The Input of Terrestrial Dissolved Organic Carbon Enhanced Bacteria Growth Efficiency on Phytoplankton-DOC and Indigenous Lake DOC: A Microcosm Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of DOC
2.2. Experimental Design
2.3. Sampling
2.4. Analysis of Total Carbon, Phosphorus, Total Proteins, and Total Carbohydrates
2.5. Stable Isotope Analysis
2.6. Calculations
2.7. Data Analysis
3. Results
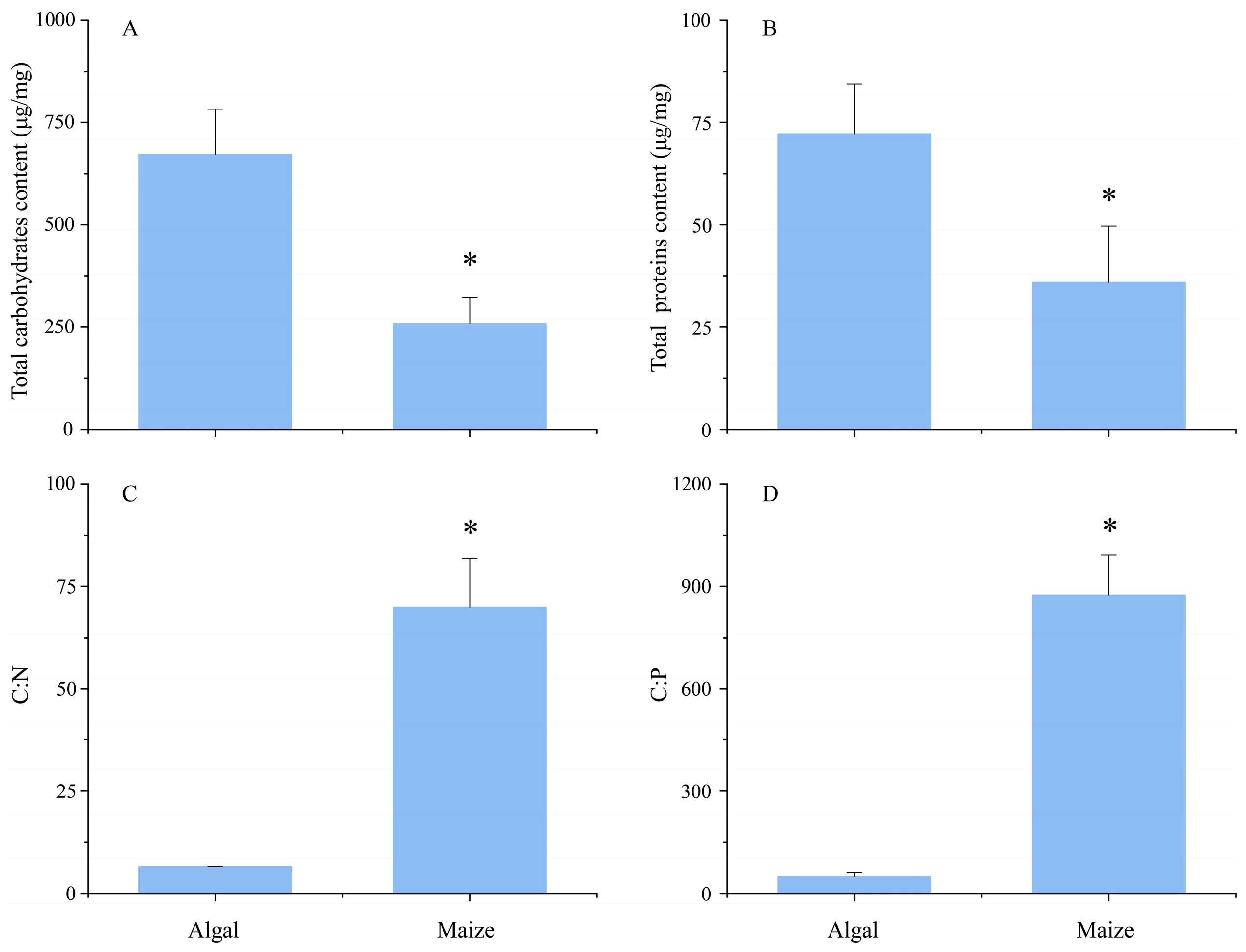
3.1. Biochemical Properties
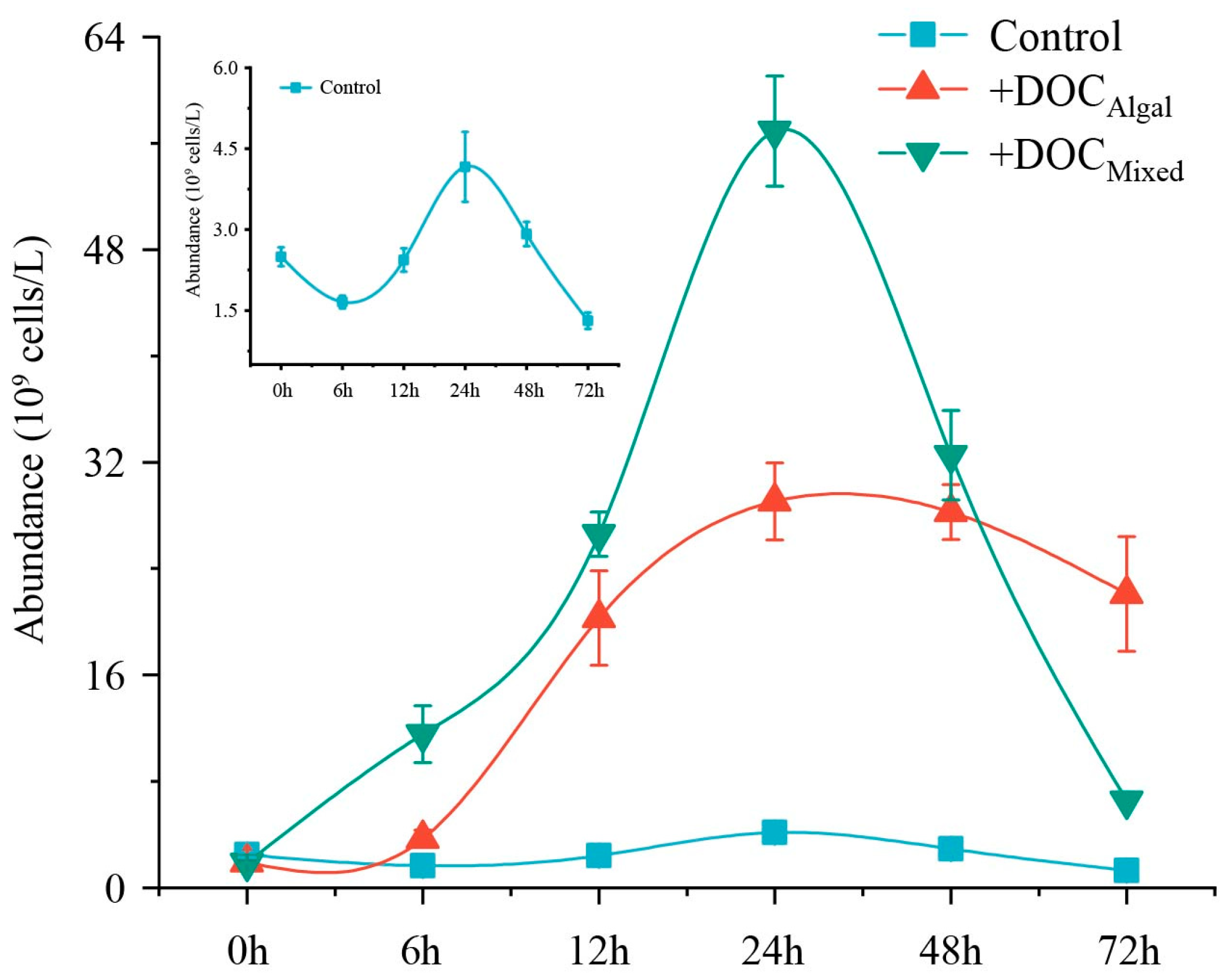
3.2. Bacteria Growth Curve
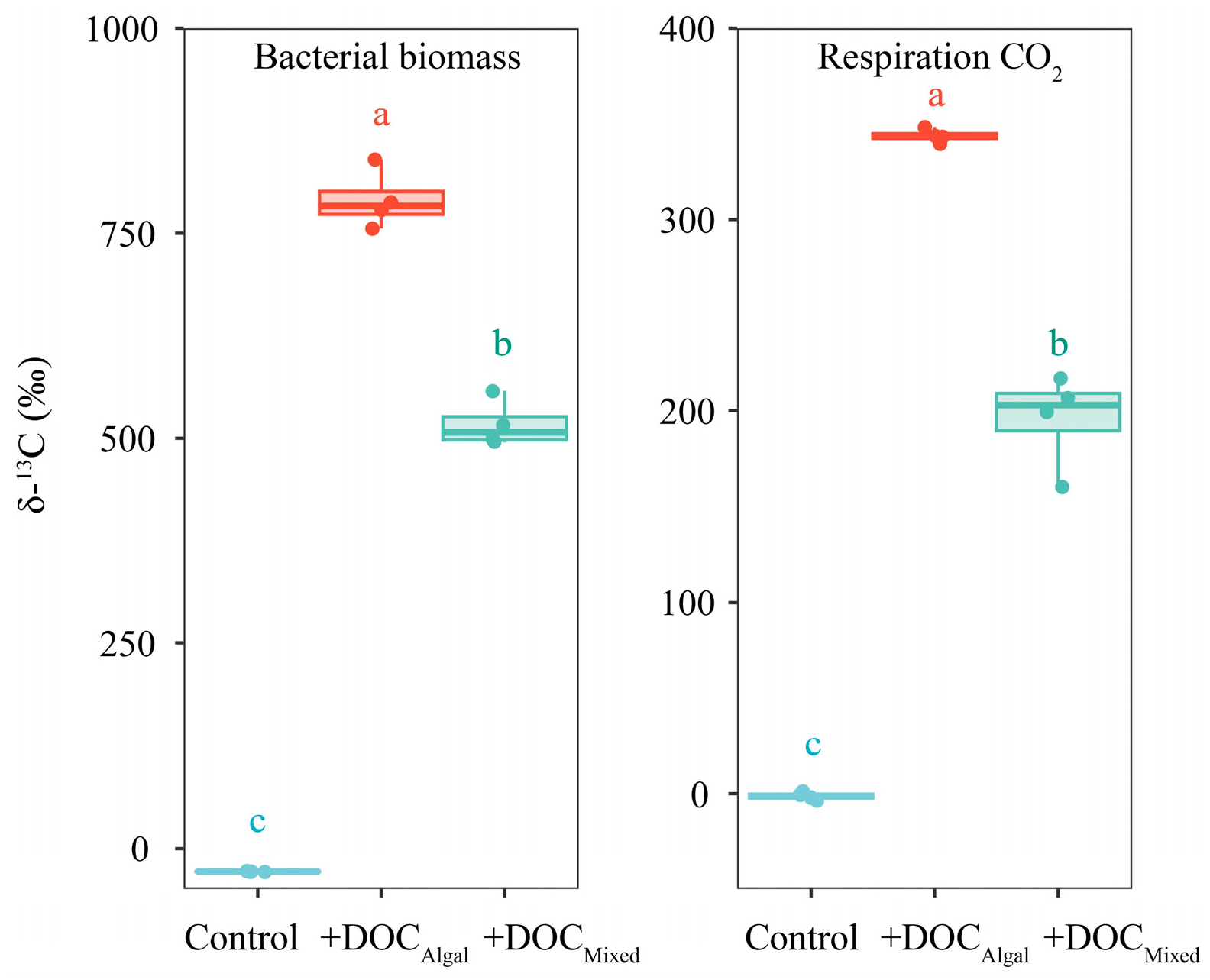
3.3. Carbon Stable Isotope Compositions
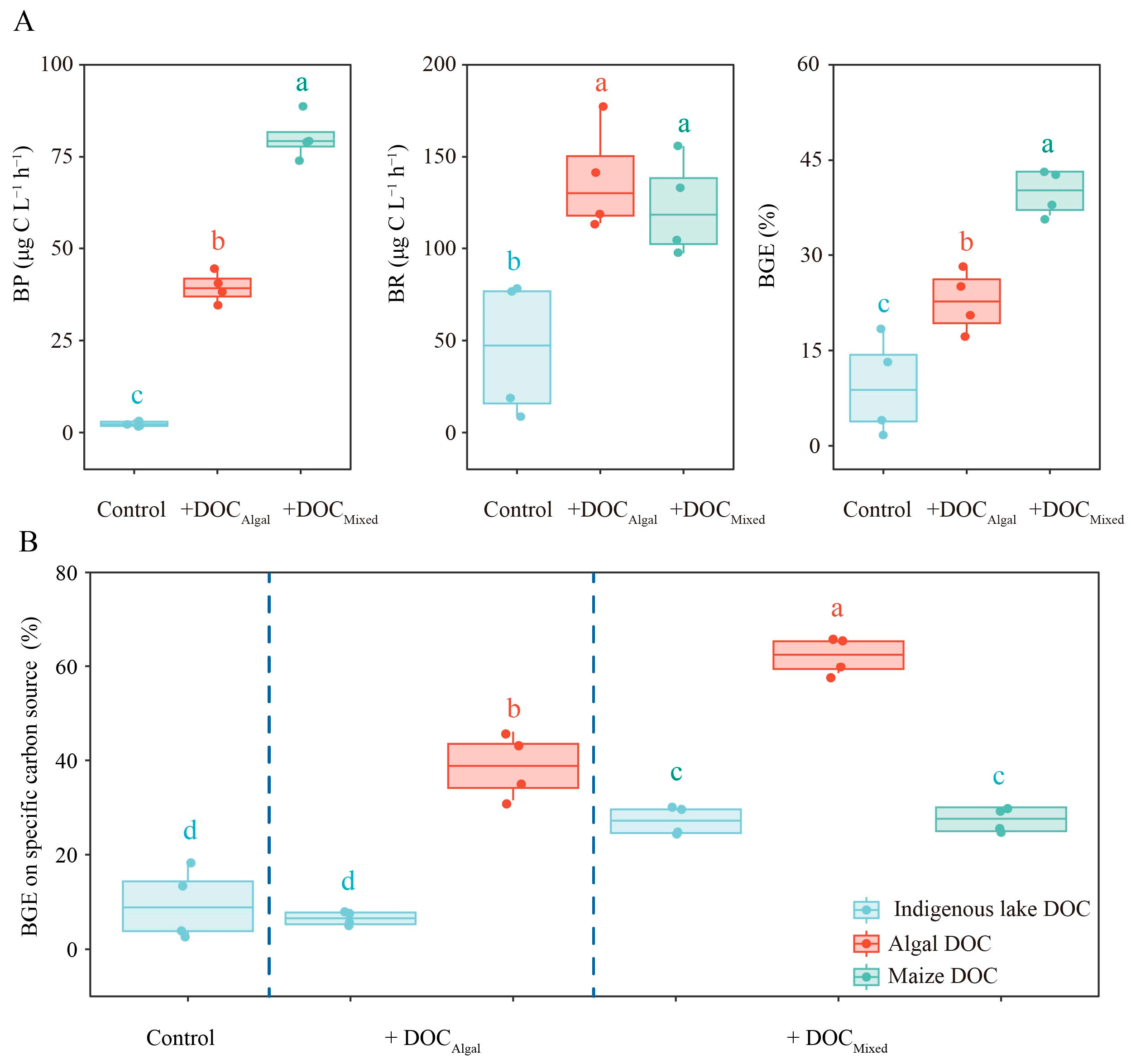
3.4. Bacteria Growth Efficiency on Different DOC
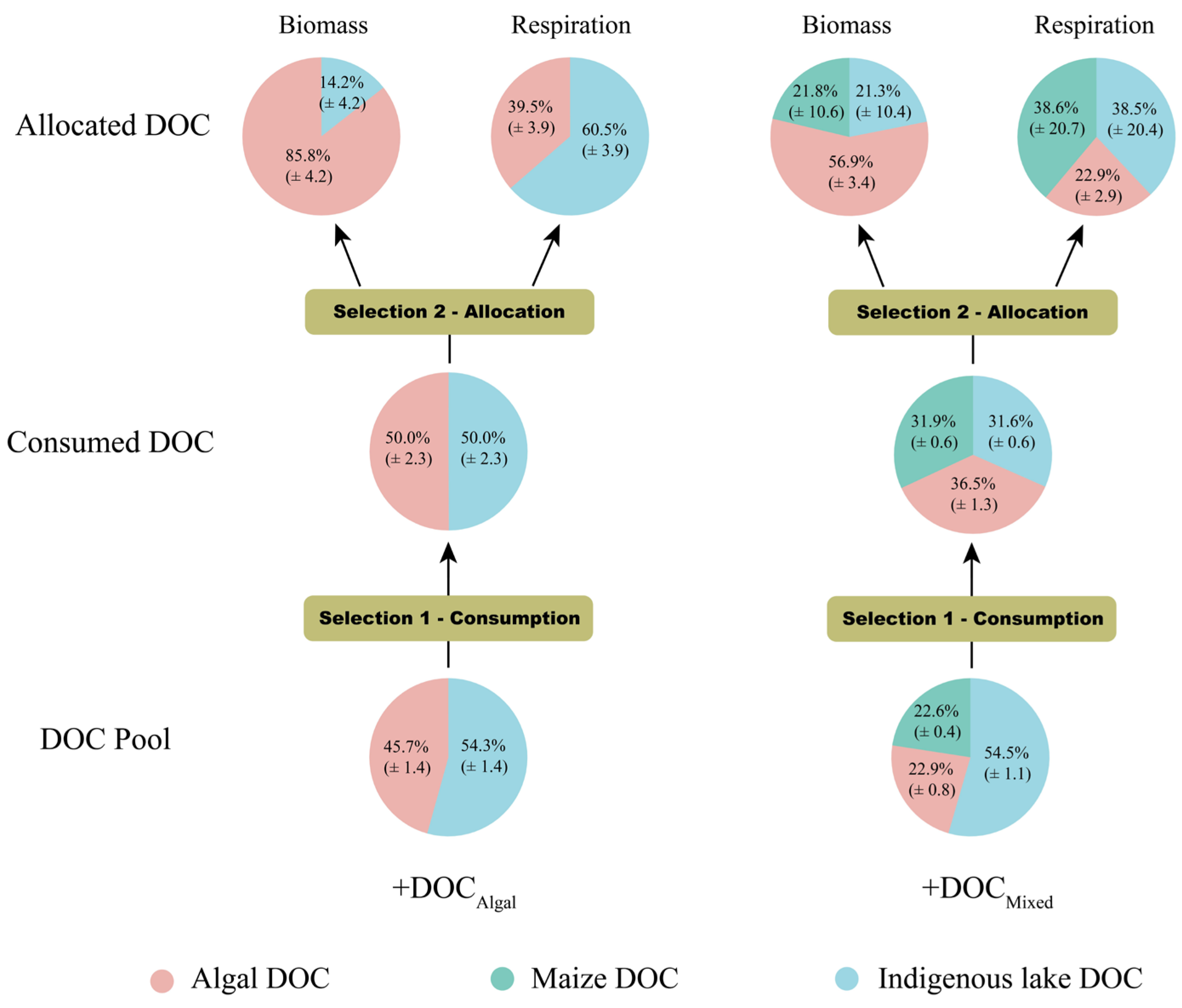
3.5. Bacteria Consuming Strategy of Different DOC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOC | Dissolved organic carbon |
| BP | Bacterial production rate |
| BR | Bacterial respiration rate |
| BGE | Bacterial growth efficiency |
| C:N | The ratio of carbon to nitrogen |
| C:P | The ratio of carbon to phosphorus |
| LMM | A linear mixed-effects model |
| δ13Cbacteria | The δ13C value of bacterial biomass |
| δ13CO2 | The δ13C value of CO2 produced by bacterial respiration |
References
