Construction Biotechnology: Integrating Bacterial Systems into Civil Engineering Practices
Abstract
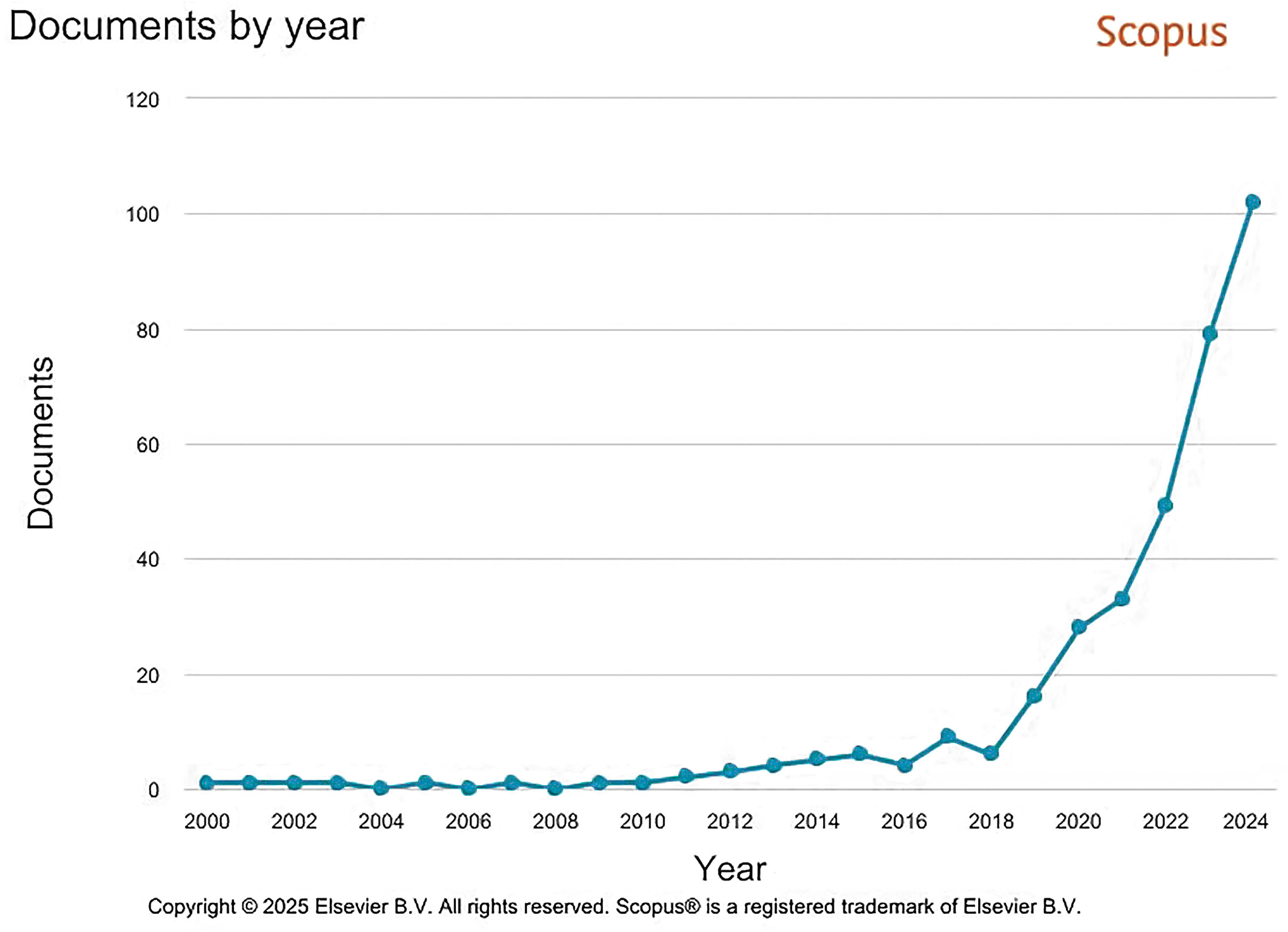
1. Introduction
1.1. Motivation for Integrating Microbiology into Civil Engineering
1.2. Historical Context and Emerging Need
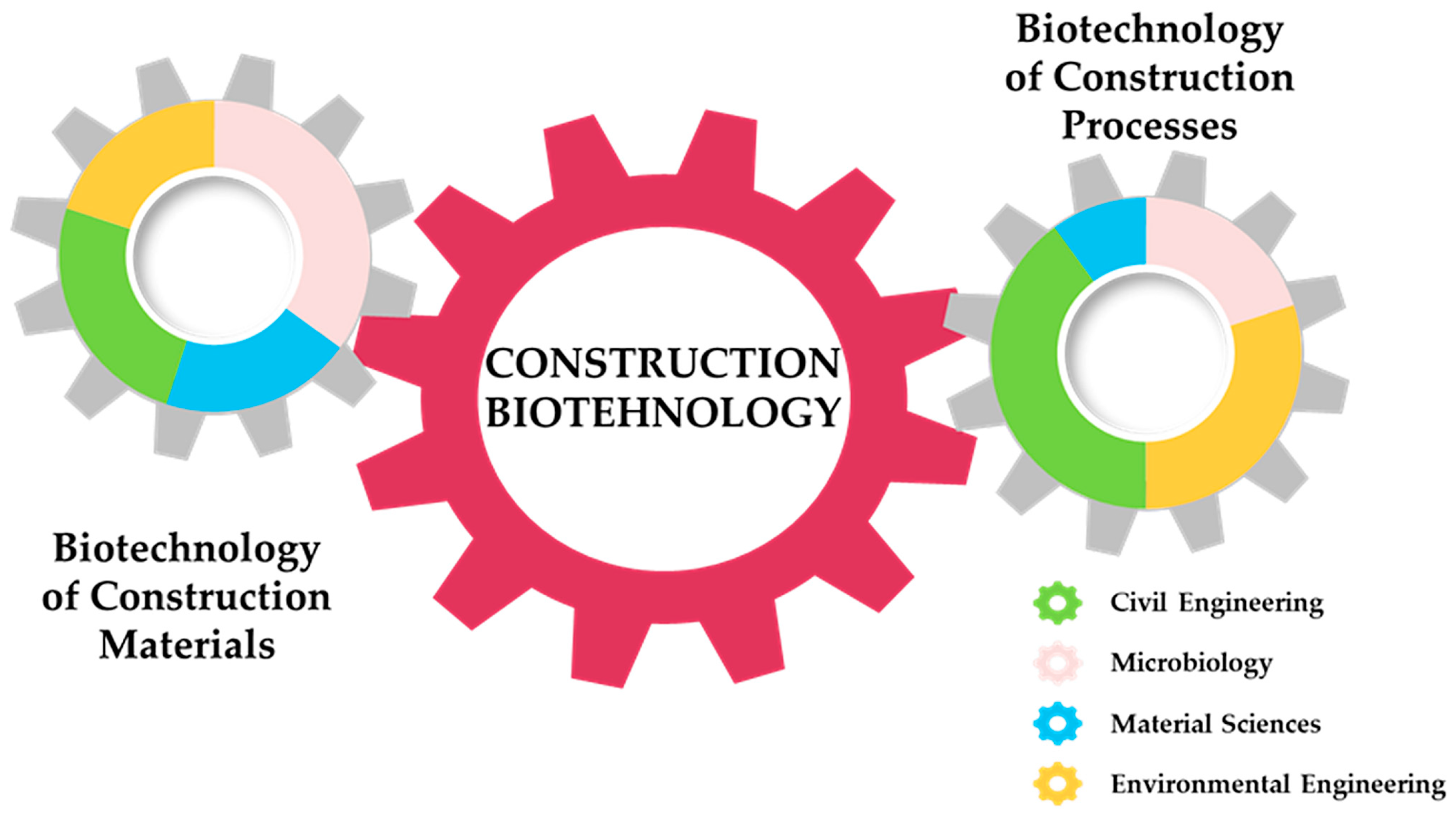
1.3. Definition and Scope of Construction Biotechnology
