Untangling the Complexity of Two-Component Signal Transduction in Bacteria
Abstract
1. Introduction
2. Canonical TCS
3. Transcriptional Regulation of TCS Genes
| HK/RR | Organism | Function Description | Transcriptional Organization | References |
|---|---|---|---|---|
| EnvZ/OmpR | E. coli | Osmoregulation | Classical operon; background transcription, upregulated under external osmotic stimuli | [12] |
| PhoR/PhoB | E. coli | Phosphate metabolism | Classical operon; transcription is constitutive and positively autoregulated under phosphate-limiting conditions | [50,51] |
| CpxA/CpxR | E. coli | Envelope stress response system | Classical operon; transcription is constitutive and positively autoregulated under envelope stress | [57,58] |
| BvgS/BvgA | B. pertussis | Virulence gene regulation | Classical operon; transcription is constitutive and positively autoregulated under virulence-promoting conditions | [52,53,59] |
| ComD/ComE | S. pneumoniae | Genetic competence | Classical operon; transcription is constitutive and positively autoregulated via competence-stimulating peptide | [55] |
| SitK/SitR | S. coelicolor | Repression of sporulation | Classical operon; transcription is conditionally activated under specific stress conditions | [54] |
| SsrA/SsrB | Salmonella | Virulence gene regulation | Nonoperonic; transcription is independent and uncoordinated from separate promoters | [56] |
4. Variations in RR Activities
| HK/RR | Organism | Function Description | Description of the RR Effector Domain and Its Activity | References |
|---|---|---|---|---|
| EnvZ/OmpR | E. coli | Osmoregulation | CTD DNA-binding domain, regulates gene expression | [60] |
| PhoR/PhoB | E. coli | Phosphate metabolism | CTD DNA-binding domain, regulates gene expression | [74] |
| SsrA/SsrB | Salmonella spp. | Virulence gene regulation | CTD DNA-binding domain, regulates gene expression | [56] |
| NtrB/NtrC | Salmonella spp. | Nitrogen regulation | CTD DNA-binding domain, oligomerizes to activate σ54-RNAP | [13,61] |
| FlgS/FlgR | C. jejuni | Flagellar gene expression | Lacks a CTD DNA-binding domain, activates transcription by interaction with σ54-RNAP | [63] |
| FlgS/FlgR | H. pylori | Flagellar biosynthesis | Lacks a CTD DNA-binding domain, activates transcription by interaction with σ54-RNAP | [64] |
| PdtaS/PdtaR | M. tuberculosis | Adaptation to stress by the regulation of RNA structure | CTD RNA-binding domain, binds nascent RNA to prevent transcriptional termination | [69] |
| CheA/CheB | E. coli | Chemotactic adaptation | CTD with enzymatic activity, the receptor methyl-esterase | [70] |
| CheA/CheY | E. coli | Flagellar motor switching | CTD involved in protein–protein interaction, binds FliM to reverse flagellar rotation | [73] |
| DivJ/DivK | C. crescentus | Controls cell cycle progression and the cell’s fate | Single domain (receiver domain), modulates DivL–CckA signaling through a protein–protein interaction | [75] |
| -/AmiR | Pseudomonas aeruginosa | Amidase operon regulation | Orphan RR, CTD RNA-binding domain, transcriptional antitermination regulator | [66,76] |
| -/NasR | Klebsiella spp. | Nitrate/nitrite assimilation | Orphan RR, CTD nitrate-activated RNA-binding domain, antiterminator | [67] |
| -/CsrA | E. coli | Global carbon metabolism, motility, biofilm formation | Orphan RR, CTD RNA-binding domain, stabilization of RNA | [68] |
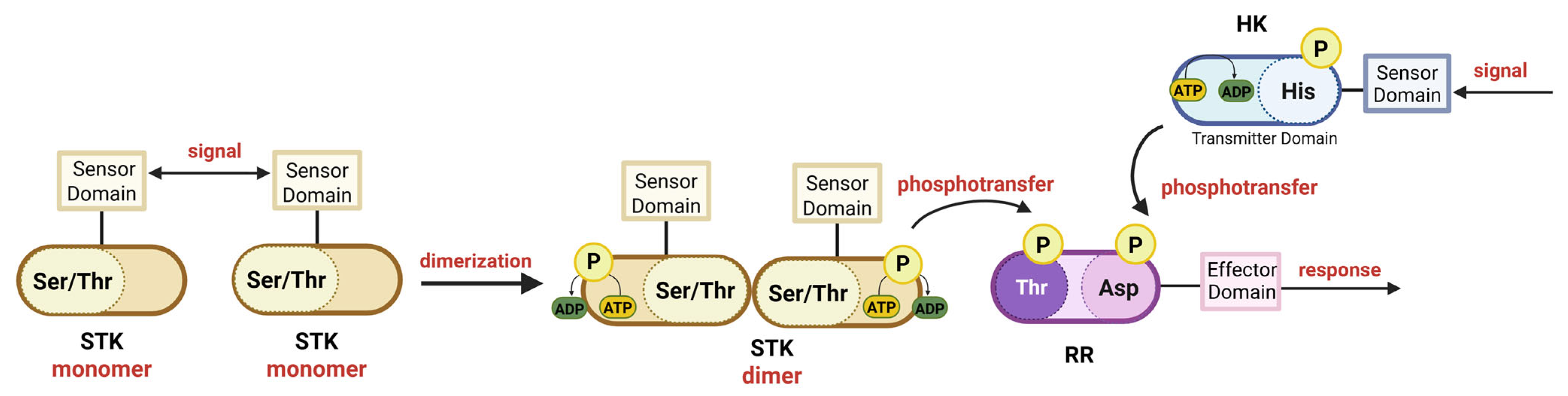
5. Regulation of RR Activity by Phosphorylation
6. Nonspecific Phosphorylation and Acetylation
| RR Name | Organism | Biological Function | Regulation by Phosphorylation | References |
|---|---|---|---|---|
| ComE | S. pneumoniae | Capsule assembly | HK-dependent; gene repression by phosphorylated RR | [79] |
| OmpR | E. coli | Osmoregulation | HK- or AcP-dependent; gene activation in both states: binds DNA when unphosphorylated and phosphorylation increases the specificity | [82] |
| PhoP | B. subtilis | Phosphate metabolism | HK-dependent; gene activation in both states: binds DNA when unphosphorylated and phosphorylation increases the specificity | [83] |
| BvgA | B. pertussis | Regulation of virulence gene expression | HK-dependent; gene activation in both states: binds DNA when unphosphorylated and phosphorylation increases the specificity | [84,85] |
| RcsB | E. coli | Envelope stress; capsule regulation | HK- or AcP-dependent; gene activation in both states: binds DNA when unphosphorylated and phosphorylation increases the specificity | [103] |
| SsrB | Salmonella spp. | SPI-2 transcription; biofilm formation | HK-dependent; stage-dependent phosphorylated and nonphosphorylated forms activate different sets of genes | [86] |
