therapy.
Second, deeper characterisation of plasmablast populations arising during flares, through single-cell technologies, BCR sequencing, and functional assays, will clarify which clones drive pathology and whether specific microbial triggers selectively expand certain autoreactive specificities [
129]. This knowledge could inform development of antigen-specific tolerogenic strategies or selective depletion approaches that spare protective immunity while eliminating pathogenic clones.
Third, the interplay between chronic dysbiosis and acute microbial triggers deserves mechanistic investigation. Does restoration of eubiosis raise the threshold for viral reactivation-triggered flares? Can barrier repair strategies reduce the frequency of plasmablast storms even without eliminating the underlying autoimmune diathesis? [
83] Interventional studies combining microbiome modulation with standard immunosuppression could address these questions.
Fourth, combination therapeutic strategies targeting multiple nodes of the plasmablast storm pathway merit exploration. The redundancy inherent in immune activation suggests that single-target approaches may have ceiling effects, while combinations targeting innate sensing (TLR inhibition, IFN-I blockade), cytokine signalling (JAK inhibition), and B-cell survival (BAFF blockade, CD20/CD19 depletion) may achieve synergistic dampening of autoreactive responses [
130]. Rational combination design guided by mechanistic understanding could improve efficacy while managing safety considerations.
Emerging technologies will transform our understanding of plasmablast biology and tissue organisation. Spatial transcriptomics enables mapping of plasmablast niches within inflamed tissues, identifying stromal support cells and understanding the architecture of ectopic lymphoid structures. Recent spatial transcriptomic analysis of lupus nephritis demonstrated plasmablast clustering near damaged tubular epithelium, suggesting direct antibody-mediated injury mechanisms [
131]. Single-cell multi-omics approaches combining scRNA-seq, BCR sequencing, and surface proteomics (CITE-seq) can simultaneously profile transcriptome, B-cell receptor repertoire, and phenotype, enabling clonal tracking of autoreactive plasmablasts from memory precursors through storm expansion. CRISPR-based functional genomic screens in primary human B cells are beginning to identify genes essential for plasmablast differentiation in inflammatory contexts, revealing potential novel therapeutic targets [
132,
133].
11. Limitations of the Plasmablast Storm Model
Several limitations of the plasmablast storm framework warrant explicit acknowledgment. Not all flares involve plasmablast storms. Some disease exacerbations may be driven primarily by T-cell-mediated mechanisms, complement activation, or innate immune pathways without prominent B-cell involvement. Seronegative disease subsets and flares occurring during effective B-cell depletion therapy illustrate that plasmablast-independent mechanisms exist.
Furthermore, evidence linking microbial triggers to plasmablast expansion remains associative rather than causal in humans. There is limited proofs that antiviral prophylaxis prevents flares or that microbiome restoration reduces plasmablast storms. Inter-individual variability in microbial exposure, genetics, and immune regulation likely determines whether plasmablast storms translate into clinical flares. Accordingly, plasmablast storms should be viewed as a dominant but not exclusive driver of autoimmune flares.
The model does not fully explain why identical microbial exposures trigger plasmablast storms in some individuals but not others. HLA associations, TLR polymorphisms, and B-cell intrinsic factors likely determine susceptibility, but integration of host genetics into the framework requires further development.
In addition, the model is best developed for systemic autoimmune diseases with circulating plasmablasts; application to organ-specific autoimmunity (e.g., type 1 diabetes, autoimmune thyroiditis) where plasmablasts may be tissue-restricted requires additional validation.
The temporal resolution of the plasmablast storm cascade remains incompletely defined. The precise kinetics linking microbial exposure, innate immune activation, plasmablast expansion, and clinical flare onset are not yet fully delineated. High-resolution longitudinal studies with frequent sampling of viral load, cytokine signatures, and B-cell subsets will be required to identify predictive windows for pre-emptive intervention.
12. Conclusions
Plasmablast storms represent a unifying concept bridging microbial immunology with autoimmune pathophysiology. Three distinct categories of microbial input, latent herpesvirus reactivation, acute respiratory/gastrointestinal infection, and chronic microbiome dysbiosis, converge on innate sensing pathways through specific PAMPs, including CpG DNA, ssRNA, LPS, and bacterial lipoglycans. TLR7/9 engagement, particularly through BCR-TLR synergy, and type I interferon signalling create inflammatory contexts permissive for rapid, extrafollicular B-cell responses that bypass normal tolerance checkpoints.
