Nosocomial Outbreak of Ralstonia pickettii Infections Likely Linked to Saline Solutions in Germany from August 2023 to March 2024—Challenges in Medical Product-Related Outbreaks
Abstract
1. Introduction
2. Material and Methods
2.1. Outbreak Case Definition
2.2. Case Findings and R. pickettii Surveillance
2.3. Whole-Genome Sequencing
2.4. Genomic Reconstruction
2.5. Bioinformatic Analysis
2.6. Environmental Investigations
2.7. Ethics
3. Results
3.1. Case Findings and Characterization
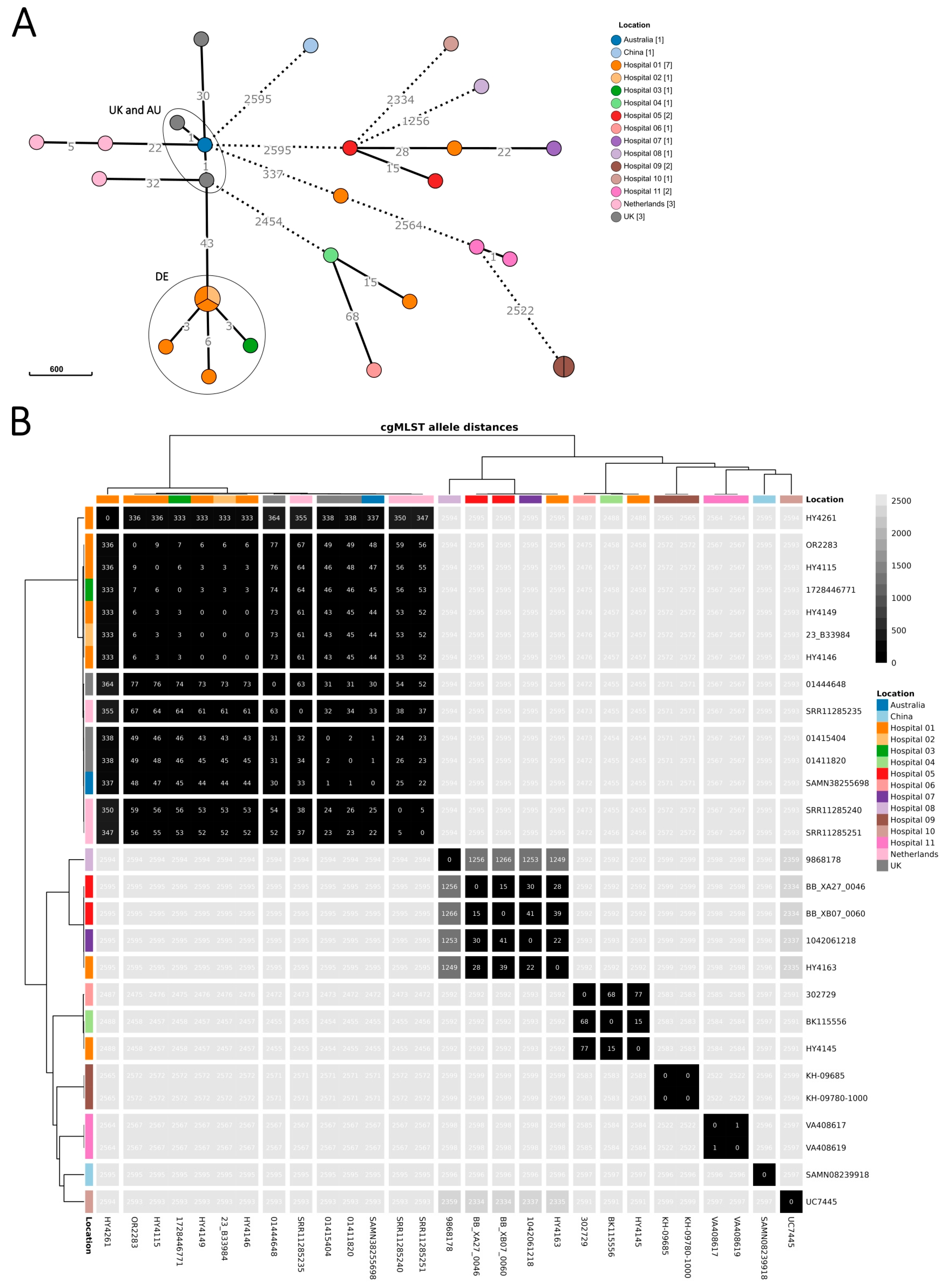
3.2. Microbiological Investigation
3.3. Environmental Investigation
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARS | Antibiotic Resistance Surveillance |
| AU | Australia |
| cgMLST | core genome multilocus sequence typing |
| EU | European Union |
| DE | Germany |
| DNA | Deoxyribonucleic acid |
| dsDNA | Double-stranded DNA |
| gDNA | genomic DNA |
| IfSG | Protection against Infection Act |
| IQR | interquartile range |
| MALDI-TOF | matrix-assisted laser desorption/ionization time-of-flight |
| MST | minimum spanning tree |
| NCBI | National Center for Biotechnology Information |
| RKI | Robert Koch Institute |
| TGA | Therapeutic Goods Administration |
| UK | United Kingdom |
References
- Ryan, M.P.; Pembroke, J.T.; Adley, C.C. Genotypic and phenotypic diversity of Ralstonia pickettii and Ralstonia insidiosa isolates from clinical and environmental sources including High-purity Water. Diversity in Ralstonia pickettii. BMC Microbiol. 2011, 11, 194. [Google Scholar] [CrossRef]
- Ryan, M.P.; Adley, C.C. Ralstonia spp.: Emerging global opportunistic pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Stelzmueller, I.; Biebl, M.; Wiesmayr, S.; Eller, M.; Hoeller, E.; Fille, M.; Weiss, G.; Lass-Floerl, C.; Bonatti, H. Ralstonia pickettii-innocent bystander or a potential threat? Clin. Microbiol. Infect. 2006, 12, 99–101. [Google Scholar] [CrossRef]
- Poty, F.; Denis, C.; Baufine-Ducrocq, H. [Nosocomial Pseudomonas pickettii infection. Danger of the use of ion-exchange resins]. Presse Med. 1987, 16, 1185–1187. [Google Scholar] [PubMed]
- Dimech, W.J.; Hellyar, A.G.; Kotiw, M.; Marcon, D.; Ellis, S.; Carson, M. Typing of strains from a single-source outbreak of Pseudomonas pickettii. J. Clin. Microbiol. 1993, 31, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Moreira, B.M.; Leobons, M.B.; Pellegrino, F.L.; Santos, M.; Teixeira, L.M.; de Andrade Marques, E.; Sampaio, J.; Pessoa-Silva, C. Ralstonia pickettii and Burkholderia cepacia complex bloodstream infections related to infusion of contaminated water for injection. J. Hosp. Infect. 2005, 60, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Bland, L.A.; Favero, M.S.; McNeil, M.M.; Davis, B.J.; Mackel, D.C.; Gravelle, C.R. Factors associated with Pseudomonas pickettii intrinsic contamination of commercial respiratory therapy solutions marketed as sterile. Appl. Environ. Microbiol. 1985, 50, 1343–1348. [Google Scholar] [CrossRef]
- Anderson, R.L.; Holland, B.W.; Carr, J.K.; Bond, W.W.; Favero, M.S. Effect of disinfectants on pseudomonads colonized on the interior surface of PVC pipes. Am. J. Public Health 1990, 80, 17–21. [Google Scholar] [CrossRef]
- Sundaram, S.; Lewis, M.; Eisenhuth, J.; Howard, G., Jr.; Larson, B. Method for qualifying microbial removal performance of 0.1 micron rated filters. Part IV: Retention of hydrogenophaga pseudoflava (ATCC 700892) and Ralstonia pickettii (ATCC 700591) by 0.2 and 0.22 micron rated filters. PDA J. Pharm. Sci. Technol. 2002, 56, 150–171. [Google Scholar]
- Wu, H.H.L.; Collier, J.; Crosby, L.; Holder, D.; Trautt, E.; Lewis, D.; Poulikakos, D.; Chinnadurai, R. Peritoneal dialysis-associated peritonitis presenting with Ralstonia pickettii infection: A novel series of three cases during the COVID-19 pandemic. Semin. Dial. 2023, 36, 70–74. [Google Scholar] [CrossRef]
- Candoni, A.; Trevisan, R.; Patriarca, F.; Silvestri, F.; Fanin, R. Pseudomonas pickettii (Biovar VA-II): A rare cause of bacteremias in haematologic patients. Eur. J. Haematol. 2001, 66, 355–356. [Google Scholar] [CrossRef]
- Lai, H.W.; Shen, Y.H.; Chien, L.J.; Tseng, S.H.; Mu, J.J.; Chan, Y.J.; Wang, F.D. Outbreak of Ralstonia pickettii bacteremia caused by contaminated saline solution in Taiwan. Am. J. Infect. Control 2016, 44, 1191–1192. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Huang, W.T.; Chen, C.P.; Sun, S.M.; Kuo, F.M.; Chan, Y.J.; Kuo, S.C.; Wang, F.D. An Outbreak of Ralstonia pickettii Bloodstream Infection Associated with an Intrinsically Contaminated Normal Saline Solution. Infect. Control Hosp. Epidemiol. 2017, 38, 444–448. [Google Scholar] [CrossRef]
- Nasir, N.; Sayeed, M.A.; Jamil, B. Ralstonia pickettii Bacteremia: An Emerging Infection in a Tertiary Care Hospital Setting. Cureus 2019, 11, e5084. [Google Scholar] [CrossRef]
- Thet, M.K.; Pelobello, M.L.F.; Das, M.; Alhaji, M.M.; Chong, V.H.; Khalil, M.A.M.; Chinniah, T.; Tan, J. Outbreak of nonfermentative Gram-negative bacteria (Ralstonia pickettii and Stenotrophomonas maltophilia) in a hemodialysis center. Hemodial. Int. 2019, 23, E83–E89. [Google Scholar] [CrossRef] [PubMed]
- Bedir Demirdag, T.; Ozkaya-Parlakay, A.; Bayrakdar, F.; Gulhan, B.; Kanik Yuksek, S.; Suzuk Yildiz, S.; Mumcuoglu, I.; Dinc, B.; Yarali, N. An outbreak of Ralstonia pickettii bloodstream infection among pediatric leukemia patients. J. Microbiol. Immunol. Infect. 2022, 55, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Menekse, S.; Haciseyitoglu, D.; Suzuk Yildiz, S.; Bayrakdar, F. An outbreak of Ralstonia pickettii bloodstream infection and clinical outcomes. J. Infect. Dev. Ctries. 2022, 16, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Rajachandran, K.; Varghese, G.S.; Kumar, J.V.; Mathew, K.T. Outbreak of Nosocomial Infection from an Unusual Source. Indian J. Crit. Care Med. 2022, 26, 1042–1044. [Google Scholar] [CrossRef]
- Mehra, K.; Khera, D.; Didel, S.; Tak, V. Outbreak of Ralstonia pickettii Blood Stream Infection in Pediatric Intensive Care Unit. Indian J. Pediatr. 2024, 91, 636. [Google Scholar] [CrossRef]
- Viana-Cardenas, E.; Triana, A.; Cardenas-Alvarez, J.; Carvajal-Diaz, E.; Mendoza, H.; Viasus, D. A large multicenter Ralstonia pickettii outbreak in critically ill patients during the COVID-19 pandemic: Epidemiological and clinical characteristics of 66 cases. J. Infect. Prev. 2024, 25, 85–88. [Google Scholar] [CrossRef]
- Marris, D.K.; Latham, D.N.; Swift, D.C.; Chaverot, S.; Ellis, D.S.; Dean, N.; Russell-Green, N.; Armstrong, B.; Ahuja, V.; Tsang, K.; et al. A multijurisdictional healthcare-associated outbreak of Ralstonia pickettii from contaminated saline, Australia 2023. Int. J. Infect. Dis. 2025, 152, 107431. [Google Scholar] [CrossRef]
- Government of Western Australia. ALERT FOR CLINICIANS—Multijurisdictional Outbreak of Ralstonia Pickettii; Government of Western Australia: Perth, Australia, 2023. [Google Scholar]
- Robert Koch-Institut. [Increase of Ralstonia pickettii]. Epidemiol. Bull. 2024, 2, 20. [Google Scholar]
- Robert Koch-Institut. [Nosocomial outbreak of Ralstonia pickettii infections]. Epidemiol. Bull. 2024, 15, 8. [Google Scholar]
- Deutsche Gesellschaft für Hygiene und Mikrobiologie. Mitteilung vom RKI: Deutsche Gesellschaft für Hygiene und Mikrobiologie. 2024. Available online: https://www.dghm.org/haeufung-von-ralstonia-pickettii/ (accessed on 16 July 2025).
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. Imeta 2023, 2, e107. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Bouras, G.; Judd, L.M.; Edwards, R.A.; Vreugde, S.; Stinear, T.P.; Wick, R.R. How low can you go? Short-read polishing of Oxford Nanopore bacterial genome assemblies. Microb. Genom. 2024, 10, 001254. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.; Weaver, A.; Stretch, R.; Jeyaratnam, D.; Mirfenderesky, M.; Elliott, D.; Patterson, C.; Williams, D.; Kenna, D.T.; Osman, K.L.; et al. Outbreak of Ralstonia pickettii associated with contamination of saline products distributed internationally, the United Kingdom, 2024. Euro Surveill. 2024, 29, 2400384. [Google Scholar] [CrossRef]
- Silva, M.; Machado, M.P.; Silva, D.N.; Rossi, M.; Moran-Gilad, J.; Santos, S.; Ramirez, M.; Carriço, J.A. chewBBACA: A complete suite for gene-by-gene schema creation and strain identification. Microb. Genom. 2018, 4, e000166. [Google Scholar] [CrossRef]
- Zhou, Z.; Alikhan, N.F.; Sergeant, M.J.; Luhmann, N.; Vaz, C.; Francisco, A.P.; Carriço, J.A.; Achtman, M. GrapeTree: Visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018, 28, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing Austria: R Core Team. 2021. Available online: https://www.R-project.org/ (accessed on 2 September 2025).
- Engler, J. tidyheatmaps: Heatmaps from Tidy Data. R Package Version 0.2.1. 2024. Available online: https://CRAN.R-project.org/package=tidyheatmaps (accessed on 2 September 2025).
- Krone, M.; Rauschenberger, V.; Blaschke, V.; Claus, H.; Kurzai, O.; Kampmeier, S. Ralstonia pickettii bloodstream infections with potential genomic link to internationally distributed contaminated saline solution, Germany, October 2023. Euro Surveill. 2024, 29, 2400010. [Google Scholar] [CrossRef] [PubMed]
- Fabricci, M.; Trinca, A.; Talotti, L.; Busetti, M.; Fotakis, E.A.; Merakou, C.; Koncan, R.; Ghiotti, A.; Negri, C.; Di Maso, V.; et al. A urokinase-associated outbreak of Ralstonia mannitolilytica bloodstream infections in haemodialysis patients in north-eastern Italy, January to April 2023. Euro Surveill. 2023, 28, 2300328. [Google Scholar] [CrossRef]
- MINISTERIAL STATEMENTS—Ralstonia [Press Release]. Australia. 2023. Available online: https://documents.parliament.qld.gov.au/speeches/spk2023/Shannon_Fentiman-Waterford-20231128-833823709569.pdf (accessed on 7 January 2024).
- New South Wales Government. Safety Notice 041/23; [Press Release]; New South Wales Government: Sydney, Australia, 2023. [Google Scholar]
- Therapeutic Goods Administration. Recall: Interpharma Sodium Chloride 0.9% 30 mL Ampoules; [Press Release]; Therapeutic Goods Administration: Canberra, Australia, 2023. [Google Scholar]
- Therapeutic Goods Administration. Safety Alert: Action Following Potential Contamination of Some Saline Products with Ralstonia pickettii; [Press Release]; Therapeutic Goods Administration: Canberra, Australia, 2023. [Google Scholar]
- Medicines and Healthcare products Regulatory Agency (MHRA). Urgent Field Safety Notice Product Recall Sodium Chloride Irrigation Solution 0.9% w/v, Sodium Chloride Eye Wash Solution 0.9% w/v, Sodium Chloride Inhalation Solution 0.9% w/v: Legency Remedies Pvt Ltd.; [Press Release]; Medicines and Healthcare Products Regulatory Agency: London, UK, 2024. [Google Scholar]
