Time-Course Gene Expression of ‘Candidatus Liberibacter solanacearum’, Prophage, and Wolbachia Genes in Bactericera cockerelli from Ingestion to in Planta Transmission
Abstract
1. Introduction
2. Materials and Methods
2.1. Potato Psyllid Insect Colony Establishment and Maintenance
2.2. Infection Rate, Transmission Efficiency, and ‘Ca. L. solanacearum’ Accumulation, or Load, in Infected Potato Psyllid Colonies
2.3. Tomato Source Plants for ‘Ca. L. solanacearum’ Acquisition-Access Period
2.4. Expression of ‘Ca. L. solanacearum’, Prophage, and Wolbachia Genes in Potato Psyllids at Different Inoculation-Access Periods Following a 48-h Acquisition-Access Period on Tomato
2.5. Selection of Genes and Loci for Reverse Transcription Quantitative PCR (RT-qPCR) Amplification
2.6. Polymerase Chain Reaction (PCR) and Reverse Transcriptase PCR Amplification, Cloning, and Sequencing
2.7. Extraction of RNA, cDNA Synthesis, and Reverse Transcriptase PCR
2.8. Reference Gene Selection for RT-qPCR amplification of ‘Ca. L. solanacearum’-Prophage and Wolbachia Genes/Loci
2.9. Quantitative Polymerase Chain Reaction Amplification
2.10. Statistical Analysis
3. Results
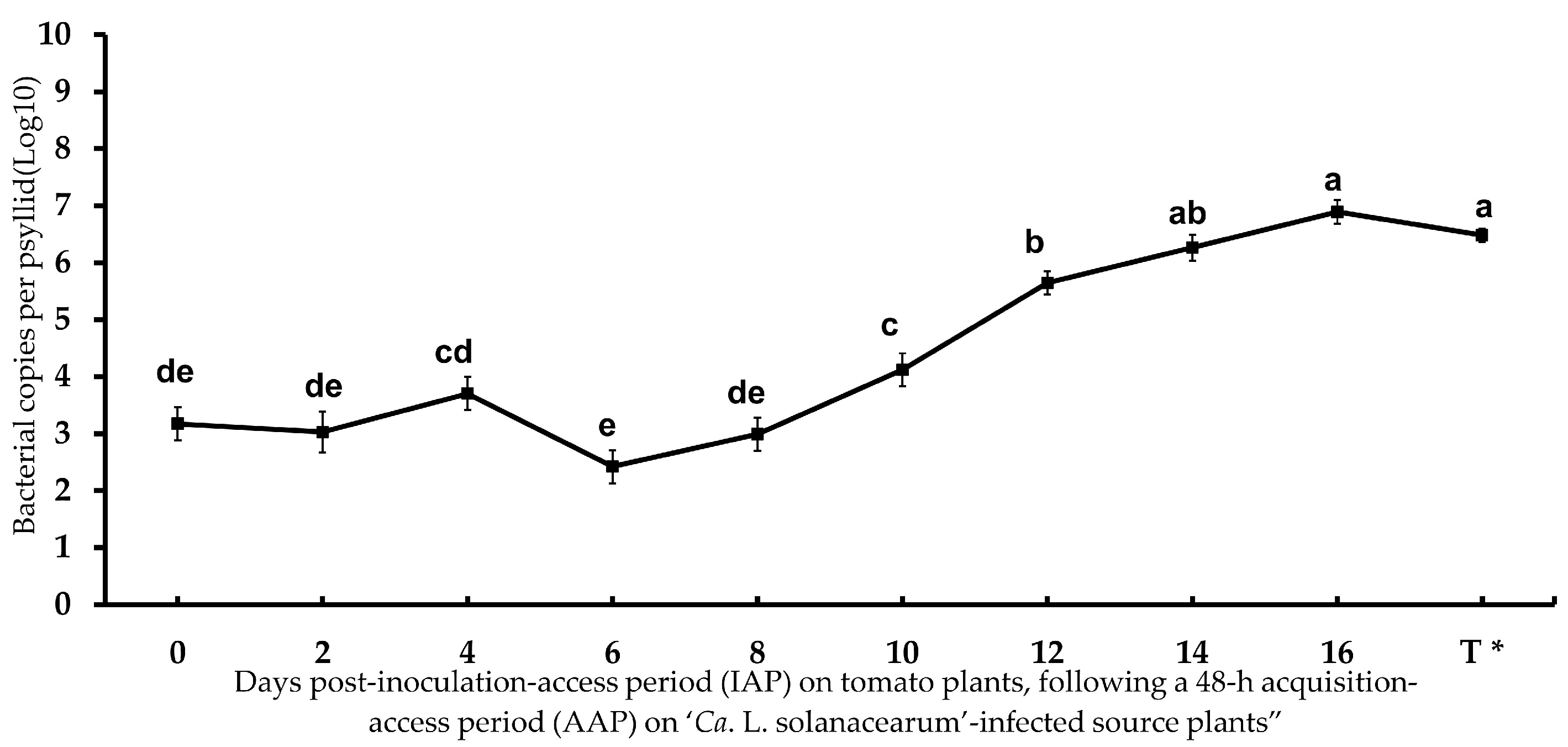
3.1. Transmission Efficiency and Infection Rate of ‘Ca. L. solanacearum’-Infected Potato Psyllid
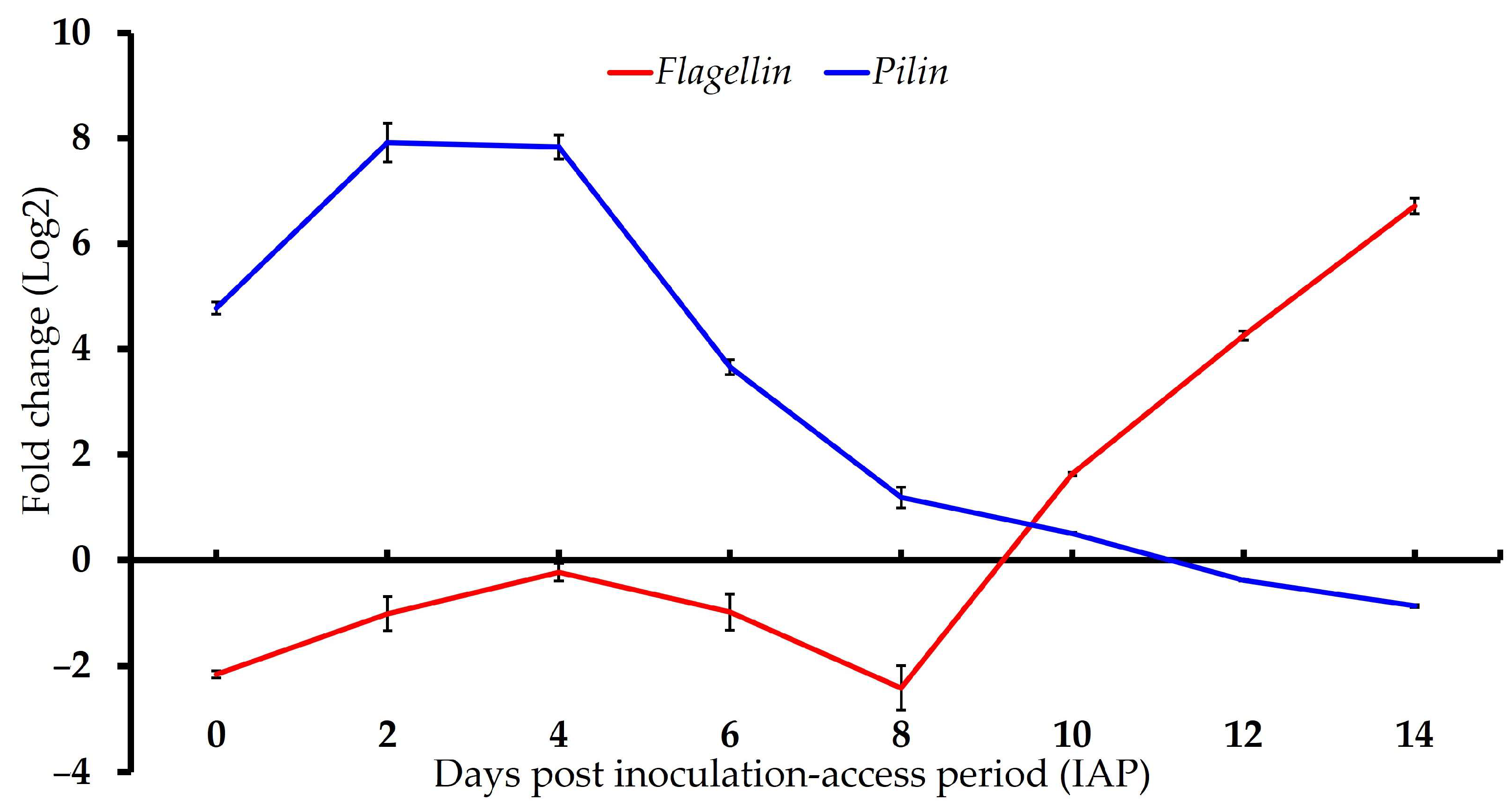
3.2. Gene Expression of ‘Ca. L. solanacearum’ Predicted Orthologs Encoding Putative Bacterial Flagella and Pili Components
3.3. Gene Expression of ‘Ca. L. solanacearum’ Predicted Orthologs Encoding Putative Virulence Effectors
3.4. Predicted ‘Ca. L. solanacearum’ Ortholog of Prophage Genes Associated with Phage Cycle Regulation
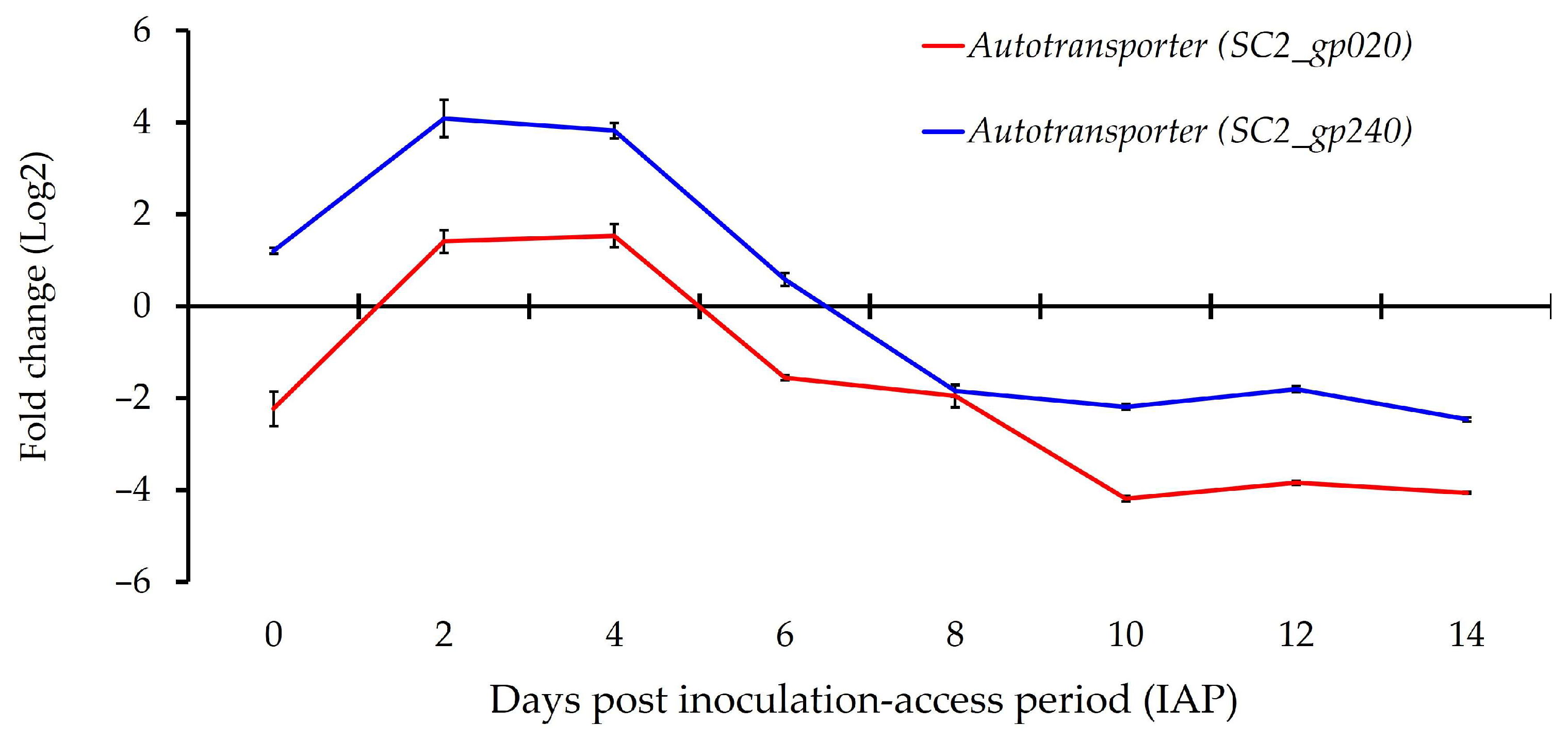
3.5. Expression of Predicted Phage Genes Associated with Psyllid–‘Ca. L. solanacearum’ Interactions
3.6. Expression of Predicted Potato Psyllid-Associated Wolbachia Genes/Loci
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkar, P.; Ghanim, M. Unravelling the pathogenesis and molecular interactions of liberibacter phytopathogens with their psyllid vectors. Agronomy 2020, 10, 1132. [Google Scholar] [CrossRef]
- Wang, N.; Pierson, E.A.; Setubal, J.C.; Xu, J.; Levy, J.G.; Zhang, Y.; Li, J.; Rangel, L.T.; Martins, J., Jr. The ‘Candidatus Liberibacter’–host interface: Insights into pathogenesis mechanisms and disease control. Annu. Rev. Phytopathol. 2017, 55, 451–482. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Achor, D.; Levy, A. Intracellular life cycle of ‘Candidatus Liberibacter asiaticus’ inside psyllid gut cells. Phytopathology 2022, 112, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Mora, V.; Ramasamy, M.; Damaj, M.B.; Irigoyen, S.; Ancona, V.; Ibanez, F.; Avila, C.A.; Mandadi, K.K. Potato zebra chip: An overview of the disease, control strategies, and prospects. Front. Microbiol. 2021, 12, 700663. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Rehman, M.; Rogan, D.; Martin, R.R.; Idris, A.M. First report of ‘Candidatus Liberibacter psyllaurous’ (synonym ‘Ca. L. solanacearum’) associated with ‘tomato vein-greening’ and ‘tomato psyllid yellows’ diseases in commercial greenhouses in Arizona. Plant Dis. 2010, 94, 376. [Google Scholar] [CrossRef]