The recurrent nature of these plasmablast storms shapes the natural history of autoimmune disease. Each acute expansion drives autoantibody diversification through somatic hypermutation and promotes epitope spreading through the release of sequestered antigens and disruption of peripheral tolerance. This progressive erosion of tolerance checkpoints establishes a vicious cycle whereby successive flares entrench autoimmunity and generate the long-lived memory B cells and plasma cells that sustain chronic disease. Chronic dysbiosis and barrier dysfunction maintain B cells near activation thresholds, creating ongoing vulnerability to future storms triggered by viral reactivation or acute infection.
Therapeutic opportunities emerge at multiple levels of this framework. Antiviral strategies may blunt herpesvirus-triggered storms in high-risk patients. Targeting innate amplification through IFN-I blockade (Anifrolumab) or TLR inhibition can prevent the inflammatory licensing of plasmablast responses. B-cell-directed therapies can eliminate precursors and pathogenic clones. Modulation of cytokine networks through JAK inhibition or IL-6 blockade dampens the signals driving plasmablast differentiation. Microbiome modulation may reduce tonic TLR stimulation and restore metabolic restraints on B-cell differentiation. By understanding plasmablast storms as the nexus where diverse microbial inputs ignite autoimmunity, we gain leverage for interventions that interrupt this cycle and modify the trajectory of chronic autoimmune disease.
Author Contributions
Conceptualization, M.S.; methodology, J.S. software, M.S. and J.S.; validation, M.S. and J.S.; formal analysis, M.S. and J.S.; investigation, M.S. and J.S.; resources, M.S. and J.S.; data curation, M.S. and J.S. writing—original draft preparation M.S. and J.S. writing—review and editing M.S. and J.S.; visualization, M.S. and J.S.; supervision, M.S. project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
List of Abbreviations
| ACPA | Anti-citrullinated protein antibodies |
| ANA | Antinuclear antibodies |
| ANCA | Anti-neutrophil cytoplasmic antibodies |
| APRIL | A proliferation-inducing ligand |
| ASC | Antibody-secreting cell |
| BAFF | B-cell activating factor |
| BCR | B-cell receptor |
| BLIMP-1 | B lymphocyte-induced maturation protein-1 |
| CAR-T | Chimeric antigen receptor T cell |
| CD | Cluster of differentiation |
| CITE-seq | Cellular indexing of transcriptomes and epitopes by sequencing |
| CMV | Cytomegalovirus |
| COVID-19 | Coronavirus disease 2019 |
| CpG | Cytosine-phosphate-guanine |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CXCR | C-X-C chemokine receptor |
| DNA | Deoxyribonucleic acid |
| DORIS | Definition of remission in SLE |
| dsDNA | Double-stranded deoxyribonucleic acid |
| EBV | Epstein–Barr virus |
| EF | Extrafollicular |
| GC | Germinal centre |
| HDAC | Histone deacetylase |
| HHV-6 | Human herpesvirus 6 |
| HLA | Human leukocyte antigen |
| IFN | Interferon |
| IFN-I | Type I interferon |
| IFN-α | Interferon-alpha |
| Ig | Immunoglobulin |
| IgG | Immunoglobulin G |
| IL | Interleukin |
| IL-6 | Interleukin-6 |
| IL-21 | Interleukin-21 |
| IRF4 | Interferon regulatory factor 4 |
| ISG | Interferon-stimulated gene |
| JAK | Janus kinase |
| LPS | Lipopolysaccharide |
| MDA5 | Melanoma differentiation-associated protein 5 |
| MPO | Myeloperoxidase |
| NET | Neutrophil extracellular trap |
| NETosis | Neutrophil extracellular trap formation |
| PAD | Peptidylarginine deiminase |
| PAMP | Pathogen-associated molecular pattern |
| pDC | Plasmacytoid dendritic cell |
| PR3 | Proteinase 3 |
| PRDM1 | PR domain zinc finger protein 1 |
| RA | Rheumatoid arthritis |
| RNA | Ribonucleic acid |
| RNP | Ribonucleoprotein |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SCFA | Short-chain fatty acid |
| scRNA-seq | Single-cell RNA sequencing |
| SLE | Systemic lupus erythematosus |
| SLEDAI | Systemic Lupus Erythematosus Disease Activity Index |
| Sm | Smith antigen |
| STAT | Signal transducer and activator of transcription |
