Acanthospermum australe Extract Inhibits the Chaperone Activity of Plasmodium falciparum Heat Shock Protein 70-1
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Collection and Extract Preparation
2.3. Gas Chromatography–Mass Spectrometry Analysis
2.4. Plasmodium Lactate Dehydrogenase (pLDH) Assay
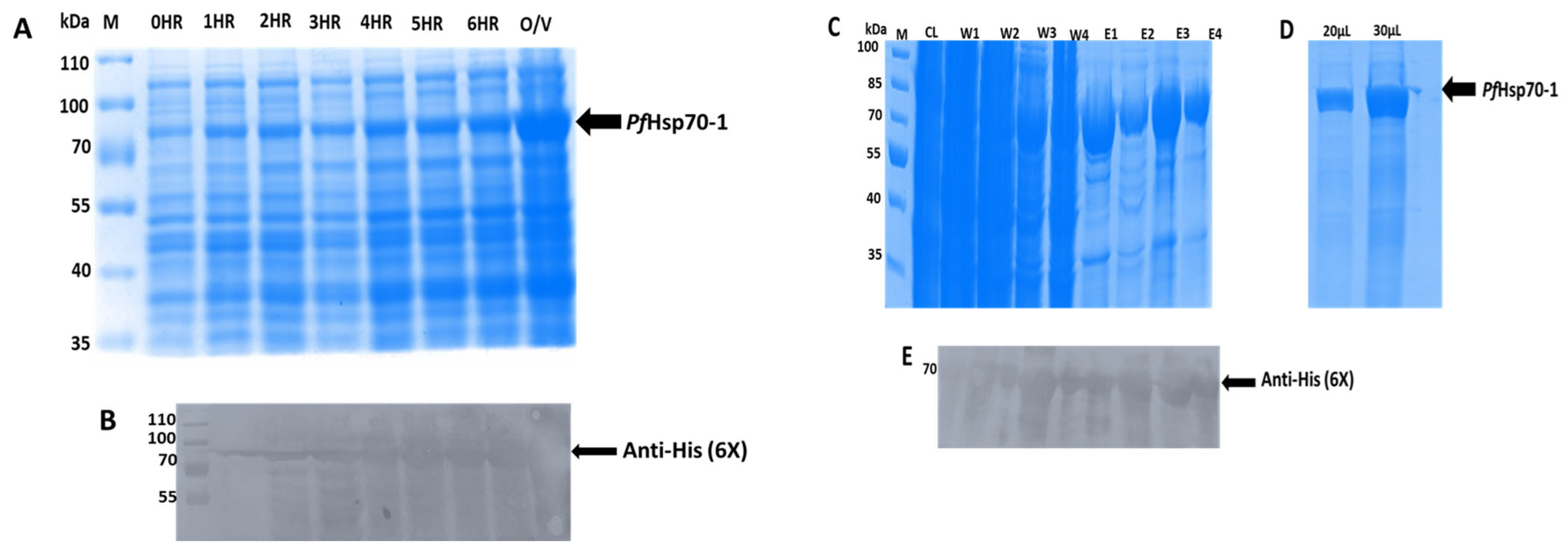
2.5. Transformation and Expression of Recombinant PfHsp70-1 Protein
2.6. Purification of Expressed PfHsp70-1 Protein
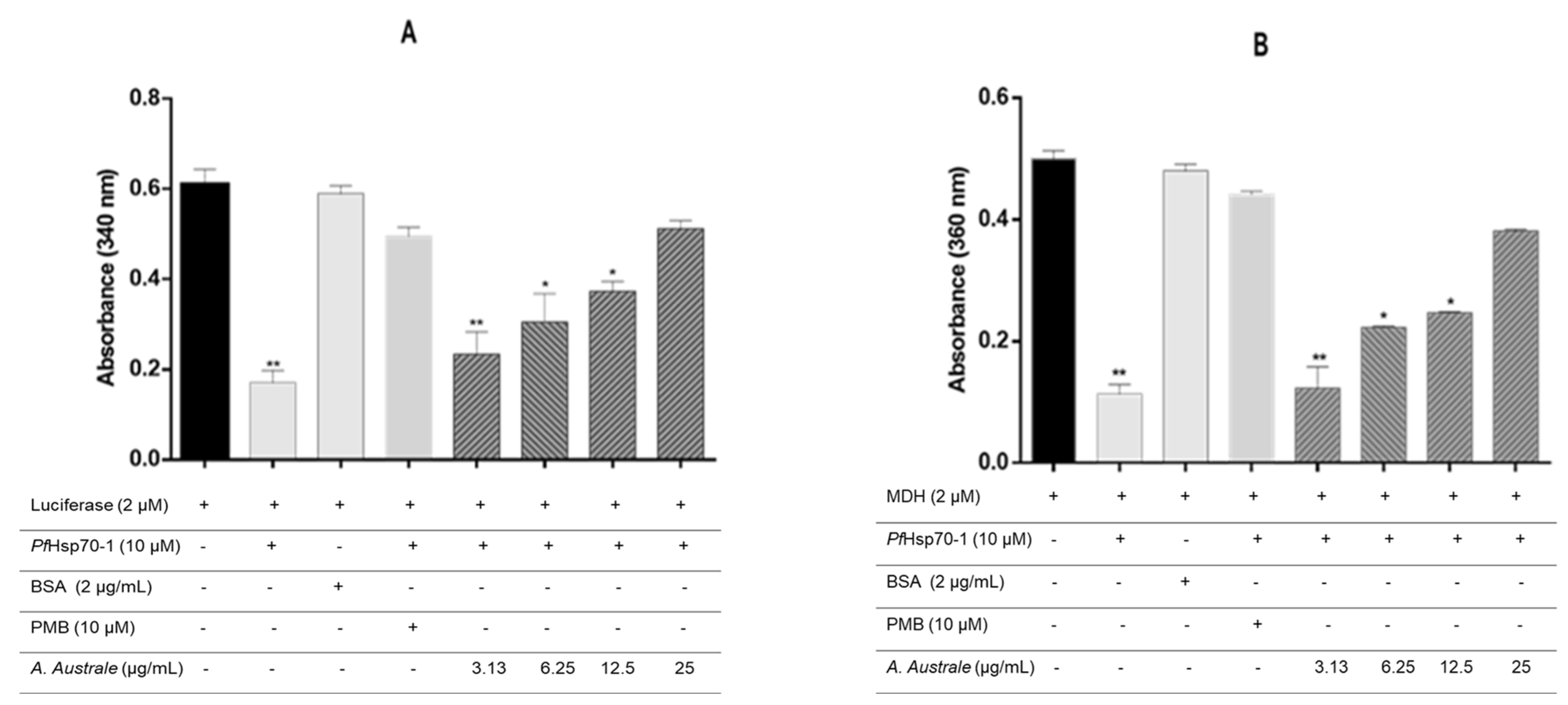
2.7. Malate Dehydrogenase and Luciferase Aggregation Assay
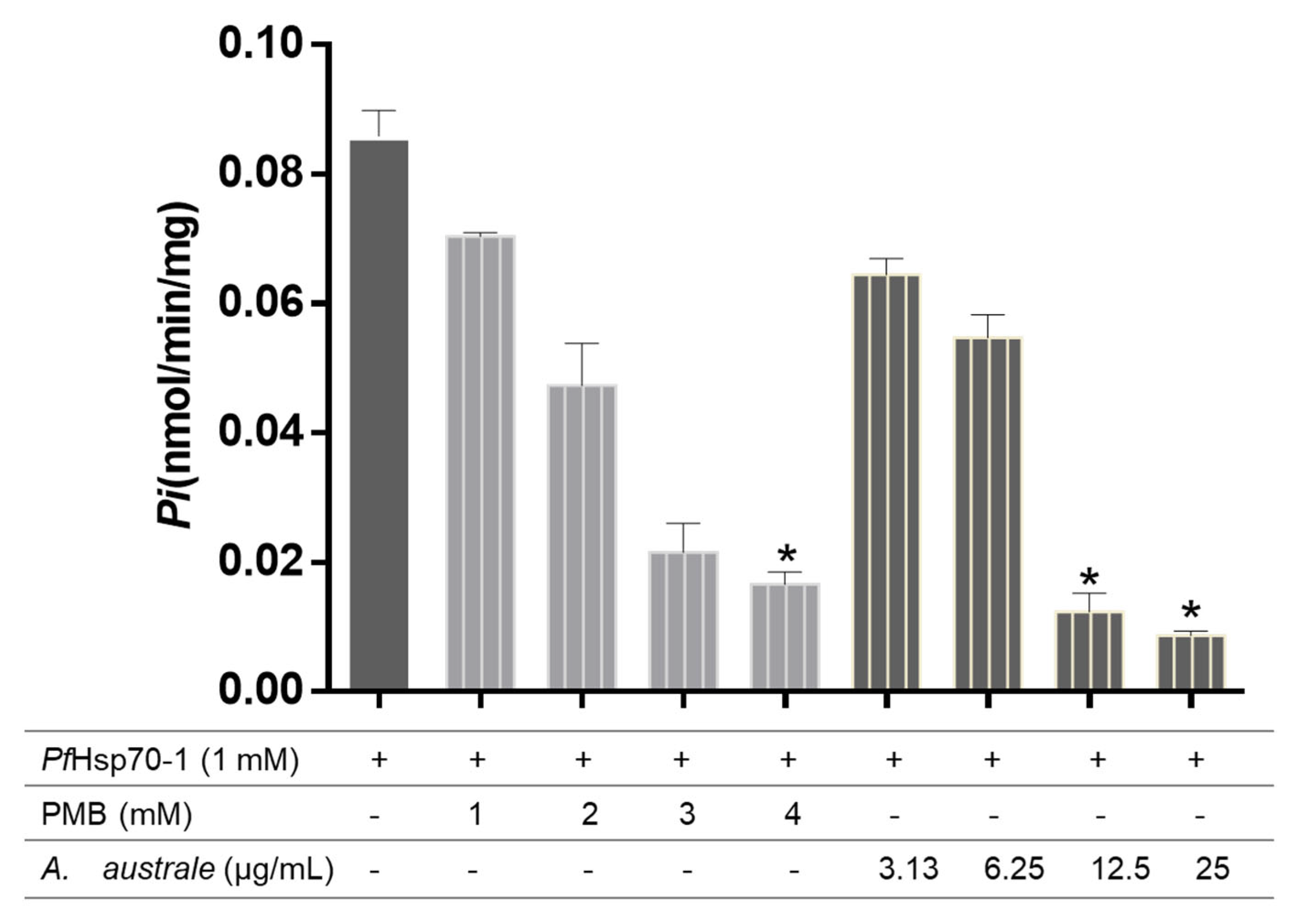
2.8. ATPase Assay
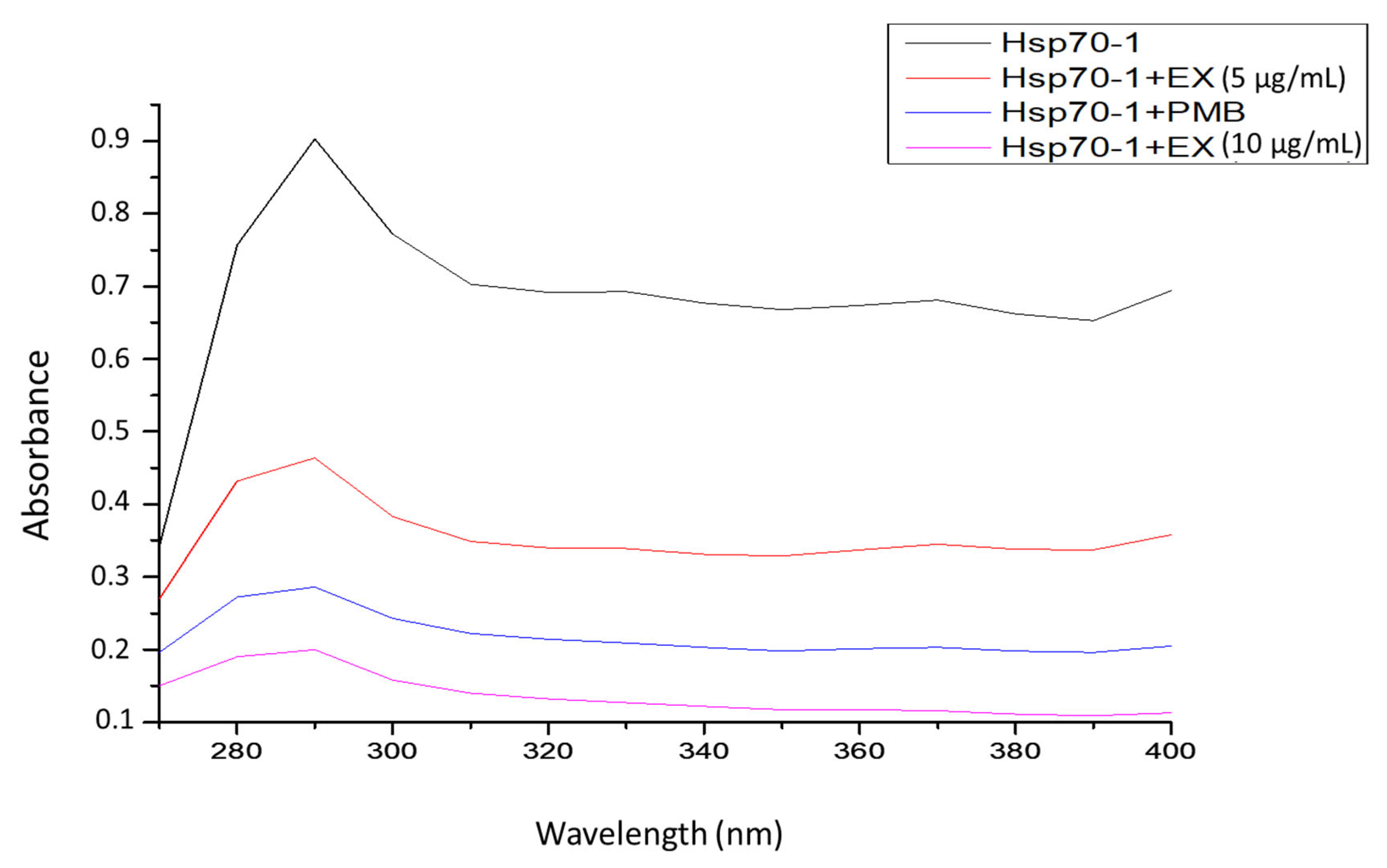
2.9. UV-Vis Spectrometer Analysis
2.10. Statistical Analysis
3. Results
3.1. Chemical Profiling of the DCM Extract Using GC-MS
3.2. Antiplasmodial Activity
3.3. The Expression and Purification of PfHs70-1 Recombinant Protein
3.4. The Extract Suppresses the Chaperone Activity of PfHsp70-1
3.5. A. australe Extract Inhibits PfHsp70-1 ATPase Activity
3.6. UV-Vis Analysis of the Interaction of PfHsp70-1 with the Plant Extract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCM | Dichloromethane |
| GAE | Gallic Acid Equivalent |
| GC-MS | Gas chromatography–mass spectrometer |
| IPTG | Isopropyl-β-D-thiogalactopyranoside |
| MDH | Malate dehydrogenase |
| PfHsp | Plasmodium falciparum heat shock protein |
| PMB | Polymyxin B |
| QE | Quercetin |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| UV-vis | Ultraviolet-visible (UV-vis) |
References