- Ammar, E.D.; George, J.; Sturgeon, K.; Stelinski, L.L.; Shatters, R.G. Asian citrus psyllid adults inoculate huanglongbing bacterium more efficiently than nymphs when this bacterium is acquired by early instar nymphs. Sci. Rep. 2020, 10, 18244. [Google Scholar] [CrossRef] [PubMed]
- Pelz-Stelinski, K.S.; Brlansky, R.H.; Ebert, T.A.; Rogers, M.E. Transmission parameters for ‘Candidatus Liberibacter asiaticus’ by Asian citrus psyllid (Hemiptera: Psyllidae). J. Econ. Entomol. 2010, 103, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N. Made for each other: Vector–pathogen interfaces in the huanglongbing pathosystem. Phytopathology 2022, 112, 26–43. [Google Scholar] [CrossRef]
- Heck, M. Insect Transmission of plant pathogens: A systems biology perspective. mSystems 2018, 3, e00168-17. [Google Scholar] [CrossRef]
- Andrade, M.; Li, J.; Wang, N. ‘Candidatus Liberibacter asiaticus’: Virulence traits and control strategies. Trop. Plant Pathol. 2020, 45, 285–297. [Google Scholar] [CrossRef]
- Cong, Q.; Kinch, L.N.; Kim, B.H.; Grishin, N.V. Predictive sequence analysis of the ‘Candidatus Liberibacter asiaticus’ proteome. PLoS ONE 2012, 7, e41071. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, L.; Hall, D.G.; Li, W.; Doddapaneni, H.; Lin, H.; Liu, L.; Vahling, C.M.; Gabriel, D.W.; Williams, K.P.; et al. Complete Genome Sequence of Citrus Huanglongbing Bacterium, ‘Candidatus Liberibacter asiaticus’ Obtained Through Metagenomics. Mol. Plant-Microbe Interact. 2009, 22, 1011–1020. [Google Scholar] [CrossRef]
- Lin, H.; Lou, B.; Glynn, J.M.; Doddapaneni, H.; Civerolo, E.L.; Chen, C.; Duan, Y.; Zhou, L.; Vahling, C.M. The Complete Genome Sequence of ‘Candidatus Liberibacter asiaticus’, the Bacterium Associated with Potato Zebra Chip Disease. PLoS ONE 2011, 6, e19135. [Google Scholar] [CrossRef]
- Thapa, S.P.; De Francesco, A.; Trinh, J.; Gurung, F.B.; Pang, Z.; Vidalakis, G.; Wang, N.; Ancona, V.; Ma, W.; Coaker, G. Genome-wide analyses of liberibacter species provides insights into evolution, phylogenetic relationships, and virulence factors. Mol. Plant Pathol. 2020, 21, 716–731. [Google Scholar] [CrossRef]
- Du, J.; Wang, Q.; Zeng, C.; Zhou, C.; Wang, X. A prophage-encoded nonclassical secretory protein of ‘Candidatus Liberibacter asiaticus’ induces a strong immune response in Nicotiana benthamiana and citrus. Mol. Plant Pathol. 2022, 23, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Nachappa, P.; Levy, J.; Pierson, E.; Tamborindeguy, C. Diversity of endosymbionts in the potato psyllid, Bactericera cockerelli (Hemiptera: Triozidae), vector of zebra chip disease of potato. Curr. Microbiol. 2011, 62, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Hunter, W.B.; Reese, J.; Morgan, J.K.; Marutani-Hert, M.; Huang, H.; Lindeberg, M. Survey of endosymbionts in the Diaphorina citri metagenome and assembly of a Wolbachia wDi draft genome. PLoS ONE 2012, 7, e50067. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Wang, Y.F. The effect of the endosymbiont Wolbachia on the behavior of insect hosts. Insect Sci. 2020, 27, 846–858. [Google Scholar] [CrossRef]