| STAT3 | Signal transducer and activator of transcription 3 |
| Tfh | T follicular helper |
| TLR | Toll-like receptor |
| TLR2 | Toll-like receptor 2 |
| TLR4 | Toll-like receptor 4 |
| TLR7 | Toll-like receptor 7 |
| TLR8 | Toll-like receptor 8 |
| TLR9 | Toll-like receptor 9 |
| TYK2 | Tyrosine kinase 2 |
| VZV | Varicella-zoster virus |
References
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef]
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Primers 2016, 2, 16039. [Google Scholar] [CrossRef] [PubMed]
- James, J.A.; Robertson, J.M. Lupus and Epstein-Barr. Curr. Opin. Rheumatol. 2012, 24, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D.; Holers, V.M. Rheumatoid arthritis pathogenesis, prediction, and prevention: An emerging paradigm shift. Arthritis Rheumatol. 2021, 73, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Mariette, X. Sjögren syndrome-associated lymphomas: An update on pathogenesis and management. Br. J. Haematol. 2015, 168, 317–327. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Primers 2021, 7, 86. [Google Scholar] [CrossRef]
- Draborg, A.H.; Duus, K.; Houen, G. Epstein-Barr virus in systemic autoimmune diseases. Clin. Dev. Immunol. 2013, 2013, 535738. [Google Scholar] [CrossRef]
- Varani, S.; Landini, M.P. Cytomegalovirus-induced immunopathology and its clinical consequences. Herpesviridae 2011, 2, 6. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. COVID-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Smatti, M.K.; Cyprian, F.S.; Nasrallah, G.K.; Al Thani, A.A.; Almishal, R.O.; Yassine, H.M. Viruses and autoimmunity: A review on the potential interaction and molecular mechanisms. Viruses 2019, 11, 762. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Jenks, S.A.; Cashman, K.S.; Woodruff, M.C.; Lee, F.E.; Sanz, I. Extrafollicular responses in humans and SLE. Immunol. Rev. 2019, 288, 136–148. [Google Scholar] [CrossRef]
- Tipton, C.M.; Fucile, C.F.; Darce, J.; Chida, A.; Ichikawa, T.; Gregoretti, I.; Schieferl, S.; Hom, J.; Jenks, S.; Feldman, R.J.; et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 2015, 16, 755–765. [Google Scholar] [CrossRef]
- Jacobi, A.M.; Odendahl, M.; Reiter, K.; Bruns, A.; Burmester, G.R.; Radbruch, A.; Valet, G.; Lipsky, P.E.; Dörner, T. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003, 48, 1332–1342. [Google Scholar] [CrossRef]
- Mei, H.E.; Yoshida, T.; Sime, W.; Hiepe, F.; Thiele, K.; Manz, R.A.; Radbruch, A.; Dörner, T. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood 2009, 113, 2461–2469. [Google Scholar] [CrossRef]
- Tellier, J.; Nutt, S.L. Plasma cells: The programming of an antibody-secreting machine. Eur. J. Immunol. 2019, 49, 30–37. [Google Scholar] [CrossRef]
- Radbruch, A.; Muehlinghaus, G.; Luger, E.O.; Inamine, A.; Smith, K.G.C.; Dörner, T.; Hiepe, F. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006, 6, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Nehar-Belaid, D.; Hong, S.; Marber, R.; Chen, G.; Bolisetty, M.; Baisch, J.; Walters, L.; Punaro, M.; Rossi, R.J.; Chung, C.-H.; et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat. Immunol. 2020, 21, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Raso, F.; Liu, S.; Simpson, M.J.; Barton, G.M.; Mayer, C.T.; Acharya, M.; Muppidi, J.R.; Marshak-Rothstein, A.; Reboldi, A. Antigen receptor signaling and cell death resistance controls intestinal humoral response zonation. Immunity 2023, 56, 2373–2387.e8. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.C.; Ramonell, R.P.; Haddad, N.S.; Anam, F.A.; Rudolph, M.E.; Walker, T.A.; Truong, A.D.; Dixit, A.N.; Han, J.E.; Cabrera-Mora, M.; et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature 2022, 611, 139–147. [Google Scholar] [CrossRef]
- Sanz, I.; Wei, C.; Jenks, S.A.; Cashman, K.S.; Tipton, C.; Woodruff, M.C.; Hom, J.; Lee, F.E.-H. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front. Immunol. 2019, 10, 2458. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2022, 40, 413–442. [Google Scholar] [CrossRef]