| AlgR | P. aeruginosa | Infection; biofilm formation | HK-dependent; stage-dependent phosphorylated and nonphosphorylated forms activate different sets of genes | [88] |
| DegU | B. subtilis | Motility; competence | HK- or AcP-dependent; phosphorylated and nonphosphorylated forms activate different sets of genes | [92,100,101] |
| FimZ | E. coli | Pili expression; pili elongation | Orphan RR; unknown phosphodonor; phosphorylated and nonphosphorylated forms activate different sets of genes | [94] |
| HnoC | S. oneidensis | Biofilm formation in response to nitric oxide | HK-dependent; the unphosphorylated protein binds as tetramer and represses transcription | [96] |
| Rrp2 | B. burgdorferi | Virulence | Orphan RR; acetyl phosphate-dependent activation | [64] |
| CheY | E. coli | Chemotactic motor switching | HK- or AcP-dependent; activated by acetylation | [104] |
| TcrX | M. tuberculosis | Acid-sensing persistence regulon | HK-dependent; acetylation modulates phosphorylation | [105] |
| MtrA | M. tuberculosis | Cell cycle/cell wall gene regulation | HK-dependent; acetylation modulates phosphorylation | [105] |
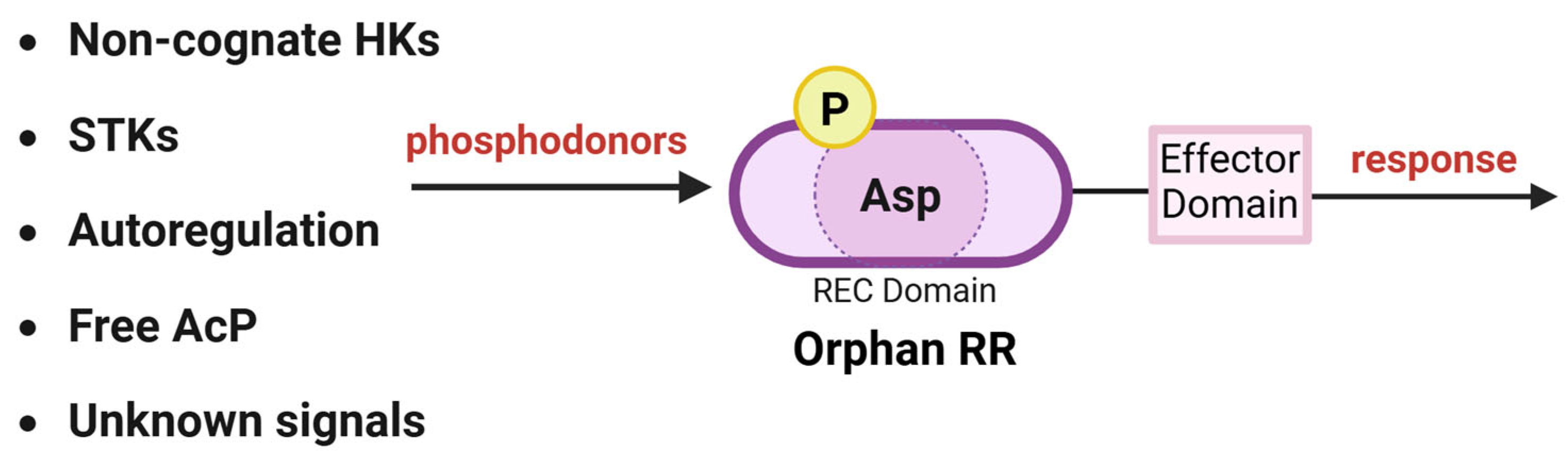
7. Orphan RRs
8. Role of Serine-Threonine Kinases in RR Phosphorylation
| RR Name | Organism | Biological Functions | STK Target Site in the RR | Effect of STK Phosphorylation | References |
|---|---|---|---|---|---|
| DosR | M. tuberculosis | Hypoxia response; latency regulon control | REC domain | Enhances DNA binding affinity | [109] |
| GraR | S. aureus | Resistance to antimicrobial peptides | DNA-binding domain | Enhances DNA binding affinity | [113,121] |
| VraR | S. aureus | Cell wall stress response and antibiotic resistance | DNA-binding domain and REC domain | Enhances DNA binding affinity | [112,113] |
| RitR | S. pneumoniae | Iron uptake and oxidative stress response | DNA-binding domain | Enhances DNA binding affinity | [113,122] |
| CovR | Streptococcus spp. | Regulation of virulence genes; immune evasion | REC domain | Inhibits Asp phosphorylation and DNA binding | [111] |
| WalR | B. subtilis | Cell wall homeostasis; control of peptidoglycan synthesis | REC domain | Modulates dimerization and DNA binding | [112] |
9. Negative Control of TCSs
| Target TCS Components | Organism | Biological Functions | Mechanism of Negative Regulation | References |
|---|---|---|---|---|
| CheY~P (HK) | E. coli | Chemotaxis | Spontaneous autodephosphorylation and deactivation | [107] |
| NtrB (HK) | E. coli | Nitrogen assimilation regulation | Increased phosphatase activity of NtrB after binding to the PII protein | [128] |
| KinA (HK) | B. subtilis | Inhibition of sporulation under unfavorable environmental conditions | Inhibition of KinA by SdA and KipI (antikinase); regulated by KipA | [125,126] |
| FixL (HK) | R. meliloti/C. crescentus | Repression of nitrogen fixation and the heme biosynthesis pathway | FixL autophosphorylation directly inhibited by FixT (SDRR) | [132,139] |
| CckA (HK) | R. sphaeroides | Regulation of cell cycle progression | Inhibition of CckA by Osp (SDRR) | [130] |
| BphP (HK) | D. radiodurans | Resistance to ionizing radiation | Red light activation of phosphatase activity | [123,124] |
| CopS (HK) | P. aeruginosa | Regulation of free copper ions in the periplasmic area | CopS phosphatase activity regulated by Cu+ (active in the absence of Cu+) | [102] |
| PhoQ (HK) | Salmonella enterica/E. coli | Regulation of the virulence-related system (phoPQ) | PhoQ phosphatase activity induced via a direct interaction with MgrB (kinase activity is inhibited) | [127] |
| EnvZ (HK) | E. coli | Part of the envelope stress response | Modulation of EnvZ activity by MzrA | [129] |
| Spo0A~P (RR) | B. subtilis | Inhibition of sporulation initiation | Specific dephosphorylation of Spo0A~P by Spo0E | [131] |
| Spo0F~P (phosphotransfer RR) | B. subtilis | Prevents sporulation during competence | Dephosphorylation of Spo0F~P by RapE | [131] |
| DegU~P (RR) | B. subtilis | Motility; biofilm formation; competence | Preferential degradation of the phosphorylated form | [133] |
| PhoP (RR) | E. coli | Maintenance of the envelope stress response and virulence regulation | ClpAP-mediated proteolysis, prevented by FtsH | [134,135] |
| phoP mRNA | E. coli | Posttranscriptional regulation of the virulence-related system (phoPQ) | Translational repression of the phoP mRNA by micA and gcvB sRNA bind | [136] |
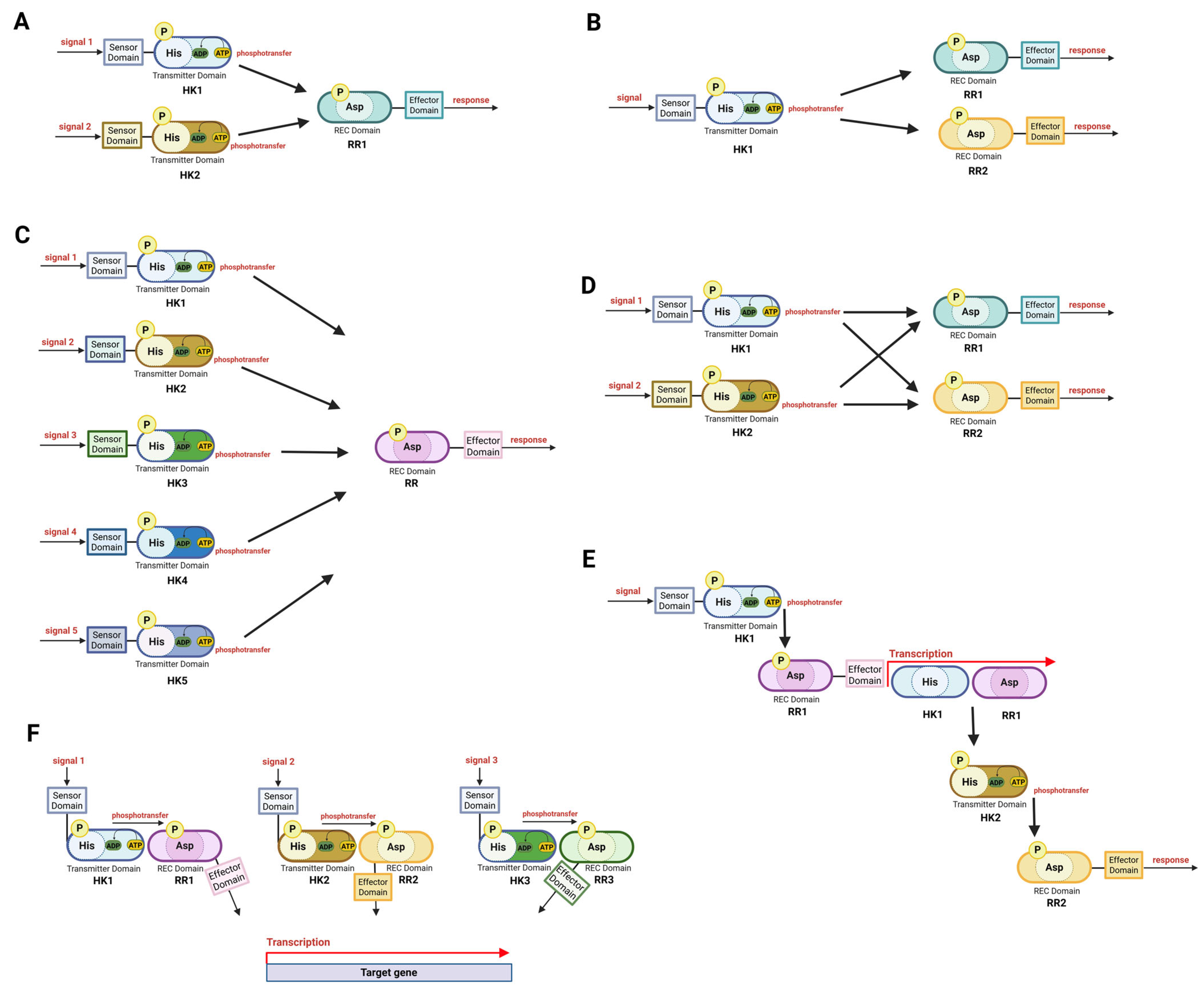
10. Cross-Talk Between TCSs
| HK/RR | Organism | Biological Functions | Type of Interaction | Mechanism | References |