- Zekar, L.; Sharman, T. Malaria (Plasmodium Falciparum); StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Haakenstad, A.; Harle, A.C.; Tsakalos, G.; Micah, A.E.; Tao, T.; Anjomshoa, M.; Cohen, J.; Fullman, N.; Hay, S.I.; Mestrovic, T.; et al. Tracking spending on malaria by source in 106 countries, 2000–2016: An economic modelling study. Lancet Infect. Dis. 2019, 19, 703–716. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2020: 20 Years of Global Progress and Challenges; World Malaria Report; WHO: Geneva, Switzerland, 2020; pp. 1–151. [Google Scholar]
- Ceravolo, I.P.; Zani, C.L.; Figueiredo, F.J.; Kohlhoff, M.; Santana, A.E.; Krettli, A.U. Aspidosperma pyrifolium, a medicinal plant from the Brazilian caatinga, displays a high antiplasmodial activity and low cytotoxicity. Malar. J. 2018, 17, 436. [Google Scholar] [CrossRef]
- Mathenge, P.G.; Low, S.K.; Vuong, N.L.; Mohamed, M.Y.F.; Faraj, H.A.; Alieldin, G.I.; Yahia, N.A.; Khan, A.; Diab, O.M.; Mohamed, Y.M.; et al. Efficacy and resistance of different artemisinin-based combination therapies: A systematic review and network meta-analysis. Parasitol. Int. 2020, 74, 101919. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.; Feitosa, L.M.; Silveira, F.F.; Boechat, N. Current antimalarial therapies and advances in the development of semi-synthetic artemisinin derivatives. An. Da Acad. Bras. De Ciências 2018, 90, 1251–1271. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J. Quinolines block every step of malaria heme crystal growth. Proc. Natl. Acad. Sci. USA 2017, 114, 7483–7485. [Google Scholar] [CrossRef] [PubMed]
- Torrente, M.P.; Shorter, J. The metazoan protein disaggregase and amyloid depolymerase system: Hsp110, Hsp70, Hsp40, and small heat shock proteins. Prion 2013, 7, 457–463. [Google Scholar] [CrossRef]
- Cockburn, I.L.; Boshoff, A.; Pesce, E.R.; Blatch, G.L. Selective modulation of plasmodial Hsp70s by small molecules with antimalarial activity. Biol. Chem. 2014, 395, 1353–1362. [Google Scholar] [CrossRef]