- MacLennan, I.C.; Toellner, K.M.; Cunningham, A.F.; Serre, K.; Sze, D.M.-Y.; Zúñiga, E.; Cook, M.C.; Vinuesa, C.G. Extrafollicular antibody responses. Immunol. Rev. 2003, 194, 8–18. [Google Scholar] [CrossRef]
- William, J.; Euler, C.; Christensen, S.; Shlomchik, M.J. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 2002, 297, 2066–2070. [Google Scholar] [CrossRef]
- Soni, C.; Wong, E.B.; Domeier, P.P.; Khan, T.N.; Satoh, T.; Akira, S.; Rahman, Z.S.M. B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J. Immunol. 2014, 193, 4400–4414. [Google Scholar] [CrossRef]
- Jenks, S.A.; Cashman, K.S.; Zumaquero, E.; Marigorta, U.M.; Patel, A.V.; Wang, X.; Tomar, D.; Woodruff, M.C.; Simon, Z.; Bugrovsky, R.; et al. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 2018, 49, 725–739.e6. [Google Scholar] [CrossRef]
- Arazi, A.; Rao, D.A.; Berthier, C.C.; Davidson, A.; Liu, Y.; Hoover, P.J.; Chicoine, A.; Eisenhaure, T.M.; Jonsson, A.H.; Li, S.; et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 2019, 20, 902–914, Erratum in Nat Immunol. 2019, 20, 1404. https://doi.org/10.1038/s41590-019-0398-x. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G.; Allen, C.D.C. B cell responses: Cell interaction dynamics and decisions. Cell 2019, 177, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Soni, C.; Reizis, B. Self-DNA at the epicenter of SLE: Immunogenic forms, regulation, and effects. Front. Immunol. 2019, 10, 1601. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kuo, H.H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell 2020, 183, 143–157.e13. [Google Scholar] [CrossRef]
- Shapiro-Shelef, M.; Calame, K. Regulation of plasma-cell development. Nat. Rev. Immunol. 2005, 5, 230–242. [Google Scholar] [CrossRef]
- Sciammas, R.; Shaffer, A.L.; Schatz, J.H.; Zhao, H.; Staudt, L.M.; Singh, H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 2006, 25, 225–236. [Google Scholar] [CrossRef]
- Ding, C.; Chen, X.; Dascani, P.; Hu, X.; Bolli, R.; Zhang, H.-G.; McLeish, K.R.; Yan, J. STAT3 signaling in B cells is critical for germinal center maintenance and contributes to the pathogenesis of murine models of lupus. J. Immunol. 2016, 196, 4477–4486. [Google Scholar] [CrossRef]
- Avery, D.T.; Deenick, E.K.; Ma, C.S.; Simpson, N.; Chew, G.Y.; Chan, T.D.; Palendira, U.; Bustamante, J.; Boisson-Dupuis, S.; Choo, S.; et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 2010, 207, 155–171. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Jacobi, A.M.; Mei, H.; Hoyer, B.F.; Mumtaz, I.M.; Thiele, K.; Radbruch, A.; Burmester, G.-R.; Hiepe, F.; Dörner, T. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2010, 69, 305–308. [Google Scholar] [CrossRef]
- Harley, J.B.; James, J.A. Epstein-Barr virus infection induces lupus autoimmunity. Bull NYU Hosp. Jt. Dis. 2006, 64, 45–50. [Google Scholar]
- Bjørnevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Thorley-Lawson, D.A. Epstein-Barr virus: Exploiting the immune system. Nat. Rev. Immunol. 2001, 1, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.J.; Hochberg, D.; Rand, W.M.; Thorley-Lawson, D.A. EBV and systemic lupus erythematosus: A new perspective. J. Immunol. 2005, 174, 6599–6607. [Google Scholar] [CrossRef]
- Kang, I.; Quan, T.; Nolasco, H.; Park, S.-H.; Hong, M.S.; Crouch, J.; Pamer, E.G.; Howe, J.G.; Craft, J. Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J. Immunol. 2004, 172, 1287–1294. [Google Scholar] [CrossRef]
- Draborg, A.H.; Jørgensen, J.M.; Müller, H.; Nielsen, C.T.; Jacobsen, S.; Iversen, L.V.; Theander, E.; Nielsen, L.P.; Houen, G.; Duus, K. Epstein-Barr virus early antigen diffuse (EBV-EA/D)-directed immunoglobulin A antibodies in systemic lupus erythematosus patients. Scand. J. Rheumatol. 2012, 41, 280–289. [Google Scholar] [CrossRef]
- Broadley, I.; Pera, A.; Morrow, G.; Davies, K.A.; Kern, F. Expansions of cytotoxic CD4+CD28- T cells drive excess cardiovascular mortality in rheumatoid arthritis and other chronic inflammatory conditions and are triggered by CMV infection. Front. Immunol. 2017, 8, 195. [Google Scholar] [CrossRef]
- Asherson, R.A.; Cervera, R. Antiphospholipid antibodies and infections. Ann. Rheum. Dis. 2003, 62, 388–393. [Google Scholar] [CrossRef]