- Jain, M.; Fleites, L.A.; Gabriel, D.W. A small Wolbachia protein directly represses phage lytic cycle genes in ‘Candidatus Liberibacter asiaticus’ within psyllids. MSphere 2017, 2, e00171-17. [Google Scholar] [CrossRef]
- Fisher, T.W.; Vyas, M.; He, R.; Nelson, W.; Cicero, J.M.; Willer, M.; Kim, R.; Kramer, R.; May, G.A.; Crow, J.A.; et al. Comparison of potato and Asian citrus psyllid adult and nymph transcriptomes identified vector transcripts with potential involvement in circulative, propagative liberibacter transmission. Pathogens 2014, 3, 875–907. [Google Scholar] [CrossRef]
- El-Desouky, A.; Shatters, R.G., Jr.; Heck, M. Huanglongbing pathogens: Acquisition, transmission and vector interactions. In Asian Citrus Psyllid Biology, Ecology and Management of the Huanglongbing Vector; CABI: Wallingford, UK, 2020; pp. 113–139. [Google Scholar] [CrossRef]
- He, R.; Levy, J.; Brown, J.K.; Wang, J.; Ambrós, S.; Zhang, S. Differential gene expression of Asian citrus psyllids infected with ‘Candidatus Liberibacter asiaticus’ reveals hyper-susceptibility to invasion by instar fourth–fifth and teneral adult stages. Front. Plant Sci. 2023, 14, 1229620. [Google Scholar] [CrossRef] [PubMed]
- Saberi, E.; Qureshi, J.A.; Brown, J.K. Differential expression of ‘Candidatus Liberibacter solanacearum’ genes and prophage loci in different life stages of potato psyllid. Sci. Rep. 2024, 14, 16248. [Google Scholar] [CrossRef] [PubMed]
- Liefting, L.W.; Sutherland, P.W.; Ward, L.I.; Paice, K.L.; Weir, B.S.; Clover, G.R.G. A new ‘Candidatus Liberibacter’ species associated with diseases of solanaceous crops. Plant Dis. 2009, 93, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Islam, M.S.; Bai, Y.; Wen, A.; Lan, S.; Gudmestad, N.C.; Civerolo, E.L. Genetic diversity of ‘Candidatus Liberibacter solanacearum’ strains in the United States and Mexico revealed by simple sequence repeat markers. Eur. J. Plant Pathol. 2012, 132, 297–308. [Google Scholar] [CrossRef]
- Swisher, K.D.; Henne, D.C.; Crosslin, J.M. Identification of a fourth haplotype of Bactericera cockerelli (Hemiptera: Triozidae) in the United States. J. Insect Sci. 2014, 14, 161. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Sengoda, V.G.; Cooper, W.R.; Swisher, K.D.; Henne, D.C.; Munyaneza, J.E. Latent period and transmission of ‘Candidatus Liberibacter solanacearum’ by the potato psyllid Bactericera cockerelli (Hemiptera: Triozidae). PLoS ONE 2014, 9, e93475. [Google Scholar] [CrossRef]
- Levy, J.; Ravindran, A.; Gross, D.; Tamborindeguy, C.; Pierson, E. Translocation of ‘Candidatus Liberibacter solanacearum’, the zebra chip pathogen, in potato and tomato. Phytopathology 2011, 101, 1285–1291. [Google Scholar] [CrossRef]
- Zhang, S.; Flores-Cruz, Z.; Zhou, L.; Kang, B.H.; Fleites, L.A.; Gooch, M.D.; Wulff, N.A.; Davis, M.J.; Duan, Y.-P.; Gabriel, D.W. ‘Ca. Liberibacter asiaticus’ carries an excision plasmid prophage and a chromosomally integrated prophage that becomes lytic in plant infections. Mol. Plant Microbe Interact. 2011, 24, 458–468. [Google Scholar] [CrossRef]
