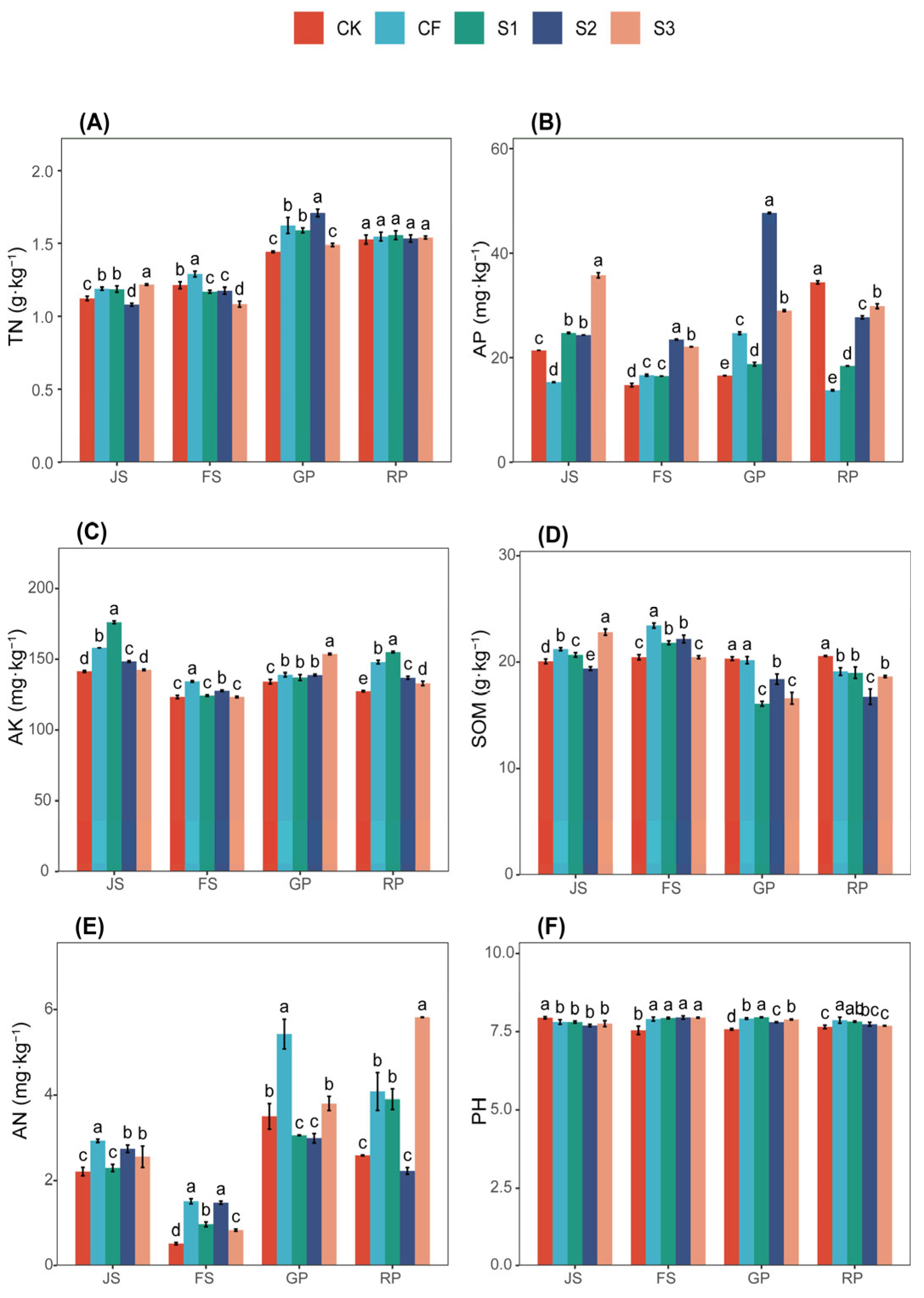
Figure 1.
Soil nutrients treated differently. (A) TN, total nitrogen; (B) AP, available phosphorus; (C) AK, available potassium; (D) SOM, soil organic matter; (E) AN, ammonium nitrogen; (F) PH. JS, jointing stage; FS, flowering stage; GP, grouting period; RP, reaping period. The letters a, b, c, etc. in the picture represent the differences among various treatments within the same period. If the letters are the same, the differences are not significant; if the letters are different, the differences are significant. CK (water applied throughout the reproductive period), CF (NF applied throughout the reproductive period), S1 (application of BS at the JS and NF at the GP), S2 (application of NF at the JS and BS at the GP), and S3 (application of biogas slurry throughout the reproductive period, excluding basal fertilizer).
Figure 1.
Soil nutrients treated differently. (A) TN, total nitrogen; (B) AP, available phosphorus; (C) AK, available potassium; (D) SOM, soil organic matter; (E) AN, ammonium nitrogen; (F) PH. JS, jointing stage; FS, flowering stage; GP, grouting period; RP, reaping period. The letters a, b, c, etc. in the picture represent the differences among various treatments within the same period. If the letters are the same, the differences are not significant; if the letters are different, the differences are significant. CK (water applied throughout the reproductive period), CF (NF applied throughout the reproductive period), S1 (application of BS at the JS and NF at the GP), S2 (application of NF at the JS and BS at the GP), and S3 (application of biogas slurry throughout the reproductive period, excluding basal fertilizer).