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin. Microbiol. Rev. 2015, 28, 313–335. [Google Scholar] [CrossRef]
- Caselli, E.; Di Luca, D. Molecular biology and clinical associations of Roseoloviruses human herpesvirus 6 and human herpesvirus 7. New Microbiol. 2007, 30, 173–187. [Google Scholar] [PubMed]
- Soldan, S.S.; Berti, R.; Salem, N.; Secchiero, P.; Flamand, L.; Calabresi, P.A.; Bhriain, S.N. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: Increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 1997, 3, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Dunn, N.; Kharlamova, N.; Fogdell-Hahn, A. The role of herpesvirus 6A and 6B in multiple sclerosis and epilepsy. Scand. J. Immunol. 2020, 92, e12984. [Google Scholar] [CrossRef] [PubMed]
- Broccolo, F.; Drago, F.; Cassina, G.; Fava, A.; Fusetti, L.; Matteoli, B.; Ceccherini-Nelli, L.; Sabbadini, M.G.; Lusso, P.; Parodi, A.; et al. Selective reactivation of human herpesvirus 6 in patients with autoimmune connective tissue diseases. J. Med. Virol. 2013, 85, 1925–1934. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. Update on varicella zoster virus vasculopathy. Curr. Infect. Dis. Rep. 2014, 16, 407. [Google Scholar] [CrossRef]
- Gilden, D.; White, T.; Khmeleva, N.; Heintzman, A.; Choe, A.; Boyer, P.J.; Grose, C.; Carpenter, J.E.; Rempel, A.; Bos, N.; et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology 2015, 84, 1948–1955. [Google Scholar] [CrossRef]
- Mitchell, B.M.; Font, R.L. Detection of varicella zoster virus DNA in some patients with giant cell arteritis. Invest Ophthalmol. Vis. Sci. 2001, 42, 2572–2577. [Google Scholar] [CrossRef]
- Melano, I.; Husni, M.E.; Piuzzi, N.S.; Foo, S.-S.; Chen, W. Emerging and underrecognized viral triggers of autoimmune inflammatory rheumatic disease flares. Nat. Rev. Rheumatol. 2025. [Google Scholar] [CrossRef]
- Ledoult, E.; Guerrier, T.; Dubucquoi, S.; Figeac, M.; Villenet, C.; Daunou, B.; Behal, H.; Pokeerbux, M.R.; Machet, T.; Koether, V.; et al. Lille COVID Research Network (LICORNE). Dual role of plasmablasts as immune regulators or amplifiers in COVID-19. Clin. Immunol. 2025, 281, 110609. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Jamil, R.; Chowdhury, A.; Mukherjee, M.; Venegas, C.; Miyasaki, K.; Zhang, K.; Patel, Z.; Salter, B.; Yuen, A.C.Y.; et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID syndrome. J. Autoimmun. 2023, 137, 103024. [Google Scholar] [CrossRef]
- Cervia-Hasler, C.; Brüningk, S.C.; Hoch, T.; Fan, B.; Muzio, G.; Thompson, R.C.; Ceglarek, L.; Meledin, R.; Westermann, P.; Emmenegger, M.; et al. Persistent complement dysregulation with signs of thromboinflammation in active long COVID. Science 2024, 383, eadg7942. [Google Scholar] [CrossRef] [PubMed]
- Wegner, N.; Wait, R.; Sroka, A.; Eick, S.; Nguyen, K.-A.; Lundberg, K.; Kinloch, A.; Culshaw, S.; Potempa, J.; Venables, P.J. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2662–2672. [Google Scholar] [CrossRef]
- Potempa, J.; Mydel, P.; Koziel, J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 606–620. [Google Scholar] [CrossRef]
- Cheng, Z.; Do, T.; Mankia, K.; Meade, J.; Hunt, L.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Emery, P.; Devine, D. Dysbiosis in the oral microbiomes of anti-CCP positive individuals at risk of developing rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 162–168. [Google Scholar] [CrossRef]
- Leadbetter, E.A.; Rifkin, I.R.; Hohlbaum, A.M.; Beaudette, B.C.; Shlomchik, M.J.; Marshak-Rothstein, A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 2002, 416, 603–607. [Google Scholar] [CrossRef]
- Marshak-Rothstein, A.; Rifkin, I.R. Immunologically active autoantigens: The role of toll-like receptors in the development of chronic inflammatory disease. Annu. Rev. Immunol. 2007, 25, 419–441. [Google Scholar] [CrossRef]
- Lau, C.M.; Broughton, C.; Tabor, A.S.; Akira, S.; Flavell, R.A.; Mamula, M.J.; Christensen, S.R.; Shlomchik, M.J.; Viglianti, G.A.; Rifkin, I.R.; et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 2005, 202, 1171–1177. [Google Scholar] [CrossRef]