|---|---|---|---|---|---|
| PhoR, CreC/PhoB | E. coli | Integrating phosphate metabolism and carbon catabolism | Many-to-one (cross-talk) | PhoB might be activated by cognate PhoR and noncognate CreC | [145,146] |
| EnvZ, AcrB/OmpR | E. coli | Regulation of porins under anaerobic and osmotic stress conditions | Many-to-one (cross-talk) | OmpR might be activated by cognate EnvZ and noncognate ArcB | [147] |
| RcsC, BarA/RcsB | S. typhimurium | Response to environmental changes and envelope stress | Many-to-one (cross-talk) | RcsB might be activated by the canonical activation pathway (phosphorelay) via RcsC (HK) and RcsD (phosphotransfer protein) or by noncognate BarA (HK) | [148] |
| VanS/PhoB, VanR | E. faecium | Signal transfer modulation during the antibiotic resistance response | One-to-many (cross-talk) | VanS might activate cognate VanR and noncognate PhoB | [149] |
| KinA–KinE/Spo0 | B. subtilis | Integration of stress signals during sporulation initiation | Many-to-one (signal integration) | Five different kinases (KinA–KinE) might phosphorylate Spo0F | [150] |
| LuxN, LuxP/LuxU | V. harveyi | Integration of quorum sensing signals | Many-to-one (signal integration) | Two different kinases (LuxN and LuxP) recognize different signaling molecules and phosphorylate LuxU | [151,152] |
| CheA, CheW/CheY, CheB | B. subtilis | Integration of signals during chemotaxis | Many-to-many (signal integration) | Two HKs: CheA and CheW can phosphorylate two RRs: CheY and CheB | [153] |
| NarQ, NarX/NarP, NarL | E. coli | Coordinated regulation of nitrate/nitrite respiration | Many-to-many (signal integration) | NarQ and NarX can phosphorylate NarP and NarL in a nitrate and nitrite concentration-dependent manner | [154] |
11. Hierarchical Actions and Cooperation of TCSs
| Primary TCS/Secondary TCS/Other Targets | Organism | Biological Functions | Mechanism | References |
|---|---|---|---|---|
| EnvZ-OmpR/SsrAB | S. typhimurium | Virulence, curli fimbria synthesis | Transcriptional activation: OmpR activates ssrA and ssrB independently | [56,155] |
| SatKR/SitKR | S. coelicolor | Growth rate regulation | Transcriptional activation: SatKR activates sitKR genes | [54] |
| NarLX/DcuSR | E. coli | Coordination of fumarate and nitrate respiration | Transcriptional repression: NarL~P blocks the transcription of dcuSR | [14] |
| PhoPQ/PmrAB | Salmonella | Resistance to antimicrobial peptides | PhoP activates PmrD, which protects PmrA~P from dephosphorylation | [157] |
| PhoPR/ResDE | B. subtilis | Integrates phosphate signals into ATP production | Transcriptional activation: PhoPR activates resDE | [156] |
| PhoPR, ResDE, Spo0AB/Pho regulon | B. subtilis | The response to phosphate deficiency is correlated with the cell cycle | Coregulation of the same genes | [158] |
| CusRS, CopRS, CzcRS/copper resistance genes | P. aeruginosa | Zinc and copper homeostasis | Coregulation of the same genes | [159] |
| PhcA, PhcB, XpsR, VsrA-VsrD, and VsrB-VsrC/Eps (viral factor) | R. solanacearum | Virulence regulation by multiple signals | Coregulation of the same genes | [160] |
| PmrAB, PhoPQ, RcsCB/Ugd (lipopolysaccharides modifying enzyme) | Salmonella | Regulation of antibiotic resistance by multiple environmental signals | Coregulation of the same genes | [161] |
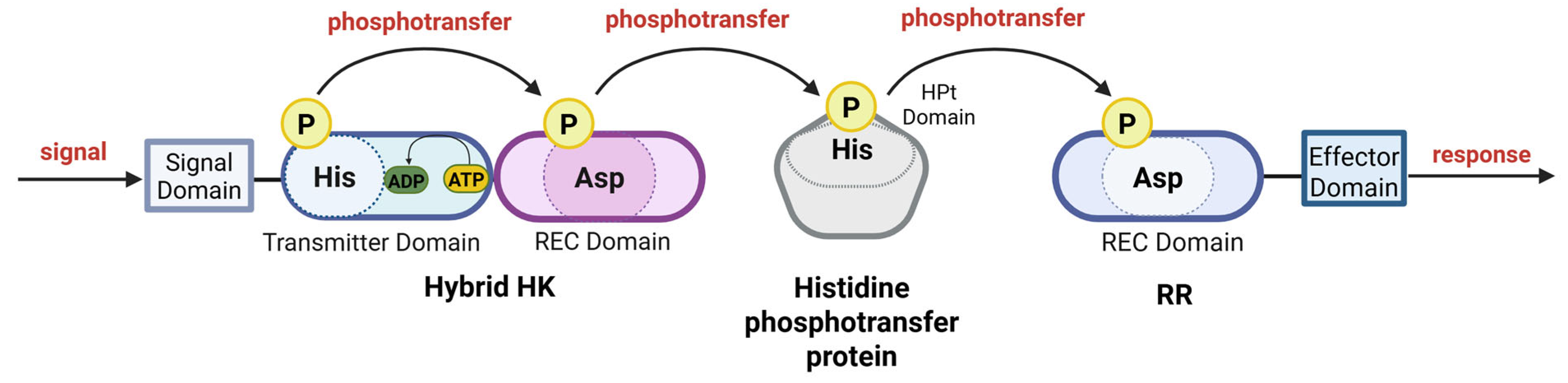
12. Phosphorelay
| HK/RR | Organism | Biological Functions | Type of Regulation | Mechanism | References |
|---|---|---|---|---|---|
| KinA-KinE/Spo0A, Spo0F | B. subtilis | Sporulation initiation | Multicomponent phosphorelay | Involves multiple regulators and phosphatases for signal integration and control | [131,169,170] |
| RcsC, RcsD (HPt)/RcsB | E. coli | Biofilm formation, motility control | Three-component phosphorelay | Involves accessory proteins like RcsF and IgaA | [171] |
| ArcB (hybrid HK)/ArcA | E. coli | Anaerobic respiration regulation | Hybrid HK phosphorelay | Involves cytoplasmic HK, responsive to the redox state | [172,173] |
| CckA (hybrid HK), ChpT (HPt)/CtrA | C. crescentus | Cell cycle regulation, DNA replication block | Hybrid HK phosphorelay | Phosphotransfer from CckA to ChpT; controls the master regulator CtrA | [174] |
| VirA (hybrid HK)/VirG | Agrobacterium tumefaciens | Plant infection (virulence gene expression) | Hybrid HK phosphorelay | Phosphorelay via an internal receiver and HPt domains in VirA | [175] |
13. Hybrid Sensor Kinases in Phosphorelays
14. Negative Control of Phosphorelays
15. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martínez-Antonio, A.; Janga, S.C.; Salgado, H.; Collado-Vides, J. Internal-sensing machinery directs the activity of the regulatory network in Escherichia coli. Trends Microbiol. 2006, 14, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.P.; Ulijasz, A.T. Regulation of transcription by eukaryotic-like serine-threonine kinases and phosphatases in Gram-positive bacterial pathogens. Virulence 2014, 5, 863–885. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Le Minh, P.; de Cima, S.; Bervoets, I.; Maes, D.; Rubio, V.; Charlier, D. Ligand binding specificity of RutR, a member of the TetR family of transcription regulators in Escherichia coli. FEBS Open Bio. 2015, 5, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Le Minh, P.; Velázquez Ruiz, C.; Vandermeeren, S.; Abwoyo, P.; Bervoets, I.; Charlier, D. Differential protein-DNA contacts for activation and repression by ArgP, a LysR-type (LTTR) transcriptional regulator in Escherichia coli. Microbiol. Res. 2018, 206, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Fiscus, V.; Meng, W.; Zheng, Z.; Zhang, L.-H.; Fuqua, C.; Chen, L. The Agrobacterium tumefaciens transcription factor BlcR is regulated via oligomerization. J. Biol. Chem. 2011, 286, 20431–20440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuber, P. Requirement of the zinc-binding domain of ClpX for Spx proteolysis in Bacillus subtilis and effects of disulfide stress on ClpXP activity. J. Bacteriol. 2007, 189, 7669–7680. [Google Scholar] [CrossRef]
- Seshasayee, A.S.N. Gene expression homeostasis and chromosome architecture. Bioarchitecture 2014, 4, 221–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dorman, C.J. Co-operative roles for DNA supercoiling and nucleoid-associated proteins in the regulation of bacterial transcription. Biochem. Soc. Trans. 2013, 41, 542–547. [Google Scholar] [CrossRef]
- Santos-Beneit, F.; Rodríguez-García, A.; Sola-Landa, A.; Martín, J.F. Cross-talk between two global regulators in Streptomyces: PhoP and AfsR interact in the control of afsS, pstS and phoRP transcription. Mol. Microbiol. 2009, 72, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.M.; Carroll, P.A.; Rahme, L.G.; Ausubel, F.M.; Calderwood, S.B. Modulation of Expression of the ToxR Regulon in Vibrio cholerae by a Member of the Two-Component Family of Response Regulators. Infect. Immun. 1998, 66, 5854–5861. [Google Scholar] [CrossRef]
- Hirakawa, H.; Kurushima, J.; Hashimoto, Y.; Tomita, H. Progress Overview of Bacterial Two-Component Regulatory Systems as Potential Targets for Antimicrobial Chemotherapy. Antibiotics 2020, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Kenney, L.J.; Anand, G.S. EnvZ/OmpR Two-Component Signaling: An Archetype System That Can Function Noncanonically. EcoSal Plus 2020, 9, 10–1128. [Google Scholar] [CrossRef]
- North, H.; McLaughlin, M.; Fiebig, A.; Crosson, S. The Caulobacter NtrB-NtrC two-component system bridges nitrogen assimilation and cell development. J. Bacteriol. 2023, 205, e00181-23. [Google Scholar] [CrossRef]
- Goh, E.-B.; Bledsoe, P.J.; Chen, L.-L.; Gyaneshwar, P.; Stewart, V.; Igo, M.M. Hierarchical Control of Anaerobic Gene Expression in Escherichia coli K-12: The Nitrate-Responsive NarX-NarL Regulatory System Represses Synthesis of the Fumarate-Responsive DcuS-DcuR Regulatory System. J. Bacteriol. 2005, 187, 4890–4899. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, K.; Fan, Y.; Fang, H.; Nie, Y.; Wu, X.-L. The Bacterial MtrAB Two-Component System Regulates the Cell Wall Homeostasis Responding to Environmental Alkaline Stress. Microbiol. Spectr. 2022, 10, e02311-22. [Google Scholar] [CrossRef]
- Jin, S.; Hui, M.; Lu, Y.; Zhao, Y. An overview on the two-component systems of Streptomyces coelicolor. World J. Microbiol. Biotechnol. 2023, 39, 78. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de la Nieta, R.; Santamaría, R.I.; Díaz, M. Two-Component Systems of Streptomyces coelicolor: An Intricate Network to Be Unraveled. Int. J. Mol. Sci. 2022, 23, 15085. [Google Scholar] [CrossRef]
- Bleul, L.; Francois, P.; Wolz, C. Two-Component Systems of S. aureus: Signaling and Sensing Mechanisms. Genes 2021, 13, 34. [Google Scholar] [CrossRef]
- Sultan, M.; Arya, R.; Kim, K.K. Roles of Two-Component Systems in Pseudomonas aeruginosa Virulence. Int. J. Mol. Sci. 2021, 22, 12152. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Abdel Aziz, M.H. Crosstalk involving two-component systems in Staphylococcus aureus signaling networks. J. Bacteriol. 2024, 206, e00418-23. [Google Scholar] [CrossRef] [PubMed]
