Changes in Probiotic Lachnospiraceae Genera Across Different Stages of COVID-19: A Meta-Analysis of 16S rRNA Microbial Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection and Obtaining the Sequencing Datasets
2.2. Sequencing Read Data Processing and Analysis
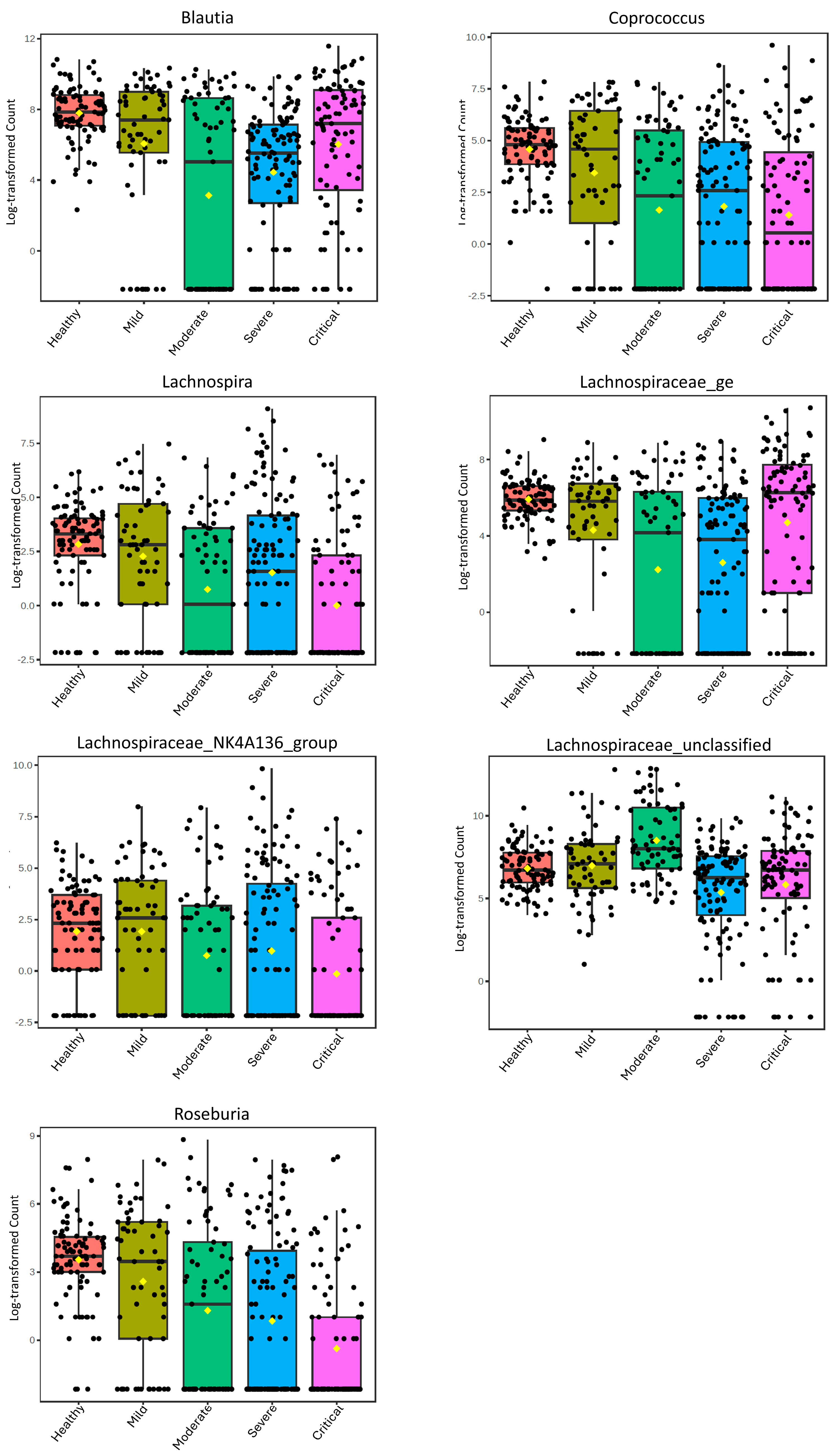
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stackebrandt, E. The Family Lachnospiraceae. In The Prokaryotes: Firmicutes and Tenericutes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 197–201. [Google Scholar]
- Liu, C.; Du, M.-X.; Abuduaini, R.; Yu, H.-Y.; Li, D.-H.; Wang, Y.-J.; Zhou, N.; Jiang, M.-Z.; Niu, P.-X.; Han, S.-S.; et al. Enlightening the taxonomy darkness of human gut microbiomes with a cultured biobank. Microbiome 2021, 9, 119. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Littmann, E.R.; Fontana, E.; Moody, T.U.; Kohout, C.E.; Gjonbalaj, M.; Eaton, V.; Seok, R.; Leiner, I.M.; Pamer, E.G. Functional and genomic variation between human-derived isolates of Lachnospiraceae reveals inter- and intra-species diversity. Cell Host Microbe 2020, 28, 134–146.e4. [Google Scholar] [CrossRef]
- Sheridan, P.O.; Martin, J.C.; Lawley, T.D.; Browne, H.P.; Harris, H.M.B.; Bernalier-Donadille, A.; Duncan, S.H.; O’TOole, P.W.; Scott, K.P.; Flint, H.J. Polysaccharide utilization loci and nutritional specialization in a dominant group of butyrate-producing human colonic Firmicutes. Microb. Genom. 2016, 2, e000043. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Hatziioanou, D.; Gherghisan-Filip, C.; Saalbach, G.; Horn, N.; Wegmann, U.; Duncan, S.H.; Flint, H.J.; Mayer, M.J.; Narbad, A. Discovery of a novel lantibiotic nisin O from Blautia obeum A2-162, isolated from the human gastrointestinal tract. Microbiology 2017, 163, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Luo, W.; Tan, B.; Nie, K.; Deng, M.; Wu, S.; Xiao, M.; Wu, X.; Meng, X.; Tong, T.; et al. Roseburia intestinalis stimulates TLR5-dependent intestinal immunity against Crohn’s disease. eBioMedicine 2022, 85, 104285. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Hexun, Z.; Miyake, T.; Maekawa, T.; Mori, H.; Yasukawa, D.; Ohno, M.; Nishida, A.; Andoh, A.; Tani, M. High abundance of Lachnospiraceae in the human gut microbiome is related to high immunoscores in advanced colorectal cancer. Cancer Immunol. Immunother. 2023, 72, 315–326. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Park, S.-C.; Kim, N.-E.; Shin, C.; Lee, S.K.; Jung, Y.; Yoon, D.; Kim, H.; Kim, S.; et al. Role of an unclassified Lachnospiraceae in the pathogenesis of type 2 diabetes: A longitudinal study of the urine microbiome and metabolites. Exp. Mol. Med. 2022, 54, 1125–1132. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chiu, M.-H.; Kek, H.-P.; Yang, M.-C.; Su, Y.-T.; Liu, H.-K.; Wu, M.-S.; Yeh, Y.-T. The reduced gut Lachnospira species is linked to liver enzyme elevation and insulin resistance in pediatric fatty liver disease. Int. J. Mol. Sci. 2024, 25, 3640. [Google Scholar] [CrossRef]
- Yin, Y.S.; Minacapelli, C.D.; Parmar, V.; Catalano, C.C.; Bhurwal, A.; Gupta, K.; Rustgi, V.K.; Blaser, M.J. Alterations of the fecal microbiota in relation to acute COVID-19 infection and recovery. Mol. Biomed. 2022, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-N.; Joo, E.-J.; Lee, C.-W.; Ahn, K.-S.; Kim, H.-L.; Park, D.-I.; Park, S.-K. Reversion of gut microbiota during the recovery phase in patients with asymptomatic or mild COVID-19: Longitudinal study. Microorganisms 2021, 9, 1237. [Google Scholar] [CrossRef]