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Solomon, C.T.; Jones, S.E.; Weidel, B.C.; Buffam, I.; Fork, M.L.; Karlsson, J.; Larsen, S.; Lennon, J.T.; Read, J.S.; Sadro, S. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: Current knowledge and future challenges. Ecosystems 2015, 18, 376–389. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, X.; Zhou, L.; Zhang, Y.; Xu, H.; Zhu, M.; Zhu, G.; Jang, K.-S.; Spencer, R.G.; Jeppesen, E. Rainstorms drive export of aromatic and concurrent bio-labile organic matter to a large eutrophic lake and its major tributaries. Water Res. 2023, 229, 119448. [Google Scholar] [CrossRef]
- Berggren, M.; Ström, L.; Laudon, H.; Karlsson, J.; Jonsson, A.; Giesler, R.; Bergström, A.K.; Jansson, M. Lake secondary production fueled by rapid transfer of low molecular weight organic carbon from terrestrial sources to aquatic consumers. Ecol. Lett. 2010, 13, 870–880. [Google Scholar] [CrossRef]
- Holgerson, M.A.; Hovel, R.A.; Kelly, P.T.; Bortolotti, L.E.; Brentrup, J.A.; Bellamy, A.R.; Oliver, S.K.; Reisinger, A.J. Integrating ecosystem metabolism and consumer allochthony reveals nonlinear drivers in lake organic matter processing. Limnol. Oceanogr. 2022, 67, S71–S85. [Google Scholar] [CrossRef]
- Mao, Z.; Han, Y.; Xun, F.; An, S.; Li, B.; Wang, Y.; Chen, H.; Wu, Q.L.; Xing, P. Warming effects on pelagic carbon metabolism is related to substrate composition and bacterioplankton community history. Water Res. 2025, 270, 122846. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.J.; Findlay, S.; Pace, M.L. Bacterial production in fresh and saltwater ecosystems: A cross-system overview. Mar. Ecol. Prog. Ser. 1988, 43, 1–10. [Google Scholar] [CrossRef]