- McLean, T.C.; Lo, R.; Tschowri, N.; Hoskisson, P.A.; Al Bassam, M.M.; Hutchings, M.I.; Som, N.F. Sensing and responding to diverse extracellular signals: An updated analysis of the sensor kinases and response regulators of Streptomyces species. Microbiology 2019, 165, 929–952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y. EvgS/EvgA, the unorthodox two-component system regulating bacterial multiple resistance. Appl. Environ. Microbiol. 2023, 89, e01577-23. [Google Scholar] [CrossRef]
- Mao, M.; He, L.; Yan, Q. An updated overview on the bacterial PhoP/PhoQ two-component signal transduction system. Front. Cell. Infect. Microbiol. 2025, 15, 1509037. [Google Scholar] [CrossRef]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-Component Signal Transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Stock, A.M. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 2009, 63, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Mitrophanov, A.Y.; Groisman, E.A. Signal integration in bacterial two-component regulatory systems. Genes Dev. 2008, 22, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Groisman, E.A. Feedback Control of Two-Component Regulatory Systems. Annu. Rev. Microbiol. 2016, 70, 103–124. [Google Scholar] [CrossRef]
- Gao, R.; Mack, T.R.; Stock, A.M. Bacterial Response Regulators: Versatile Regulatory Strategies from Common Domains. Trends Biochem. Sci. 2007, 32, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, C.; Liu, M.; Liu, Y.; Jiang, L. Targeting New Functions and Applications of Bacterial Two-Component Systems. Chembiochem 2024, 25, e202400392. [Google Scholar] [CrossRef]
- Cruz-Bautista, R.; Ruíz-Villafán, B.; Romero-Rodríguez, A.; Rodríguez-Sanoja, R.; Sánchez, S. Trends in the two-component system’s role in the synthesis of antibiotics by Streptomyces. Appl. Microbiol. Biotechnol. 2023, 107, 4727–4743. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Huang, C.; Zhou, X.; Zhou, S.; Deng, Y. Engineering two-component systems for advanced biosensing: From architecture to applications in biotechnology. Biotechnol. Adv. 2024, 75, 108404. [Google Scholar] [CrossRef]
- Alvarez, A.F.; Georgellis, D. Environmental adaptation and diversification of bacterial two-component systems. Curr. Opin. Microbiol. 2023, 76, 102399. [Google Scholar] [CrossRef]
- Agrawal, R.; Sahoo, B.K.; Saini, D.K. Cross-talk and specificity in two-component signal transduction pathways. Futur. Microbiol. 2016, 11, 685–697. [Google Scholar] [CrossRef]
- Stupar, M.; Furness, J.; De Voss, C.J.; Tan, L.; West, N.P. Two-component sensor histidine kinases of Mycobacterium tuberculosis: Beacons for niche navigation. Mol. Microbiol. 2022, 117, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Inouye, M. The HAMP Linker in Histidine Kinase Dimeric Receptors Is Critical for Symmetric Transmembrane Signal Transduction. J. Biol. Chem. 2004, 279, 48152–48158. [Google Scholar] [CrossRef]
- Singh, M.; Berger, B.; Kim, P.S.; Berger, J.M.; Cochran, A.G. Computational learning reveals coiled coil-like motifs in histidine kinase linker domains. Proc. Natl. Acad. Sci. USA 1998, 95, 2738–2743. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Vallurupalli, P.; Vu, A.; Lee, K.; Sun, S.; Bai, W.-J.; Wu, C.; Zhou, H.; Shea, J.-E.; Kay, L.E.; et al. The Linker between the Dimerization and Catalytic Domains of the CheA Histidine Kinase Propagates Changes in Structure and Dynamics That Are Important for Enzymatic Activity. Biochemistry 2014, 53, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Ponting, C.P. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol. Lett. 1999, 176, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.L.; Zhulin, I.B. PAS domains: Internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 1999, 63, 479–506. [Google Scholar] [CrossRef]
- Ishii, E.; Eguchi, Y. Diversity in Sensing and Signaling of Bacterial Sensor Histidine Kinases. Biomolecules 2021, 11, 1524. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.A.; Andrianova, E.P.; Zhulin, I.B. Diverse domain architectures of CheA histidine kinase, a central component of bacterial and archaeal chemosensory systems. Microbiol. Spectr. 2023, 12, e03464-23. [Google Scholar] [CrossRef]
- Weiss, V.; Kramer, G.; Dünnebier, T.; Flotho, A. Mechanism of regulation of the bifunctional histidine kinase NtrB in Escherichia coli. J. Mol. Microbiol. Biotechnol. 2002, 4, 229–233. [Google Scholar]
- Wright, G.S.A.; Saeki, A.; Hikima, T.; Nishizono, Y.; Hisano, T.; Kamaya, M.; Nukina, K.; Nishitani, H.; Nakamura, H.; Yamamoto, M.; et al. Architecture of the complete oxygen-sensing FixL-FixJ two-component signal transduction system. Sci. Signal. 2018, 11, eaaq0825. [Google Scholar] [CrossRef] [PubMed]