- Moreira-Rosário, A.; Marques, C.; Pinheiro, H.; Araújo, J.R.; Ribeiro, P.; Rocha, R.; Mota, I.; Pestana, D.; Ribeiro, R.; Pereira, A.; et al. Gut microbiota diversity and C-reactive protein are predictors of disease severity in COVID-19 patients. Front. Microbiol. 2021, 12, 705020. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, J.; Zhang, X.; Dai, Z.; Liu, M.; Zhang, H.; Zhang, N.; Lei, R.; Luo, P.; Zhang, J. Large-scale genetic correlation studies explore the causal relationship and potential mechanism between gut microbiota and COVID-19-associated risks. BMC Microbiol. 2024, 24, 292. [Google Scholar] [CrossRef]
- De Maio, F.; Ianiro, G.; Coppola, G.; Santopaolo, F.; Abbate, V.; Bianco, D.M.; Del Zompo, F.; De Matteis, G.; Leo, M.; Nesci, A.; et al. Improved gut microbiota features after the resolution of SARS-CoV-2 infection. Gut Pathog. 2021, 13, 62. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, H.; Cui, G.; Lu, H.; Wang, L.; Luo, H.; Chen, X.; Ren, H.; Sun, R.; Liu, W.; et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut 2021, 70, 1253–1265. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef]
- Groussin, M.; Poyet, M.; Sistiaga, A.; Kearney, S.M.; Moniz, K.; Noel, M.; Hooker, J.; Gibbons, S.M.; Segurel, L.; Froment, A.; et al. Elevated rates of horizontal gene transfer in the industrialized human microbiome. Cell 2021, 184, 2053–2067.e18. [Google Scholar] [CrossRef]
- Abellan-Schneyder, I.; Matchado, M.S.; Reitmeier, S.; Sommer, A.; Sewald, Z.; Baumbach, J.; List, M.; Neuhaus, K.; Tringe, S.G. Primer, pipelines, parameters: Issues in 16S rRNA gene sequencing. mSphere 2021, 6, 1–22. [Google Scholar] [CrossRef]
- Mizrahi-Man, O.; Davenport, E.R.; Gilad, Y.; White, B.A. Taxonomic classification of bacterial 16S rRNA genes using short sequencing reads: Evaluation of effective study designs. PLoS ONE 2013, 8, 18–23. [Google Scholar] [CrossRef]
- Taufer, C.R.; da Silva, J.; Rampelotto, P.H. In Silico Analysis of Probiotic Bacteria Changes Across COVID-19 Severity Stages. Microorganisms 2024, 12, 2353. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Cher, G.L.Y.; Abid, M.B. Role of Gut Microbiome in COVID-19: An Insight Into Pathogenesis and Therapeutic Potential. Front. Immunol. 2021, 12, 765965. [Google Scholar] [CrossRef] [PubMed]
- Broderick, D.; Marsh, R.; Waite, D.; Pillarisetti, N.; Chang, A.B.; Taylor, M.W. Realising respiratory microbiomic meta-analyses: Time for a standardised framework. Microbiome 2023, 11, 57. [Google Scholar] [CrossRef]
- Albrich, W.C.; Ghosh, T.S.; Ahearn-Ford, S.; Mikaeloff, F.; Lunjani, N.; Forde, B.; Suh, N.; Kleger, G.-R.; Pietsch, U.; Frischknecht, M.; et al. A high-risk gut microbiota configuration associates with fatal hyperinflammatory immune and metabolic responses to SARS-CoV-2. Gut Microbes 2022, 14, 2073131. [Google Scholar] [CrossRef]
- Gaibani, P.; D’Amico, F.; Bartoletti, M.; Lombardo, D.; Rampelli, S.; Fornaro, G.; Coladonato, S.; Siniscalchi, A.; Re, M.C.; Viale, P.; et al. The gut microbiota of critically Ill patients with COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 670424. [Google Scholar] [CrossRef]
- Galperine, T.; Choi, Y.; Pagani, J.L.; Kritikos, A.; Papadimitriou-Olivgeris, M.; Méan, M.; Scherz, V.; Opota, O.; Greub, G.; Guery, B.; et al. Temporal changes in fecal microbiota of patients infected with COVID-19: A longitudinal cohort. BMC Infect. Dis. 2023, 23, 537. [Google Scholar] [CrossRef]
- Rafiqul Islam, S.M.; Foysal, M.J.; Hoque, M.N.; Mehedi, H.M.H.; Rob, M.A.; Salauddin, A.; Tanzina, A.Y.; Biswas, S.; Noyon, S.H.; Siddiki, A.M.A.M.Z.; et al. Dysbiosis of Oral and Gut Microbiomes in SARS-CoV-2 Infected Patients in Bangladesh: Elucidating the Role of Opportunistic Gut Microbes. Front. Med. 2022, 9, 821777. [Google Scholar] [CrossRef]
- Reinold, J.; Farahpour, F.; Fehring, C.; Dolff, S.; Konik, M.; Korth, J.; van Baal, L.; Hoffmann, D.; Buer, J.; Witzke, O.; et al. A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates with Severe COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 747816. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D.; Bandopadhyay, P.; Ray, Y.; Paul, S.R.; Sarif, J.; D’Rozario, R.; Lahiri, A.; Das, S.; Bhowmick, D.; Chatterjee, S.; et al. Association of gut microbial dysbiosis with disease severity, response to therapy and disease outcomes in Indian patients with COVID-19. Gut Pathog. 2023, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, X.; Jiang, G.; Tang, H.; Ming, S.; Tang, L.; Lu, J.; Guo, C.; Shan, H.; Huang, X. Altered oral and gut microbiota and its association with SARS-CoV-2 viral load in COVID-19 patients during hospitalization. NPJ Biofilms Microbiomes 2021, 7, 61. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Pezzini, M.F.; Rampelotto, P.H.; Dall’AGnol, J.; Guerreiro, G.T.S.; Longo, L.; Uribe, N.D.S.; Lange, E.C.; Álvares-Da-Silva, M.R.; Joveleviths, D. Changes in the gut microbiota of rats after exposure to the fungicide Mancozeb. Toxicol. Appl. Pharmacol. 2023, 466, 115459. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Tang, X. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China. Glob. Health Med. 2020, 2, 66–72. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 cytokine storm: What we know so far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Qin, N.; Zheng, B.; Yao, J.; Guo, L.; Zuo, J.; Wu, L.; Zhou, J.; Liu, L.; Guo, J.; Ni, S.; et al. Influence of H7N9 virus infection and associated treatment on human gut microbiota. Sci. Rep. 2015, 5, 14771. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.; Sencio, V.; Raise, A.; Huillet, E.; Layec, S.; Deruyter, L.; Heumel, S.; Auger, S.; Robert, V.; Langella, P.; et al. Description of a Newly Isolated Blautia faecis Strain and Its Benefit in Mouse Models of Post-Influenza Secondary Enteric and Pulmonary Infections. Nutrients 2022, 14, 1478. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, S.; Wu, L.; Cao, H.; Fan, Y.; Wang, X.; Yu, Z.; Zhou, M.; Gao, R.; Wang, J. Coprococcus eutactus screened from healthy adolescent attenuates chronic restraint stress-induced depression-like changes in adolescent mice: Potential roles in the microbiome and neurotransmitter modulation. J. Affect. Disord. 2024, 356, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shan, S.; Shi, J.; Li, H.; An, N.; Li, S.; Cui, K.; Guo, H.; Li, Z. Coprococcus eutactus, a Potent Probiotic, Alleviates Colitis via Acetate-Mediated IgA Response and Microbiota Restoration. J. Agric. Food Chem. 2022, 70, 13494–13506. [Google Scholar] [CrossRef]
- Escudeiro, P.; Henry, C.S.; Dias, R.P. Functional characterization of prokaryotic dark matter: The road so far and what lies ahead. Curr. Res. Microb. Sci. 2022, 3, 100159. [Google Scholar] [CrossRef]
- Ke, S.; Weiss, S.T.; Liu, Y.-Y. Dissecting the role of the human microbiome in COVID-19 via metagenome-assembled genomes. Nat. Commun. 2022, 13, 5235. [Google Scholar] [CrossRef]
- Li, S.; Yang, S.; Zhou, Y.; Disoma, C.; Dong, Z.; Du, A.; Zhang, Y.; Chen, Y.; Huang, W.; Chen, J.; et al. Microbiome Profiling Using Shotgun Metagenomic Sequencing Identified Unique Microorganisms in COVID-19 Patients With Altered Gut Microbiota. Front. Microbiol. 2021, 12, 636296. [Google Scholar] [CrossRef]
- Eleftheriotis, G.; Tsounis, E.P.; Aggeletopoulou, I.; Dousdampanis, P.; Triantos, C.; Mouzaki, A.; Marangos, M.; Assimakopoulos, S.F. Alterations in gut immunological barrier in SARS-CoV-2 infection and their prognostic potential. Front. Immunol. 2023, 14, 1129190. [Google Scholar] [CrossRef]
- Tsounis, E.P.; Triantos, C.; Konstantakis, C.; Marangos, M.; Assimakopoulos, S.F. Intestinal barrier dysfunction as a key driver of severe COVID-19. World J. Virol. 2023, 12, 68–90. [Google Scholar] [CrossRef]
- Raj, S.T.; Bruce, A.W.; Anbalagan, M.; Srinivasan, H.; Chinnappan, S.; Rajagopal, M.; Khanna, K.; Chandramoorthy, H.C.; Mani, R.R. COVID-19 influenced gut dysbiosis, post-acute sequelae, immune regulation, and therapeutic regimens. Front. Cell Infect. Microbiol. 2024, 14, 1384939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Descamps, H.C.; Herrmann, B.; Wiredu, D.; Thaiss, C.A. The path toward using microbial metabolites as therapies. eBioMedicine 2019, 44, 747–754. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Okin, D.; Drew, D.A.; Battista, V.M.; Jesudasen, S.J.; Kuntz, T.M.; Bhosle, A.; Thompson, K.N.; Reinicke, T.; Lo, C.-H.; et al. Metagenomic assessment of gut microbial communities and risk of severe COVID-19. Genome Med. 2023, 15, 12. [Google Scholar] [CrossRef]
- Sehli, S.; Allali, I.; Chahboune, R.; Bakri, Y.; Al Idrissi, N.; Hamdi, S.; Nejjari, C.; Amzazi, S.; Ghazal, H. Metagenomics Approaches to Investigate the Gut Microbiome of COVID-19 Patients. Bioinform. Biol. Insights 2021, 15, 11779322211015192. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Dillon, M.R.; Zhang, Y.; Rideout, J.R.; Bolyen, E.; Li, H.; Albert, P.S.; Caporaso, J.G.; Arumugam, M. q2-longitudinal: Longitudinal and paired-sample analyses of microbiome data. mSystems 2018, 3, e00219-18. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Hsu, T.; Sirota-Madi, A.; Shafquat, A.; Abu-Ali, G.; Morgan, X.C.; Huttenhower, C. Sequencing and beyond: Integrating molecular “omics” for microbial community profiling. Nat. Rev. Microbiol. 2015, 13, 360–372. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Butcher, J.; Stintzi, A.; Figeys, D. Advancing functional and translational microbiome research using meta-omics approaches. Microbiome 2019, 7, 154. [Google Scholar] [CrossRef]
- Cen, X.; Wang, F.; Huang, X.; Jovic, D.; Dubee, F.; Yang, H.; Li, Y. Towards precision medicine: Omics approach for COVID-19. Biosaf. Health 2023, 5, 78–88. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, S.; Wang, P.; Chen, X.; Bi, J.; Cheng, L.; Zhang, X. A comprehensive review of the analysis and integration of omics data for SARS-CoV-2 and COVID-19. Brief. Bioinform. 2022, 23, bbab446. [Google Scholar] [CrossRef]
- Muñoz-Fontela, C.; Dowling, W.E.; Funnell, S.G.P.; Gsell, P.-S.; Riveros-Balta, A.X.; Albrecht, R.A.; Andersen, H.; Baric, R.S.; Carroll, M.W.; Cavaleri, M.; et al. Animal models for COVID-19. Nature 2020, 586, 509–515. [Google Scholar] [CrossRef]
- Batista, K.S.; de Albuquerque, J.G.; de Vasconcelos, M.H.A.; Bezerra, M.L.R.; Barbalho, M.B.d.S.; Pinheiro, R.O.; Aquino, J.d.S. Probiotics and prebiotics: Potential prevention and therapeutic target for nutritional management of COVID-19? Nutr. Res. Rev. 2023, 36, 181–198. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Aolymat, I.; Al-Holy, M.; Ayyash, M.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Osaili, T.; Apostolopoulos, V.; Liu, S.-Q.; Shah, N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. npj Sci. Food 2020, 4, 17. [Google Scholar] [CrossRef]
- Su, Q.; Lau, R.I.; Liu, Q.; Li, M.K.; Mak, J.W.Y.; Lu, W.; Lau, I.S.; Lau, L.H.; Yeung, G.T.; Cheung, C.P.; et al. The gut microbiome associates with phenotypic manifestations of post-acute COVID-19 syndrome. Cell Host Microbe 2024, 32, 651–660.e4. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Xia, Y.; Sun, J. Microbiome and intestinal pathophysiology in post-acute sequelae of COVID-19. Genes Dis. 2024, 11, 100978. [Google Scholar] [CrossRef] [PubMed]
- Horsley, A.R.; Pearmain, L.; Knight, S.; Schindler, N.; Wang, R.; Bennett, M.; Robey, R.C.; Davies, J.C.; Djukanović, R.; Heaney, L.G.; et al. Large scale clinical trials: Lessons from the COVID-19 pandemic. BMJ Open Respir. Res. 2022, 9, e001226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lau, R.I.; Liu, Q.; Su, Q.; Chan, F.K.L.; Ng, S.C. Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 323–337. [Google Scholar] [CrossRef] [PubMed]
| Autor; Year | Accession Number | Country | Type of Study | NGS Technology | N | Groups |
|---|---|---|---|---|---|---|
| Albrich et al., 2022 [28] | PRJEB50040 | Switzerland and Ireland | Cohort | MiSeq | 98 | 8 mild, 24 moderate, and 66 severe |
| Gaibani et al., 2021 [29] | PRJNA700830 | Italy | Case–control | MiSeq | 69 | COVID-19 |
| Galperine et al., 2023 [30] | PRJEB61722 | Switzerland | Cohort | MiSeq | 57 | 42 severe, 15 critical |
| Rafiqul Islam et al., 2022 [31] | PRJNA767939 | Bangladesh | Cross-section | MiSeq | 37 | 15 healthy, 22 COVID-19 |
| Reinold et al., 2021 [32] | PRJNA747262 | Germany | Cross-section | NovaSeq 6000 | 212 | 95 negative, 44 mild, 35 moderate, 26 severe, 12 critical |
| Talukdar et al., 2023 [33] | PRJNA895415 | India | Cohort | MiSeq | 52 | 7 mild, 45 severe |
| Wu et al., 2021 [34] | PRJNA684070 | China | Case–control | NovaSeq 6000 | 56 | 32 healthy, 5 mild, 16 moderate, 3 severe |
| Genus | Study | Reference Database | Statistical Analysis | p Values | FDR | Healthy | Mild | Moderate | Severe | Critical | LDA Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blautia | PRJEB50040 | NR | Mann–Whitney | 0.00042096 | - | - | - | - | - | - | - |
| Standard Protocol | Silva v. 138 | Lefse | 0.082563 | 0.49618 | - | 528.38 | 568.08 | 324.02 | - | 2.09 | |
| PRJEB61722 | EzBioCloud | NBZIMM | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | 0.67677 | 0.87127 | 187.86 | 272.73 | 1.64 | ||||
| PRJNA747262 | Greengenes 13.8 | Lefse | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | 0.27837 | 0.62404 | 1093.1 | 1159.1 | 1336.7 | 852.62 | 1301.8 | 2.39 | |
| PRJNA895415 | Silva v. 138 | Lefse | 0.0018892 | 0.027866 | - | - | - | - | - | 5.21 | |
| Standard Protocol | Silva v. 138 | Lefse | 0.036234 | 0.37246 | - | 468.71 | - | 168.31 | - | 2.18 | |
| PRJNA684070 | Greengenes 13.8 | Lefse | NR | NR | NR | NR | NR | NR | NR | NR | |
| Standard Protocol | Silva v. 138 | Lefse | - | - | - | - | - | - | - | - | |
| Coprococcus | PRJEB50040 | NR | Mann–Whitney | 0.003043936 | - | - | - | - | - | - | - |
| Standard Protocol | Silva v. 138 | Lefse | 0.11478 | 0.54002 | - | 31.5 | 16.417 | 15.909 | - | 0.944 | |
| PRJEB61722 | EzBioCloud | NBZIMM | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | 0.16682 | 0.66966 | - | - | - | 64.762 | 33 | −1.23 | |
| PRJNA747262 | Greengenes 13.8 | Lefse | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | 0.22521 | 0.59503 | 122.15 | 216.5 | 199.11 | 152 | 137.83 | 1.68 | |
| PRJNA895415 | Silva v. 138 | Lefse | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | 0.18663 | 0.7538 | - | 41.429 | - | 23.978 | - | 0.988 | |
| PRJNA684070 | Greengenes 13.8 | Lefse | NR | NR | NR | NR | NR | NR | NR | NR | |
| Standard Protocol | Silva v. 138 | Lefse | - | - | - | - | - | - | - | - | |
| Lachnospira | PRJEB50040 | NR | Mann–Whitney | 7.98309 × 10−5 | - | - | - | - | - | - | - |
| Standard Protocol | Silva v. 138 | Lefse | 0.0048226 | 0.1136 | - | 21.875 | 33.083 | 11.455 | - | 1.07 | |
| PRJEB61722 | EzBioCloud | NBZIMM | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | 0.0057487 | 0.35032 | - | - | - | 146.17 | 22 | −1.8 | |
| PRJNA747262 | Greengenes 13.8 | Lefse | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | 0.21381 | 0.58498 | 42.895 | 72.091 | 65.114 | 54.346 | 29.083 | 1.35 | |
| PRJNA895415 | Silva v. 138 | Lefse | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | 0.012363 | 0.21696 | - | 83.714 | - | 10.4 | - | 1.58 | |
| PRJNA684070 | Greengenes 13.8 | Lefse | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | - | - | - | - | - | - | - | - | |
| Roseburia | PRJEB50040 | NR | Mann–Whitney | 0.000113838 | - | - | - | - | - | - | - |
| Standard Protocol | Silva v. 138 | Lefse | 0.0087579 | 0.15472 | - | 8.625 | 19.583 | 20.879 | - | 0.853 | |
| PRJEB61722 | EzBioCloud | NBZIMM | NR | NR | - | - | - | NR | NR | NR | |
| Standard Protocol | Silva v. 138 | Lefse | 0.039976 | 0.58486 | - | - | - | 67.048 | 4.7333 | −1.51 | |
| PRJNA747262 | Greengenes 13.8 | Lefse | <0.05 | NR | NR | NR | NR | NR | NR | >3.5 | |
| Standard Protocol | Silva v. 138 | Lefse | 0.042058 | 0.27312 | 78.274 | 119.34 | 175.77 | 114.12 | 43.917 | 1.83 | |
| PRJNA895415 | Silva v. 138 | Lefse | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | 0.25183 | 0.76757 | - | 21.571 | - | 9.3333 | - | 0.852 | |
| PRJNA684070 | Greengenes 13.8 | Lefse | - | - | - | - | - | - | - | - | |
| Standard Protocol | Silva v. 138 | Lefse | - | - | - | - | - | - | - | - |
| Genus | Study | Reference Database | Statistical Analysis | p Values | FDR | Healthy | COVID-19 | LDA Score |
|---|---|---|---|---|---|---|---|---|
| Blautia | PRJNA767939 | NCBI | Kruskal–Wallis | - | - | - | - | - |
| Standard Protocol | Silva v. 138 | LEfSe | 0.81092 | 0.89347 | 18.067 | 36.045 | −1 | |
| Coprococcus | PRJNA767939 | NCBI | Kruskal–Wallis | - | - | - | - | - |
| Standard Protocol | Silva v. 138 | LEfSe | 0.39419 | 0.57006 | 3.1333 | 2.9091 | 0.0462 | |
| Lachnospira | PRJNA767939 | NCBI | Kruskal–Wallis | - | - | - | - | - |
| Standard Protocol | Silva v. 138 | LEfSe | 0.035134 | 0.12232 | 9.6 | 13.091 | −0.439 | |
| Roseburia | PRJNA767939 | NCBI | Kruskal–Wallis | - | - | - | - | - |
| Standard Protocol | Silva v. 138 | LEfSe | 0.001058 | 0.016576 | 3.4 | 0.81818 | 0.36 |
| Genus | Study | Reference Database | Statistical Analysis | p Values | FDR | Healthy | Mild | Moderate | Severe | Critical | LDA Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lachnospiraceae_FCS020_group | PRJEB50040 | Silva v. 138 | Lefse | 0.2352 | 0.65374 | - | 4.5 | 1.2917 | 1.3485 | - | 0.416 |
| Lachnospiraceae_FCS020_group | PRJEB61722 | Silva v. 138 | Lefse | 0.27561 | 0.71539 | - | - | - | 1.7619 | 0.8 | −0.171 |
| Lachnospiraceae_FCS020_group | PRJNA895415 | Silva v. 138 | Lefse | 0.09367 | 0.61012 | - | - | 2.2857 | 0.84444 | - | 0.236 |
| Lachnospiraceae_ge | PRJEB50040 | Silva v. 138 | Lefse | 0.0072566 | 0.14405 | - | 80.125 | 56 | 50.924 | - | 1.19 |
| Lachnospiraceae_ge | PRJEB61722 | Silva v. 138 | Lefse | 0.82062 | 0.911 | - | - | - | 157.19 | 126.67 | −1.21 |
| Lachnospiraceae_ge | PRJNA747262 | Silva v. 138 | Lefse | 0.094113 | 0.44042 | 249.07 | 307.32 | 392.63 | 357.81 | 640.75 | 2.29 |
| Lachnospiraceae_ge | PRJNA895415 | Silva v. 138 | Lefse | 0.012753 | 0.21696 | - | - | 74.857 | 19.733 | - | 1.46 |
| Lachnospiraceae_NK3A20_group | PRJNA747262 | Silva v. 138 | Lefse | 0.22463 | 0.59503 | 1.5368 | 1.9773 | 1.0571 | 0.038462 | 0.66667 | 0.294 |
| Lachnospiraceae_NK4A136_group | PRJEB61722 | Silva v. 138 | Lefse | 0.0001754 | 0.044201 | - | - | - | 155.52 | 20.267 | −1.84 |
| Lachnospiraceae_NK4A136_group | PRJNA747262 | Silva v. 138 | Lefse | 0.00019874 | 0.010158 | 30.895 | 82 | 105.94 | 102.08 | 68 | 1.59 |
| Lachnospiraceae_NK4B4_group | PRJNA747262 | Silva v. 138 | Lefse | 0.00020731 | 0.010158 | 0 | 0.22727 | 0.17143 | 1.3462 | 0.41667 | 0.224 |
| Lachnospiraceae_XPB1014_group | PRJNA747262 | Silva v. 138 | Lefse | 0.25132 | 0.60649 | 0.021053 | 0.022727 | 0 | 0 | 0.41667 | 0.0822 |
| Lachnospiraceae_ND3007_group | PRJEB50040 | Silva v. 138 | Lefse | 0.2958 | 0.68442 | - | 28 | 11.083 | 5.7424 | - | 1.08 |
| Lachnospiraceae_ND3007_group | PRJEB61722 | Silva v. 138 | Lefse | 0.34702 | 0.71539 | - | - | - | 7.8333 | 7.0667 | −0.141 |
| Lachnospiraceae_ND3007_group | PRJNA747262 | Silva v. 138 | Lefse | 0.7409 | 0.84526 | 41.263 | 51.932 | 35.314 | 41.115 | 35.5 | 0.969 |
| Lachnospiraceae_ND3007_group | PRJNA895415 | Silva v. 138 | Lefse | 0.032752 | 0.35878 | - | - | 2.8571 | 0.77778 | - | 0.31 |
| Lachnospiraceae_UCG_001 | PRJEB50040 | Silva v. 138 | Lefse | 0.0651 | 0.45349 | - | 1 | 1.2083 | 0.86364 | - | 0.0691 |
| Lachnospiraceae_UCG_001 | PRJEB61722 | Silva v. 138 | Lefse | 0.058343 | 0.58486 | - | - | - | 8.5952 | 0.13333 | −0.719 |
| Lachnospiraceae_UCG_001 | PRJNA747262 | Silva v. 138 | Lefse | 0.15103 | 0.52394 | 9.1053 | 7.2955 | 6.7429 | 9.1923 | 1.75 | 0.674 |
| Lachnospiraceae_UCG_002 | PRJEB61722 | Silva v. 138 | Lefse | 0.016965 | 0.47234 | - | - | - | 0 | 0.46667 | 0.0911 |
| Lachnospiraceae_UCG_002 | PRJNA747262 | Silva v. 138 | Lefse | 0.82311 | 0.89087 | 0.021053 | 0.022727 | 0 | 0 | 0 | 0.00491 |
| Lachnospiraceae_UCG_003 | PRJEB50040 | Silva v. 138 | Lefse | 0.61272 | 0.86598 | - | 0 | 0 | 0.24242 | - | 0.0497 |
| Lachnospiraceae_UCG_004 | PRJEB50040 | Silva v. 138 | Lefse | 0.019059 | 0.20522 | - | 7.875 | 1.75 | 1.5909 | - | 0.617 |
| Lachnospiraceae_UCG_004 | PRJEB61722 | Silva v. 138 | Lefse | 0.023851 | 0.47234 | - | - | - | 28.357 | 5.8 | −1.09 |
| Lachnospiraceae_UCG_004 | PRJEB61722 | Silva v. 138 | Lefse | 0.023851 | 0.47234 | - | - | - | 28.357 | 5.8 | −1.09 |
| Lachnospiraceae_UCG_004 | PRJNA895415 | Silva v. 138 | Lefse | 0.012537 | 0.21696 | - | - | 4.7143 | 1.3333 | - | 0.43 |
| Lachnospiraceae_UCG_006 | PRJEB50040 | Silva v. 138 | Lefse | 1.1569 × 10−5 | 0.0024526 | - | 0.5 | 0 | 0 | - | 0.0969 |
| Lachnospiraceae_UCG_006 | PRJNA747262 | Silva v. 138 | Lefse | 0.24373 | 0.60649 | 0.042105 | 0.25 | 0.17143 | 0.11538 | 0.75 | 0.132 |
| Lachnospiraceae_UCG_010 | PRJEB50040 | Silva v. 138 | Lefse | 0.0004688 | 0.033128 | - | 19.25 | 4.0417 | 3.9848 | - | 0.936 |
| Lachnospiraceae_UCG_010 | PRJNA747262 | Silva v. 138 | Lefse | 0.1599 | 0.52394 | 31.411 | 47.409 | 47.514 | 39 | 54.25 | 1.09 |
| Lachnospiraceae_unclassified | PRJEB50040 | Silva v. 138 | Lefse | 0.37074 | 0.76342 | - | 397 | 281 | 340.27 | - | 1.77 |
| Lachnospiraceae_unclassified | PRJEB61722 | Silva v. 138 | Lefse | 0.05358 | 0.58486 | - | - | - | 404.71 | 137.8 | −2.13 |
| Lachnospiraceae_unclassified | PRJNA747262 | Silva v. 138 | Lefse | 0.77331 | 0.86605 | 524.83 | 499.14 | 536.26 | 492.65 | 452.67 | 1.63 |
| Lachnospiraceae_unclassified | PRJNA895415 | Silva v. 138 | Lefse | 0.15071 | 0.68777 | - | - | 290.14 | 175.8 | - | 1.76 |
| Lachnospiraceae_unclassified | PRJNA684070 | Silva v. 138 | Lefse | 0.0035627 | 0.037853 | - | - | 4001.5 | 4506.3 | 157.67 | 3.34 |
| Genus | Study | Reference Database | Statistical Analysis | p Values | FDR | Healthy | COVID-19 | LDA Score |
|---|---|---|---|---|---|---|---|---|
| Lachnospiraceae_ge | Standard Protocol | Silva v. 138 | LEfSe | 0.48465 | 0.66996 | 0.93333 | 4.0909 | −0.411 |
| Lachnospiraceae_NK3A20_group | Standard Protocol | Silva v. 138 | LEfSe | 0.27423 | 0.48637 | 0.93333 | 0.090909 | 0.153 |
| Lachnospiraceae_UCG_004 | Standard Protocol | Silva v. 138 | LEfSe | 0.031072 | 0.1211 | 1.1333 | 0 | 0.195 |
| Lachnospiraceae_unclassified | Standard Protocol | Silva v. 138 | LEfSe | 0.26464 | 0.47838 | 8.8667 | 147.45 | −1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taufer, C.R.; da Silva, J.; Rampelotto, P.H. Changes in Probiotic Lachnospiraceae Genera Across Different Stages of COVID-19: A Meta-Analysis of 16S rRNA Microbial Data. Microorganisms 2025, 13, 2061. https://doi.org/10.3390/microorganisms13092061
Taufer CR, da Silva J, Rampelotto PH. Changes in Probiotic Lachnospiraceae Genera Across Different Stages of COVID-19: A Meta-Analysis of 16S rRNA Microbial Data. Microorganisms. 2025; 13(9):2061. https://doi.org/10.3390/microorganisms13092061
Chicago/Turabian StyleTaufer, Clarissa Reginato, Juliana da Silva, and Pabulo Henrique Rampelotto. 2025. "Changes in Probiotic Lachnospiraceae Genera Across Different Stages of COVID-19: A Meta-Analysis of 16S rRNA Microbial Data" Microorganisms 13, no. 9: 2061. https://doi.org/10.3390/microorganisms13092061
APA StyleTaufer, C. R., da Silva, J., & Rampelotto, P. H. (2025). Changes in Probiotic Lachnospiraceae Genera Across Different Stages of COVID-19: A Meta-Analysis of 16S rRNA Microbial Data. Microorganisms, 13(9), 2061. https://doi.org/10.3390/microorganisms13092061







