Diagnostic Challenges of Cyclosporiasis in Chronic Diarrhea: A Case Study
Abstract
1. Introduction
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | Inflammatory Bowel Disease |
| UC | Ulcerative colitis |
| PCR | Polymerase chain reaction |
| DNA | Deoxyribonucleic acid |
| TNF | Tumor Necrosis Factor |
| JAK | Janus Kinase inhibitors |
| RBC | Red Blood Cell |
| CRP | C-reactive protein |
| MCV | Mean Corpuscular Volume |
| CT | Computed Tomography |
| BMI | Body Mass Index |
| Hb | Hemoglobin |
| CMV | Cytomegalovirus |
Appendix A
| Diagnostic Procedure | Details |
|---|---|
| Blood tests | Complete blood count (CBC) C-reactive protein (CRP) Renal function (creatinine, urea) Liver panel (AST, ALT, ALP, GGT, bilirubin, total protein, albumin) All were assessed using automated analyzers. Fecal calprotectin was measured using a quantitative ELISA (cut-off < 50 μg/g) [14,16]. |
| Serological tests | Viral serology included HBsAg Total anti-HBc Anti-HCV HIV-1/2 antigen/antibody (p24/Ab) Screening for latent tuberculosis was performed using an interferon-gamma release assay (IGRA, QuantiFERON test) [17,18]. |
| Stool examinations | Stool was performed for Salmonella spp., Shigella spp., pathogenic Escherichia coli, and Yersinia enterocolitica. Clostridioides difficile toxins A and B were detected by enzyme immunoassay. Parasitological examination included three stool samples collected at 48 h intervals. Ziehl–Neelsen staining was performed to identify oocysts of Cyclospora cayetanensis under light microscopy [1,4,22]. |
| Endoscopy | Gastroscopy was performed using standard high-definition video endoscopes. Colonoscopy and rectosigmoidoscopy were carried out using standard high-definition video endoscopes. Disease activity was graded using the Mayo endoscopic subscore (0–3), and disease extent was classified according to the Montreal classification [14,15,23]. |
| Histopathology | Biopsies from the colon and duodenum were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E). Colonic mucosa was evaluated for crypt architectural distortion, mucosal atrophy, and inflammatory infiltrates (plasma cells, eosinophils), in line with ECCO consensus criteria for ulcerative colitis [23,24]. Duodenal biopsies were assessed for intraepithelial lymphocytes and graded according to the Marsh classification. |
| Imaging | Contrast-enhanced computed tomography (CT) of the abdomen and pelvis was performed to assess colonic wall thickening, loss of haustration, and mesenteric lymph nodes. Abdominal ultrasound was used to evaluate bowel wall thickness and lymphadenopathy. |
References
- Flisiak, R. Chapter 83: Cyklosporoza. In Choroby Zakaźne i Pasożytnicze; Flisiak, R., Ed.; PZWL: Lublin, Poland, 2020; Volume IV, pp. 1027–1030. [Google Scholar]
- Virella, G. Chapter 45. Cyklosporoza. In Mikrobiologia i Choroby Zakaźne; Heczko, P.B., Ed.; MedPharm: Wrocław, Poland, 2000; pp. 435–439. [Google Scholar]
- Li, J.; Wang, R.; Chen, Y.; Xiao, L.; Zhang, L. Cyclospora cayetanensis infection in humans: Biological characteristics, clinical features, epidemiology, detection method and treatment. Parasitology 2020, 147, 160–170. [Google Scholar] [CrossRef]
- Kajfasz, P. Rzadkie Zespoły Biegunkowe Wywołane Przez Pierwotniaki. Gastroenterologia Praktyczna. 2022. Available online: https://gastroenterologia-praktyczna.pl/a2181/Rzadkie-zespoly-biegunkowe-wywolane-przez-pierwotniaki-Cyklosporoza.html/ (accessed on 16 September 2025).