Figure 2.
Soil enzyme activity under different treatments. (A) Catalase, CAT; (B) Urease, UE; (C) Sucrase, SC; (D) Neutral phosphatase, NP. The letters a, b, c, etc. in the picture represent the differences among various treatments within the same period. If the letters are the same, the differences are not significant; if the letters are different, the differences are significant. CK (water applied throughout the reproductive period), CF (NF applied throughout the reproductive period), S1 (application of BS at the JS and NF at the GP), S2 (application of NF at the JS and BS at the GP), and S3 (application of biogas slurry throughout the reproductive period, excluding basal fertilizer); JS, jointing stage; FS, flowering stage; GP, grouting period; RP, reaping period.
Figure 2.
Soil enzyme activity under different treatments. (A) Catalase, CAT; (B) Urease, UE; (C) Sucrase, SC; (D) Neutral phosphatase, NP. The letters a, b, c, etc. in the picture represent the differences among various treatments within the same period. If the letters are the same, the differences are not significant; if the letters are different, the differences are significant. CK (water applied throughout the reproductive period), CF (NF applied throughout the reproductive period), S1 (application of BS at the JS and NF at the GP), S2 (application of NF at the JS and BS at the GP), and S3 (application of biogas slurry throughout the reproductive period, excluding basal fertilizer); JS, jointing stage; FS, flowering stage; GP, grouting period; RP, reaping period.
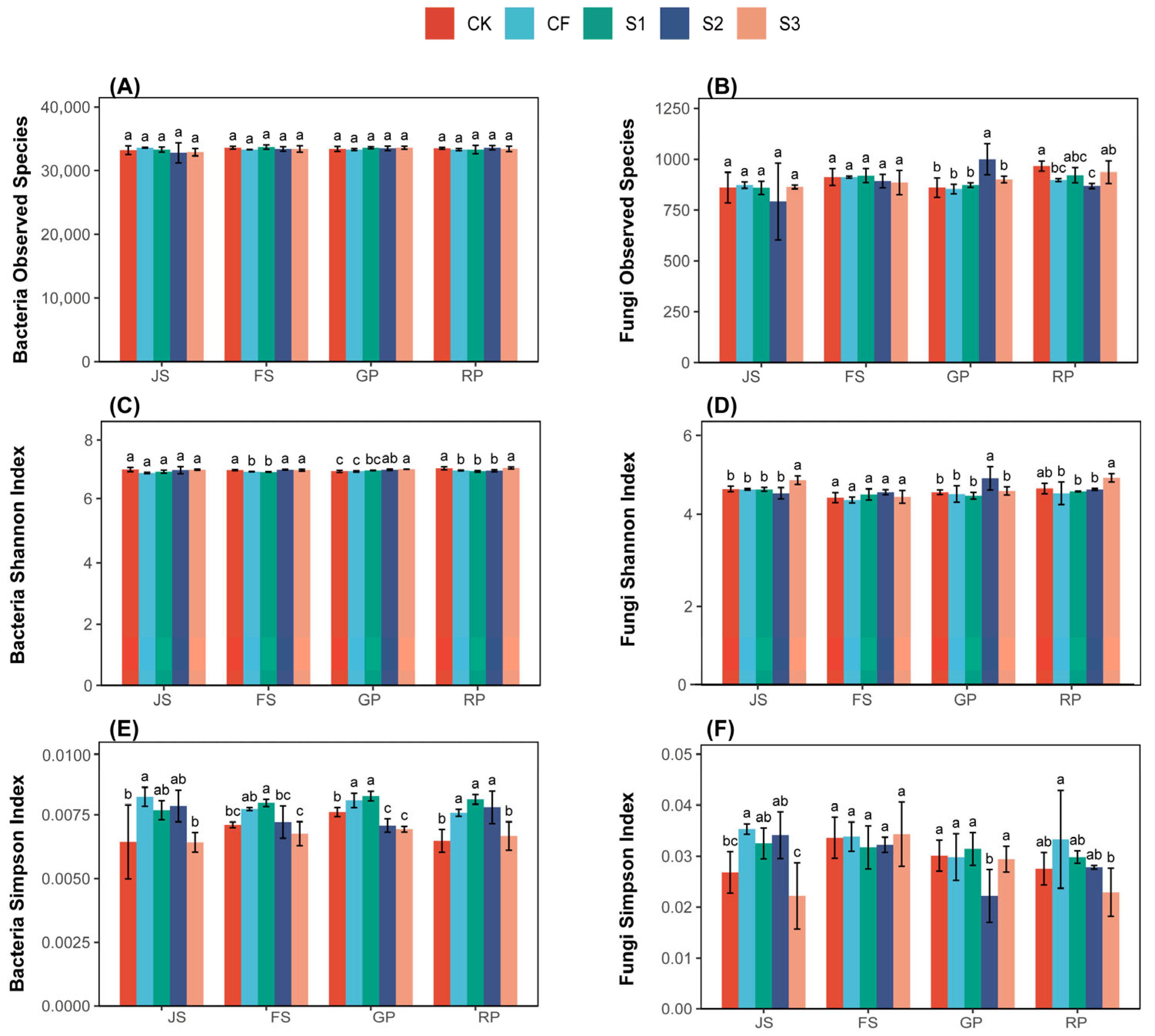
Figure 3.
Soil microbial diversity under different treatments. Bacteria observed species (A), shannon index (B) and simpson index (C). Fungi observed species (D), shannon index (E) and simpson index (F). The letters a, b, c. in the picture represent the differences among various treatments within the same period. If the letters are the same, the differences are not significant; if the letters are different, the differences are significant. CK (water applied throughout the reproductive period), CF (NF applied throughout the reproductive period), S1 (application of BS at the JS and NF at the GP), S2 (application of NF at the JS and BS at the GP), and S3 (application of biogas slurry throughout the reproductive period, excluding basal fertilizer). JS, jointing stage; FS, flowering stage; GP, grouting period; RP, reaping period.
Figure 3.
Soil microbial diversity under different treatments. Bacteria observed species (A), shannon index (B) and simpson index (C). Fungi observed species (D), shannon index (E) and simpson index (F). The letters a, b, c. in the picture represent the differences among various treatments within the same period. If the letters are the same, the differences are not significant; if the letters are different, the differences are significant. CK (water applied throughout the reproductive period), CF (NF applied throughout the reproductive period), S1 (application of BS at the JS and NF at the GP), S2 (application of NF at the JS and BS at the GP), and S3 (application of biogas slurry throughout the reproductive period, excluding basal fertilizer). JS, jointing stage; FS, flowering stage; GP, grouting period; RP, reaping period.
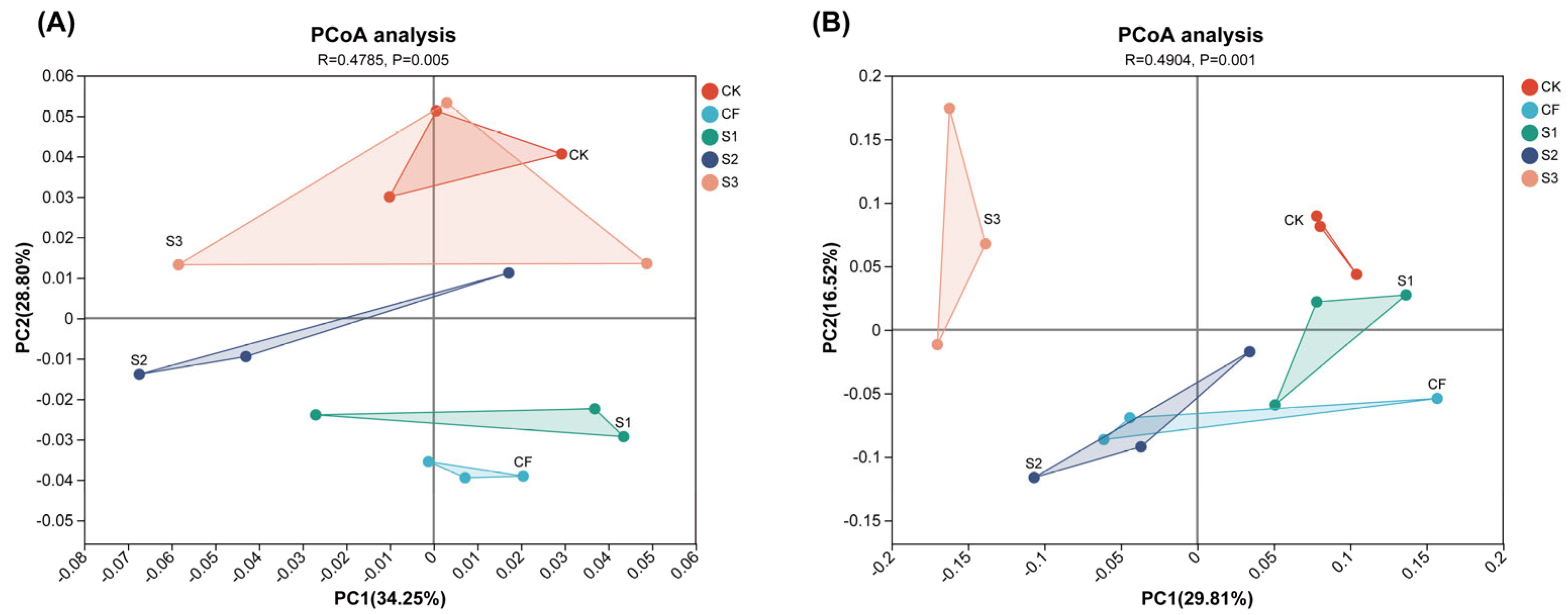
Figure 4.
Principal component analysis of soil bacterial community (A) and fungal community (B) under different fertilization strategies.
Figure 4.
Principal component analysis of soil bacterial community (A) and fungal community (B) under different fertilization strategies.
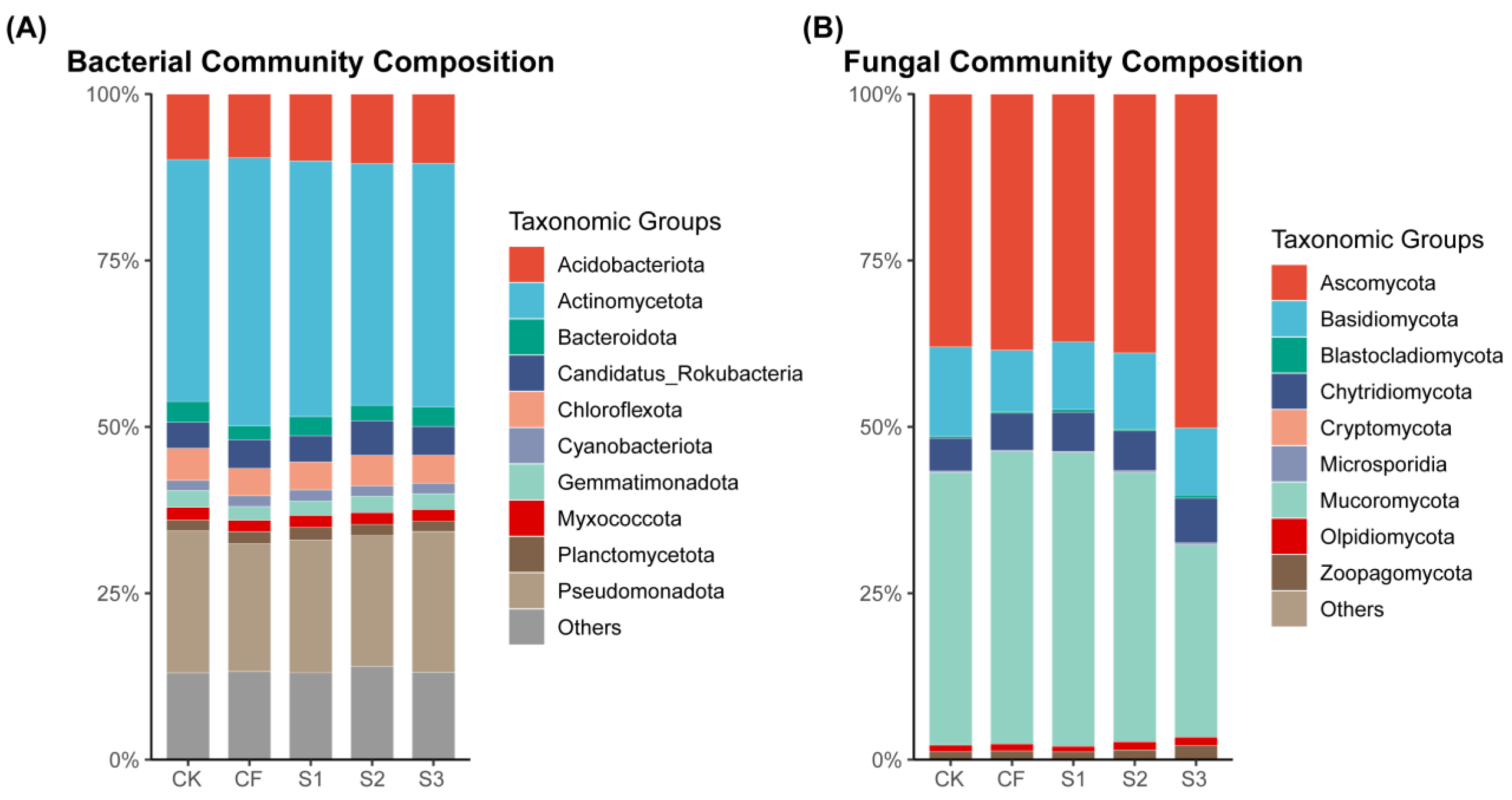
Figure 5.
Soil bacterial community phylum abundance (A) and fungal community phylum abundance (B) under different fertilization strategies.
Figure 5.
Soil bacterial community phylum abundance (A) and fungal community phylum abundance (B) under different fertilization strategies.
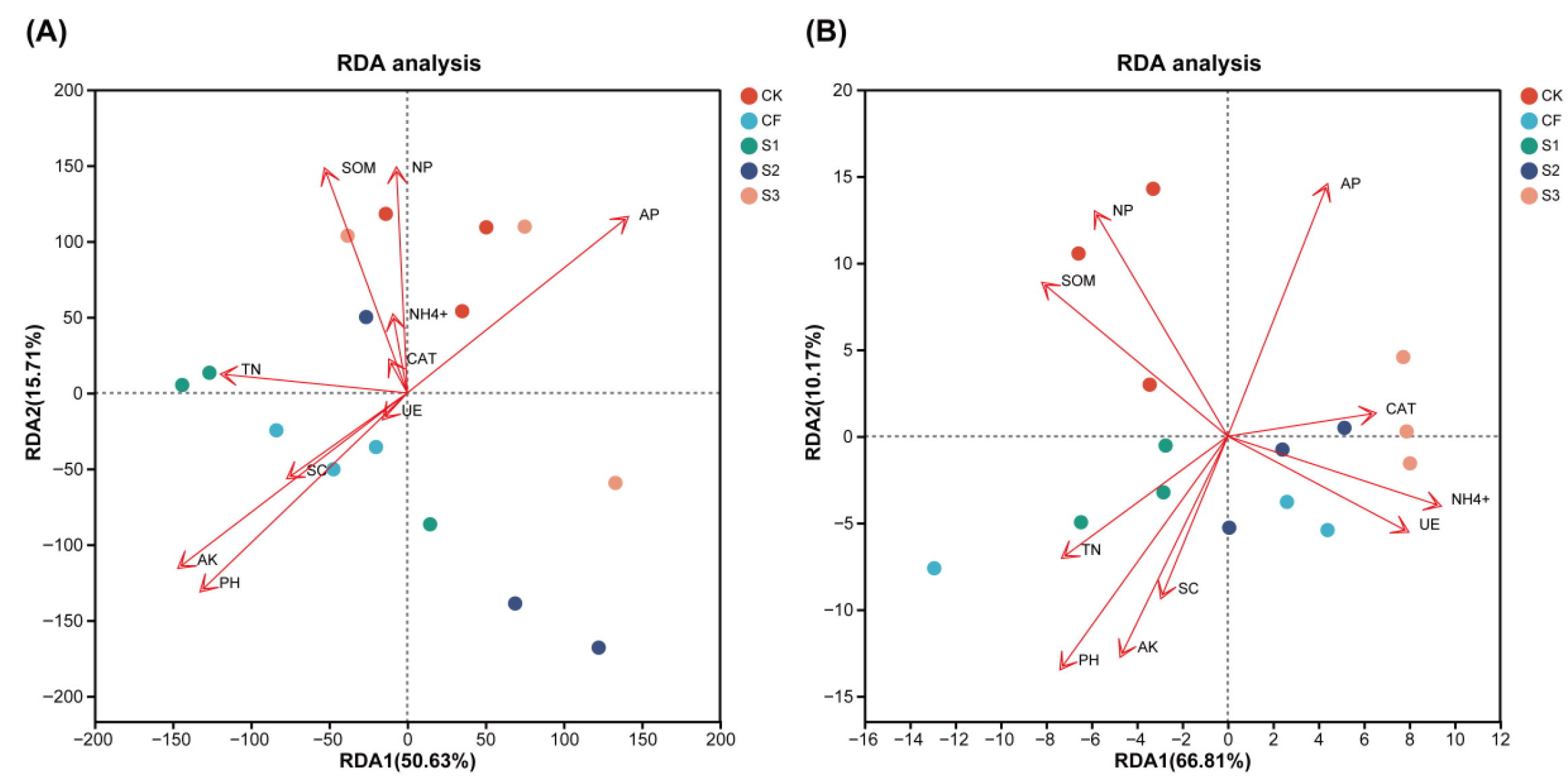
Figure 6.
Redundancy analysis (RDA) ordination diagrams describing the relationship between bacterial (A) and fungal (B) communities and wheat soil properties. TN, total nitrogen; AP, fast-acting phosphorus; AK, fast-acting potassium; NH4+, ammonium nitrogen; SOM, soil organic matter; CAT, catalase activity; UE, urease activity; NP, neutral phosphatase activity; SC, sucrase activity.
Figure 6.
Redundancy analysis (RDA) ordination diagrams describing the relationship between bacterial (A) and fungal (B) communities and wheat soil properties. TN, total nitrogen; AP, fast-acting phosphorus; AK, fast-acting potassium; NH4+, ammonium nitrogen; SOM, soil organic matter; CAT, catalase activity; UE, urease activity; NP, neutral phosphatase activity; SC, sucrase activity.
Figure 7.
Correlations between soil nutrients, microorganisms, and wheat yield (red strings indicate positive correlations, blue strings indicate negative correlations, and the width of the strings reflects the strength of the correlation).
Figure 7.
Correlations between soil nutrients, microorganisms, and wheat yield (red strings indicate positive correlations, blue strings indicate negative correlations, and the width of the strings reflects the strength of the correlation).
Figure 8.
Spearman’s correlation between relative abundance of soil bacterial and fungal phyla and fertilization strategies (* for p < 0.05, ** for p < 0.01, *** for p < 0.001).
Figure 8.
Spearman’s correlation between relative abundance of soil bacterial and fungal phyla and fertilization strategies (* for p < 0.05, ** for p < 0.01, *** for p < 0.001).
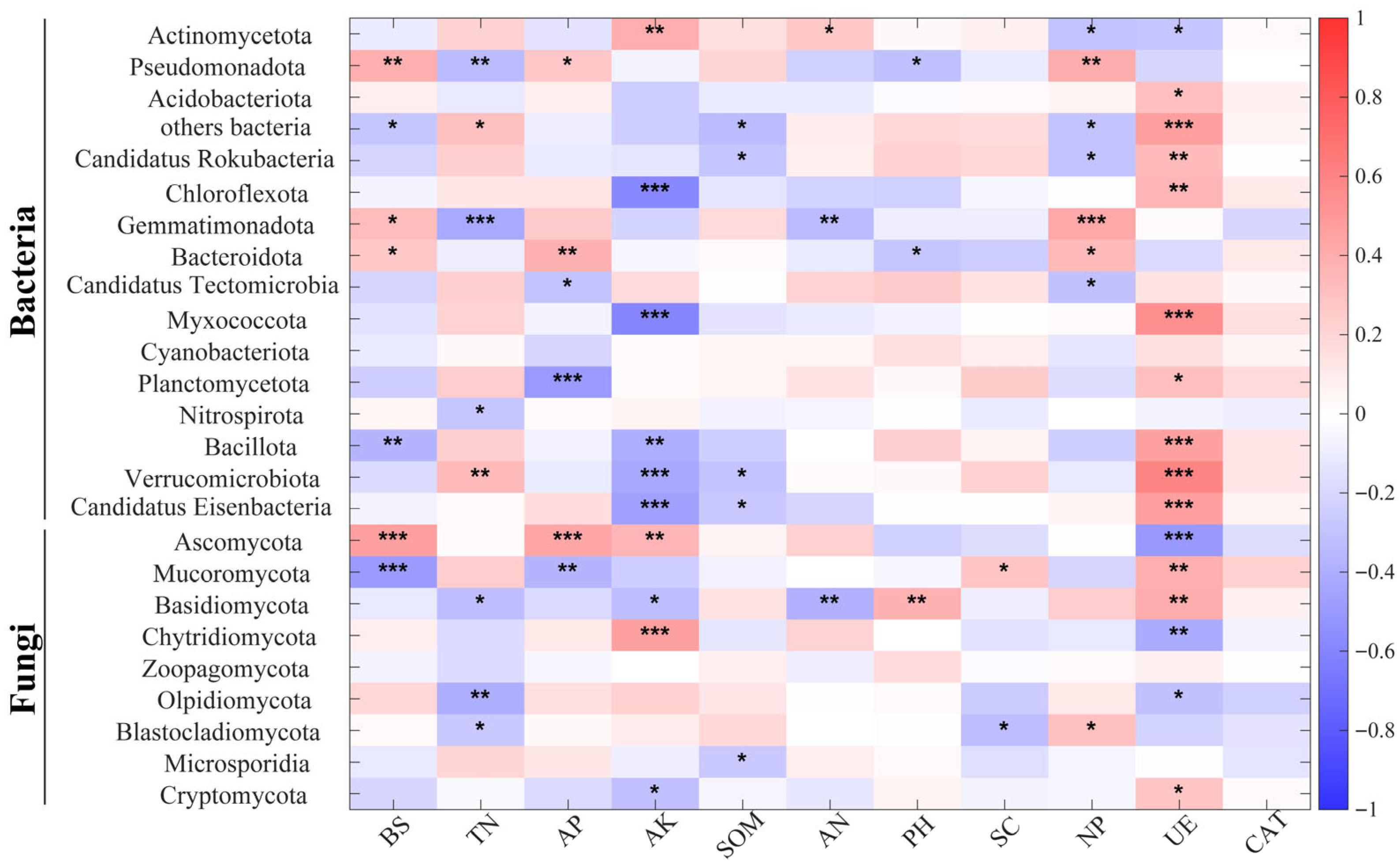
Figure 9.
Spearman’s correlation between relative abundance of soil bacterial and fungal phyla and environmental factors (* for p < 0.05, ** for p < 0.01, *** for p < 0.001).
Figure 9.
Spearman’s correlation between relative abundance of soil bacterial and fungal phyla and environmental factors (* for p < 0.05, ** for p < 0.01, *** for p < 0.001).
Figure 10.
Partial least squares path model (PLS—SEM) analysis of the soil properties and soil microorganisms affected by biogas slurry under different fertilization strategies (the blue arrow represents a significant positive impact, the red arrow represents a significant negative impact, and the implementation represents a direct impact relationship; the dashed line represents an indirect impact relationship (A) as S1, (B) as S2, and (C) as S3).
Figure 10.
Partial least squares path model (PLS—SEM) analysis of the soil properties and soil microorganisms affected by biogas slurry under different fertilization strategies (the blue arrow represents a significant positive impact, the red arrow represents a significant negative impact, and the implementation represents a direct impact relationship; the dashed line represents an indirect impact relationship (A) as S1, (B) as S2, and (C) as S3).
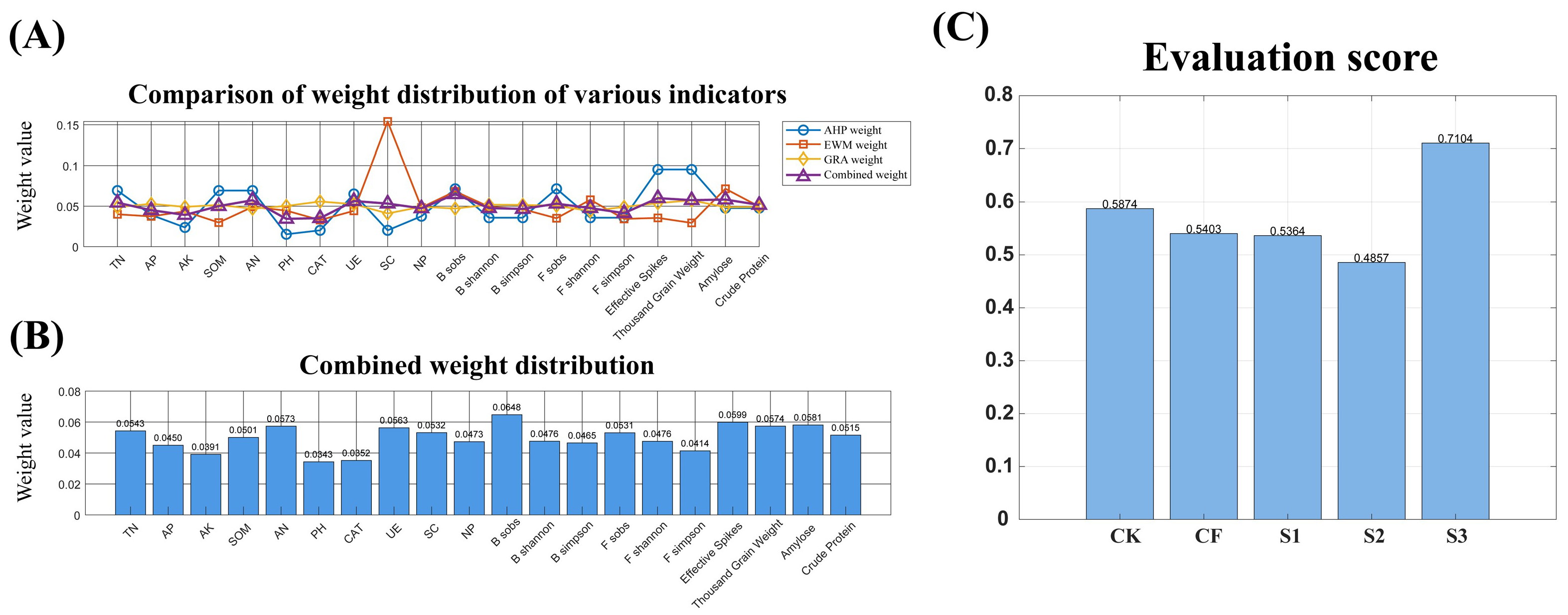
Figure 11.
Comparison of weight distribution of various indicators (A), Combined weight distribution (B), Evaluation score (C) using the AHP-EWM-GRA-WGRA evaluation method.
Figure 11.
Comparison of weight distribution of various indicators (A), Combined weight distribution (B), Evaluation score (C) using the AHP-EWM-GRA-WGRA evaluation method.
Table 1.
Main properties of soil.
Table 1.
Main properties of soil.
| pH | TN (g·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | SOM (g·kg−1) | AN (mg·kg−1) |
|---|
| 7.87 ± 0.032 | 1.24 ± 0.0055 | 31.33 ± 0.089 | 142.33 ± 0.58 | 22.64 ± 0.29 | 2.94 ± 0.088 |
Table 2.
Main properties of biogas slurry.
Table 2.
Main properties of biogas slurry.
| pH | Total Solids Concentration | Volatile Solids | AP (mg·L−1) | AK (g·L−1) | TN (mg·L−1) | SOM (g·L−1) |
|---|
| 8.35 | 0.43% | 34.92% | 18.08 | 0.84 | 375.54 | 1.11 |
Table 3.
The amount of biogas digestate and fertilizer used in different treatments.
Table 3.
The amount of biogas digestate and fertilizer used in different treatments.
| Treatment | JS | GP |
|---|
| Water L·ha−1 | Amount of Nitrogen Fertilizer kg·ha−1 | Amount of Biogas Digestate L·ha−1 | Water L·ha−1 | Amount of Nitrogen Fertilizer kg·ha−1 | Amount of Biogas Digestate L·ha−1 |
|---|
| CK | 33,960 | 0 | 0 | 33,960 | 0 | 0 |
| CF | 33,960 | 150 | 0 | 33,960 | 150 | 0 |
| S1 | 16,980 | 0 | 16,980 | 33,960 | 150 | 0 |
| S2 | 33,960 | 150 | 0 | 16,980 | 0 | 2264 |
| S3 | 16,980 | 0 | 16,980 | 16,980 | 0 | 16,980 |
Table 4.
Hierarchical analysis method evaluation system.
Table 4.
Hierarchical analysis method evaluation system.
| Decision-Making Objective (A) | Decision-Making Factors (B) | Decision-Making Indicators (C) |
|---|
| Optimal fertilization strategy plan for replacing nitrogen fertilizer with biogas slurry (A) | Soil nutrients (B1) | TN (C1) |
| | | TN (C2) |
| | | AP (C3) |
| | | AK (C3) |
| | | SOM (C4) |
| | | AN (C5) |
| | | PH (C6) |
| | Soil enzyme activity (B2) | CAT (C7) |
| | | UE (C8) |
| | | SC (C9) |
| | | NP (C10) |
| | Soil bacterial diversity (B3) | B_sobs (C11) |
| | | B_shannon (C12) |
| | | B_simpson (C13) |
| | Soil fungal diversity (B4) | F_sobs (C14) |
| | | F_shannon (C15) |
| | | F_simpson (C16) |
| | Wheat yield and quality (B5) | Effective Spikes (C17) |
| | | Thousand-Grain Weight (C18) |
| | | Amylose (C19) |
| | | Crude Protein (C20) |
Table 5.
Saaty 1–9 ratio-scale and method-scale values.
Table 5.
Saaty 1–9 ratio-scale and method-scale values.
| Relative Importance | Definition |
|---|
| 1 | Equally important |
| 3 | Slightly more important |
| 5 | Significantly more important |
| 7 | Clearly more important |
| 9 | Absolutely more important |
| 2, 4, 6, 8 | Intermediate value between two adjacent judgments |
| 1/3 | Slightly less important |
| 1/5 | Significantly less important |
| 1/7 | Absolutely less important |
| 1/9 | Absolutely less important |
| 1/2, 1/4, 1/6, 1/8 | Intermediate value between two adjacent judgments |
Table 6.
Effect of different fertilization programs on yield indicators of wheat.
Table 6.
Effect of different fertilization programs on yield indicators of wheat.
| Treat | Number of Effective
Spikes m−2 | Thousand-Grain
Weight g | Amylose
mg·g−1 | Crude Protein
g·kg−1 | Yield
kg·ha−1 |
|---|
| CK | 452.00 ± 43.27 a | 47.20 ± 0.46 a | 615.12 ± 18.02 b | 131.39 ± 1.14 b | 8970.67 ± 896.19 a |
| CF | 505.33 ± 34.02 a | 50.38 ± 0.82 a | 514.73 ± 3.33 c | 133.59 ± 1.74 b | 9632.57 ± 720.53 a |
| S1 | 481.33 ± 85.54 a | 49.07 ± 3.50 a | 521.93 ± 3.34 c | 123.12 ± 2.25 c | 9120 ± 617.19 a |
| S2 | 468.00 ± 13.86 a | 49.28 ± 3.66 a | 528.91 ± 2.76 c | 138.58 ± 2.01 a | 8768 ± 532.59 a |
| S3 | 498.67 ± 29.48 a | 50.65 ± 1.83 a | 635.13 ± 14.24 a | 124.31 ± 4.06 c | 9461.33 ± 352.48 a |
Table 7.
A—B Consistency check and local weight values.
Table 7.
A—B Consistency check and local weight values.
| A—B | Soil Nutrition | Soil Enzyme Activities | Bacterial Diversity | Fungal Diversity | Wheat Quality | Local Weight Values |
|---|
| Soil nutrition | 1 | 2 | 2 | 2 | 1 | 0.2857 |
| Soil enzyme activities | 1/2 | 1 | 1 | 1 | 1/2 | 0.1429 |
| Bacterial diversity | 1/2 | 1 | 1 | 1 | 1/2 | 0.1429 |
| Fungal diversity | 1/2 | 1 | 1 | 1 | 1/2 | 0.1429 |
| Wheat quality | 1 | 2 | 2 | 2 | 1 | 0.2857 |
Table 8.
B1—C Consistency check and local weight values.
Table 8.
B1—C Consistency check and local weight values.
| B1—C | TN | AP | AK | SOM | AN | PH | Local Weight Values |
|---|
| TN | 1 | 2 | 3 | 1 | 1 | 4 | 0.2423 |
| AP | 1/2 | 1 | 2 | 1/2 | 1/2 | 3 | 0.1369 |
| AK | 1/3 | 1/2 | 1 | 1/3 | 1/3 | 2 | 0.0829 |
| SOM | 1 | 2 | 3 | 1 | 1 | 4 | 0.2423 |
| AN | 1 | 2 | 3 | 1 | 1 | 4 | 0.2423 |
| PH | 1/4 | 1/3 | 1/2 | 1/4 | 1/4 | 1 | 0.0534 |
Table 9.
B2—C Consistency check and local weight values.
Table 9.
B2—C Consistency check and local weight values.
| B2—C | CAT | UE | SC | NP | Local Weight Values |
|---|
| CAT | 1 | 1/3 | 1 | 1/2 | 0.1409 |
| UE | 3 | 1 | 3 | 2 | 0.4554 |
| SC | 1 | 1/3 | 1 | 1/2 | 0.1409 |
| NP | 2 | 1/2 | 2 | 1 | 0.2628 |
Table 10.
B3—C Consistency check and local weight values.
Table 10.
B3—C Consistency check and local weight values.
| B3—C | B_sobs | B_shannon | B_simpson | Local Weight Values |
|---|
| B_sobs | 1 | 2 | 2 | 0.5 |
| B_shannon | 1/2 | 1 | 1 | 0.25 |
| B_simpson | 1/2 | 1 | 1 | 0.25 |
Table 11.
B4—C Consistency check and local weight values.
Table 11.
B4—C Consistency check and local weight values.
| B4—C | F_sobs | F_shannon | F_simpson | Local Weight Values |
|---|
| F_sobs | 1 | 2 | 2 | 0.50 |
| F_shannon | 1/2 | 1 | 1 | 0.25 |
| F_simpson | 1/2 | 1 | 1 | 0.25 |
Table 12.
B5—C Consistency check and local weight values.
Table 12.
B5—C Consistency check and local weight values.
| B5—C | Effective Spikes | Thousand-Grain Weight | Amylose | Crude Protein | Local Weight Values |
|---|
| Effective Spikes | 1 | 1 | 2 | 2 | 0.3333 |
| Thousand-Grain Weight | 1 | 1 | 2 | 2 | 0.3333 |
| Amylose | 1/2 | 1/2 | 1 | 1 | 0.1667 |
| Crude Protein | 1/2 | 1/2 | 1 | 1 | 0.1667 |