- Zininga, T.; Anokwuru, C.P.; Sigidi, M.T.; Tshisikhawe, M.P.; Ramaite, I.I.; Traoré, A.N.; Hoppe, H.; Shonhai, A.; Potgieter, N. Extracts obtained from Pterocarpus angolensis DC and Ziziphus mucronata exhibit antiplasmodial activity and inhibit heat shock protein 70 (Hsp70) function. Molecules 2017, 22, 1224. [Google Scholar] [CrossRef]
- Daniyan, M.O.; Przyborski, J.M.; Shonhai, A. Partners in mischief: Functional networks of heat shock proteins of Plasmodium falciparum and their influence on parasite virulence. Biomolecules 2019, 9, 295. [Google Scholar] [CrossRef]
- Mallmann, R.; Ethur, E.M.; Bianchetti, P.; Faleiro, D.; Hoehne, L.; Goettert, M.I. Effectiveness of aqueous and hydroalcoholic extracts of Acanthospermum australe (Loefl.) Kuntze against diarrhea-inducing bacteria. Braz. J. Biol. 2018, 78, 619–624. [Google Scholar] [CrossRef]
- Nethengwe, M.F.; Opoku, A.R.; Dludla, P.V.; Madida, K.T.; Shonhai, A.; Smith, P.; Singh, M. Larvicidal, antipyretic and antiplasmodial activity of some Zulu medicinal plants. J. Med. Plants Res. 2012, 6, 1255–1262. [Google Scholar] [CrossRef]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Gibbins, B.L.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium failciparum drugs sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar] [CrossRef]
- Salomane, N.; Pooe, O.J.; Simelane, M.B. Iso-mukaadial acetate and ursolic acid acetate inhibit the chaperone activity of Plasmodium falciparum heat shock protein 70-1. Cell Stress Chaperones 2021, 26, 685–693. [Google Scholar] [CrossRef]