- Dubey, B.N.; Agustoni, E.; Böhm, R.; Kaczmarczyk, A.; Mangia, F.; von Arx, C.; Jenal, U.; Hiller, S.; Plaza-Menacho, I.; Schirmer, T. Hybrid histidine kinase activation by cyclic di-GMP-mediated domain liberation. Proc. Natl. Acad. Sci. USA 2020, 117, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Abriata, L.A.; Albanesi, D.; Dal Peraro, M.; de Mendoza, D. Signal Sensing and Transduction by Histidine Kinases as Unveiled through Studies on a Temperature Sensor. Acc. Chem. Res. 2017, 50, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, A.; Vazquez, D.B.; Almada, J.C.; Inda, M.E.; Drusin, S.I.; Villalba, J.M.; Moreno, D.M.; Ruysschaert, J.M.; Cybulski, L.E. A Transmembrane Histidine Kinase Functions as a pH Sensor. Biomolecules 2020, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Bhate, M.P.; Molnar, K.S.; Goulian, M.; DeGrado, W.F. Signal Transduction in Histidine Kinases: Insights from New Structures. Structure 2015, 23, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Kansari, M.; Idiris, F.; Szurmant, H.; Kubař, T.; Schug, A. Mechanism of activation and autophosphorylation of a histidine kinase. Commun. Chem. 2024, 7, 196. [Google Scholar] [CrossRef]
- Sankhe, G.D.; Raja, R.; Singh, D.P.; Bheemireddy, S.; Rana, S.; Athira, P.J.; Dixit, N.M.; Saini, D.K. Sequestration of histidine kinases by non-cognate response regulators establishes a threshold level of stimulation for bacterial two-component signaling. Nat. Commun. 2023, 14, 4483. [Google Scholar] [CrossRef]
- Gao, R.; Stock, A.M. Temporal hierarchy of gene expression mediated by transcription factor binding affinity and activation dynamics. mBio 2015, 6, e00686-15. [Google Scholar] [CrossRef]
- Hoffer, S.M.; Westerhoff, H.V.; Hellingwerf, K.J.; Postma, P.W.; Tommassen, J. Autoamplification of a two-component regulatory system results in “learning” behavior. J. Bacteriol. 2001, 183, 4914–4917. [Google Scholar] [CrossRef] [PubMed]
- Deora, R.; Bootsma, H.J.; Miller, J.F.; Cotter, P.A. Diversity in the Bordetella virulence regulon: Transcriptional control of a Bvg-intermediate phase gene. Mol. Microbiol. 2001, 40, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.R.; Miller, J.F.; Falkow, S. Autogenous regulation of the Bordetella pertussis bvgABC operon. Proc. Natl. Acad. Sci. USA 1990, 87, 3763–3767. [Google Scholar] [CrossRef] [PubMed]
- Gongerowska-Jac, M.; Szafran, M.J.; Mikołajczyk, J.; Szymczak, J.; Bartyńska, M.; Gierlikowska, A.; Biały, S.; Elliot, M.A.; Jakimowicz, D.; van Wezel, G.P. Global Chromosome Topology and the Two-Component Systems in Concerted Manner Regulate Transcription in Streptomyces. mSystems 2021, 6, e0114221. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Granadel, C.; Campo, N.; Hénard, V.; Prudhomme, M.; Claverys, J.-P. Expression and maintenance of ComD–ComE, the two-component signal-transduction system that controls competence of Streptococcus pneumoniae. Mol. Microbiol. 2010, 75, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Walthers, D.; Oropeza, R.; Kenney, L.J. The response regulator SsrB activates transcription and binds to a region overlapping OmpR binding sites at Salmonella pathogenicity island 2. Mol. Microbiol. 2004, 54, 823–835. [Google Scholar] [CrossRef]
- Choudhary, K.S.; Kleinmanns, J.A.; Decker, K.; Sastry, A.V.; Gao, Y.; Szubin, R.; Seif, Y.; Palsson, B.O.; Silva-Rocha, R. Elucidation of Regulatory Modes for Five Two-Component Systems in Escherichia coli Reveals Novel Relationships. mSystems 2020, 5, e00980-20. [Google Scholar] [CrossRef] [PubMed]
- De Wulf, P.; McGuire, A.M.; Liu, X.; Lin, E.C.C. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 2002, 277, 26652–26661. [Google Scholar] [CrossRef]
- Roy, C.R.; Falkow, S. Identification of Bordetella pertussis regulatory sequences required for transcriptional activation of the fhaB gene and autoregulation of the bvgAS operon. J. Bacteriol. 1991, 173, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Walthers, D.; Tran, V.K.; Kenney, L.J. Interdomain Linkers of Homologous Response Regulators Determine Their Mechanism of Action. J. Bacteriol. 2003, 185, 317–324. [Google Scholar] [CrossRef]
- Weiss, D.S.; Batut, J.; Klose, K.E.; Keener, J.; Kustu, S. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell 1991, 67, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Vanatta, D.K.; Shukla, D.; Lawrenz, M.; Pande, V.S. A network of molecular switches controls the activation of the two-component response regulator NtrC. Nat. Commun. 2015, 6, 7283. [Google Scholar] [CrossRef] [PubMed]
- Brahmachary, P.; Dashti, M.G.; Olson, J.W.; Hoover, T.R. Helicobacter pylori FlgR is an enhancer-independent activator of sigma54-RNA polymerase holoenzyme. J. Bacteriol. 2004, 186, 4535–4542. [Google Scholar] [CrossRef]