- Tang, Y.; Su, L.; Xu, R.; Wang, S.; Su, Y.; Liu, Z.; Yu, J.; Dumont, H.J.; Jeppesen, E. Response of zooplankton to inputs of terrestrial dissolved organic matter: Food quality constraints induced by microbes. Limnol. Oceanogr. 2023, 68, 709–722. [Google Scholar] [CrossRef]
- Del Giorgio, P.A.; Cole, J.J. Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 1998, 29, 503–541. [Google Scholar] [CrossRef]
- Guillemette, F.; Leigh McCallister, S.; Del Giorgio, P.A. Selective consumption and metabolic allocation of terrestrial and algal carbon determine allochthony in lake bacteria. ISME J. 2016, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Kritzberg, E.S.; Cole, J.J.; Pace, M.M.; Granéli, W. Bacterial growth on allochthonous carbon in humic and nutrient-enriched lakes: Results from whole-lake 13 C addition experiments. Ecosystems 2006, 9, 489–499. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M.; Hadwen, W.L.; Roelke, D.L.; Growns, I.O.; Rohlfs, A.M. Terrestrial dissolved organic carbon subsidizes estuarine zooplankton: An in situ mesocosm study. Limnol. Oceanogr. 2016, 61, 254–267. [Google Scholar] [CrossRef]
- Berggren, M.; Laudon, H.; Haei, M.; Ström, L.; Jansson, M. Efficient aquatic bacterial metabolism of dissolved low-molecular-weight compounds from terrestrial sources. ISME J. 2010, 4, 408–416. [Google Scholar] [CrossRef]
- Kritzberg, E.S.; Cole, J.J.; Pace, M.L.; Granéli, W.; Bade, D.L. Autochthonous versus allochthonous carbon sources of bacteria: Results from whole-lake 13C addition experiments. Limnol. Oceanogr. 2004, 49, 588–596. [Google Scholar] [CrossRef]
- Del Giorgio, P.A.; Cole, J.J.; Cimbleris, A. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature 1997, 385, 148–151. [Google Scholar] [CrossRef]
- Karlsson, J.; Jansson, M.; Jonsson, A. Respiration of allochthonous organic carbon in unproductive forest lakes determined by the Keeling plot method. Limnol. Oceanogr. 2007, 52, 603–608. [Google Scholar] [CrossRef]
- Attermeyer, K.; Hornick, T.; Kayler, Z.; Bahr, A.; Zwirnmann, E.; Grossart, H.-P.; Premke, K. Enhanced bacterial decomposition with increasing addition of autochthonous to allochthonous carbon without any effect on bacterial community composition. Biogeosciences 2014, 11, 1479–1489. [Google Scholar] [CrossRef]
- Guillemette, F.; McCallister, S.L.; del Giorgio, P.A. Differentiating the degradation dynamics of algal and terrestrial carbon within complex natural dissolved organic carbon in temperate lakes. J. Geophys. Res.-Biogeo. 2013, 118, 963–973. [Google Scholar] [CrossRef]
- McKnight, D.M.; Aiken, G.R. Sources and age of aquatic humus. In Aquatic Humic Substances: Ecology and Biogeochemistry; Springer: Heidelberg, Germany, 1998; pp. 9–39. [Google Scholar]
- Allesson, L.; Andersen, T.; Dörsch, P.; Eiler, A.; Wei, J.; Hessen, D.O. Phosphorus availability promotes bacterial DOC-mineralization, but not cumulative CO2-production. Front. Microbiol. 2020, 11, 569879, Erratum in Front. Microbiol. 2020, 11, 614974. [Google Scholar]
- Farjalla, V.F.; Azevedo, D.A.; Esteves, F.A.; Bozelli, R.L.; Roland, F.; Enrich-Prast, A. Influence of hydrological pulse on bacterial growth and DOC uptake in a clear-water Amazonian lake. Microb. Ecol. 2006, 52, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Kritzberg, E.S.; Cole, J.J.; Pace, M.M.; Granéli, W. Does autochthonous primary production drive variability in bacterial metabolism and growth efficiency in lakes dominated by terrestrial C inputs? Aquat. Microb. Ecol. 2005, 38, 103–111. [Google Scholar] [CrossRef]
- Morana, C.; Sarmento, H.; Descy, J.-P.; Gasol, J.M.; Borges, A.V.; Bouillon, S.; Darchambeau, F. Production of dissolved organic matter by phytoplankton and its uptake by heterotrophic prokaryotes in large tropical lakes. Limnol. Oceanogr. 2014, 59, 1364–1375. [Google Scholar] [CrossRef]
- Danger, M.; Cornut, J.; Chauvet, E.; Chavez, P.; Elger, A.; Lecerf, A. Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: A case of aquatic priming effect? Ecology 2013, 94, 1604–1613. [Google Scholar] [CrossRef]
- Guenet, B.; Danger, M.; Abbadie, L.; Lacroix, G. Priming effect: Bridging the gap between terrestrial and aquatic ecology. Ecology 2010, 91, 2850–2861. [Google Scholar] [CrossRef]
- Guenet, B.; Danger, M.; Harrault, L.; Allard, B.; Jauset-Alcala, M.; Bardoux, G.; Benest, D.; Abbadie, L.; Lacroix, G. Fast mineralization of land-born C in inland waters: First experimental evidences of aquatic priming effect. Hydrobiologia 2014, 721, 35–44. [Google Scholar] [CrossRef]
- Farjalla, V.F.; Marinho, C.C.; Faria, B.M.; Amado, A.M.; Esteves, F.d.A.; Bozelli, R.L.; Giroldo, D. Synergy of fresh and accumulated organic matter to bacterial growth. Microb. Ecol. 2009, 57, 657–666. [Google Scholar] [CrossRef]
- Fonte, E.S.; Amado, A.M.; Meirelles-Pereira, F.; Esteves, F.A.; Rosado, A.S.; Farjalla, V.F. The combination of different carbon sources enhances bacterial growth efficiency in aquatic ecosystems. Microb. Ecol. 2013, 66, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.M.; Wagner, K.; Burns, N.R.; Herberg, E.R.; Wanek, W.; Kaplan, L.A.; Battin, T.J. No evidence of aquatic priming effects in hyporheic zone microcosms. Sci. Rep. 2014, 4, 5187. [Google Scholar] [CrossRef]
- Attermeyer, K.; Tittel, J.; Allgaier, M.; Frindte, K.; Wurzbacher, C.; Hilt, S.; Kamjunke, N.; Grossart, H.-P. Effects of light and autochthonous carbon additions on microbial turnover of allochthonous organic carbon and community composition. Microb. Ecol. 2015, 69, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Drake, T.W.; Raymond, P.A.; Spencer, R.G. Terrestrial carbon inputs to inland waters: A current synthesis of estimates and uncertainty. Limnol. Oceanogr. Lett. 2018, 3, 132–142. [Google Scholar] [CrossRef]
- Waichman, A.V. Autotrophic carbon sources for heterotrophic bacterioplankton in a floodplain lake of central Amazon. Hydrobiologia 1996, 341, 27–36. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y.; Tang, X.; Zhang, Y.; Jang, K.-S.; Szekely, A.J.; Jeppesen, E. Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter. Water Res. 2021, 190, 116776. [Google Scholar] [CrossRef]
- Theil-Nielsen, J.; Søndergaard, M. Bacterial carbon biomass calculated from biovolumes. Arch. Hydrobiol. 1998, 141, 195–207. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Parnell, A.C.; Inger, R. Simmr: A Stable Isotope Mixing Model. R Package Version 0.3. 2016. Available online: https://CRAN.R-project.org/package=simmr (accessed on 13 June 2023).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P.; Bolker, M.B. Package ‘lme4’. Convergence 2015, 12, 2. [Google Scholar]
- Lenth, R.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.5. 4. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 10 March 2025).
- Clarke, A. Energy flow in growth and production. Trends Ecol. Evol. 2019, 34, 502–509. [Google Scholar] [CrossRef]
- Amon, R.M.; Benner, R. Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 1996, 41, 41–51. [Google Scholar] [CrossRef]
- Thorp, J.H.; Delong, M.D. Dominance of autochthonous autotrophic carbon in food webs of heterotrophic rivers. Oikos 2002, 96, 543–550. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Gulf Professional Publishing: London, UK, 2001. [Google Scholar]
- Cimbleris, A.C.; Kalff, J. Planktonic bacterial respiration as a function of C:N:P ratios across temperate lakes. Hydrobiologia 1998, 384, 89–100. [Google Scholar] [CrossRef]
- Koch, A.L. What size should a bacterium be? A question of scale. Annu. Rev. Microbiol. 1996, 50, 317–348. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Guan, S.; Shi, H. Quantitative connection between cell size and growth rate by phospholipid metabolism. Cells 2020, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Cotner, J.B.; Wetzel, R.G. Uptake of dissolved inorganic and organic phosphorus compounds by phytoplankton and bacterioplankton. Limnol. Oceanogr. 1992, 37, 232–243. [Google Scholar] [CrossRef]
- Han, Y.; Li, B.; Xun, F.; Gao, P.; Mao, Z.; Tao, Y.; Xing, P. Metabolism of exogenous and endogenous dissolved organic carbon for bacterioplankton production and respiration in reservoirs. Lake Sci. 2022, 34, 162–173. [Google Scholar]
- LaBrie, R.; Lapierre, J.F.; Maranger, R. Contrasting patterns of labile and semilabile dissolved organic carbon from continental waters to the open ocean. J. Geophys. Res. Biogeosciences 2020, 125, e2019JG005300. [Google Scholar] [CrossRef]
- Ostapenia, A.; Parparov, A.; Berman, T. Lability of organic carbon in lakes of different trophic status. Freshw. Biol. 2009, 54, 1312–1323. [Google Scholar] [CrossRef]
- Schmid, M.W.; van Moorsel, S.J.; Hahl, T.; De Luca, E.; De Deyn, G.B.; Wagg, C.; Niklaus, P.A.; Schmid, B. Effects of plant community history, soil legacy and plant diversity on soil microbial communities. J. Ecol. 2021, 109, 3007–3023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Zhang, H.; Liu, Z.; Jeppesen, E.; Gao, J.; Tang, Y. The Input of Terrestrial Dissolved Organic Carbon Enhanced Bacteria Growth Efficiency on Phytoplankton-DOC and Indigenous Lake DOC: A Microcosm Study. Microorganisms 2025, 13, 2081. https://doi.org/10.3390/microorganisms13092081
Jin Z, Zhang H, Liu Z, Jeppesen E, Gao J, Tang Y. The Input of Terrestrial Dissolved Organic Carbon Enhanced Bacteria Growth Efficiency on Phytoplankton-DOC and Indigenous Lake DOC: A Microcosm Study. Microorganisms. 2025; 13(9):2081. https://doi.org/10.3390/microorganisms13092081
Chicago/Turabian StyleJin, Zong’an, Huiping Zhang, Zhengwen Liu, Erik Jeppesen, Jian Gao, and Yali Tang. 2025. "The Input of Terrestrial Dissolved Organic Carbon Enhanced Bacteria Growth Efficiency on Phytoplankton-DOC and Indigenous Lake DOC: A Microcosm Study" Microorganisms 13, no. 9: 2081. https://doi.org/10.3390/microorganisms13092081
APA StyleJin, Z., Zhang, H., Liu, Z., Jeppesen, E., Gao, J., & Tang, Y. (2025). The Input of Terrestrial Dissolved Organic Carbon Enhanced Bacteria Growth Efficiency on Phytoplankton-DOC and Indigenous Lake DOC: A Microcosm Study. Microorganisms, 13(9), 2081. https://doi.org/10.3390/microorganisms13092081