- Deng, W.; Nickle, D.C.; Learn, G.H.; Maust, B.; Mullins, J.I. ViroBLAST: A stand-alone BLAST web server for flexible queries of multiple databases and user’s datasets. Bioinformatics 2007, 23, 2334–2336. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.; Lynch, K.H.; Dennis, J.J.; Wishart, D.S. PHAST: A fast phage search tool. Nucleic Acids Res. 2011, 39 (Suppl. S2), W347–W352. [Google Scholar] [CrossRef] [PubMed]
- Azhahianambi, P.; Ghosh, S.; Kumar, C.A.; Suryanarayana, V.V.S. Cost effectiveness of colony lysis and colony PCR methods for screening of recombinant. Indian J. Exp. Biol. 2008, 46, 731–735. [Google Scholar]
- Manzano Galdeano, D.; Moreira Granato, L.; De Souza Pacheco, I.; Antonio Machado, M. RNAi tools applied to hemipteran insects that are vectors of plant pathogens. In Revisão Anual de Patologia de Plantas; RAPP: Passo Fundo, Brazil, 2018; Chapter 4; pp. 51–68. [Google Scholar]
- Yan, Q.; Sreedharan, A.; Wei, S.; Wang, J.; Pelz-Stelinski, K.; Folimonova, S.; Wang, N. Global gene expression changes in ‘Candidatus Liberibacter asiaticus’ during the transmission in distinct hosts between plant and insect. Mol. Plant Pathol. 2013, 14, 391–404. [Google Scholar] [CrossRef]
- Gutzwiller, F.; Carmo, C.R.; Miller, D.E.; Rice, D.W.; Newton, I.L.G.; Hawley, R.S.; Teixeira, L.; Bergman, C.M. Dynamics of Wolbachia pipientis gene expression across the Drosophila melanogaster life cycle. G3 2015, 5, 2843–2856. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2^(-ΔΔCT) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rahman, M.M.; Govindarajulu, Z. A modification of the test of Shapiro and Wilk for normality. J. Appl. Stat. 1997, 24, 219–236. [Google Scholar] [CrossRef]
- Zheng, Z.; Bao, M.; Wu, F.; Chen, J.; Deng, X. Predominance of single prophage carrying a CRISPR/cas system in ‘Candidatus Liberibacter asiaticus’ strains in southern China. PLoS ONE 2016, 11, e0146422. [Google Scholar] [CrossRef]
- Sengoda, V.G.; Buchman, J.L.; Henne, D.C.; Pappu, H.R.; Munyaneza, J.E. ‘Candidatus Liberibacter solanacearum’ titer over time in Bactericera cockerelli (Hemiptera: Triozidae) after acquisition from infected potato and tomato plants. J. Econ. Entomol. 2013, 106, 1964–1972. [Google Scholar] [CrossRef]
- Cooper, W.R.; Sengoda, V.G.; Munyaneza, J.E. Localization of ‘Candidatus Liberibacter solanacearum’ (Rhizobiales: Rhizobiaceae) in Bactericera cockerelli (Hemiptera: Triozidae). Ann. Entomol. Soc. Am. 2014, 107, 204–210. [Google Scholar] [CrossRef]
- Darolt, J.C.; De Moura, F.; Bento, M.; Merlin, B.L. The genome of ‘Candidatus Liberibacter asiaticus’ is highly transcribed when infecting the gut of Diaphorina citri. Front. Microbiol. 2021, 12, 687725. [Google Scholar] [CrossRef]