- Shonhai, A.; Botha, M.; de Beer, T.A.; Boshoff, A.; Blatch, G.L. Structure-function study of a Plasmodium falciparum Hsp70 using three-dimensional modelling and in vitro analyses. Protein Pept. Lett. 2008, 15, 1117–1125. [Google Scholar] [CrossRef]
- Opoku, F.; Govender, P.P.; Pooe, O.J.; Simelane, M.B. Evaluating iso-mukaadial acetate and ursolic acid acetate as plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase inhibitors. Biomolecules 2019, 9, 861. [Google Scholar] [CrossRef]
- Cockburn, I.L.; Pesce, E.R.; Pryzborski, J.M.; Davies-Coleman, M.T.; Clark, P.G.; Keyzers, R.A. Screening for small molecule modulators of Hsp70 chaperone activity using protein aggregation suppression assays: Inhibition of the plasmodial chaperone PfHsp70-1. Biol. Chem. 2011, 392, 431–438. [Google Scholar] [CrossRef]
- Mabate, B.; Zininga, T.; Ramatsui, L.; Makumire, S.; Achilonu, I.; Dirr, H.W.; Shonhai, A. Structural and biochemical characterization of Plasmodium falciparum Hsp70-x reveals functional versatility of its C-terminal EEVN motif. Proteins Struct. Funct. Bioinform. 2018, 86, 1189–1201. [Google Scholar] [CrossRef]
- Dey, P.; Goyary, D.; Chattopadhyay, P.; Kishor, S.; Karmakar, S.; Verma, A. Evaluation of larvicidal activity of Piper longum leaf against the dengue vector, Aedes aegypti, malarial vector, Anopheles stephensi and filariasis vector, Culex quinquefasciatus. S. Afr. J. Bot. 2020, 132, 482–490. [Google Scholar] [CrossRef]
- Starlin, T.; Prabha, P.S.; Thayakumar, B.K.A.; Gopalakrishnan, V.K. Screening and GC-MS profiling of ethanolic extract of Tylophora pauciflora. Bioinformation 2019, 15, 425. [Google Scholar] [CrossRef]
- Carey, A.F.; Wang, G.; Su, C.Y.; Zwiebel, L.J.; Carlson, J.R. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 2010, 464, 66–71. [Google Scholar] [CrossRef]