- Brown, G.J.; Cañete, P.F.; Wang, H.; Medhavy, A.; Bones, J.; Roco, J.A.; He, Y.; Qin, Y.; Cappello, J.; Ellyard, J.I.; et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 2022, 605, 349–356. [Google Scholar] [CrossRef]
- Souyris, M.; Cenac, C.; Azar, P.; Daviaud, D.; Canivet, A.; Grunenwald, S.; Pienkowski, C.; Chaumeil, J.; Mejía, J.E.; Guéry, J.-C. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 2018, 3, eaap8855. [Google Scholar] [CrossRef] [PubMed]
- Rönnblom, L.; Pascual, V. The innate immune system in SLE: Type I interferons and dendritic cells. Lupus 2008, 17, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Jego, G.; Palucka, A.K.; Blanck, J.P.; Chalouni, C.; Pascual, V.; Bhardwaj, N. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 2003, 19, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.-C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef]
- Cassese, G.; Arce, S.; Hauser, A.E.; Lehnert, K.; Moewes, B.; Mostarac, M.; Muehlinghaus, G.; Szyska, M.; Radbruch, A.; Manz, R. APlasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 2003, 171, 1684–1690. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Mackay, F.; Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 2009, 9, 491–502. [Google Scholar] [CrossRef]
- Zegarra-Ruiz, D.F.; El Beidaq, A.; Iñiguez, A.J.; Lubrano Di Ricco, M.; Manfredo Vieira, S.; Ruff, W.E.; Mubiru, D.; Fine, R.L.; Sterpka, J.; Greiling, T.M.; et al. A diet-sensitive commensal Lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe 2019, 25, 113–127.e6. [Google Scholar] [CrossRef]
- Azzouz, D.; Omarbekova, A.; Heguy, A.; Schwudke, D.; Gisch, N.; Rovin, B.H.; Caricchio, R.; Buyon, J.P.; Alekseyenko, A.V.; Silverman, G.J. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann. Rheum. Dis. 2019, 78, 947–956. [Google Scholar] [CrossRef]
- Manfredo Vieira, S.; Hiltensperger, M.; Kumar, V.; Zegarra-Ruiz, D.; Dehner, C.; Khan, N.; Costa, F.R.C.; Tiniakou, E.; Greiling, T.; Ruff, W.; et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018, 359, 1156–1161, Erratum in Science 2018, 360, 6388. https://doi.org/10.1126/science.aar7201. [Google Scholar] [CrossRef]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, E.; Kim, S.W.; Suda, W.; Kawasumi, M.; Onawa, S.; Taguchi-Atarashi, N.; Morita, H.; Taylor, T.D.; Hattori, M.; Ohno, H. Gut microorganisms act together to exacerbate inflammation in spinal cords. Nature 2020, 585, 102–106. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky gut as a danger signal for autoimmune diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef]
- Ogunrinde, E.; Zhou, Z.; Luo, Z.; Alekseyenko, A.; Li, Q.-Z.; Macedo, D.; Kamen, D.L.; Oates, J.C.; Gilkeson, G.S.; Jiang, W. A link between plasma microbial translocation, microbiome, and autoantibody development in first-degree relatives of systemic lupus erythematosus patients. Arthritis Rheumatol. 2019, 71, 1858–1868. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef]
- Pitzalis, C.; Jones, G.W.; Sherlock, J.P.; Jones, S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 2014, 14, 447–462. [Google Scholar] [CrossRef]
- Hauser, A.E.; Debes, G.F.; Arce, S.; Cassese, G.; Hamann, A.; Radbruch, A.; Manz, R.A. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J. Immunol. 2002, 169, 1277–1282. [Google Scholar] [CrossRef]
- Odendahl, M.; Jacobi, A.; Hansen, A.; Feist, E.; Hiepe, F.; Burmester, G.R.; Lipsky, P.E.; Radbruch, A.; Dörner, T. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 2000, 165, 5970–5979. [Google Scholar] [CrossRef]
- Moser, K.; Tokoyoda, K.; Radbruch, A.; MacLennan, I.; Manz, R.A. Stromal niches, plasma cell differentiation and survival. Curr. Opin. Immunol. 2006, 18, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Mietzner, B.; Tsuiji, M.; Scheid, J.; Velinzon, K.; Tiller, T.; Abraham, K.; Gonzalez, J.B.; Pascual, V.; Stichweh, D.; Wardemann, H.; et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc. Natl. Acad. Sci. USA 2008, 105, 9727–9732. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, M.R.; McClain, M.T.; Rubertone, M.V.; Scofield, R.H.; Dennis, G.J.; James, J.A.; Harley, J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003, 349, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Di Noia, J.M.; Bhattacharya, D. Activation-induced cytidine deaminase: Structure, functions, and regulation. Annu. Rev. Biochem. 2007, 76, 1–22. [Google Scholar] [CrossRef]