- Andrade, M.O.; Pang, Z.; Achor, D.S.; Wang, H.; Yao, T.; Singer, B.H.; Wang, N. The flagella of ‘Candidatus Liberibacter asiaticus’ and its movement in planta. Mol. Plant Pathol. 2020, 21, 109–123. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, A.; Lovelace, A.H.; Shaw, D.; Qiu, M.; Wang, Y.; Gurung, F.; Ancona, V.; Wang, C.; Levy, A.; Jiang, T.; et al. Transcriptome profiling of ‘Candidatus Liberibacter asiaticus’ in citrus and psyllids. Phytopathology 2022, 112, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Cicero, J.M.; Fisher, T.W.; Qureshi, J.A.; Stansly, P.A.; Brown, J.K. Colonization and intrusive invasion of potato psyllid by ‘Candidatus Liberibacter solanacearum’. Phytopathology 2017, 107, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.; Wang, N. The tad pilus apparatus of ‘Candidatus Liberibacter asiaticus’ and its regulation by VisNR. Mol. Plant Microbe Interact. 2019, 32, 1175–1187. [Google Scholar] [CrossRef]
- Xu, Q.; Rawlings, N.D.; Farr, C.L.; Chiu, H.-J.; Grant, J.C.; Jaroszewski, L.; Klock, H.E.; Knuth, M.W.; Miller, M.D.; Weekes, D.; et al. Structural and sequence analysis of Imelysin-like proteins implicated in bacterial iron uptake. PLoS ONE 2011, 6, e21875. [Google Scholar] [CrossRef]
- Ravindran, A.; Saenkham, P.; Levy, J.; Tamborindeguy, C.; Lin, H.; Gross, D.C.; Pierson, E. Characterization of the serralysin-like gene of ‘Candidatus Liberibacter solanacearum’ associated with potato zebra chip disease. Phytopathology 2018, 108, 327–335. [Google Scholar] [CrossRef]
- Hansen, A.K.; Skidmore, I.H. Psyllids, it’s what’s on the inside that counts: Community cross talk facilitates prophage interactions. mSphere 2017, 2, e00227-17. [Google Scholar] [CrossRef]
- Ding, F.; Allen, V.; Luo, W.; Zhang, S.; Duan, Y. Molecular mechanisms underlying heat or tetracycline treatments for citrus HLB control. Hortic. Res. 2018, 5, 1. [Google Scholar] [CrossRef]
- Fu, S.M.; Hartung, J.; Zhou, C.Y.; Su, H.N.; Tan, J.; Li, Z.A. Ultrastructural changes and putative phage particles observed in sweet orange leaves infected with ‘Candidatus Liberibacter asiaticus’. Plant Dis. 2015, 99, 320–324. [Google Scholar] [CrossRef]
- Jain, M.; Fleites, L.A.; Gabriel, D.W. Prophage-encoded peroxidase in ‘Candidatus Liberibacter asiaticus’ is a secreted effector that suppresses plant defenses. Mol. Plant-Microbe Interact. 2015, 28, 1330–1337. [Google Scholar] [CrossRef]
- Thompson, S.M.; Thompson, S.M.; Johnson, C.P.; Lu, A.Y.; Frampton, R.A.; Sullivan, K.L.; Fiers, M.W.E.J.; Crowhurst, R.N.; Pitman, A.R.; Scott, I.A.W.; et al. Genomes of ‘Candidatus Liberibacter solanacearum’ haplotype A from New Zealand and the United States suggest significant genome plasticity in the species. Phytopathology 2015, 105, 863–871. [Google Scholar] [CrossRef]
- Hao, G.; Boyle, M.; Zhou, L.; Duan, Y. The intracellular citrus Huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ encodes two novel autotransporters. PLoS ONE 2013, 8, e68921. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Huston, S.L.; Killiny, N.; Igo, M.M. XatA, an AT-1 autotransporter important for the virulence of Xylella fastidiosa Temecula1. Microbiologyopen 2012, 1, 33–45. [Google Scholar] [CrossRef]