- Youssef, A.M.; Maaty, D.A.; Al-Saraireh, Y.M. Phytochemical Analysis and Profiling of Antitumor Compounds of Leaves and Stems of Calystegia silvatica (Kit.) Griseb. Molecules 2023, 28, 630. [Google Scholar] [CrossRef]
- Kuley, E.; Kuscu, M.M.; Durmus, M.; Ucar, Y. Inhibitory activity of Co-microencapsulation of cell free supernatant from Lactobacillus plantarum with propolis extracts towards fish spoilage bacteria. LWT 2021, 146, 111433. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Viljoen, A.M. Pharmacological interactions of essential oil constituents on the in vitro growth of Plasmodium falciparum. S. Afr. J. Bot. 2010, 76, 662–667. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A.M. The biological activities of 20 naturally identical essential oil constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Weathers, P.J.; Towler, M.; Hassanali, A.; Lutgen, P.; Engeu, P.O. Dried-leaf Artemisia annua: A practical malaria therapeutic for developing countries. World J. Pharmacol. 2014, 3, 39. [Google Scholar] [CrossRef]
- Motshudi, M.C.; Olaokun, O.O.; Mkolo, N.M. Evaluation of GC× GC-TOF-MS untargeted metabolomics, cytotoxicity and antimicrobial activity of leaf extracts of Artemisia afra (Jacq.) purchased from three local vendors. J. King Saud Univ.Sci. 2021, 33, 101422. [Google Scholar] [CrossRef]
- Singh, N.; Mansoori, A.; Jiwani, G.; Solanke, A.U.; Thakur, T.K.; Kumar, R.; Chaurasiya, M.; Kumar, A. Antioxidant and antimicrobial study of Schefflera vinosa leaves crude extracts against rice pathogens. Arab. J. Chem. 2021, 14, 103243. [Google Scholar] [CrossRef]
- Claessens, A.K.; Ibragimova, K.I.; Geurts, S.M.; Bos, M.E.; Erdkamp, F.L.; Tjan-Heijnen, V.C. The role of chemotherapy in the treatment of advanced breast cancer: An overview for clinical practice. Crit. Rev. Oncol. Hematol. 2020, 153, 102988. [Google Scholar] [CrossRef]
- Murugesan, K.; Mulugeta, K.; Hailu, E.; Tamene, W.; Yadav, S.A. Insights for integrative medicinal potentials of Ethiopian Kale (Brassica carinata): Investigation of antibacterial, antioxidant potential and phytocompounds composition of its leaves. Chin. Herb. Med. 2021, 13, 250–254. [Google Scholar] [CrossRef]
- Jabeen, N.; Khan, I.H.; Javaid, A. Fungicidal potential of leaf extracts of Datura metel L. to control Sclerotium rolfii Sacc. Allelopath. J. 2022, 56, 59–68. [Google Scholar] [CrossRef]
- Suryanti, V.; Kusumaningsih, T.; Marliyana, S.D.; Setyono, H.A.; Trisnawati, E.W. Identification of active compounds and antioxidant activity of teak (Tectona grandis) leaves. J. Biol. Divers. 2020, 21. [Google Scholar] [CrossRef]
- Mailafiya, M.M.; Yusuf, A.J.; Abdullahi, M.I.; Aleku, G.A.; Ibrahim, I.A.; Yahaya, M.; Abubakar, H.; Sanusi, A.; Adamu, H.W.; Alebiosu, C.O. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J. Med. Plants Econ. Dev. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Yang, J.; Ma, X.; Zheng, S.; Deng, S.; Huang, Y.; Yang, X.; Zhao, P. Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food Nutr. Res. 2017, 61, 1364117. [Google Scholar] [CrossRef]
- Waiganjo, B.; Moriasi, G.; Onyancha, J.; Elias, N.; Muregi, F. Antiplasmodial and cytotoxic activities of extracts of selected medicinal plants used to treat malaria in Embu County, Kenya. J. Parasitol. Res. 2020, 2020, 8871375. [Google Scholar] [CrossRef]
- Solana, J.C.; Bernardo, L.; Moreno, J.; Aguado, B.; Requena, J.M. The Astonishing Large Family of HSP40/DnaJ Proteins Existing in Leishmania. Genes 2022, 13, 742. [Google Scholar] [CrossRef]
- Aitken, A.; Learmonth, M.P. Protein determination by UV absorption. In The Protein Protocols Handbook; Humana Press: Totowa, NJ, USA, 2002; pp. 3–6. [Google Scholar]
| Peak Number | Retention Time (s) | Compound Name | Peak Area % | Molecular Formula | Molecular Weight (g/mol) | Bioactivities | Reference(s) |
|---|---|---|---|---|---|---|---|
| 1 | 1496.4 | n-Hexadecanoic acid | 0.98394 | C16H32O2 | 18 | Larvicidal, anticancer, hemolytic | [20,21] |
| 2 | 1635.8 | Octadecanoic acid | 0.91725 | C18H36O2 | 25 | Hepato-protective, antioxidant, larvicidal | [21,22] |
| 3 | 1826.6 | (S,Z)-Heptadeca-1,9-dien-4,6-diyn-3-ol | 0.53102 | C17H24O | 244 | Antitumor | [23] |
| 4 | 1844.7 | 1,6-Heptadien-4-ol | 1.9095 | C7H12O | 776 | Antimicrobial | [24] |
| 5 | 1990.8 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl- | 0.22794 | C15H26O | 698 | Anti-parasitic, skin-repellent, antimalarial | [25,26,27,28] |
| 6 | 2032.7 | 5-Benzofuranacetic acid, 6-ethenyl-2,4,5,6,7,7a-hexahydro-3,6-dimethyl-à-methylene-2-oxo-, methyl ester | 22.564 | C16H20O4 | 705 | Anticonvulsant, anti-inflammatory, antimicrobial | [29] |
| 7 | 2075.1 | Methyl 10,12-pentacosadiynoate | 2.3019 | C26H44O2 | 729 | Antimicrobial | [30] |
| 8 | 2122.7 | Fluoxymesterone | 2.6697 | C20H29FO3 | 561 | Anticancer | [31] |
| 9 | 2124.6 | Estra-1,3,5(10)-trien-17á-ol | 2.7174 | C18H24O | 645 | Anti-estrogenic | [32] |
| 10 | 2162.9 | 1-Eicosanol | 0.57624 | C20H42O | 916 | Antioxidant, fungicidal | [33,34] |
| 11 | 2250.9 | Stigmasterol | 0.56558 | C29H48O | 869 | Antibacterial, antifungal, anticancer | [35,36] |
| Extract | Concentration (µg/mL) | IC50 (µg/mL) | |
|---|---|---|---|
| 10 | 50 | ||
| A. australe | 72.09 ± 3.84 ** | 94.84 ± 3.07 ** | 1.3 ± 0.11 |
| Chloroquine | - | - | 3.12 |
| Samples | Maximum Absorbance | |
|---|---|---|
| 290 nm | 400 nm | |
| PfHsp70-1 only (1 mM) | 0.897 | 0.69 |
| PfHsp70-1 with PMB (10 mM) | 0.283 | 0.198 |
| PfHsp70-1 with A. australe (5 µg/mL) | 0.461 | 0.357 |
| PfHsp70-1 with A. australe (10 µg/mL) | 0.203 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koza, N.A.; Myeza, N.N.; Hoppe, H.; Mosa, R.A.; Kappo, A.P.; Simelane, M.B.C.; Opoku, A.R. Acanthospermum australe Extract Inhibits the Chaperone Activity of Plasmodium falciparum Heat Shock Protein 70-1. Microorganisms 2025, 13, 2195. https://doi.org/10.3390/microorganisms13092195
Koza NA, Myeza NN, Hoppe H, Mosa RA, Kappo AP, Simelane MBC, Opoku AR. Acanthospermum australe Extract Inhibits the Chaperone Activity of Plasmodium falciparum Heat Shock Protein 70-1. Microorganisms. 2025; 13(9):2195. https://doi.org/10.3390/microorganisms13092195
Chicago/Turabian StyleKoza, Ntombikhona Appear, Ntokozo Nkosinathi Myeza, Heinrich Hoppe, Rebamang Anthony Mosa, Abidemi Paul Kappo, Mthokozisi Blessing Cedric Simelane, and Andrew Rowland Opoku. 2025. "Acanthospermum australe Extract Inhibits the Chaperone Activity of Plasmodium falciparum Heat Shock Protein 70-1" Microorganisms 13, no. 9: 2195. https://doi.org/10.3390/microorganisms13092195
APA StyleKoza, N. A., Myeza, N. N., Hoppe, H., Mosa, R. A., Kappo, A. P., Simelane, M. B. C., & Opoku, A. R. (2025). Acanthospermum australe Extract Inhibits the Chaperone Activity of Plasmodium falciparum Heat Shock Protein 70-1. Microorganisms, 13(9), 2195. https://doi.org/10.3390/microorganisms13092195