- Pawłowski, Z.S.; Stefaniak, J. Chapter 27: Cyklosporoza. In Parazytologia Kliniczna w Ujęciu Wielodyscyplinarnym; PZWL: Warszawa, Poland, 2004; pp. 226–229. [Google Scholar]
- Shields, J.M.; Olson, B.H. Cyclospora cayetanensis: A review of an emerging parasitic coccidian. Int. J. Parasitol. 2003, 33, 371–391. [Google Scholar] [CrossRef]
- Herwaldt, B.L.; Ackers, M.L. An outbreak in 1996 of cyclosporiasis associated with imported raspberries. The Cyclospora Working Group. N. Engl. J. Med. 1997, 336, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Toruner, M.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Orenstein, R.; Sandborn, W.J.; Colombel, J.; Egan, L.J. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008, 134, 929–936. [Google Scholar] [CrossRef]
- Gong, S.S.; Fan, Y.H.; Han, Q.Q.; Lv, B.; Xu, Y. Nested case-control study on risk factors for opportunistic infections in patients with inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, M.; Kunisaki, R.; Yoshimura, N.; Takeuchi, Y.; Watanabe, M. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J. Gastroenterol. 2013, 48, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Purohit, T.; Razonable, R.; Loftus, E.V., Jr. Opportunistic infections due to inflammatory bowel disease therapy. Inflamm. Bowel Dis. 2014, 20, 196–212. [Google Scholar] [CrossRef]
- Beaugerie, L.; Rahier, J.F.; Kirchgesner, J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1324–1335. [Google Scholar] [CrossRef]
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology 2018, 155, 337–346. [Google Scholar] [CrossRef]
- Bryant, P.A.; Baddley, J.W. Opportunistic Infections in Biological Therapy, Risk and Prevention. Rheum. Dis. Clin. N. Am. 2017, 43, 27–41. [Google Scholar] [CrossRef]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European evidence-based consensus on the prevention, diagnosis, and management of opportunistic infections in inflammatory bowel disease. J. Crohn’s Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Ellul, P.; Greuter, T.; Rahier, J.F.; Verstockt, B.; Abreu, C.; Albuquerque, A.; Allocca, M.; Esteve, M.; Farraye, F.A.; et al. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 879–913. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Facciorusso, A.; Dulai, P.S.; Jairath, V.; Sandborn, W.J. Comparative risk of serious infections with biologic and/or immunosuppressive therapy in patients with inflammatory bowel diseases: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 69–81. [Google Scholar] [CrossRef]
- Cohen, R.D.; Bhayat, F.; Blake, A.; Travis, S. The Safety Profile of Vedolizumab in Ulcerative Colitis and Crohn’s Disease: Four years of global post-marketing data. J. Crohns Colitis 2020, 14, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Yazdanpanah, Y.; Colombel, J.F.; Travis, S.P. The European (ECCO) consensus on opportunistic infections in IBD. J. Crohns Colitis 2009, 3, 47–91. [Google Scholar] [CrossRef]