- Rojas, C.M.; Ham, J.H.; Deng, W.; Doyle, J.J.; Collmer, A. HecA, a member of a class of adhesins produced by diverse pathogenic bacteria, contributes to the attachment, aggregation, epidermal cell killing, and virulence phenotypes of Erwinia chrysanthemi EC16 on Nicotiana clevelandii seedlings. Proc. Natl. Acad. Sci. USA 2002, 99, 13142–13147. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Bonilla, S.I.; Manalo, A.M.; Pelz-Stelinski, K.S. Complete de novo assembly of Wolbachia endosymbiont of Diaphorina citri Kuwayama (Hemiptera: Liviidae) using long-read genome sequencing. Sci. Rep. 2022, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Vostokova, E.V.; Dranenko, N.O.; Gelfand, M.S.; Bochkareva, O.O. Genome rearrangements drive evolution of ANK genes in Wolbachia. bioRxiv 2023. [Google Scholar] [CrossRef]
- Campanacci, V.; Mukherjee, S.; Roy, C.R.; Cherfils, J. Structure of the Legionella effector AnkX reveals the mechanism of phosphocholine transfer by the FIC domain. EMBO J. 2013, 32, 1469–1477. [Google Scholar] [CrossRef]
- Allgood, S.C.; Romero Dueñas, B.P.; Noll, R.R.; Pike, C.; Lein, S.; Neunuebel, M.R. Legionella effector AnkX disrupts host cell endocytic recycling in a phosphocholination-dependent manner. Front. Cell. Infect. Microbiol. 2017, 7, 397. [Google Scholar] [CrossRef]
- Furniss, R.C.D.; Slater, S.; Frankel, G.; Clements, A. Enterohaemorrhagic E. coli modulates an ARF6:Rab35 signaling axis to prevent recycling endosome maturation during infection. J. Mol. Biol. 2016, 428, 3399–3407. [Google Scholar] [CrossRef]
- Ghanim, M.; Achor, D.; Ghosh, S.; Kontsedalov, S.; Lebedev, G.; Levy, A. ‘Candidatus Liberibacter asiaticus’ accumulates inside endoplasmic reticulum associated vacuoles in the gut cells of Diaphorina citri. Sci. Rep. 2017, 7, 16945. [Google Scholar] [CrossRef]
- Ghosh, S.; Jassar, O.; Kontsedalov, S.; Lebedev, G.; Wang, C.; Turner, D.; Levy, A.; Ghanim, M. A transcriptomics approach reveals putative interaction of ‘Candidatus Liberibacter solanacearum’ with the endoplasmic reticulum of its psyllid vector. Insects 2019, 10, 279. [Google Scholar] [CrossRef]
- Cooper, W.R.; Swisher Grimm, K.D.; Angelella, G.M.; Mustafa, T. Acquisition and transmission of ‘Candidatus Liberibacter solanacearum’ differs among Wolbachia-infected and -uninfected haplotypes of Bactericera cockerelli. Plant Dis. 2023, 107, 2440–2445. [Google Scholar] [CrossRef]
| 1-‘Candidatus Liberibacter solanacearum’ Chromosomal, Prophage Genes (Loci) | |||
| Locus ID a | ‘Ca. L. asiaticus’ Homolog b | Homolog c | Gene (Locus) Annotation |
| AZch1 | CLIBASIA_01645 | DJ66_RS00660 | bacteriophage repressor protein C1 |
| AZch2 | CLIBASIA_02905 | DJ66_RS03455 | LuxR transcriptional regulator |
| AZch4 | CLIBASIA_01345 | DJ66_RS04950 | serralysin |
| AZch5 | CLIBASIA_02610 | DJ66_RS04795 | imelysin domain protein |
| AZch6 | CLIBASIA_02090 | DJ66_RS00075 | flagellin; FliC |
| AZch8 | CLIBASIA_03095 | DJ66_RS03635 | Major fimbrial protein; Flp1 |
| AZch9 | CLIBASIA_04145 | DJ66_RS02195 | TolC; transporter |
| AZch10 | CLIBASIA_04540 | DJ66_RS00995 | ‘Ca. Liberibacter’-genus-specific-protein |