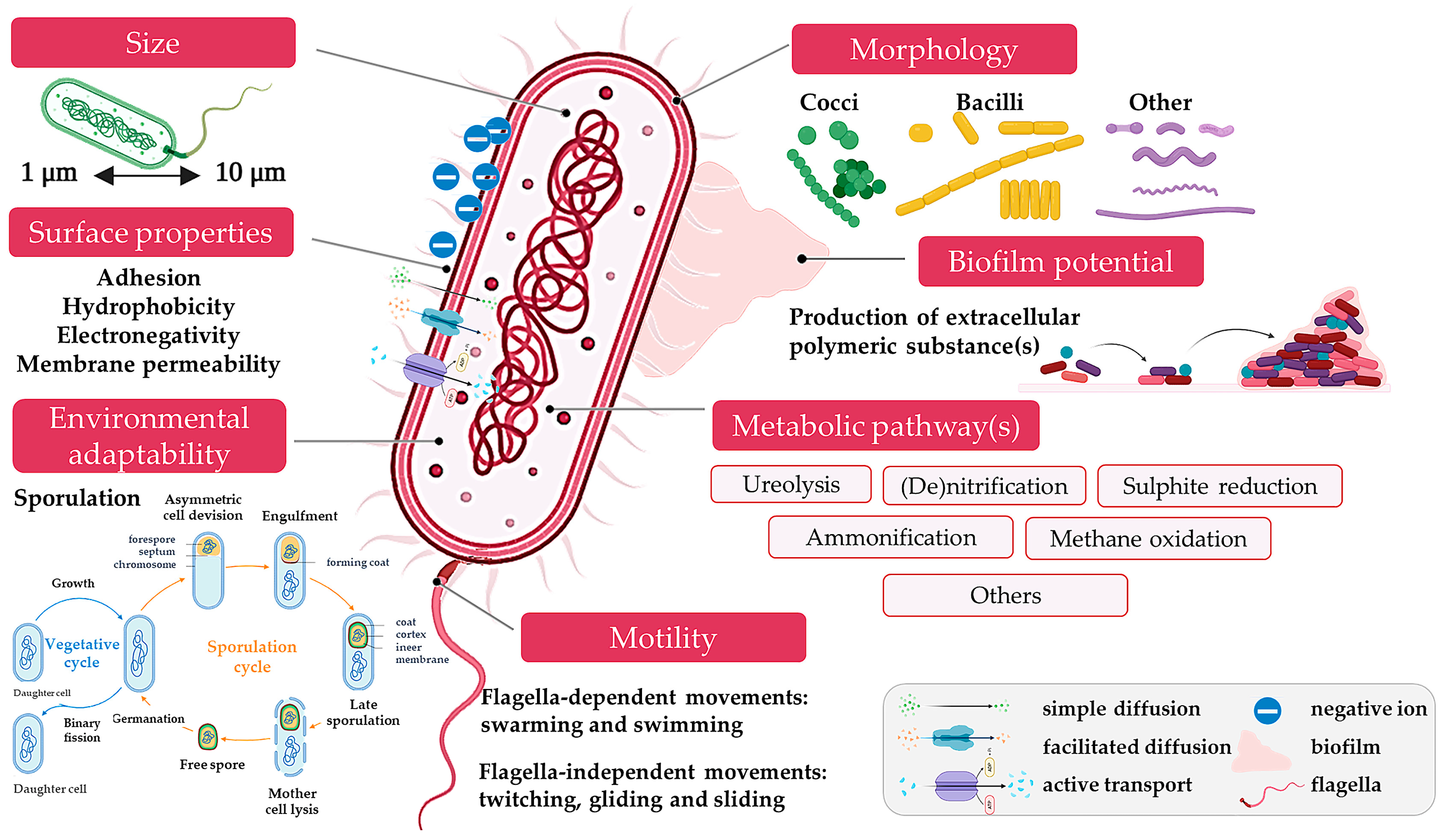
2. Bacterial Contribution and Mechanisms Relevant to Civil Engineering
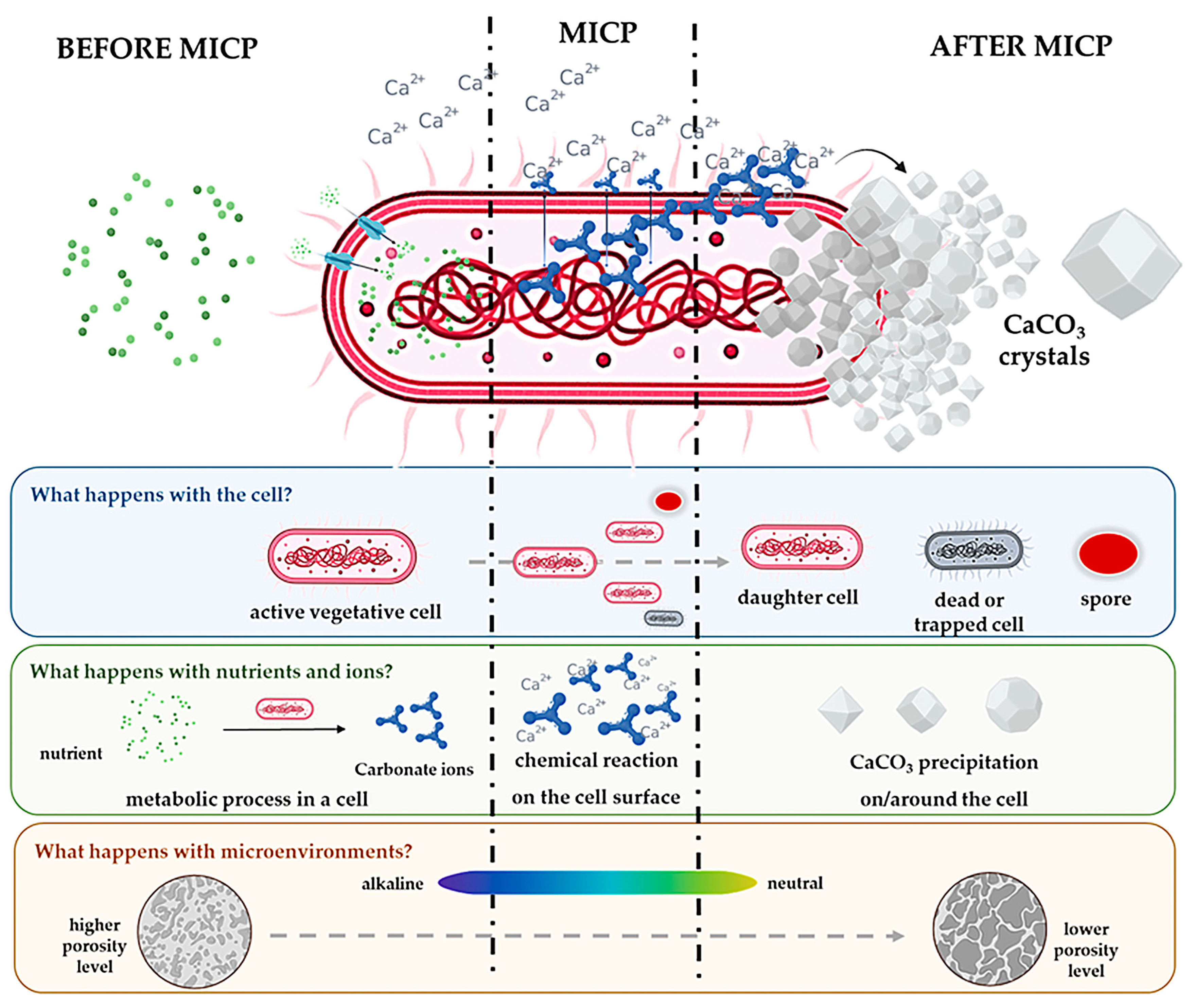
2.1. Microbial-Induced Carbonate Precipitation
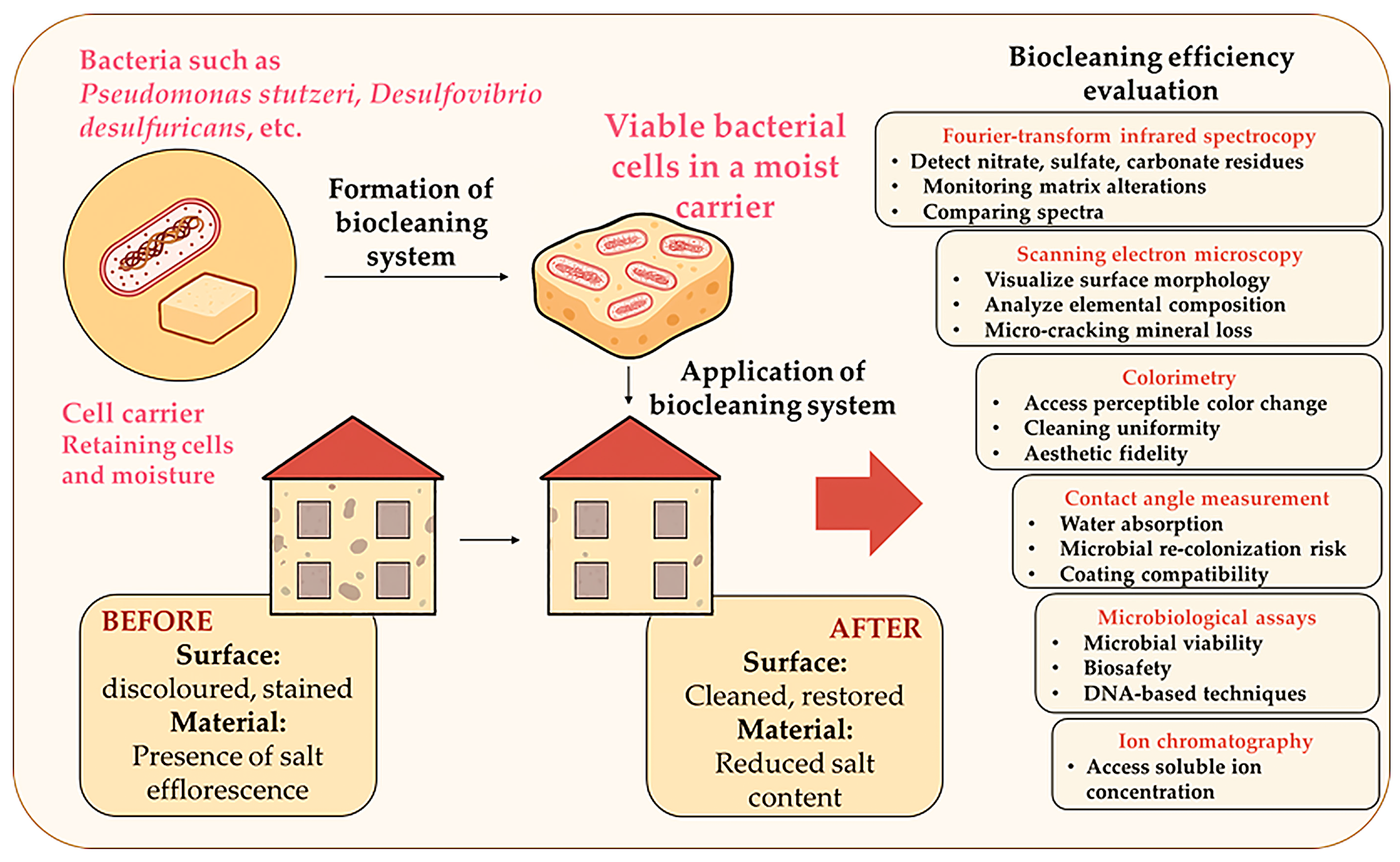
2.2. Biocleaning
- Denitrification: Bacteria such as P. stutzeri reduce nitrates that form efflorescence on frescos and limestone façades.
- Desulfation: Sulphate-reducing bacteria (SRB) remove gypsum or black crusts resulting from atmospheric pollution.
- Enzymatic biodegradation: Specific bacterial enzymes catalyse the breakdown of binders, adhesives, and organic films on painted surfaces.
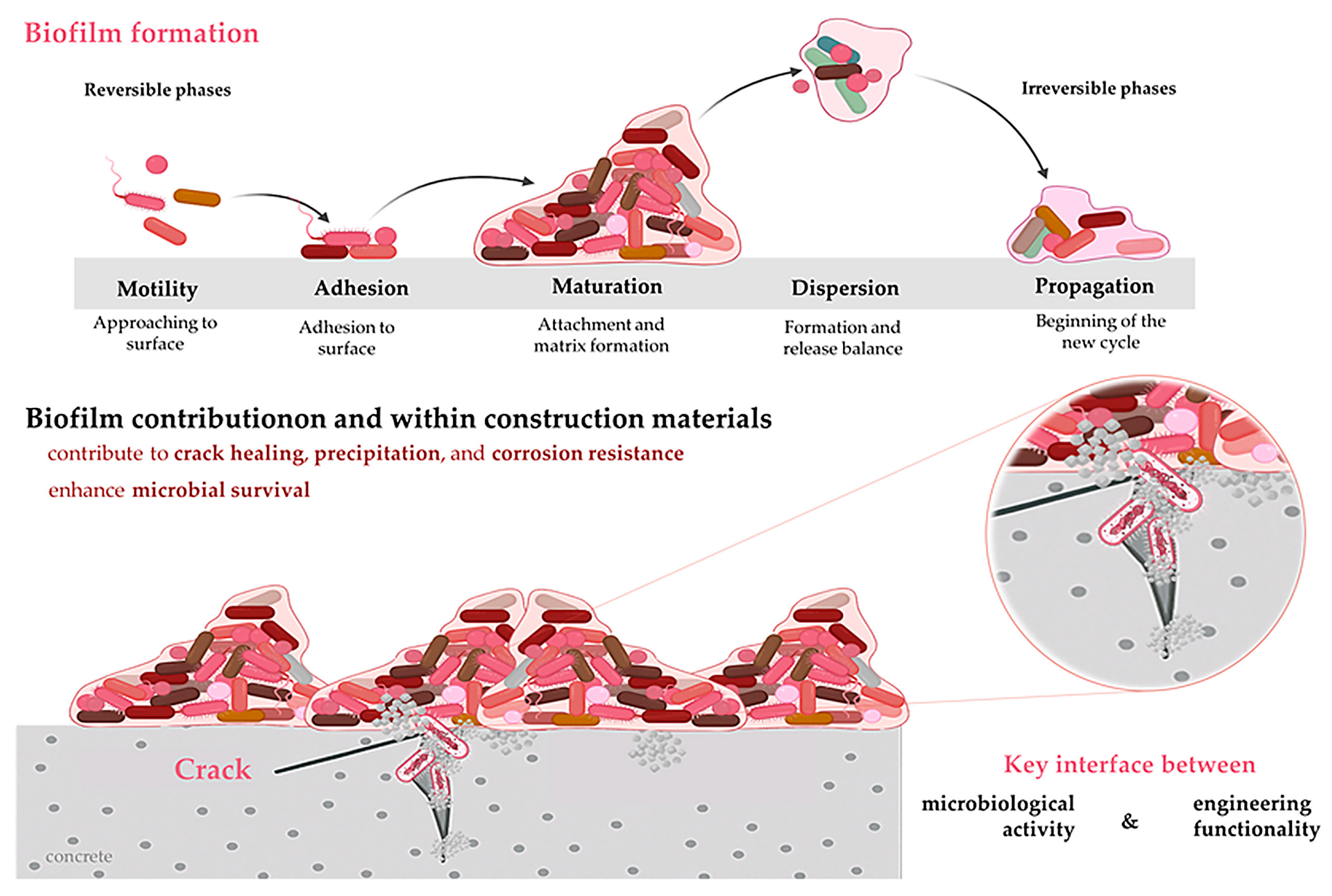
2.3. Biofilm Formation and Material Interactions
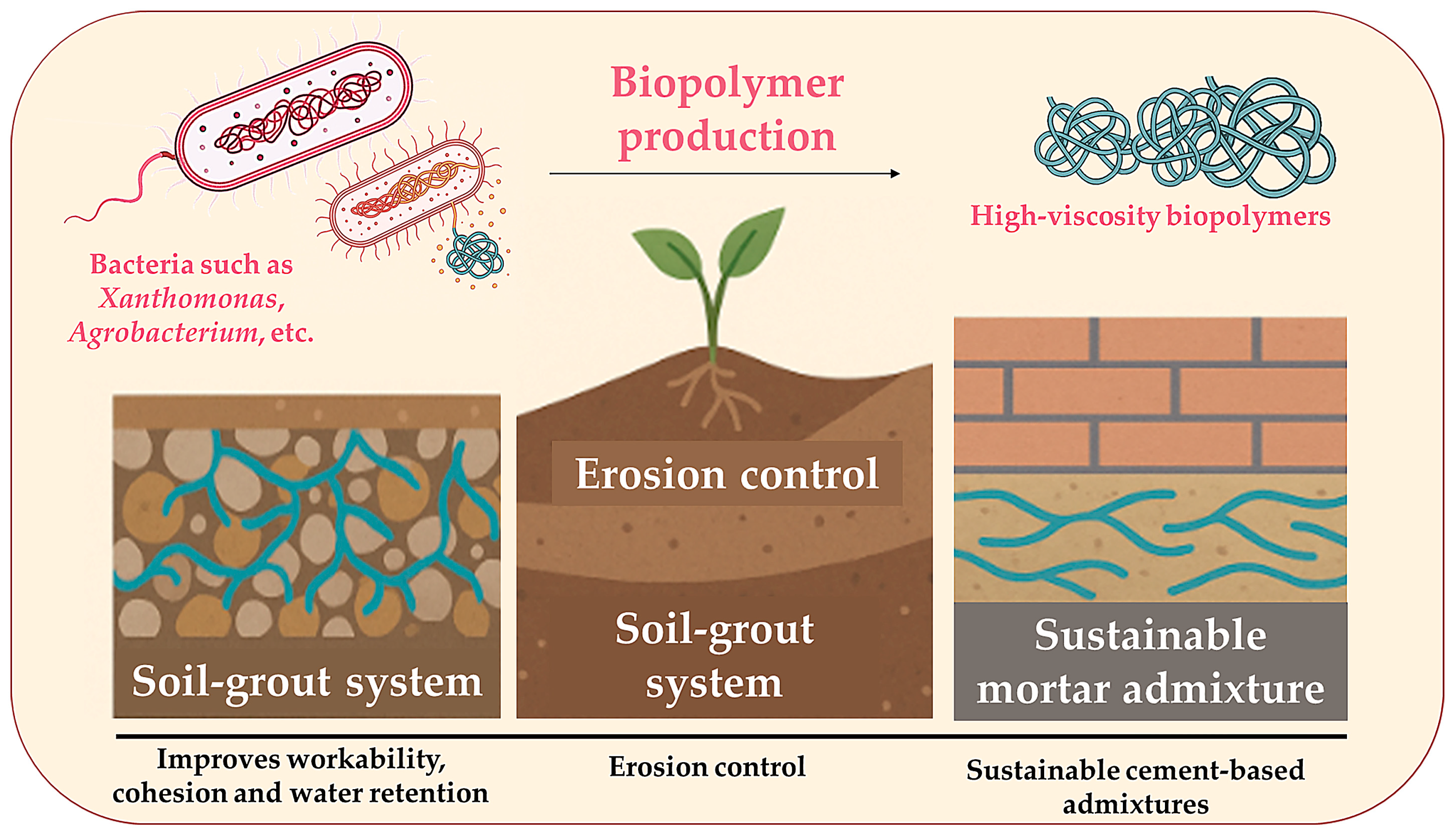
2.4. Biopolymer Production
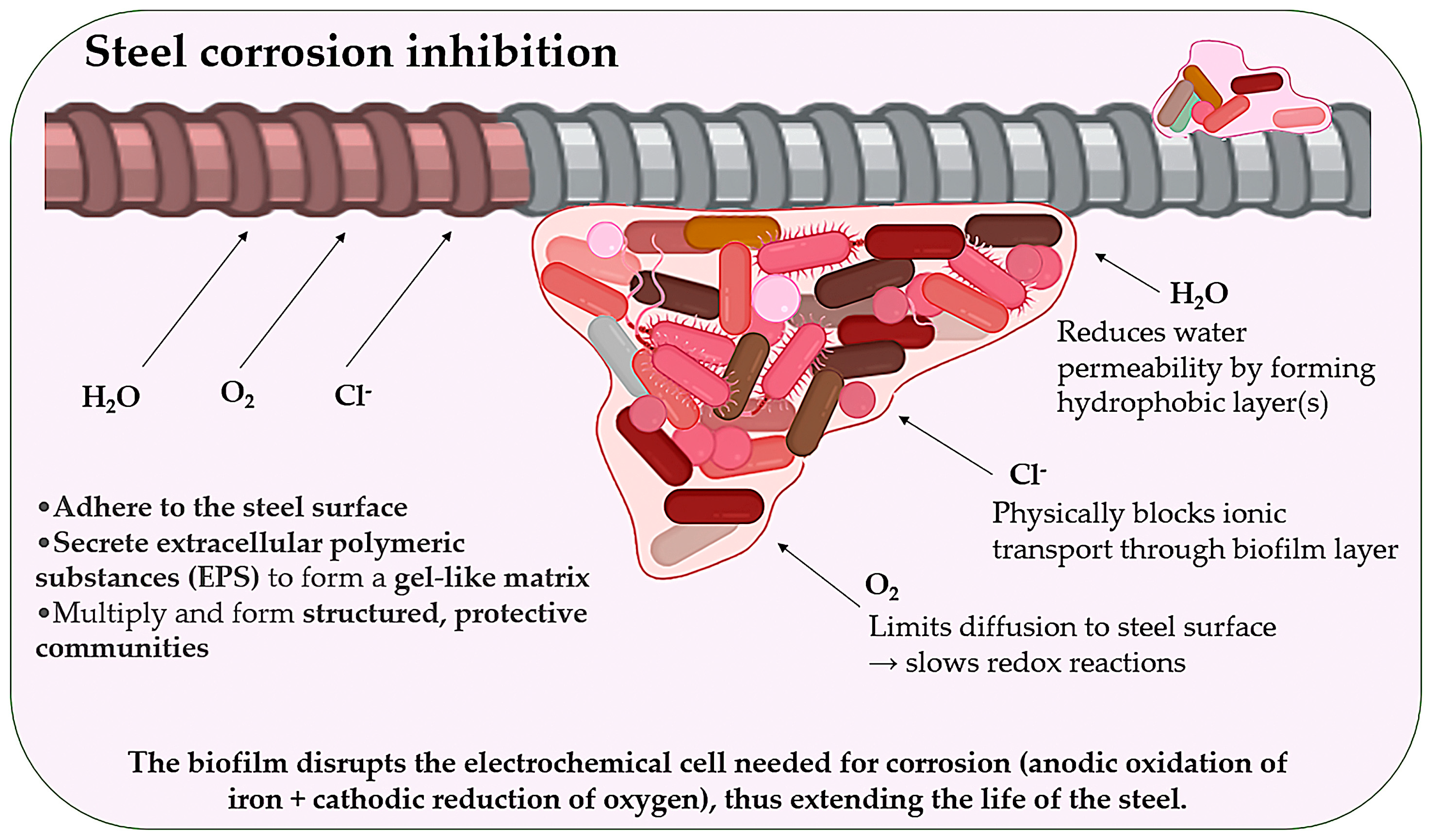
2.5. Corrosion Inhibition via Biofilm Barriers
2.6. Genetically Engineered Bacterial Systems
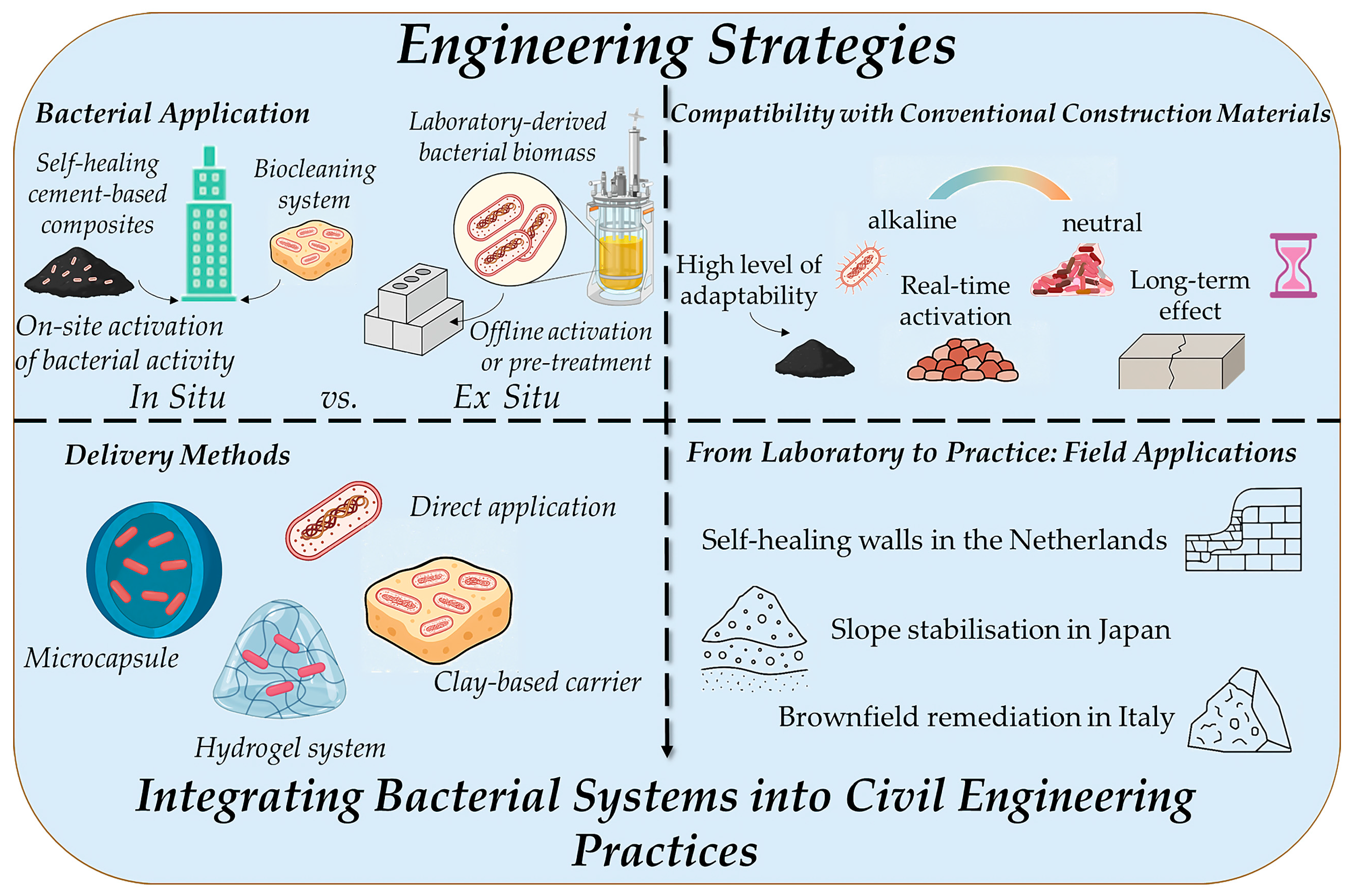
3. Engineering Integration Strategies
3.1. In Situ vs. Ex Situ Bacterial Application
3.2. Delivery Methods for Achieving Timed Bacterial Response
- Hydrogels and smart polymers that degrade or swell in response to pH shifts, moisture ingress, or mechanical damage, enabling timed or site-specific nutrient and bacterial release [106]. These materials form a responsive membrane that supports bacterial viability and metabolic activation when cracks form or water enters the system.
- Expanded clays and lightweight aggregates, which serve as bacterial shelters within concrete. Their porous internal structure allows bacteria to remain dormant for extended periods while shielding them from high alkalinity and mechanical stress [107]. Upon exposure to moisture or oxygen, bacterial activation can proceed.
- Microcapsules engineered with biofilm-inducing triggers, such as surface charge variation, temperature cues, or quorum-sensing mechanisms, help ensure that bacterial activity is initiated only in critical zones, such as crack interfaces or porous microenvironments [103].
3.3. Compatibility with Conventional Construction Materials
3.4. From Laboratory to Practice: Field Applications
4. Challenges and Limitations
4.1. Biological Variability and Environmental Sensitivity
4.2. Longevity and Durability in Real-World Conditions
4.3. Regulatory and Safety Considerations
4.4. Economic Feasibility and Scalability
4.5. Biosafety
5. Future Outlook
5.1. Synthetic Biology and Engineered Bacteria
5.2. Digital Tools and Modelling in Bioconstruction
5.3. Integration into Green Building Certifications
5.4. Cross-Disciplinary Research Initiatives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masniari, O.; Koestoer, R.H. Sustainable Building Materials for Green Construction: A review. Int. Res. J. Adv. Eng. Sci. 2024, 9, 68–72. [Google Scholar]
- Bhagatkar, P.M.; Lamba, A. An Empirical Review of Innovative Soil Improvement Techniques in Geotechnical Engineering. Mesopotamian J. Civ. Eng. 2024, 2024, 42–53. [Google Scholar] [CrossRef]
- Qin, K.; Zheng, Z.; Wang, J.; Pan, H.; Tang, R. Biomineralization strategy: From material manufacturing to biological regulation. Giant 2024, 19, 100317. [Google Scholar] [CrossRef]
- Jamaldar, A.; Salimi, M.; Payan, M. Application of Natural and Synthetic Fibers in Bio-Based Earthen Composites: A State-of-the-Art Review. Results Eng. 2025, 25, 103732. [Google Scholar] [CrossRef]
- Thasnee, S.; Miguel, C.A.; Yazmin, M.V.; Ortega Del Rosario, M.D.L.A. Human-Centered and Regenerative Design: Leveraging Biomaterials for Climate-Responsive Built Environment. In Sustainable Built Environment for People and Society; InTechOpen: Rijeka, Croatia, 2025. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- van Paassen, L.A.; Ghose, R.; van der Linden, T.J.M.; van der Star, W.R.L.; van Loosdrecht, M.C.M. Quantifying bio-mediated ground improvement by ureolysis. Geotechnique 2010, 60, 473–479. [Google Scholar]
- DeJong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities, and challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef]
- Stabnikov, V.; Ivanov, V.; Chu, J. Construction Biotechnology: A new area of biotechnological research and applications. World J. Microbiol. Biotechnol. 2015, 31, 1303–1314. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikov, V. Construction Biotechnology: Biogeochemistry, Microbiology and Biotechnology of Construction Materials and Processes; Springer: Singapore, 2017. [Google Scholar]
- Ivanov, V.; Chu, J.; Stabnikov, V. Basics of Construction Microbial Biotechnology. In Biotechnologies and Biomimetics for Civil Engineering; Pacheco Torgal, F., Labrincha, J., Diamanti, M., Yu, C.P., Lee, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Ivanov, V.; Chu, J. Applications of Microorganisms to Geotechnical Engineering for Bioclogging and Biocementation of Soil in Situ. Rev. Environ. Sci. Bio/Technol. Nat. Resour. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Upscaling effects of soil improvement by microbially induced calcite precipitation by injection of ureolytic bacteria. J. Geotech. Geoenviron. Eng. 2014, 140, 04014068. [Google Scholar]
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors affecting efficiency of microbially induced calcite precipitation. J. Geotech. Geoenviron. Eng. 2012, 138, 992–1001. [Google Scholar] [CrossRef]
- Crowther, T.; Rappuoli, R.; Corinaldesi, C. Scientists’ call to action: Microbes, planetary health, and the Sustainable Development Goals. Cell 2024, 187, 5195–5216. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Kosoe, E.A. Integrating Green Infrastructure into Sustainable Agriculture to Enhance Soil Health, Biodiversity, and Microclimate Resilience. Sustainability 2025, 17, 3838. [Google Scholar] [CrossRef]
- Barberán, A.; Chávez, D.; Cajas, A.; Egas, M.C.; Criollo, M.; Pineda, J.; País-Chanfrau, J.M.; Trujillo, L.E. A new area of application and research in bio-processes: Biotechnologies in civil construction. Bionatura 2020, 5, 1072–1077. [Google Scholar] [CrossRef]
- Mishra, P.N.; Jain, S.; Bore, T.; Chang, I.; Kwon, Y.-M.; Wang, Y.; Dash, H.R.; Kumar, A.; Tiwari, S.; Jiang, N.; et al. Biological perspectives in geotechnics: Application and monitoring. J. Rock Mech. Geotech. Eng. 2024, 16, 2854–2878. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Huang, Y.; You, L.; Dai, Z. Engineering living materials by synthetic biology. Biophys Rev. 2023, 4, 011305. [Google Scholar] [CrossRef]
- Gilbert, C.; Tang, T.C.; Ott, W.; Dorr, B.A.; Shaw, W.M.; Sun, G.L.; Lu, T.K.; Ellis, T. Living materials with programmable functionalities grown from engineered microbial co-cultures. Nat. Mater. 2021, 20, 691–700. [Google Scholar] [CrossRef]
- Rajadesingu, S.; Palani, N.; Celestina Mendonce, K.C.; Vijayakumar, P.; Monisha, P.; Ayyadurai, S. State-of-the-art review on advancements of eco-friendly bacterial-infused self-healing concrete for sustainable constructions. J. Build. Eng. 2024, 91, 109669. [Google Scholar] [CrossRef]
- Payan, M.; Sangdeh, M.K.; Salami, M.; Ranjbar, P.Z.; Arabani, M.; Hosseinpour, I. A comprehensive review on the application of microbially induced calcite precipitation (MICP) technique in soil erosion mitigation as a sustainable and environmentally friendly approach. Results Eng. 2024, 24, 103235. [Google Scholar] [CrossRef]
- Wong, G.C.L.; Antani, J.D.; Lele, P.P.; Chen, J.; Nan, B.; Kühn, M.J.; Persat, A.; Bru, J.-L.; Høyland-Kroghsbo, N.M.; Siryaporn, A. Roadmap on emerging concepts in the physical biology of bacterial biofilms: From surface sensing to community formation. Phys. Biol. 2021, 18, 051501. [Google Scholar] [CrossRef]
- Bridier, A.; Piard, J.C.; Pandin, C.; Labarthe, S.; Dubois-Brissonnet, F.; Briandet, R. Spatial organization plasticity as an adaptive driver of surface microbial communities. Front. Microbiol. 2017, 8, 1364. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Contribution of bacterial cells as nucleation centers in microbiologically induced CaCO3 precipitation—A mathematical modeling approach. J. Basic Microbiol. 2021, 61, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Volke, D.C.; Nikel, P.I. Getting bacteria in shape: Synthetic morphology approaches for the design of efficient microbial cell factories. Adv. Biosyst. 2018, 2, 1800111. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Tomic, A.; Ranitovic, A.; Cvetkovic, D.; Markov, S. Operating parameter optimization of cell surface hydrophobicity test for ureolytic bacteria. J. Serbian Chem. Soc. 2020, 82, 533–545. [Google Scholar] [CrossRef]
- Seifan, M.; Berenjian, A. Application of microbially induced calcium carbonate precipitation in designing bio self-healing concrete. World J. Microbiol. Biotechnol. 2018, 34, 168. [Google Scholar] [CrossRef]
- Olufemi Odeyemi, O.; Adeniyi Alaba, P. Microbiologically Influenced Corrosion in Oil Fields: Mechanisms, Detection, and Mitigation Strategies; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Microbiologically influenced corrosion: Looking to the future. Int. Microbiol. 2005, 8, 169–180. [Google Scholar]
- Jiang, G.; Keller, J.; Bond, P.L. Determining the long-term effects of microbial corrosion on concrete sewers. Water Res. 2016, 88, 703–713. [Google Scholar]
- Šovljanski, O.; Tomić, A.; Markov, S. Relationship between Bacterial Contribution and Self-Healing Effect of Cement-Based Materials. Microorganisms 2022, 10, 1399. [Google Scholar] [CrossRef]
- Qin, W.Q.; Liu, Y.F.; Liu, J.F.; Zhou, L.; Yang, S.Z. Space environment-induced biofilm structural adaptations in Bacillus subtilis. Microb. Biotech. 2025, 18, e70111. [Google Scholar]
- Gerbersdorf, S.U.; Wieprecht, S. Biostabilization of cohesive sediments: Revisiting the role of abiotic conditions, physiology and diversity of microbes, polymeric secretion, and biofilm architecture. Geobiology 2015, 13, 68–97. [Google Scholar] [CrossRef] [PubMed]
- Toley, B.J.; Forbes, N.S. Motility is critical for effective distribution and accumulation of bacteria in tumor tissue. Integr. Biol. 2012, 4, 165–176. [Google Scholar] [CrossRef]
- Šovljanski, O.; Milović, T.; Bulatović, V.; Erceg, T.; Stanojev, J.; Bajac, B.; Tomić, A. Insights into self-healing capacity of cement matrix containing high-efficiency bacteria under challenging conditions. J. Buil. Eng. 2024, 98, 111094. [Google Scholar] [CrossRef]
- Šovljanski, O.; Bulatović, V.; Milović, T.; Grahovac, J.; Erceg, T.; Dramićanin, M.; Tomić, A. Bioaugmentation of Industrial Wastewater and Formation of Bacterial–CaCO3 Coupled System for Self-Healing Cement. Buildings 2024, 14, 4011. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Kan, F.-K.; Basri, H.F.; Silini, M.O.E.; Rajasekar, A. Enhanced MICP for Soil Improvement and Heavy Metal Remediation: Insights from Landfill Leachate-Derived Ureolytic Bacterial Consortium. Microorganisms 2025, 13, 174. [Google Scholar] [CrossRef]
- Fouladi, A.S.; Arulrajah, A.; Chu, J.; Horpibulsuk, S. Application of Microbially Induced Calcite Precipitation (MICP) technology in construction materials: A comprehensive review of waste stream contributions. Cons. Build. Eng. 2024, 388, 131546. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Zhang, P.; Wang, Q.; Pan, W.; Wang, S.; Xie, X. Research status, hot spots, difficulties and future development direction of microbial geoengineering. J. Road Eng. 2024, 4, 234–255. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially Induced Cementation to Control Sand Response to Undrained Shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Anbu, P.; Kang, C.H.; Shin, Y.J.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus 2016, 5, 250. [Google Scholar] [CrossRef]
- Šovljanski, O.; Pezo, L.; Stanojev, J.; Bajac, B.; Kovač, S.; Tóth, E.; Ristić, I.; Tomić, A.; Ranitović, A.; Cvetković, D.; et al. Comprehensive Profiling of Microbiologically Induced CaCO3 Precipitation by Ureolytic Bacillus Isolates from Alkaline Soils. Microorganisms 2021, 9, 1691. [Google Scholar] [CrossRef]
- Jonkers, H.M. Bacteria-based self-healing concrete. Heron 2011, 56, 1–12. [Google Scholar]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Deeba, S.; Ammasi, A.K. State-of-the-art review on self-healing in mortar, concrete, and composites. Case Stud. Constr. Mat. 2024, 20, e03298. [Google Scholar] [CrossRef]
- Shilar, F.; Ganachari, S.; Patil, V. A comprehensive review on the strength, durability, and microstructural analysis of bacterial concrete. Structures 2024, 68, 107078. [Google Scholar] [CrossRef]
- Carter, M.S.; Tuttle, M.J.; Mancini, J.A.; Martineau, R.; Hung, C.; Gupta, M.K. Microbially Induced Calcium Carbonate Precipitation by Sporosarcina pasteurii: A Case Study in Optimizing Biological CaCO3 Precipitation. Appl. Environ. Microbiol. 2023, 89, e01794-22. [Google Scholar] [CrossRef]
- Diana, N.A.; Soemitro, R.A.A.; Ekaputri, J.J.; Satrya, T.R.; Warnana, D.D. Biogrouting with microbial-induced carbonate precipitation (MICP) for improving the physical and mechanical properties of granular soils potential liquefaction. MethodsX 2025, 14, 103246. [Google Scholar] [CrossRef] [PubMed]
- Šovljanski, O.; Pezo, L.; Grahovac, J.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Best-performing Bacillus strains for microbiologically induced CaCO3 precipitation: Screening of relative influence of operational and environmental factors. J. Biotech. 2022, 350, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Baidya, P.; Dahal, B.K.; Pandit, A.; Joshi, D. Bacteria-Induced Calcite Precipitation for Engineering and Environmental Applications. Adv. Mater. Sci. Eng. 2023, 2023, 613209. [Google Scholar] [CrossRef]
- Proksch, G. Engineered Living Materials (ELMs) for the Built Environment. In Proceedings of the ACSA 112th Annual Meeting: Disrupters on the Edge, Vancouver, BC, Canada, 14–16 March 2024. [Google Scholar]
- Nagy, B.; Kustermann, A. Rehabilitation of Porous Building Components and Masonry by MICP Injection Method. Buildings 2023, 13, 1273. [Google Scholar] [CrossRef]
- Tian, A. Advances in Construction Materials and Structural Engineering: A Comprehensive Review. Eng. Arch. 2025. Preprint. [Google Scholar]
- Gebru, K.A.; Kidanemariam, T.G.; Gebretinsae, H.K. Bio-cement production using microbially induced calcite precipitation (MICP) method: A review. Chem. Eng. Sci. 2021, 238, 116610. [Google Scholar] [CrossRef]
- Cappitelli, F.; Toniolo, L.; Sansonetti, A.; Gulotta, D.; Ranalli, G.; Zanardini, E.; Sorlini, C. Advantages of Using Microbial Technology over Traditional Chemical Technology in Removal of Black Crusts from Stone Surfaces of Historical Monuments. Appl. Environ. Microbiol. 2007, 73, 5671–5675. [Google Scholar] [CrossRef]
- Vučetić, S.; Čjepa, D.; Miljević, B.; Bergh, J.M.v.d.; Šovljanski, O.; Tomić, A.; Nikolić, E.; Markov, S.; Hiršenberger, H.; Ranogajec, J. Bio-Stimulated Surface Healing of Historical and Compatible Conservation Mortars. Materials 2023, 16, 642. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, G.; Alfano, G.; Belli, C.; Lustrato, G.; Colombini, M.P.; Bonaduce, I.; Zanardini, E.; Abbruscato, P.; Cappitelli, F.; Sorlini, C. Biotechnology applied to cultural heritage: Biorestoration of frescoes using viable bacterial cells and enzymes. J. Appl. Microbiol. 2005, 98, 75–83. [Google Scholar]
- Ranalli, G.; Chiavarini, M.; Guidetti, V.; Marsala, F.; Matteini, M.; Zanardini, E.; Sorlini, C. The use of microorganisms for the removal of nitrates and organic substances on artistic stoneworks. In Proceedings of the 8th International Congress of Deterioration and Conservation of Stone, Berlin, Germany, 30 September–4 October 1996; pp. 1415–1420. [Google Scholar]
- Bosch-Roig, P.; Lustrato, G.; Zanardini, E.; Ranalli, G. Biocleaning of Cultural Heritage stone surfaces and frescoes: Which delivery system can be the most appropriate? Ann. Microbiol. 2014, 65, 1227–1241. [Google Scholar] [CrossRef]
- Ruginescu, R.; Enache, M.; Popescu, O.; Gomoiu, I.; Cojoc, R.; Batrinescu-Moteau, C.; Maria, G.; Dumbravician, M.; Neagu, S. Characterization of some salt-tolerant bacterial hydrolases with potential utility in cultural heritage bio-cleaning. Microorganisms 2022, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Ranalli, G.; Zanardini, E.; Andreotti, A.; Colombini, M.P.; Corti, C.; Bosch-Roig, P.; De Nuntiis, P.; Lustrato, G.; Mandrioli, P.; Rampazzi, L.; et al. Hi-tech restoration by two steps biocleaning process of Triumph of Death fresco at the Camposanto Monumental Cemetery (Pisa, Italy). J. Appl. Microbiol. 2018, 125, 800–812. [Google Scholar] [CrossRef]
- Vidaković, A.; Šovljanski, O.; Vučurović, D.; Racić, G.; Đilas, M.; Ćurčić, N.; Markov, S. Novel denitrifying bacteria Pseudomonas stutzeri strain D1—From isolation to the biomass production. Chem. Ind. Chem. Eng. Q. 2019, 25, 403–415. [Google Scholar] [CrossRef]
- Tomić, A.; Vučetić, S.; Šovljanski, O.; Pezo, L.; Ranogajec, J.; Markov, S. Effective bioactive systems for nitrate removal from building materials. Constr. Build. Mat. 2022, 338, 127514. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Flemming, H.C.; van Hullebusch, E.D.; Little, B.J.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. Microbial extracellular polymeric substances in the environment, technology and medicine. Nat. Rev. Microbiol. 2025, 23, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Eswari, J.S.; Mahapatra, C. Revolutionizing concrete: Unveiling bio-concrete’s advantages and challenges in self-healing through microbial-induced calcium carbonate precipitation. Sustain. Mater. Technol. 2025, 45, e01465. [Google Scholar] [CrossRef]
- Castro-Alonso, M.J.; Lilia Ernestina, M.H.; Sanchez-Muñoz, M.A.; Mariel Rubi, M.F.; Narayanasamy, R.; Nagamani, B. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Doctolero, J.Z.S.; Beltran, A.B.; Uba, M.O.; Tigue, A.A.S.; Promentilla, M.A.B. Self-Healing Biogeopolymers Using Biochar-Immobilized Spores of Pure- and Co-Cultures of Bacteria. Minerals 2020, 10, 1114. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Zhang, J.; Liu, B.; Guohao, X.; Xiaoyi, T.; Haoying, G.; Changjie, S.; Runhao, L.; Xiaona, X.; Weilin, L.; et al. Synergistic effect of composite bacteria on self-healing process of concrete crack. Case Stud. Constr. Mater. 2024, 20, e03028. [Google Scholar] [CrossRef]
- Luhar, S.; Luhar, I.; Shaikh, F.U.A. A Review on the Performance Evaluation of Autonomous Self-Healing Bacterial Concrete: Mechanisms, Strength, Durability, and Microstructural Properties. J. Compos. Sci. 2022, 6, 23. [Google Scholar] [CrossRef]
- Bagga, M.; Hamley-Bennett, C.; Alex, A. Advancements in bacteria based self-healing concrete and the promise of modelling. Constr. Build. Mater. 2022, 358, 129412. [Google Scholar] [CrossRef]
- Galishnikova, V.; Elroba, S.; Mahadi, M.; Dayoub, N.; Sakna, A.; Fakhratov, M. Bacteria-Based concrete crack healing: A review of crack healing effecting factors and size. AIP Conf. Proc. 2022, 2559, 050010. [Google Scholar] [CrossRef]
- Kanwal, M.; Adnan, F.; Khushnood, R.A.; Jalil, A.; Khan, H.A.; Wattoo, A.G.; Rasheed, S. Biomineralization and corrosion inhibition of steel in simulated bio-inspired self-healing concrete. J. Build. Eng. 2024, 82, 108224. [Google Scholar] [CrossRef]
- Elgendy, I.; Elkaliny, N.; Saleh, H.; Darwish, G.; Almostafa, M.; Metwally, K.; Yahya, G.; Mahmoud, J. Bacteria-powered self-healing concrete: Breakthroughs, challenges, and future prospects. J. Ind. Microbiol. Biotech. 2025, 52, kuae051. [Google Scholar]
- Tang, J.; Xu, J. Application of microbial precipitation in self-healing concrete: A review on the protection strategies for bacteria. Const. Build. Mat. 2021, 306, 124950. [Google Scholar] [CrossRef]
- Shasiya, P.S.; Simi Pushpan, K.; Nair, A.B. Biopolymers as Engineering Materials. In Handbook of Biopolymers; Thomas, S., AR, A., Jose Chirayil, C., Thomas, B., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Yermagambetova, A.; Tazhibayeva, S.; Takhistov, P.; Tyussyupova, B.; Tapia-Hernández, J.A.; Musabekov, K. Microbial Polysaccharides as Functional Components of Packaging and Drug Delivery Applications. Polymers 2024, 16, 2854. [Google Scholar] [CrossRef]
- Kavazanjian, E.; Iglesias, E.; Karatas, I. Biopolymer soil stabilization for wind erosion control. In Proceedings of the 17th International Conference on Soil Mechanics and Geotechnical Engineering, Reykjavik, Iceland, 1–6 September 2019; pp. 881–884. [Google Scholar]
- Verma, D.K.; Niamah, A.K.; Patel, A.R.; Thakur, M.; Sandhu, K.S.; Chávez-González, M.L.; Shah, N.; Aguilar, C.N. Chemistry and Microbial Sources of Curdlan with Potential Application and Safety Regulations as Prebiotic in Food and Health. Food Res. Int. 2020, 133, 109136. [Google Scholar] [CrossRef]
- Lim, A.; Atmaja, P.C.; Rustiani, S. Bio-mediated soil improvement of loose sand with fungus. J. Rock Mech. Geotech. Eng. 2020, 12, 180–187. [Google Scholar] [CrossRef]
- Fatehi, H.; Ong, D.E.L.; Yu, J.; Chang, I. Biopolymers as Green Binders for Soil Improvement in Geotechnical Applications: A Review. Geosciences 2021, 11, 291. [Google Scholar] [CrossRef]
- Žižlavský, T.; Vyšvařil, M.; Rovnaníková, P. Characterization of aerial lime-based mortars with addition of biopolymers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 379, 012006. [Google Scholar] [CrossRef]
- Pinaeva, L.; Noskov, R. Biodegradable biopolymers: Real impact to environment pollution. Sci. Total Environ. 2024, 947, 174445. [Google Scholar] [CrossRef]
- Moreno, M.; Morris, W.; Alvarez, M.G.; Duffó, G. Corrosion of reinforcing steel in simulated concrete pore solutions: Effect of carbonation and chloride content. Corros. Sci. 2004, 46, 2681–2699. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yao, W.; Chen, Q.; Zhu, H.; Shah, S. Understanding the formation and structure of bio-mineralization for self-healing of marine concrete: An experimental and thermodynamic approach. Cem. Concr. Compos. 2024, 145, 105369. [Google Scholar] [CrossRef]
- Zheng, J.; Bi, Z.; Xu, J.; Chen, Q.; Zhu, H. Biomineralized coating inhibiting corrosion of reinforcement in cracked concrete: A study in simulated aggressive environments. J. Build. Eng. 2025, 101, 111889. [Google Scholar] [CrossRef]
- Kanwal, M.; Khushnood, R.A.; Shahid, M.; Wattoo, A.G. An integrated and eco-friendly approach for corrosion inhibition and microstructural densification of reinforced concrete by immobilizing Bacillus subtilis in pyrolytic sugarcane-bagasse. J. Clean. Prod. 2022, 355, 131785. [Google Scholar] [CrossRef]
- Lou, Y.; Chang, W.; Cui, T. Microbiologically influenced corrosion inhibition mechanisms in corrosion protection: A review. Bioelectrochemistry 2021, 141, 107883. [Google Scholar] [CrossRef]
- Shen, Y.; Dong, Y.; Yang, Y. Study of pitting corrosion inhibition effect on aluminum alloy in seawater by biomineralized film. Bioelectrochemistry 2020, 132, 107408. [Google Scholar] [CrossRef]
- Guo, N.; Wang, Y.; Hui, X. Marine bacteria inhibit corrosion of steel via synergistic biomineralization. J. Mater. Sci. Technol. 2021, 66, 82–90. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Tan, Y.; Meng, G.; Liu, H.; Cheng, Y.; Liu, H. Characterizations of the biomineralization film caused by marine Pseudomonas stutzeri and its mechanistic effects on X80 pipeline steel corrosion. J. Mater. Sci. Technol. 2022, 125, 15–28. [Google Scholar] [CrossRef]
- Bittihn, P.; Din, M.O.; Tsimring, L.S.; Hasty, J. Rational engineering of synthetic microbial systems: From single cells to consortia. Curr. Opin. Microbiol. 2018, 45, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.; Courchesne, N.D.; Duraj-Thatte, A.; Praveschotinunt, P.; Joshi, N.S. Engineered Living Materials: Prospects and Challenges for Using Biological Systems to Direct the Assembly of Smart Materials. Adv. Mater. 2018, 30, e1704847. [Google Scholar] [CrossRef] [PubMed]
- Deter, H.S.; Lu, T. Engineering microbial consortia with rationally designed cellular interactions. Curr. Opin. Biotechnol. 2022, 76, 102730. [Google Scholar] [CrossRef]
- Huo, L.; Cheng, H.; Kong, Q.; Chen, X. Bond-Slip Monitoring of Concrete Structures Using Smart Sensors—A Review. Sensors 2019, 19, 1231. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, A.A.; Firoozi, A.A.; Oyejobi, D.O.; Avudaiappan, S.; Flores, E.S. Emerging trends in sustainable building materials: Technological innovations, enhanced performance, and future directions. Res. Eng. 2024, 24, 103521. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Barciela, P.; Prieto, M.A. In Situ and Ex Situ Bioremediation of Different Persistent Soil Pollutants as Agroecology Tool. Processes 2024, 12, 2223. [Google Scholar] [CrossRef]
- Lofrano, G.; Libralato, G.; Carotenuto, M.; Meriç, S. A critical review of advanced remediation technologies for contaminated marine sediments. Mar. Pollut. Bull. 2017, 109, 20–30. [Google Scholar]
- Feng, C.; Zong, X.; Cui, B.; Guo, H.; Zhang, W.; Zhu, J. Application of Carrier Materials in Self-Healing Cement-Based Materials Based on Microbial-Induced Mineralization. Crystals 2022, 12, 797. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Shao, M.W. Long-term viability of encapsulated microbial agents in construction materials. Eng. Biol. 2023, 7, 22–34. [Google Scholar]
- Elazzazy, A.M.; Baeshen, M.N.; Alasmi, K.M.; Alqurashi, S.I.; Desouky, S.E.; Khattab, S.M.R. Where Biology Meets Engineering: Scaling Up Microbial Nutraceuticals. Microorganisms 2025, 13, 566. [Google Scholar] [CrossRef]
- Mirsalami, S.M.; Mirsalami, M. Advances in genetically engineered microorganisms: Transforming food production through precision fermentation and synthetic biology. Future Foods 2025, 11, 100601. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamade, L. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhang, L.; Zhao, J. Smart capsules for microbial self-healing in cementitious materials. Smart Mater. Civ. Eng. 2022, 5, 215–233. [Google Scholar]
- Sadanov, A.K.; Baimakhanova, B.B.; Orasymbet, S.E.; Ratnikova, I.A.; Turlybaeva, Z.Z.; Baimakhanova, G.B.; Amitova, A.A.; Omirbekova, A.A.; Aitkaliyeva, G.S.; Kossalbayev, B.D.; et al. Engineering Useful Microbial Species for Infrastructure Applications. Microorganisms 2025, 13, 599. [Google Scholar] [CrossRef]
- Harimoto, R.; Takahashi, K.; Yamaguchi, T. Development of pH-sensitive microbial release systems for crack-responsive concrete. J. Adv. Concr. Technol. 2022, 20, 1–11. [Google Scholar]
- Gebremedhin, K.G.; Eryürük, S.H. Field applications of microbial biogrouting in geotechnical engineering. Geotech. Front. 2024, 39, 52–68. [Google Scholar]
- Oz, C.; Güler, M.; Yildiz, S. Engineered microcapsules for microbial activation in smart concrete systems. Constr. Build. Mater. 2021, 279, 122486. [Google Scholar]
- Milović, T.; Bulatović, V.; Pezo, L.; Dramićanin, M.; Tomić, A.; Pezo, M.; Šovljanski, O. Enhancing Compressive Strength of Cement by Indigenous Individual and Co-Culture Bacillus Bacteria. Materials 2024, 17, 4975. [Google Scholar] [CrossRef]
- Tziviloglou, E.; Van Tittelboom, K.; Palin, D.; Wang, J.; Sierra-Beltrán, M.G.; Erşan, Y.Ç.; Mors, R.; Wiktor, V.; Jonkers, H.M.; Schlangen, E.; et al. Bio-Based Self-Healing Concrete: From Research to Field Application. In Self-Healing Materials; Advances in Polymer Science; Hager, M., van der Zwaag, S., Schubert, U., Eds.; Springer: Cham, Germany, 2016; Volume 273. [Google Scholar]
- Qian, C.; Zheng, T.; Zhang, X.; Su, Y. Application of microbial self-healing concrete: Case study. Constr. Build. Mater. 2021, 290, 123226. [Google Scholar] [CrossRef]
- Sierra Beltran, M.G.; Jonkers, H.M.; Mera-Ortiz, W. Field application of self-healing concrete with natural fibres as linings for irrigation canals in Ecuador. In Proceedings of the 5th International Conference on Self-Healing Materials, Durham, NC, USA, 22–24 June 2015; Reichert, M., Ed.; Duke University: Durham, NC, USA; pp. 1–4. [Google Scholar]
- Davies, R.; Teall, O.; Pilegis, M.; Kanellopoulos, A.; Sharma, T.; Jefferson, A.; Gardner, D.; Al-Tabbaa, A.; Paine, K.; Lark, R. Large Scale Application of Self-Healing Concrete: Design, Construction, and Testing. Front. Mater. 2018, 5, 2018. [Google Scholar] [CrossRef]
- Lawson, C.E.; Harcombe, W.R.; Hatzenpichler, R.; Lindemann, S.R.; Löffler, F.E.; O’Malley, M.A.; Martín, H.G.; Pfleger, B.F.; Raskin, L.; Venturelli, O.S.; et al. Common principles and best practices for engineering microbiomes. Nat. Rev. Microbiol. 2019, 17, 725–741. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Mungray, A.K.; Arkatkar, A.; Kumar, S.S. Recent advancement in scaling-up applications of microbial fuel cells: From reality to practicability. Sustain. Energy Technol. Assess. 2021, 45, 101226. [Google Scholar] [CrossRef]
- Wehrs, M.; Tanjore, D.; Eng, T.; Lievense, J.; Pray, T.R.; Mukhopadhyay, A. Engineering Robust Production Microbes for Large-Scale Cultivation. Trends Microbiol. 2019, 27, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.Y.; Mal, J.; Sandak, A.; Luo, L.; Jian, J.; Pradhan, N. Advances in microbial self-healing concrete: A critical review of mechanisms, developments, and future directions. Sci. Total Environ. 2024, 947, 174553. [Google Scholar] [CrossRef]
- Beskopylny, A.N.; Shcherban’, E.M.; Stel’makh, S.A.; Shilov, A.A.; Chernil’nik, A.; El’shaeva, D.; Chistyakov, V.A. Analysis of the Current State of Research on Bio-Healing Concrete (Bioconcrete). Materials 2024, 17, 4508. [Google Scholar] [CrossRef]
- Salami, B.A.; Bahraq, A.A.; ul Haq, M.M.; Ojelade, O.A.; Taiwo, R.; Wahab, S.; Adewumi, A.A.; Ibrahim, M. Polymer-enhanced concrete: A comprehensive review of innovations and pathways for resilient and sustainable materials. Next Mater. 2024, 4, 100225. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Chen, Z.; Dong, Y.; Jiang, Y.; Hua, J.; Liu, Y.; Osman, A.I.; Farghali, M.; Huang, L.; et al. Biomaterials technology and policies in the building sector: A review. Environ. Chem. Lett. 2024, 22, 715–750. [Google Scholar] [CrossRef]
- Fernández-Cabezón, L.; Cros, A.; Nikel, P. Evolutionary Approaches for Engineering Industrially Relevant Phenotypes in Bacterial Cell Factories. Biotech. J. 2019, 14, 1800439. [Google Scholar]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Liu, S.; Xu, W. Engineered Living Materials-Based Sensing and Actuation. Front. Sens. 2020, 1, 2020. [Google Scholar]
- Coppola, F.; Fratianni, F.; Bianco, V.; Wang, Z.; Pellegrini, M.; Coppola, R.; Nazzaro, F. New Methodologies as Opportunities in the Study of Bacterial Biofilms, Including Food-Related Applications. Microorganisms 2025, 13, 1062. [Google Scholar] [CrossRef]
- Mohseni, P.; Ghorbani, A. Exploring the synergy of artificial intelligence in microbiology: Advancements, challenges, and future prospects. CSB Rep. 2014, 1, 100005. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Yu, P.; Yang, L. A Review of Artificial Intelligence in Enhancing Architectural Design Efficiency. Appl. Sci. 2025, 15, 1476. [Google Scholar] [CrossRef]
- Armstrong, R. Towards the microbial home: An overview of developments in next-generation sustainable architecture. Microb. Biotechnol. 2023, 16, 1112–1130. [Google Scholar] [CrossRef] [PubMed]
- AlAli, M.; Beheiry, S.; Atabay, S. Strategies for the Design and Construction of Nature-Inspired & Living Laboratory (NILL 1.0)TM Buildings. Biomimetics 2024, 9, 441. [Google Scholar] [CrossRef]
- Hakio, K.; Mattelmäki, T. Future Skills of Design for Sustainability: An Awareness-Based Co-Creation Approach. Sustainability 2019, 11, 5247. [Google Scholar] [CrossRef]
- Delgado Corrales, B.; Kaiser, R.; Nerlich, P.; Agraviador, A.; Sherry, A. Chapter Three—BioMateriOME: To understand microbe-material interactions within sustainable, living architectures. Adv. Appl. Microbiol. 2013, 122, 77–126. [Google Scholar]
- Chayaamor-Heil, N.; Perricone, V.; Gruber, P.; Guéna, F. Bioinspired, biobased and living material designs: A review of recent research in architecture and construction. Bioinspir. Biomim. 2023, 18, 041001. [Google Scholar] [CrossRef] [PubMed]
| Mechanism | Bacterial Action | Applications | Key Bacteria | Environmental Considerations | Related Ref. |
|---|---|---|---|---|---|
| Ureolysis-induced carbonate precipitation | Ureolytic bacteria hydrolyse urea → carbonate → CaCO3 | Soil stabilisation self-healing concrete surface hardening crack repair corrosion inhibition | S. pasteurii | increase in pH value ammonia toxicity porous media distribution | [28,39,42] |
| Denitrification-induced carbonate Precipitation | Facultative anaerobic bacteria reduce nitrate to N2 and carbonate | Anaerobic soil zones deep foundations ureolysis alternative | P. denitrificans, Paracoccus spp. | Anaerobic, nitrate-rich zones | [38,53] |
| Sulphate Reduction and Biogenic Metal Precipitation | Sulphate-reducing bacteria → sulphide → metal sulphides | Heavy metal immobilisation corrosion control industrial remediation | Desulfovibrio spp. | Anaerobic, sulphur-rich environments | [53] |
| Photosynthetic Carbonate Precipitation | Cyanobacteria photosynthesis ↑; pH → carbonate precipitation | Living building skins CO2 sequestration light-exposed panels | Synechococcus, Gloeocapsa, Spirulina | Light-exposed, alkaline surfaces | [54] |
| MICP-Related Technique | Bioconsolidation | Biocementation | Bioaggregation/Biogrounting |
|---|---|---|---|
| Definition | Bacterial-induced hardening of porous substrates via in situ mineral precipitation | Binding of granular particles via bacterial mineral precipitation at contact points | Particle cohesion via secretion of extracellular polymeric substances (EPS) |
| Mechanism | Filling of pores and voids with bacterially precipitated minerals | Targeted CaCO3 deposition between soil grains | EPS-mediated flocculation and particle cohesion |
| Primary Bacterial Activity | MICP | MICP | MICP with (strong) EPS production |
| Main Materials Involved | Soils, sandstones, concrete, natural stone, heritage materials | Granular soils (sand, silt), cement-treated zones | Clay, silts, organic soils, and sludge |
| Engineering Applications | Stone conservation, monument repair, crack sealing in cement-based materials, etc. | Soil stabilisation, liquefaction mitigation, ground improvement, self-healing system for cement-based materials, etc. | Slope stabilisation, erosion control, and moisture retention |
| Key Benefits | Non-invasive, improves durability and permeability | Increased shear strength, stiffness, and erosion resistance | Improves cohesion, water retention, and supports vegetation |
| Environmental Sensitivity | Moderate (depends on pH, nutrients, and moisture) | High (sensitive to pH, flow, saturation) | Low to moderate (depends on bacterial growth and EPS production) |
| Typical Bioagent | Bacillus spp. S. pasteurii | S. pasteurii Pseudomonas spp. | Pseudomonas spp. Azotobacter spp., Bacillus subtilis |
| Related References | [55] | [56] | [51,57] |
| Case Study | Bacteria | Target Material/Deposit | Carrier Medium | Application Method | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Camposanto Monumentale, Italy | P. stutzeri | Nitrate efflorescence | Carbogel-based gel | Topical gel layer for 12–24 h | Validated on high-value frescoes; nitrate removal was successful | [58] |
| Stone church façade, Spain | Desulfovibrio desulfuricans | Gypsum/black crust (sulfated pollutants) | Agar-gel matrix | Surface patching with gauze sheets for 60 h | Restoration-grade stone surfaces showed no damage | [62] |
| Triumph of Death fresco, Italy | P. stutzeri A29 | Animal glue | Hydrogel in cellulose matrix | Surface patching (biotretment for 3 h; prolonged baterial treatmen (6 h) also tested; | Preliminary trials confirmed efficiency with low residue | [63] |
| Brick heritage walls, Romania | Halotolerant Bacillus spp. | Mixed salt and pollution crusts | Dry gel with moisture activation | Compressed dry pad application for 30 days | Adapted for large-scale outdoor use | [64] |
| Bacterial Biopolymer | Producing Microorganism | Key Properties | Applications | Ref. |
|---|---|---|---|---|
| Xanthan gum | Xanthomonas campestris | High viscosity, shear-thinning, stable in extreme pH | Soil stabilisation, erosion control, and eco-grouting | [81] |
| Curdlan | Agrobacterium spp. | Forms gels upon heating, with high water retention | Moisture control, sustainable binders | [82] |
| Alginate | Pseudomonas spp. Azotobacter spp. | Ion-sensitive gelation, strong water-binding | Crack sealing, biocementing agent | [83] |
| Dextran | Leuconostoc spp. | Cohesive, improves soil aggregation | Soil cohesion enhancement, dust suppression | [84] |
| Gellan gum | Sphingomonas elodea | Thermally stable, forms rigid gels | Admixture in self-healing mortars | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šovljanski, O.; Tomić, A.; Milović, T.; Bulatović, V.; Ranitović, A.; Cvetković, D.; Markov, S. Construction Biotechnology: Integrating Bacterial Systems into Civil Engineering Practices. Microorganisms 2025, 13, 2051. https://doi.org/10.3390/microorganisms13092051
Šovljanski O, Tomić A, Milović T, Bulatović V, Ranitović A, Cvetković D, Markov S. Construction Biotechnology: Integrating Bacterial Systems into Civil Engineering Practices. Microorganisms. 2025; 13(9):2051. https://doi.org/10.3390/microorganisms13092051
Chicago/Turabian StyleŠovljanski, Olja, Ana Tomić, Tiana Milović, Vesna Bulatović, Aleksandra Ranitović, Dragoljub Cvetković, and Siniša Markov. 2025. "Construction Biotechnology: Integrating Bacterial Systems into Civil Engineering Practices" Microorganisms 13, no. 9: 2051. https://doi.org/10.3390/microorganisms13092051
APA StyleŠovljanski, O., Tomić, A., Milović, T., Bulatović, V., Ranitović, A., Cvetković, D., & Markov, S. (2025). Construction Biotechnology: Integrating Bacterial Systems into Civil Engineering Practices. Microorganisms, 13(9), 2051. https://doi.org/10.3390/microorganisms13092051