- Steyaert, S.; Peeters, C.; Wieme, A.D.; Muyldermans, A.; Vandoorslaer, K.; Spilker, T.; Wybo, I.; Piérard, D.; LiPuma, J.J.; Vandamme, P.; et al. Novel Ralstonia species from human infections: Improved matrix-assisted laser desorption/ionization time-of-flight mass spectrometry-based identification and analysis of antimicrobial resistance patterns. Microbiol. Spectr. 2024, 12, e0402123. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Confirmed (n = 6) | Probable (n = 7) | Excluded (n = 12) | Total (n = 25) |
|---|---|---|---|---|
| Age in years | ||||
| Median (IQR a) | 68 (64–69) | 69 (65–73) | 67 (45–77) | 68 (60–73) |
| Unknown—n | 1 | 0 | 8 | 9 |
| Sex—n (%) | ||||
| Female | 2 (40%) | 3 (43%) | 1 (17%) | 6 (33%) |
| Male | 3 (60%) | 4 (57%) | 5 (83%) | 12 (67%) |
| Unknown | 1 | 0 | 6 | 7 |
| Material—n (%) | ||||
| Autologous stem cells | 1 (17%) | 0 | 0 | 1 (4%) |
| Bronchoalveolar lavage | 0 | 0 | 1 (8%) | 1 (4%) |
| Blood | 4 (67%) | 7 (100%) | 2 (17%) | 13 (52%) |
| Cerebrospinal fluid | 0 | 0 | 3 (25%) | 3 (12%) |
| Joint/bone | 0 | 0 | 2 (17%) | 2 (8%) |
| Screening, not further specified | 0 | 0 | 2 (17%) | 2 (8%) |
| Urine | 0 | 0 | 2 (17%) | 2 (8%) |
| Wound | 1 (17%) | 0 | 0 | 1 (4%) |
| Manufacturer | Product | Lot | Hospital 1 (n Cases = 4) | Hospital 2 (n Cases = 1) | Hospital 3 (n Cases = 1) |
|---|---|---|---|---|---|
| Manufacturer A | x | x | x | ||
| Product 1 | Lot 1–3 | x | - | x | |
| Product 1 | Lot 4 | - | x | x | |
| Product 1 | Lot 5 | x | x | - | |
| Product 2 | Lot 6, 7 | x | - | x | |
| Product 3 | Lot 8–10 | x | - | x | |
| Manufacturer B | x | x | x | ||
| Product 4 | x | x | x | ||
| Product 4 | Lot 11 | x | x | - | |
| Manufacturer C | x | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandfort, M.; von Laer, A.; Kampmeier, S.; Eichhorn, I.; Wolf, S.A.; Driller, M.; Bajić, V.; Sedaghatjoo, S.; Fuchs, S.; Engelhart, S.; et al. Nosocomial Outbreak of Ralstonia pickettii Infections Likely Linked to Saline Solutions in Germany from August 2023 to March 2024—Challenges in Medical Product-Related Outbreaks. Microorganisms 2025, 13, 2102. https://doi.org/10.3390/microorganisms13092102
Sandfort M, von Laer A, Kampmeier S, Eichhorn I, Wolf SA, Driller M, Bajić V, Sedaghatjoo S, Fuchs S, Engelhart S, et al. Nosocomial Outbreak of Ralstonia pickettii Infections Likely Linked to Saline Solutions in Germany from August 2023 to March 2024—Challenges in Medical Product-Related Outbreaks. Microorganisms. 2025; 13(9):2102. https://doi.org/10.3390/microorganisms13092102
Chicago/Turabian StyleSandfort, Mirco, Anja von Laer, Stefanie Kampmeier, Inga Eichhorn, Silver A. Wolf, Maximilian Driller, Vladimir Bajić, Somayyeh Sedaghatjoo, Stephan Fuchs, Steffen Engelhart, and et al. 2025. "Nosocomial Outbreak of Ralstonia pickettii Infections Likely Linked to Saline Solutions in Germany from August 2023 to March 2024—Challenges in Medical Product-Related Outbreaks" Microorganisms 13, no. 9: 2102. https://doi.org/10.3390/microorganisms13092102
APA StyleSandfort, M., von Laer, A., Kampmeier, S., Eichhorn, I., Wolf, S. A., Driller, M., Bajić, V., Sedaghatjoo, S., Fuchs, S., Engelhart, S., Mutters, N. T., Cónsul-Tejero, E. P., Zündorf, J., Eckmanns, T., Hogardt, M., & Haller, S. (2025). Nosocomial Outbreak of Ralstonia pickettii Infections Likely Linked to Saline Solutions in Germany from August 2023 to March 2024—Challenges in Medical Product-Related Outbreaks. Microorganisms, 13(9), 2102. https://doi.org/10.3390/microorganisms13092102