- Richardson, C.; Chida, A.S.; Engleman, E.; Sanz, I. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J. Immunol. 2013, 191, 4926–4939. [Google Scholar] [CrossRef]
- Vanderlugt, C.L.; Miller, S.D. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat. Rev. Immunol. 2002, 2, 85–95. [Google Scholar] [CrossRef]
- McClain, M.T.; Heinlen, L.D.; Dennis, G.J.; Roebuck, J.; Harley, J.B.; James, J.A. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat. Med. 2005, 11, 85–89. [Google Scholar] [CrossRef]
- Rosen, A.; Bhattacharya, D. Autoantigens as partners in initiation and propagation of autoimmune rheumatic diseases. Annu. Rev. Immunol. 2016, 34, 395–420. [Google Scholar] [CrossRef]
- Suurmond, J.; Diamond, B. Autoantibodies in systemic autoimmune diseases: Specificity and pathogenicity. J. Clin. Investig. 2015, 125, 2194–2202. [Google Scholar] [CrossRef]
- Weisel, F.; Shlomchik, M. Memory B cells of mice and humans. Annu. Rev. Immunol. 2017, 35, 255–284. [Google Scholar] [CrossRef]
- Tiller, T.; Tsuiji, M.; Yurasov, S.; Velinzon, K.; Nussenzweig, M.C.; Wardemann, H. Autoreactivity in human IgG+ memory B cells. Immunity 2007, 26, 205–213. [Google Scholar] [CrossRef]
- Manz, R.A.; Thiel, A.; Radbruch, A. Lifetime of plasma cells in the bone marrow. Nature 1997, 388, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Hiepe, F.; Dorner, T.; Hauser, A.E.; Hoyer, B.F.; Mei, H.; Radbruch, A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 2011, 7, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.W.; Dooley, M.A.; Hogan, S.L.; Anolik, J.; Looney, J.; Sanz, I.; Clarke, S.H. A novel subset of memory B cells is enriched in autoreactivity and correlates with adverse outcomes in SLE. Clin. Immunol. 2008, 126, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Nguyen, D.C.; Joyner, C.J.; Saney, C.L.; Tipton, C.M.; Andrews, J.; Lonial, S.; Kim, C.; Hentenaar, I.; Kosters, A.; et al. Understanding heterogeneity of human bone marrow plasma cell maturation and survival pathways by single-cell analyses. Cell Rep. 2023, 42, 112682. [Google Scholar] [CrossRef]
- Razonable, R.R. Antiviral drugs for viruses other than human immunodeficiency virus. Mayo Clin. Proc. 2011, 86, 1009–1026. [Google Scholar] [CrossRef]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S.; et al. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef]
- Furie, R.; Morand, E.F.; Bruce, I.N.; Manzi, S.; Kalunian, K.C.; Vital, E.M.; Ford, T.L.; Guber, R.; Pineda, L.; Tummala, R.; et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1 and TULIP-2): A randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019, 1, e208–e219. [Google Scholar] [CrossRef]
- Witte, T.; Fernandez-Ruiz, R.; Abramova, N.; Weinelt, D.; Moreau, F.; Klopp-Schulze, L.; Shaw, J.; Denis, D.; Wenzel, J. Enpatoran, a first-in-class, selective, orally administered toll-like receptor 7/8 inhibitor, in systemic and cutaneous lupus erythematosus: Results from a randomised, placebo-controlled phase Ib study. Lupus Sci. Med. 2025, 12, e001705. [Google Scholar] [CrossRef]
- Tanaka, Y.; Luo, Y.; O’Shea, J.J.; Nakayamada, S. Janus kinase-targeting therapies in rheumatology: A mechanisms-based approach. Nat. Rev. Rheumatol. 2022, 18, 133–145. [Google Scholar] [CrossRef]
- Lee, F.E.H.; Sanz, I. B cell depletion in autoimmune disease: Advances beyond anti-CD20 therapy. Nat. Rev. Rheumatol. 2024, 20, 25–40. [Google Scholar]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W.; et al. A phase III, randomized, placebo-controlled study of belimumab in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Rovin, B.H.; Houssiau, F.F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.-C.; Santiago, M.B.; et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.A.; Rovin, B.H.; Garg, J.P.; Santiago, M.B.; Aroca-Martínez, G.; Zuta Santillán, A.E.; Alvarez, D.; Navarro Sandoval, C.; Lila, A.M.; Tumlin, J.A.; et al. Efficacy and safety of obinutuzumab in active lupus nephritis. N. Engl. J. Med. 2025, 392, 1471–1483. [Google Scholar] [CrossRef]