- Boll, J.M.; Hendrixson, D.R. A specificity determinant for phosphorylation in a response regulator prevents in vivo cross-talk and modification by acetyl phosphate. Proc. Natl. Acad. Sci. USA 2011, 108, 20160–20165. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.A.; Perego, M. Transcription Antitermination by a Phosphorylated Response Regulator and Cobalamin-Dependent Termination at a B12 Riboswitch Contribute to Ethanolamine Utilization in Enterococcus faecalis. J. Bacteriol. 2011, 193, 2575–2586. [Google Scholar] [CrossRef]
- Wilson, S.A.; Wachira, S.J.; Norman, R.A.; Pearl, L.H.; Drew, R.E. Transcription antitermination regulation of the Pseudomonas aeruginosa amidase operon. EMBO J. 1996, 15, 5907–5916. [Google Scholar] [CrossRef]
- Boudes, M.; Lazar, N.; Graille, M.; Durand, D.; Gaidenko, T.A.; Stewart, V.; van Tilbeurgh, H. The structure of the NasR transcription antiterminator reveals a one-component system with a NIT nitrate receptor coupled to an ANTAR RNA-binding effector. Mol. Microbiol. 2012, 85, 431–444. [Google Scholar] [CrossRef]
- Gutiérrez, P.; Li, Y.; Osborne, M.J.; Pomerantseva, E.; Liu, Q.; Gehring, K. Solution Structure of the Carbon Storage Regulator Protein CsrA from Escherichia coli. J. Bacteriol. 2005, 187, 3496–3501. [Google Scholar] [CrossRef]
- Davenport, H.; Rohde, K. Elucidating the Role of an Atypical Anti-Termination Two-Component System in Mycobacterial Gene Regulation and Pathogenesis. FASEB J. 2019, 33, lb357–lb357. [Google Scholar] [CrossRef]
- Djordjevic, S.; Goudreau, P.N.; Xu, Q.; Stock, A.M.; West, A.H. Structural basis for methylesterase CheB regulation by a phosphorylation-activated domain. Proc. Natl. Acad. Sci. USA 1998, 95, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, S.; Liu, P.; Cheng, S.-T.; Qian, G.; Wang, S.; Fu, Y.; Qian, W.; Sun, W. The PdeK-PdeR two-component system promotes unipolar localization of FimX and pilus extension in Xanthomonas oryzae pv. oryzicola. Sci. Signal. 2021, 14, eabi9589. [Google Scholar] [CrossRef]
- Sarkar, M.K.; Paul, K.; Blair, D. Chemotaxis signaling protein CheY binds to the rotor protein FliN to control the direction of flagellar rotation in Escherichia coli. Proc. Natl. Acad. Sci. USA 2010, 107, 9370–9375. [Google Scholar] [CrossRef]
- Sourjik, V.; Berg, H.C. Binding of the Escherichia coli response regulator CheY to its target measured in vivo by fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. USA 2002, 99, 12669–12674. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, P.; Swapna, G.; Stock, A.M. Mechanism of Activation for Transcription Factor PhoB Suggested by Different Modes of Dimerization in the Inactive and Active States. Structure 2005, 13, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, S.J.; Huntwork, S.; Viollier, P.H.; Ryan, K.R. DivL Performs Critical Cell Cycle Functions in Caulobacter crescentus Independent of Kinase Activity. J. Bacteriol. 2007, 189, 8308–8320. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, B.P.; Norman, R.A.; Wan, P.T.C.; Roe, S.M.; Barrett, T.E.; Drew, R.E.; Pearl, L.H. Crystal structure and induction mechanism of AmiC–AmiR: A ligand-regulated transcription antitermination complex. EMBO J. 1999, 18, 5175–5186. [Google Scholar] [CrossRef] [PubMed]
- Mattison, K.; Kenney, L.J. Phosphorylation Alters the Interaction of the Response Regulator OmpR with Its Sensor Kinase EnvZ. J. Biol. Chem. 2002, 277, 11143–11148. [Google Scholar] [CrossRef] [PubMed]
- Ames, S.K.; Frankema, N.; Kenney, L.J. C-terminal DNA binding stimulates N-terminal phosphorylation of the outer membrane protein regulator OmpR from Escherichia coli. Proc. Natl. Acad. Sci USA 1999, 96, 11792–11797. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, X.; Wang, X.; Wang, L.; Zhang, J.; Yin, Y. ComE, an Essential Response Regulator, Negatively Regulates the Expression of the Capsular Polysaccharide Locus and Attenuates the Bacterial Virulence in Streptococcus pneumoniae. Front. Microbiol. 2017, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Kenney, L.J. Structure/function relationships in OmpR and other winged-helix transcription factors. Curr. Opin. Microbiol. 2002, 5, 135–141. [Google Scholar] [CrossRef]
- Desai, S.K.; Kenney, L.J. To ∼ P or Not to ∼ P? Non-canonical activation by two-component response regulators. Mol. Microbiol. 2017, 103, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Head, C.G.; Tardy, A.; Kenney, L.J. Relative binding affinities of OmpR and OmpR-phosphate at the ompF and ompC regulatory sites. J. Mol. Biol. 1998, 281, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hulett, F.M. Bacillus subtilis PhoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. J. Bacteriol. 1997, 179, 6302–6310. [Google Scholar] [CrossRef]