| AZch15 | CLIBASIA_RS00350 | DJ66_RS05900 | recA |
| AZch16 | CLIBASIA_RS03560 | DJ66_RS00260 | 16S rRNA |
| AZch17 | CLIBASIA_03525 | DJ66_RS04070 | gyrB; DNA gyrase subunit B |
| AZph6 | SC2_gp065 | DJ66_RS00645 | Integrase/recombinase |
| AZph11 | SC2_gp125 | DJ66_RS01515 | Phage-related repressor protein C2 |
| AZph13 | SC1/2_gp195 | DJ66_RS05295 | recB-like nuclease |
| AZph15 | SC1/2_gp200 | DJ66_RS00565 | Putative phage antirepressor |
| AZph17 | SC1/2_gp210 | DJ66_RS05280 | DNA polymerase A |
| AZph19 | SC2_gp240 | Unknown | Autotransporter adhesion gene |
| AZph20 | SC2_gp020 | DJ66_RS05330 | Autotransporter; cell wall associated biofilm |
| 2-Wolbachia endosymbiont of Bactericera cockerelli genes (loci) | |||
| LocusID d | SeqID | Wo_ DC e | Gene/locus annotation |
| AZWo1 | BcGS_20518 | HGO53_00485 | J domain-containing protein (DnaJ) |
| AZWo2 | BcAN_16274 | FK497_06755 | Phosphocholine transferase (AnkX) |
| AZWo3 | BcAN_05576 | FK497_06755 | Phosphocholine transferase (AnkX) |
| AZWo5 | BcGS_23202 | FK497_04725 | Core conserved bacterial protein (FtsZ) |
| AZWo6 | No accession No. f | WDIAC_RS0101550 | Wolbachia repressor protein g |
| IAP (Day) 1 | Transmission Rate% 2 | ‘Ca. L. solanacearum’ Copy No. (Log10) 3 |
|---|---|---|
| 2 | 0.00% (0/30) | 3.03 |
| 4 | 0.00% (0/30) | 3.70 |
| 6 | 0.00% (0/30) | 2.42 |
| 8 | 0.0% (0/30) | 2.99 |
| 10 | 0.0% (0/30) | 4.12 |
| 12 | 13.3% (4/30) | 5.65 |
| 14 | 26.6% (8/30) | 6.26 |
| 16 | 30.0% (9/30) | 6.89 |
| Locus ID (Gene/Locus Name) 1 | Fold Change (Log2 Ratio ± SE) 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Inoculation-Access Period Days After 48-h Acquisition-Access Period | ||||||||
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | |
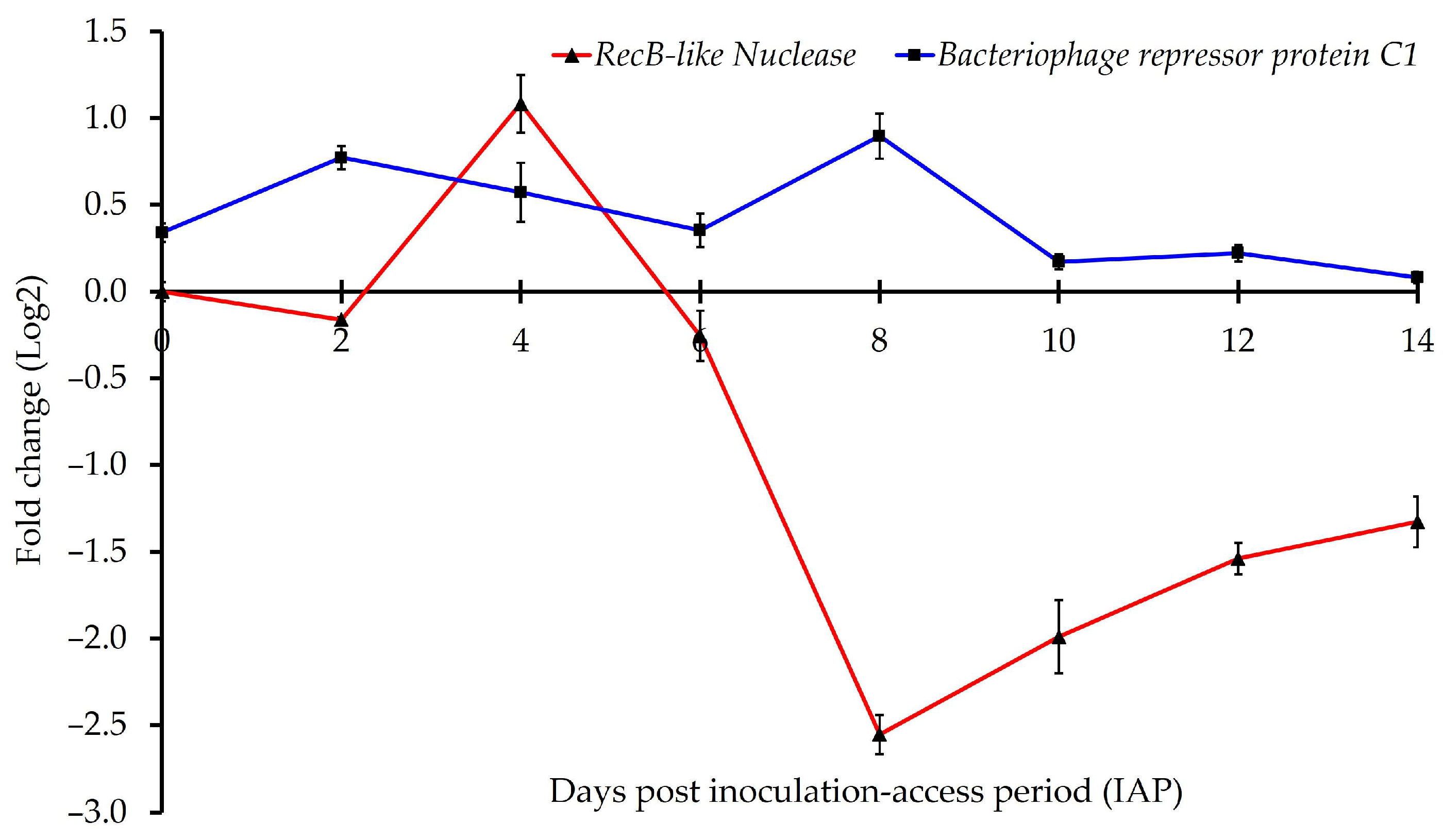
| AZch1 (Repressor protein C1) | 0.34 ± 0.07 b | 0.77 ± 0.12 cd | 0.57 ± 0.21 c | 0.36 ± 0.10 b | 0.90 ± 0.17 d | 0.17 ± 0.11 ab ϕ | 0.22 ± 0.05 ab ϕ | 0.08 ± 0.07 a ϕ |
| AZch2 (LuxR transcriptional regulator) | ND | ND | ND | ND | ND | 0.81 ± 0.2 a | 1.78 ± 0.45 b | 4.31 ± 0.05 c |
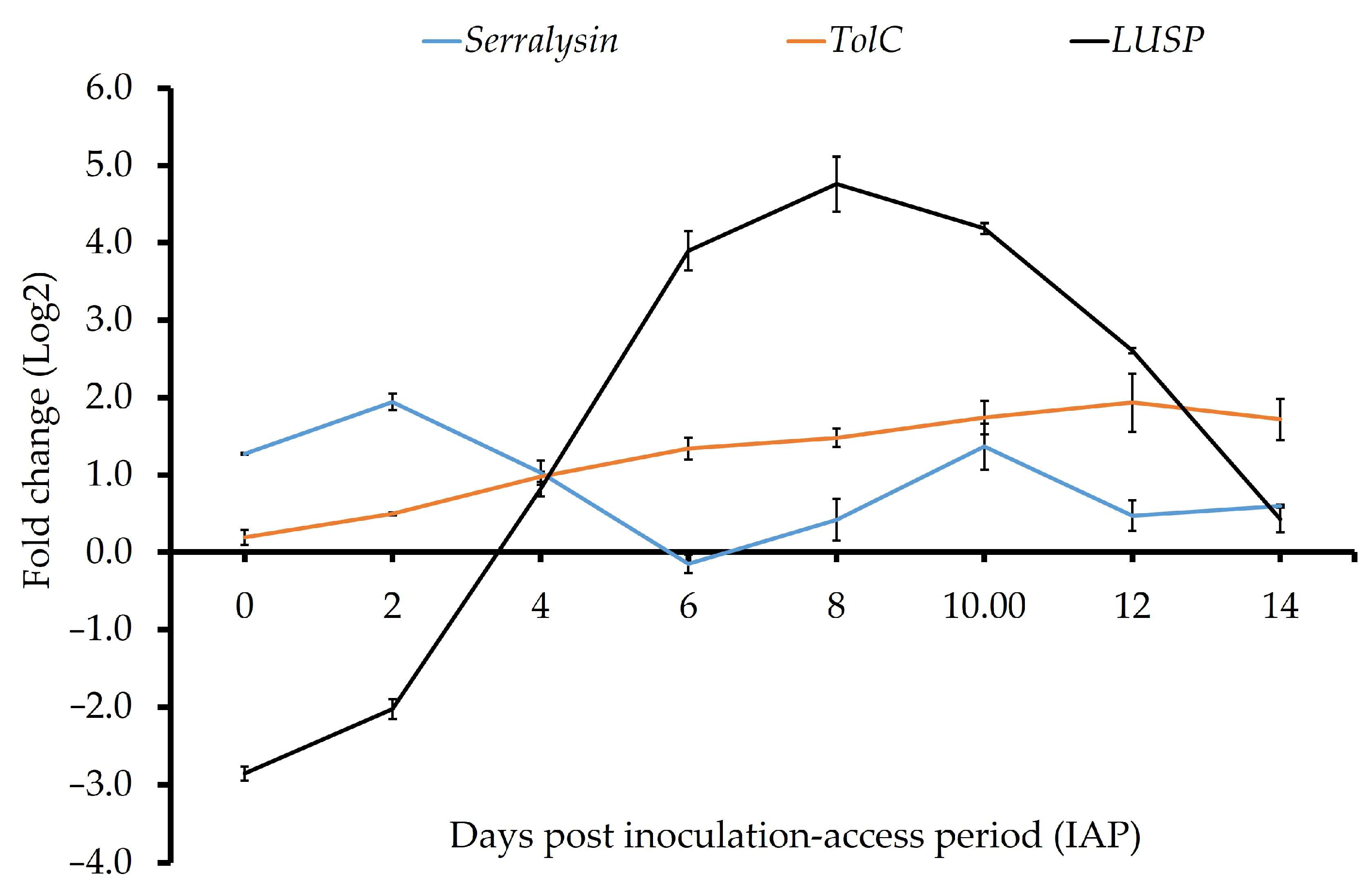
| AZch4 (Serralysin) | 1.27 ± 0.01 cd | 1.94 ± 0.11 e | 1.03 ± 0.16 c | −0.15 ± 0.12 a ϕ | 0.42 ± 0.27 b ϕ | 1.37 ± 0.30 d | 0.47 ± 0.20 b ϕ | 0.60 ± 0.02 b |
| AZch5 (Imelysin domain protein) | ND | ND | ND | ND | ND | −0.18 ± 0.07 a ϕ | 0.64 ± 0.06 b | 3.07 ± 0.04 c |
| AZch6 (Flagellin; FliC) | −2.16 ± 0.12 a | −1.01 ± 0.65 b | −0.23 ± 0.31 c | −0.98 ± 0.35 b | −2.41 ± 0.66 a | 1.63 ± 0.04 d | 4.26 ± 0.08 e | 6.71 ± 0.38 f |
| AZch8 (Major fimbrial protein; Flp3) | 4.78 ± 0.19 f | 7.91 ± 0.43 g | 7.83 ± 0.37 g | 3.66 ± 0.28 e | 1.18 ± 0.31 d | 0.5 ± 0.05 c | −0.38 ± 0.05 b | −0.87 ± 0.05 a |
| AZch9 (TolC; transporter) | 0.19 ± 0.09 a | 0.50 ± 0.02 a | 0.98 ± 0.07 b | 1.34 ± 0.14 bc | 1.48 ± 0.12 c | 1.74 ± 0.22 cd | 1.93 ± 0.38 d | 1.71 ± 0.27 cd |
| AZch10 (‘Ca. Liberibacter’ specific protein) | −2.85 ± 0.09 a | −2.03 ± 0.13 bc | 0.82 ± 0.09 d | 3.90 ± 0.25 f | 4.76 ± 0.35 f | 4.18 ± 0.07 g | 2.61 ± 0.04 e | 0.43 ± 0.17 c ϕ |
| AZph6 (Integrase/recombinase) | ND | ND | ND | ND | ND | −4.99 ± 0.14 a | −3.98 ± 0.18 b | −2.85 ± 0.11 c |
| AZph11 (phage-repressor protein C2) | −1.88 ± 0.18 c | −0.93 ± 0.39 d | −1.78 ± 0.44 c | −3.01 ± 0.21 b | −3.00 ± 0.41 b | −3.67 ± 0.02 a | −3.06 ± 0.06 b | −3.34 ± 0.02 ab |
| AZph13 (recB-like Nuclease) | 0.00 ± 0.09 a ϕ | −0.16 ± 0.02 a | 1.08 ± 0.17 e | −0.26 ± 0.15 a ϕ | −2.55 ± 0.24 a | −1.99 ± 0.30 b | −1.54 ± 0.22 c | −1.33 ± 0.27 c |
| AZph15 (Putative phage antirepressor) | ND | ND | ND | ND | −3.9 ± 0.34 c | −6.33 ± 0.02 a | −5.96 ± 0.05 b | −6.25 ± 0.03 ab |
| AZph17 (DNA polymerase A) | −2.85 ± 0.30 c | −2.63 ± 0.31 c | −3.00 ± 0.49 c | −4.08 ± 0.05 b | −4.65 ± 0.45 b | −7.29 ± 0.10 a | −7.55 ± 0.84 a | −7.21 ± 0.01 a |
| AZph19 (Putative autotransporter) | 1.21 ± 0.11 d | 4.08 ± 0.48 e | 3.82 ± 0.39 e | 0.58 ± 0.36 c | −1.84 ± 0.23 b | −2.19 ± 0.17 ab | −1.8 ± 0.07 b | −2.46 ± 0.11 a |
| AZph20 (Putative autotransporter) | −2.23 ± 0.97 b | 1.41 ± 0.34 c | 1.53 ± 0.51 c | −1.55 ± 0.12 b | −1.95 ± 0.64 b | −4.19 ± 0.15 a | −3.84 ± 0.08 a | −4.06 ± 0.04 a |
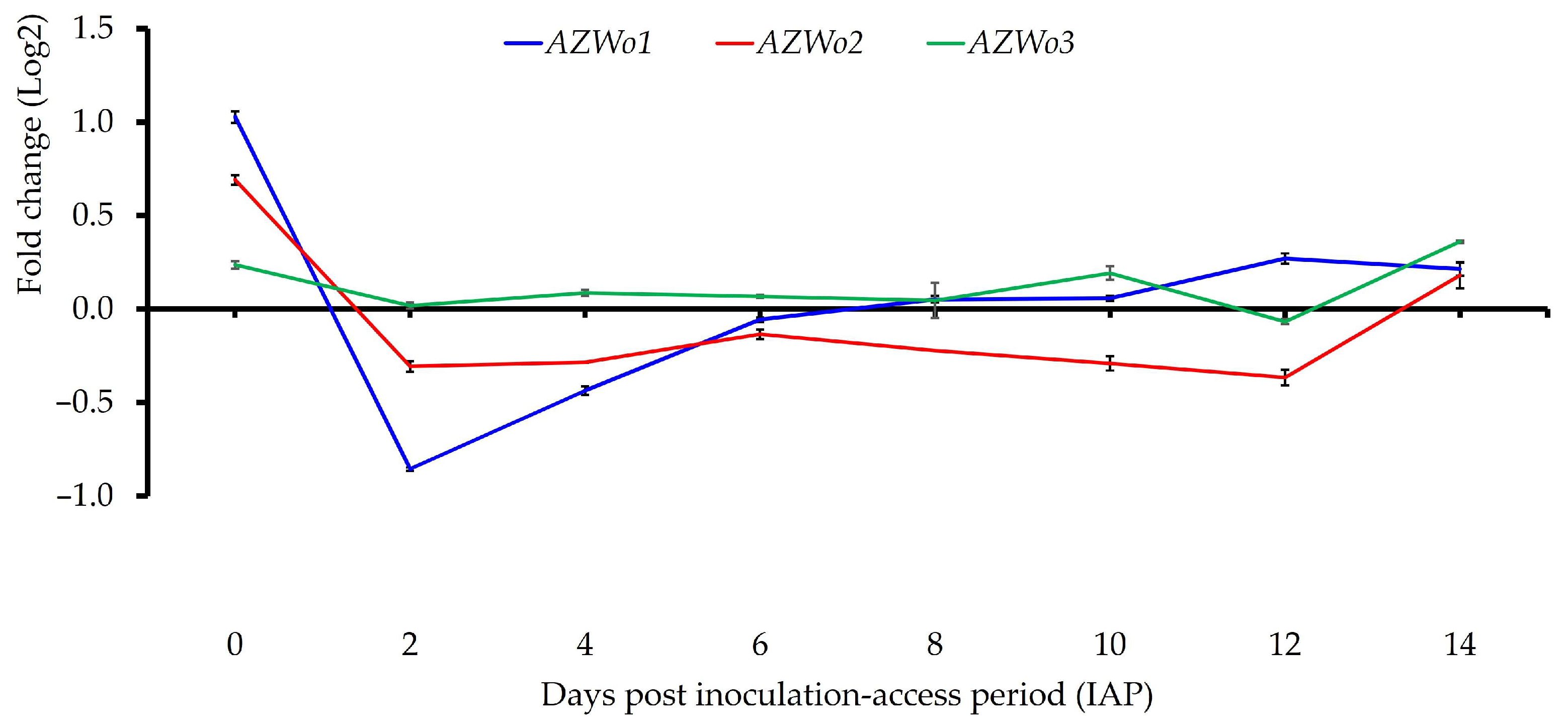
| AZWo1 (J domain-containing protein) | 1.03 ± 0.05 e | −0.86 ± 02 a | −0.44 ± 0.04 b | −0.06 ± 0.02 b | 0.05 ± 0.03 c ϕ | 0.06 ± 0.02 c ϕ | 0.27 ± 0.05 d | 0.23 ± 0.08 cd ϕ |
| AZWo2 (Phosphocholine transferase) | 0.69 ± 0.04 c | −0.3±0.05 a | −0.29±0.00 a | −0.13±0.05 a | −0.13±0.05 a | −0.22±0.07 a | −0.37±0.04 a | 0.21±0.04 b |
| AZWo3 (Phosphocholine transferase) | 0.24 ± 0.03 c | 0.02 ± 0.03 b ϕ | 0.09 ± 0.03 b ϕ | 0.07 ± 0.02 ab ϕ | 0.05 ± 0.16 ab ϕ | 0.19 ± 0.06 c ϕ | −0.07 ± 0.02 a ϕ | 0.36 ± 0.01 d |
| AZWo6 (Wolbachia repressor protein) | 0.01 ± 0.12 b ϕ | 0.63 ± 0.04 c | −0.58 ± 0.02 a | 0.73 ± 0.01 cd | 0.81 ± 0.05 cde | 0.89 ± 0.03 de | 1.65 ± 0.16 e | 1.03 ± 0.08 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saberi, E.; Qureshi, J.A.; Brown, J.K. Time-Course Gene Expression of ‘Candidatus Liberibacter solanacearum’, Prophage, and Wolbachia Genes in Bactericera cockerelli from Ingestion to in Planta Transmission. Microorganisms 2025, 13, 2120. https://doi.org/10.3390/microorganisms13092120
Saberi E, Qureshi JA, Brown JK. Time-Course Gene Expression of ‘Candidatus Liberibacter solanacearum’, Prophage, and Wolbachia Genes in Bactericera cockerelli from Ingestion to in Planta Transmission. Microorganisms. 2025; 13(9):2120. https://doi.org/10.3390/microorganisms13092120
Chicago/Turabian StyleSaberi, Esmaeil, Jawwad A. Qureshi, and Judith K. Brown. 2025. "Time-Course Gene Expression of ‘Candidatus Liberibacter solanacearum’, Prophage, and Wolbachia Genes in Bactericera cockerelli from Ingestion to in Planta Transmission" Microorganisms 13, no. 9: 2120. https://doi.org/10.3390/microorganisms13092120
APA StyleSaberi, E., Qureshi, J. A., & Brown, J. K. (2025). Time-Course Gene Expression of ‘Candidatus Liberibacter solanacearum’, Prophage, and Wolbachia Genes in Bactericera cockerelli from Ingestion to in Planta Transmission. Microorganisms, 13(9), 2120. https://doi.org/10.3390/microorganisms13092120