- Kansal, R.; Richardson, N.; Neeli, I.; Khawaja, S.; Chamberlain, D.; Ghani, M.; Ghani, Q.-U.-A.; Balazs, L.; Beranova-Giorgianni, S.; Giorgianni, F.; et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 2019, 11, eaav1648. [Google Scholar] [CrossRef]
- Mackensen, A.; Müller, F.; Mougiakakos, D.; Böltz, S.; Wilhelm, A.; Aigner, M.; Völkl, S.; Simon, D.; Kleyer, A.; Munoz, L.; et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 2022, 28, 2124–2132, Erratum in Nat. Med. 2023, 29, 2956. https://doi.org/10.1038/s41591-022-02017-5. [Google Scholar] [CrossRef]
- Hagen, M.; Bucci, L.; Böltz, S.; Nöthling, D.M.; Rothe, T.; Anoshkin, K.; Raimondo, M.G.; Tur, C.; Wirsching, A.; Wacker, J.; et al. Grieshaber-Bouyer R.BCMA-Targeted T-Cell-Engager Therapy for Autoimmune Disease. N. Engl. J. Med. 2024, 391, 867–869. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Krönke, G.; Völkl, S.; Kretschmann, S.; Aigner, M.; Kharboutli, S.; Böltz, S.; Manger, B.; Mackensen, A.; Schett, G. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 2021, 385, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Boeltz, S.; Knitza, J.; Aigner, M.; Völkl, S.; Kharboutli, S.; Reimann, H.; Taubmann, J.; Kretschmann, S.; Rösler, W.; et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet 2023, 401, 815–818. [Google Scholar] [CrossRef]
- Müller, F.; Böltz, S.; Mougiakakos, D.; Mackensen, A. CAR T-cell therapy in autoimmune diseases. N. Engl. J. Med. 2024, 390, 1628–1629. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Dahan, S.; Segal, Y.; Shoenfeld, Y. Dietary factors in rheumatic autoimmune diseases: A recipe for therapy? Nat. Rev. Rheumatol. 2017, 13, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Pamer, E.G. Microbiome-based therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Humby, F.; Bombardieri, M.; Manzo, A.; Kelly, S.; Blades, M.C.; Kirkham, B.; Spencer, J.; Pitzalis, C. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009, 6, e1. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Mariette, X. B cells in the pathogenesis of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2018, 14, 133–145. [Google Scholar] [CrossRef]
- Specks, U.; Merkel, P.A.; Seo, P.; Spiera, R.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.M.; St. Clair, E.W.; Fessler, B.J.; Ding, L.; et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N. Engl. J. Med. 2013, 369, 417–427. [Google Scholar] [CrossRef]
- Greenberg, S.A. Type 1 interferons and myositis. Arthritis Res. Ther. 2010, 12, S4. [Google Scholar] [CrossRef]
- Crow, M.K.; Olferiev, M.; Kirou, K.A. Type I interferons in autoimmune disease. Annu. Rev. Pathol. 2019, 14, 369–393. [Google Scholar] [CrossRef]
- Papillion, A.; Bhattacharya, D. Tissue-resident memory B cells: Lost or found in translation? Annu. Rev. Immunol. 2023, 41, 85–108. [Google Scholar]
- Pisetsky, D.S.; Lipsky, P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 16, 565–579. [Google Scholar] [CrossRef]
- Danaher, P.; Hasle, N.; Nguyen, E.D.; Roberts, J.E.; Rosenwasser, N.; Rickert, C.; Hsieh, E.W.Y.; Hayward, K.; Okamura, D.M.; Alpers, C.E.; et al. Childhood-onset lupus nephritis is characterized by complex interactions between kidney stroma and infiltrating immune cells. Sci. Transl. Med. 2024, 16, eadl1666. [Google Scholar] [CrossRef]
- Schäfer, P.S.L.; Dimitrov, D.; Stark, S.G.; Theis, F.J.; Saez-Rodriguez, J. Integrating single-cell multi-omics and prior biological knowledge for a functional characterization of the immune system. Nat Immunol. 2024, 25, 405–417. [Google Scholar] [CrossRef]
- Kleshchevnikov, V.; Shmatko, A.; Dann, E.; Aivazidis, A.; King, H.W.; Li, T.; Elmentaite, R.; Lomakin, A.; Kedlian, V.; Gayoso, A.; et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat. Biotechnol. 2022, 40, 661–671. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |