Genomic and Transcriptomic Profiling of a Highly Virulent Plesiomonas shigelloides Strain: Insights into Pathogenicity and Host Immune Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Identification
2.2. Physicochemical Property Measurement and Antibiotic Susceptibility Test
2.3. Whole-Genome Sequencing and Genome Annotation
2.4. Bacterial and Fish Preparation
2.5. Infection and Sample Collection
2.6. Histopathological Analysis
2.7. Reverse Transcription Quantitative PCR (RT-qPCR) and Statistical Analysis
2.8. Transcriptome Sequencing and Analysis
3. Results
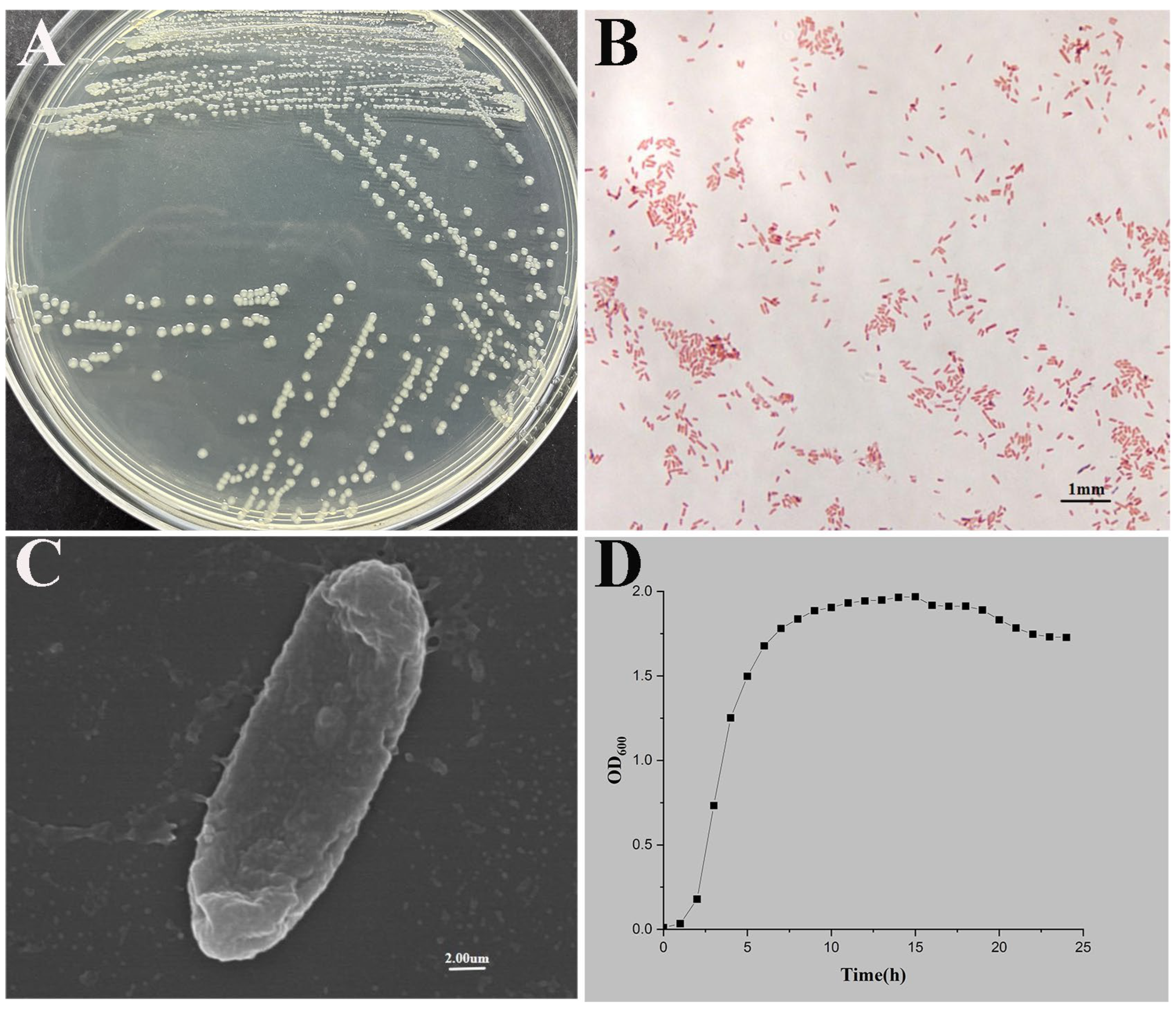
3.1. Bacterial Isolation and Identification
3.2. Physicochemical Characteristics of the Isolated Bacteria
3.3. Antibiotic Susceptibility Test
3.4. Whole-Genome Sequencing and Genome Annotation
3.5. Pathogenicity Analysis
3.6. Histopathological Analysis
3.7. Liver Immune-Related Gene Expression Levels
3.8. Transcriptome Sequencing and Analysis of DEGs
3.9. GO Enrichment Analysis of DEGs
3.10. KEGG Pathways Enrichment Analysis of DEGs
3.11. Analysis of KEGG Pathway for DEGs Associated with Immunity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, L.; Liu, S.Y.; Cheng, X.W.; Tao, Y.T.; Yang, T.; Li, P.P.; Wang, Z.X.; Shao, D.G.; Zhang, D.F. Isolation, identification and genomic analysis of Plesiomonas shigelloides isolated from diseased Percocypris pingi (Tchang, 1930). Am. J. Biochem. Biotechnol. 2017, 13, 226–232. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Liu, Y.; Yan, L.H.; Yan, Q.G.; Wen, X.T.; Cao, S.J.; Huang, Y.; Huang, X.B.; Ma, X.P.; Han, X.F.; et al. Identification and pathogenicity of Plesiomonas shigelloides from Acipenser dabryanus in China. Aquac. Res. 2021, 52, 2286–2293. [Google Scholar] [CrossRef]
- Ekundayo, T.C.; Okoh, A.I. Antimicrobial resistance in freshwater Plesiomonas shigelloides isolates: Implications for environmental pollution and risk assessment. Environ. Pollut. 2020, 257, 113493. [Google Scholar] [CrossRef]
- Shinohara, T.; Okamoto, K.; Koyano, S.; Otani, A.; Yamashita, M.; Wakimoto, Y.; Jubishi, D.; Hashimoto, H.; Ikeda, M.; Harada, S.; et al. Plesiomonas shigelloides septic shock following ingestion of Dojo Nabe (Loach Hotpot). Open Forum Infect. Dis. 2021, 8, ofab401. [Google Scholar] [CrossRef] [PubMed]
- Yaikhan, T.; Singkhamanan, K.; Dechathai, T.; Chukamnerd, A.; Chusri, S.; Pomwised, R.; Wonglapsuwan, M.; Surachat, K. Genome-based alert on a clinical Plesiomonas shigelloides PSU59 from Thailand: Resistance and virulence features. Infect. Genet. Evol. 2025, 132, 105764. [Google Scholar] [CrossRef]
- Larsen, A.M.; Mohammed, H.H.; Arias, C.R. Characterization of the gut microbiota of three commercially valuable warm water fish species. J. Appl. Microbiol. 2014, 116, 1396–1404. [Google Scholar] [CrossRef]
- Hu, Q.; Lin, Q.; Shi, C.; Fu, X.; Li, N.; Liu, L.; Wu, S. Isolation and identification of a pathogenic Plesiomonas shigelloides from diseased grass carp. Wei Sheng Wu Xue Bao 2014, 54, 229–235. (In Chinese) [Google Scholar]
- Chen, H.J.; Zhao, Y.L.; Chen, K.X.; Wei, Y.L.; Luo, H.R.; Li, Y.M.; Liu, F.; Zhu, Z.Y.; Hu, W.; Luo, D.J. Isolation, identification, and investigation of pathogenic bacteria from common carp (Cyprinus carpio) naturally infected with Plesiomonas shigelloides. Front. Immunol. 2022, 13, 872896. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.K.; Bera, A.K.; Paria, P.; Das, A.; Parida, P.K.; Kumari, S.; Bhowmick, S.; Das, B.K. Identification and pathogenicity of Plesiomonas shigelloides in Silver Carp. Aquaculture 2018, 493, 314–318. [Google Scholar] [CrossRef]
- Deng, D.; Mu, Z.Z.; Lv, X.Y.; Jiang, X.C.; Zhou, J.; Guo, H.Z.; Zhang, W.B.; Lu, Y.S.; Wu, J.P.; Du, H.; et al. Pathogenicity of Plesiomonas shigelloides and Citrobacter freundii isolated from the endangered Chinese sturgeon (Acipenser sinensis). Microb. Pathog. 2022, 173, 105818. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Chen, X.M.; Wan, J.W.; Yang, Z.N.; Tian, J.X.; Qian, A.D.; Wang, G.Q. A northern snakehead (Channa argus) model of intestinal inflammation induced by Aeromonas hydrophila: Construction and transcriptome analysis. Aquaculture 2024, 580, 740323. [Google Scholar] [CrossRef]
- Siddaiah, G.M.; Kumar, R.; Kumari, R.; Chandan, N.K.; Debbarma, J.; Damle, D.K.; Das, A.; Giri, S.S. Dietary fishmeal replacement with Hermetia illucens (Black soldier fly, BSF) larvae meal affected production performance, whole body composition, antioxidant status, and health of snakehead (Channa striata) juveniles. Anim. Feed. Sci. Technol. 2023, 297, 115597. [Google Scholar] [CrossRef]
- Kong, Y.D.; Li, M.; Chu, G.S.; Liu, H.J.; Shan, X.F.; Wang, G.Q.; Han, G.H. The positive effects of single or conjoint administration of lactic acid bacteria on Channa argus: Digestive enzyme activity, antioxidant capacity, intestinal microbiota and morphology. Aquaculture 2021, 531, 735852. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L.; McIver, C.J. Plesiomonas shigelloides Revisited. Clin. Microbiol. Rev. 2016, 29, 349–374. [Google Scholar] [CrossRef]
- Ciznár, I.; Hostacka, A.; Gonzalez-Rey, C.; Krovacek, K. Potential virulence-associated properties of Plesiomonas shigelloides strains. Folia Microbiol. 2004, 49, 543–548. [Google Scholar] [CrossRef]
- Salerno, A.; Cižnár, I.; Krovacek, K.; Conte, M.; Dumontet, S.; González-Rey, C.; Pasquale, V. Phenotypic characterization and putative virulence factors of human, animal and environmental isolates of Plesiomonas shigelloides. Folia Microbiol. 2010, 55, 641–647. [Google Scholar] [CrossRef]
- Johnson, C.I.; Martinello, P.; Collier, F. Haematoxylin and eosin staining of osmium-fixed tissue in epoxy sections. Med. Lab. Sci. 1982, 39, 371–375. [Google Scholar] [PubMed]
- Zhang, P.; Yao, H.; Ji, L.; Chen, L.; Xu, D.; Yan, W. Pathogenic characteristics of an aggregated diarrhea event caused by Plesiomonas shigelloides from stream. PLoS ONE 2024, 19, e0301623. [Google Scholar] [CrossRef]
- Jiao, S.Q.; Shen, Z.Q.; Fang, Q.Y.; Liu, X.R.; Hao, Y.K.; Kong, Y.D.; Peng, S.B.; Li, M.; Wang, G.Q. Toxic effects of microplastics on freshwater fish (Channa argus): Mechanisms of inflammation, apoptosis, and autophagy. Aquat. Toxicol. 2025, 286, 107450. [Google Scholar] [CrossRef]
- Chen, X.M.; Zhang, J.S.; Li, M.Y.; Tian, J.X.; Niu, X.T.; Shan, X.F.; Luo, S.; Wang, G.Q.; Qian, A.D. Liver transcriptome analysis and identification of differentially expressed immune gene response to Aeromonas veronii infection in Channa argus. Aquac. Int. 2023, 31, 1195–1211. [Google Scholar] [CrossRef]
- Cui, Z.W.; Li, D.Q.; Zhao, F.; Tan, A.P.; Deng, Y.T.; Lai, Y.T.; Huang, Z.B.; Jiang, L. Molecular characterization and functional analysis of IL-18 in snakehead (Channa argus) during Aeromonas schubertii and Nocardia seriolae infections. Mol. Immunol. 2021, 137, 212–220. [Google Scholar] [CrossRef]
- Ou, J.; Luo, W.S.; Zhong, Z.R.; Xie, Q.; Wang, F.; Xiong, N.X.; Luo, S.W. Manganese-superoxide dismutase (MnSOD) rescues redox balance and mucosal barrier function in midgut of hybrid fish (Carassius cuvieri♀ × Carassius auratus red var♂) infected with Aeromonas hydrophila and Edwardsiella tarda. Reprod. Breed. 2023, 3, 108–117. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Kovács, B.; Kuunya, R.; Mustafa, E.O.A.; Abbo, A.S.H.; Pál, K. Antibiotic Resistance in Aquaculture: Challenges, Trends Analysis, and Alternative Approaches. Antibiotics 2025, 14, 598. [Google Scholar] [CrossRef]
- Olivares, P.J.; Alvarez-Ortega, C.; Alcalde, R.M.; Martínez, J.L. Metabolic Compensation of Fitness Costs Is a General Outcome for Antibiotic-Resistant Pseudomonas aeruginosa Mutants Overexpressing Efflux Pumps. mBio 2017, 8, e00500-17. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Estanbouli, H.; Liao, C.; Kim, W.; Monk, J.M.; Rahman, R.; Kamboj, M.; Palsson, B.O.; Qiu, W.; Xavier, J.B. Systems-level analysis of NalD mutation, a recurrent driver of rapid drug resistance in acute Pseudomonas aeruginosa infection. PLoS Comput. Biol. 2019, 15, e1007562. [Google Scholar] [CrossRef]
- Acierno, C.; Barletta, F.; Nevola, R.; Rinaldi, L.; Sasso, F.C.; Adinolfi, L.E.; Caturano, A. Metabolic Rewiring of Bacterial Pathogens in Response to Antibiotic Pressure-A Molecular Perspective. Int. J. Mol. Sci. 2025, 26, 5574. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, D.; Wang, H.; Zhao, X.; Jiang, X.; Zhu, L.; Zhang, J.; Li, L.; Kong, X.; Pei, C. Mixed Infection in Common Carp (Cyprinus carpio) Caused by Aeromonas veronii, Aeromonas hydrophila, Plesiomonas shigelloides, and Citrobacter freundii. Animals 2025, 15, 805. [Google Scholar] [CrossRef]
- Li, D.; Cui, Z.; Zhao, F.; Zhu, X.; Tan, A.; Deng, Y.; Lai, Y.; Huang, Z. Characterization of snakehead (Channa argus) interleukin-21: Involvement in immune defense against two pathogenic bacteria, in leukocyte proliferation, and in activation of JAK-STAT signaling pathway. Fish Shellfish. Immunol. 2022, 123, 207–217. [Google Scholar] [CrossRef]
- Ndjoh, J.; Ntsama Junie Annick, M.; Etone, C.; Brian Ngokwe, Z.; Akena Ndeng, S.; Ngoulma, R.; Eno Belinga, L.; Ama Moor, V. The influence of the menstrual cycle on inflammatory markers: The cytokines Il-1β, IL-6, and TNF-α in the gingival crevicular fluid. J. Periodontal Implant. Sci. 2025, 55, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yang, M.; Perrett, S.; Wu, S. Single-molecule study of the dynamics of the molecular chaperone Hsp70 during the functional cycle. Biochem. Soc. Trans. 2025, 53, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.Z.; Ren, S.W.; Xiao, Y.F.; Tao, S.C.; Liu, J.M.; Yuan, X.Q.; Chen, X.H.; Mu, Y.N. SOCS3 acts as a potential negative regulator in the antiviral response of large yellow croaker (Larimichthys crocea) by interacting with STAT1. Water Biol. Secur. 2024, 3, 100270. [Google Scholar] [CrossRef]
- Wang, C.; Shu, Q.S.; Zeng, N.Y.; Xie, S.L.; Zou, J.X.; Tang, H.J.; Zhou, A.G. Immune response for acute Aeromonas hydrophila infection in two distinct color morphs of northern snakehead, Channa argus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 52, 101321. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Z.H.; Xia, G.K.; Lu, Z.F.; Li, X.Y.; Shen, Y.; Zhou, F.; Zhong, X.T.; Zhang, L.Q.; Wang, Z.Q. Identification of the mapkk gene family in large yellow croaker (Larimichthys crocea): Involved in the regulation of immune responses to Aeromonas hydrophila and Pseudomonas plecoglossicida infections. Fish Shellfish. Immunol. 2025, 166, 110595. [Google Scholar] [CrossRef]
- Sun, C.N.; Zhu, M.X.; Wang, L.Y.; Wen, H.S.; Qi, X.; Li, C.; Zhang, X.Y.; Sun, D.L.; Li, Y. Comprehensive genome-wide identification and functional characterization of mapk gene family in northern snakeheads (Channa argus). Fish Shellfish. Immunol. 2025, 157, 110076. [Google Scholar] [CrossRef]
- Veríssimo, A.; Castro, L.F.C.; Muñoz-Mérida, A.; Almeida, T.; Gaigher, A.; Neves, F.; Flajnik, M.F.; Ohta, Y. An Ancestral Major Histocompatibility Complex Organization in Cartilaginous Fish: Reconstructing MHC Origin and Evolution. Mol. Biol. Evol. 2023, 40, msad262. [Google Scholar] [CrossRef]
- Stanley, T.R.; Guisbert, K.S.K.; Perez, S.M.; Oneka, M.; Kernin, I.; Higgins, N.R.; Lobo, A.; Subasi, M.M.; Carroll, D.J.; Turingan, R.G.; et al. Stress response gene family expansions correlate with invasive potential in teleost fish. J. Exp. Biol. 2022, 225, 243–263. [Google Scholar] [CrossRef]
- Yang, J.; Tian, T.; Xiao, K.; Zeng, Q.K.; Tan, C.; Du, H.J. Pathogenic infection and immune-related gene expression of Chinese sturgeon (Acipenser sinensis) challenged by Citrobacter freundii. Dev. Comp. Immunol. 2021, 114, 103872. [Google Scholar] [CrossRef]
- Schischlik, F.; Jäger, R.; Rosebrock, F.; Hug, E.; Schuster, M.; Holly, R.; Fuchs, E.; Milosevic Feenstra, J.D.; Bogner, E.; Gisslinger, B.; et al. Mutational landscape of the transcriptome offers putative targets for immunotherapy of myeloproliferative neoplasms. Blood 2019, 134, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.G.; Xie, S.L.; Sun, D.; Liu, S.L.; Zhang, C.N.; Sun, Z.L.; Zhang, Y.; Chen, Y.F.; Zou, J.X. Expression of HSP70 family mRNAs in albino northern snakehead, Channa argus: Response to extreme temperature stress and bacterial infection. Fish Shellfish. Immunol. 2020, 104, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Xu, S.; Li, Y.W.; Xu, D.D.; Mo, Z.Q.; Li, J.Z.; Dan, X.M.; Luo, X.C. Role of major histocompatibility complex II antigen-presentation pathway genes in orange-spotted grouper infected with Cryptocaryon irritans. J. Fish Dis. 2020, 43, 1541–1552. [Google Scholar] [CrossRef]









| Gene Name | Forward Primer (5′-3′) | Backward Primer (5′-3′) | Size (bp) | Accession No. |
|---|---|---|---|---|
| IL-6 | CAGGTGATGAGGAGGTGGAG | TGAAGTTGGAGGCAGGACAT | 186 | XM_067503143.1 |
| IL-1β | GACACGATGCGATTCCTATTCT | CACTGGGCAGTCTTCTCGGA | 143 | XM_067519725.1 |
| IL-21 | ATATTGAGGACTGCTGCT | TGACTTGTAAGGCTTCTGT | 115 | XM_067519487.1 |
| STAT1 | AAGCACCTCCTCTCCAACTC | ACACAGCCTTGACTTTGAGC | 163 | XM_067501576.1 |
| HSP70 | TGTCATGGATGCAGCTCAGA | AGACTGACACCTGGTAACCG | 173 | XM_067492141.1 |
| β-actin | GTCTTCCCCTCCATCGTCG | TGGTCACAATACCGTGCTCG | 145 | XM_067476706.1 |
| Characteristic | P. shigelloides CA-HZ1 | Characteristic | P. shigelloides CA-HZ1 |
|---|---|---|---|
| Arabinitol | − | Mannitol | − |
| Glucose | + | Sucrose | − |
| Arabinose | − | L-RhaMnose | − |
| Hydrogen sulfide | − | Esculin | − |
| Citrate | − | Malonate | − |
| V-P test | − | Maltose | + |
| D-Xylose | − | Inositol | + |
| Nitrate reduction | + | Oxidase | + |
| Indole | + | Dulcitol | − |
| Sorbitol | − | Urea | − |
| Antibiotic | Concentration (μg/Piece) | Test Diameter of the Inhibition Zone (mm) | Sensitivity |
|---|---|---|---|
| Amikacin | 30 | 15 | I |
| Gentamicin | 120 | 19 | S |
| Tobramycin | 10 | 20 | S |
| Kanamycin | 30 | 11 | R |
| Streptomycin | 10 | 13 | I |
| Erythromycin | 15 | 7 | R |
| Medemycin | 30 | 6 | R |
| Norfloxacin | 10 | 27 | S |
| Levofloxacin | 5 | 31 | S |
| Ofloxacin | 5 | 32 | S |
| Ciprofloxacin | 5 | 32 | S |
| Polymyxin B | 300 | 15 | S |
| Clindamycin | 2 | 6 | R |
| Clarithromycin | 15 | 11 | R |
| Nitrofurantoin | 300 | 26 | S |
| Tetracycline | 30 | 27 | S |
| Aztreonam | 30 | 20 | S |
| Minocycline | 30 | 27 | S |
| Penicillin | 10 u | 13 | R |
| Oxacillin | 1 | 0 | R |
| Ampicillin | 10 u | 25 | S |
| Spectinomycin | 100 | 21 | S |
| Piperacillin | 100 | 0 | R |
| Cefoxitin | 30 | 25 | S |
| Cefazolin | 30 | 25 | S |
| Ceftofur | 30 | 20 | S |
| Cefotaxime | 30 | 27 | S |
| Cefepime | 30 | 25 | S |
| Cefuroxim | 30 | 29 | S |
| Ceftazidime | 30 | 24 | S |
| Ceftriaxone | 30 | 19 | I |
| Cefoperazone | 75 | 25 | S |
| Vancomycin | 30 | 21 | S |
| Pediatric compound sulfamethoxazole tablets | 23.75/1.25 | 23 | S |
| Chloramphenicol | 30 | 35 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gu, S.; Lv, W.; Chen, J.; Xue, M.; Liu, S.; Mao, J.; Chen, G. Genomic and Transcriptomic Profiling of a Highly Virulent Plesiomonas shigelloides Strain: Insights into Pathogenicity and Host Immune Response. Microorganisms 2025, 13, 2168. https://doi.org/10.3390/microorganisms13092168
Wang Z, Gu S, Lv W, Chen J, Xue M, Liu S, Mao J, Chen G. Genomic and Transcriptomic Profiling of a Highly Virulent Plesiomonas shigelloides Strain: Insights into Pathogenicity and Host Immune Response. Microorganisms. 2025; 13(9):2168. https://doi.org/10.3390/microorganisms13092168
Chicago/Turabian StyleWang, Zhixiu, Shaoxuan Gu, Wen Lv, Jiayi Chen, Min Xue, Suli Liu, Jiaming Mao, and Guohong Chen. 2025. "Genomic and Transcriptomic Profiling of a Highly Virulent Plesiomonas shigelloides Strain: Insights into Pathogenicity and Host Immune Response" Microorganisms 13, no. 9: 2168. https://doi.org/10.3390/microorganisms13092168
APA StyleWang, Z., Gu, S., Lv, W., Chen, J., Xue, M., Liu, S., Mao, J., & Chen, G. (2025). Genomic and Transcriptomic Profiling of a Highly Virulent Plesiomonas shigelloides Strain: Insights into Pathogenicity and Host Immune Response. Microorganisms, 13(9), 2168. https://doi.org/10.3390/microorganisms13092168