- Abanyie, F.; Harvey, R.R.; Harris, J.R.; Wiegand, R.E.; Gaul, L.; Desvignes-Kendrick, M.; Irvin, K.; Williams, I.; Hall, R.L.; Herwaldt, B.; et al. 2013 multistate outbreaks of Cyclospora cayetanensis infections associated with fresh produce: Focus on the Texas investigations. Epidemiol. Infect. 2015, 143, 3451–3458. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Feagan, B.G. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Dignass, A.; Eliakim, R.; Magro, F.; Maaser, C.; Chowers, Y.; Geboes, K.; Mantzaris, G.; Reinisch, W.; Colombel, J.F.; Vermeire, S.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis. J. Crohns Colitis 2012, 6, 991–1030. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO guidelines on the therapeutics in ulcerative colitis: Medical treatment. J. Crohns Colitis. 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut–liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Mullish, B.H.; Kelly, C.; Fischer, M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019, 394, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castaño-Rodríguez, N. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohns Colitis 2017, 11, 1180–1199. [Google Scholar] [CrossRef]
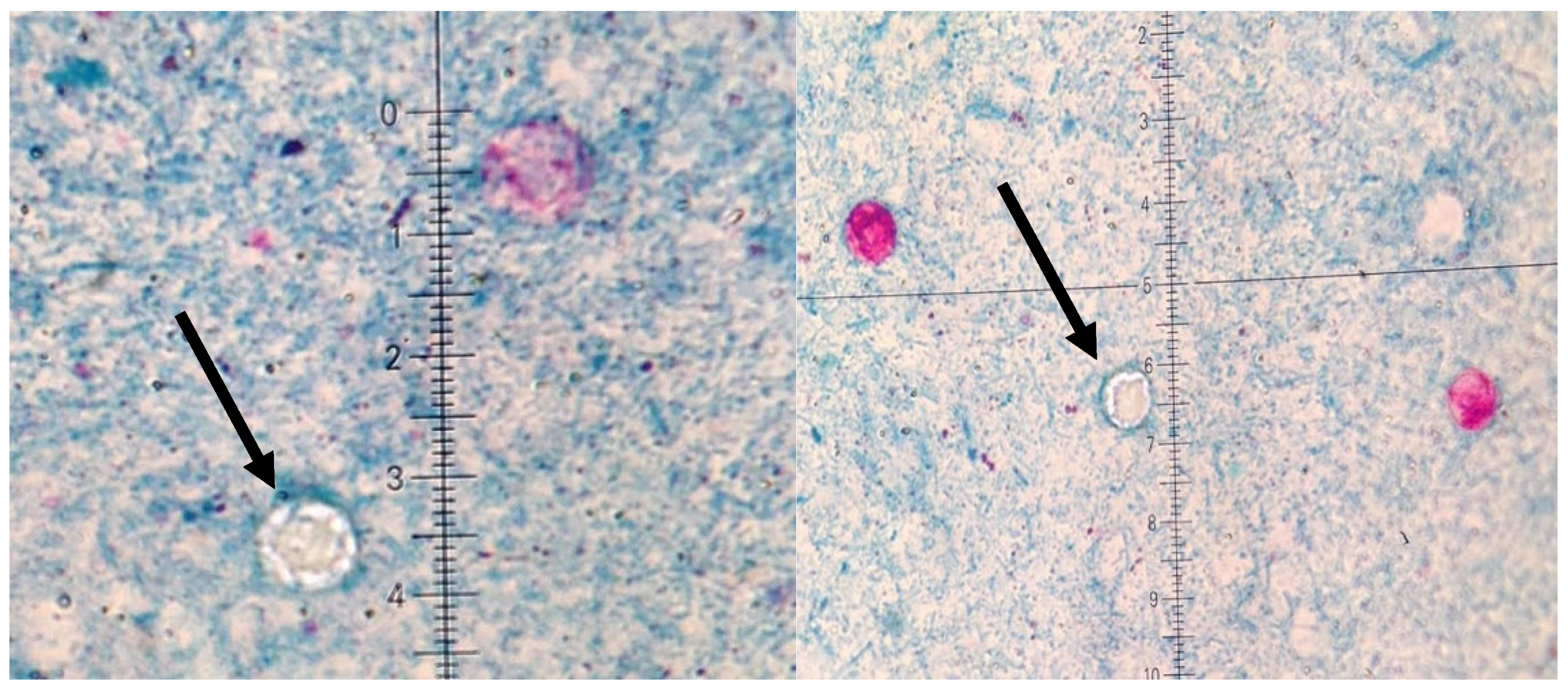
| Endoscopy | Images | Histopathology |
|---|---|---|
Gastroscopy:
| 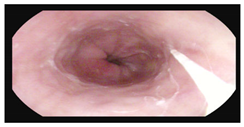  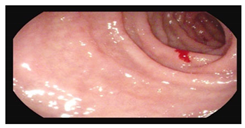 |
|
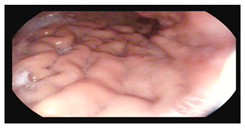
Colonoscopy (Mayo subscore 1):
| 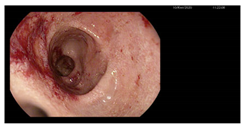 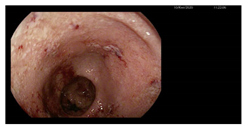 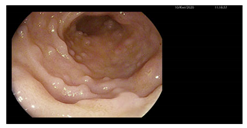 |
|
| Parameter/Time | UC Diagnosis | Before 1st Dose Vedolizumab | Before 2nd Dose | Before 4th Dose | Before 5th Dose | Cyclospora Infection | 8 Weeks Post Infection | 12 Weeks Post Infection |
|---|---|---|---|---|---|---|---|---|
| WBC (3.90–11.00) [×103/μL)] | 13.17 | 11.85 | 11.20 | 9.02 | 9.09 | 14.37 | 14.70 | 12.14 |
| RBC (3.50–5.20) [×106/μL] | 4.31 | 3.32 | 3.12 | 3.53 | 3.11 | 3.31 | 3.42 | 3.62 |
| Hgb (12.0–15.6) [g/dL] | 8.6 | 7.2 | 6.9 | 8.2 | 6.8 | 6.7 | 9.4 | 10.5 |
| MCV (80.0–99.0) [fL] | 68.7 | 74.7 | 73.7 | 74.8 | 74.0 | 68.0 | 91.8 | 94.5 |
| PLT (130–400) [×103/μL)] | 665 | 667 | 607 | 483 | 521 | 614 | 519 | 547 |
| Fe (37–145) [μg/dL] | 56 | - | - | - | - | 8 | - | - |
| CRP (<5.0) [mg/L] | 5.8 | 6.1 | 4.9 | 18.2 | 2.9 | 99.4 | 6.8 | 4.0 |
| Creatynin (0.50–0.90) [mg/dL] | 1.16 | 1.07 | 1.04 | 0.96 | 0.93 | 3.38 | 0.93 | 1.03 |
| Calprotectin (<50) [μg/g] | >800.00 | - | - | - | - | 190.00 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banasik, E.; Dobrowolska, A.; Woźnicka-Leśkiewicz, L.; Eder, P. Diagnostic Challenges of Cyclosporiasis in Chronic Diarrhea: A Case Study. Microorganisms 2025, 13, 2209. https://doi.org/10.3390/microorganisms13092209
Banasik E, Dobrowolska A, Woźnicka-Leśkiewicz L, Eder P. Diagnostic Challenges of Cyclosporiasis in Chronic Diarrhea: A Case Study. Microorganisms. 2025; 13(9):2209. https://doi.org/10.3390/microorganisms13092209
Chicago/Turabian StyleBanasik, Estera, Agnieszka Dobrowolska, Lucyna Woźnicka-Leśkiewicz, and Piotr Eder. 2025. "Diagnostic Challenges of Cyclosporiasis in Chronic Diarrhea: A Case Study" Microorganisms 13, no. 9: 2209. https://doi.org/10.3390/microorganisms13092209
APA StyleBanasik, E., Dobrowolska, A., Woźnicka-Leśkiewicz, L., & Eder, P. (2025). Diagnostic Challenges of Cyclosporiasis in Chronic Diarrhea: A Case Study. Microorganisms, 13(9), 2209. https://doi.org/10.3390/microorganisms13092209