- Boucher, P.E.; Murakami, K.; Ishihama, A.; Stibitz, S. Nature of DNA binding and RNA polymerase interaction of the Bordetella pertussis BvgA transcriptional activator at the fha promoter. J. Bacteriol. 1997, 179, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Zu, T.; Manetti, R.; Rappuoli, R.; Scarlato, V. Differential binding of BvgA to two classes of virulence genes of Bordetella pertussis directs promoter selectivity by RNA polymerase. Mol. Microbiol. 1996, 21, 557–565. [Google Scholar] [CrossRef]
- Desai, S.K.; Winardhi, R.S.; Periasamy, S.; Dykas, M.M.; Jie, Y.; Kenney, L.J. The horizontally-acquired response regulator SsrB drives a Salmonella lifestyle switch by relieving biofilm silencing. eLife 2016, 5, e10747. [Google Scholar] [CrossRef] [PubMed]
- Huesa, J.; Giner-Lamia, J.; Pucciarelli, M.G.; Paredes-Martínez, F.; García-del Portillo, F.; Marina, A.; Casino, P. Structure-based analyses of Salmonella RcsB variants unravel new features of the Rcs regulon. Nucleic Acids Res. 2021, 49, 2357–2374. [Google Scholar] [CrossRef]
- Rubio-Canalejas, A.; Admella, J.; Pedraz, L.; Torrents, E. Pseudomonas aeruginosa Nonphosphorylated AlgR Induces Ribonucleotide Reductase Expression under Oxidative Stress Infectious Conditions. mSystems 2023, 8, e01005-22. [Google Scholar] [CrossRef] [PubMed]
- Walthers, D.; Carroll, R.K.; Navarre, W.W.; Libby, S.J.; Fang, F.C.; Kenney, L.J. The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol. Microbiol. 2007, 65, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Amati, G.; Bisicchia, P.; Galizzi, A. DegU-P represses expression of the motility fla-che operon in Bacillus subtilis. J. Bacteriol. 2004, 186, 6003–6014. [Google Scholar] [CrossRef]
- Hsueh, Y.-H.; Cozy, L.M.; Sham, L.-T.; Calvo, R.A.; Gutu, A.D.; Winkler, M.E.; Kearns, D.B. DegU-phosphate activates expression of the anti-sigma factor FlgM in Bacillus subtilis. Mol. Microbiol. 2011, 81, 1092–1108. [Google Scholar] [CrossRef]
- Mishra, A.; Hughes, A.C.; Amon, J.D.; Rudner, D.Z.; Wang, X.; Kearns, D.B. SwrA-mediated multimerization of DegU and an upstream activation sequence enhance flagellar gene expression in Bacillus subtilis. J. Mol. Biol. 2023, 436, 168419. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, T.; Schiklang, T.; Dragoš, A.; Dietel, A.-K.; Kost, C.; Kovács, Á.T. Impaired competence in flagellar mutants of Bacillus subtilis is connected to the regulatory network governed by DegU. Environ. Microbiol. Rep. 2018, 10, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Kojima, F.; Miyake, Y.; Yoshimura, M.; Ishijima, N.; Iyoda, S.; Sekine, Y.; Yamanaka, Y.; Yamamoto, K. Regulation of constant cell elongation and Sfm pili synthesis in Escherichia coli via two active forms of FimZ orphan response regulator. Genes Cells 2022, 27, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.C.I.; Downey, J.S.; Kreth, J.; Qi, F.; Shi, W.; Cvitkovitch, D.G.; Goodman, S.D. Oligomerization of the Response Regulator ComE from Streptococcus mutans Is Affected by Phosphorylation. J. Bacteriol. 2012, 194, 1127–1135. [Google Scholar] [CrossRef]
- Plate, L.; Marletta, M.A. Phosphorylation-dependent derepression by the response regulator HnoC in the Shewanella oneidensis nitric oxide signaling network. Proc. Natl. Acad. Sci. USA 2013, 110, E4648–E4657. [Google Scholar] [CrossRef]
- Bang, I.S.; Audia, J.P.; Park, Y.K.; Foster, J.W. Autoinduction of the ompR response regulator by acid shock and control of the Salmonella enterica acid tolerance response. Mol. Microbiol. 2002, 44, 1235–1250. [Google Scholar] [CrossRef]
- Almada, J.C.; Bortolotti, A.; Porrini, L.; Albanesi, D.; Miguel, V.; Cybulski, L. Allosteric coupling activation mechanism in histidine kinases. Sci. Rep. 2025, 15, 14682. [Google Scholar] [CrossRef]
- McCleary, W.R.; Stock, J.B.; Ninfa, A.J. Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 1993, 175, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol. Microbiol. 2007, 66, 395–409. [Google Scholar] [CrossRef]
- Cairns, L.S.; Martyn, J.E.; Bromley, K.; Stanley-Wall, N.R. An alternate route to phosphorylating DegU of Bacillus subtilis using acetyl phosphate. BMC Microbiol. 2015, 15, 78. [Google Scholar] [CrossRef]
- Novoa-Aponte, L.; Xu, C.; Soncini, F.C.; Argüello, J.M. The Two-Component System CopRS Maintains Subfemtomolar Levels of Free Copper in the Periplasm of Pseudomonas aeruginosa Using a Phosphatase-Based Mechanism. mSphere 2020, 5, e01193-20. [Google Scholar] [CrossRef]
- Hu, L.I.; Chi, B.K.; Kuhn, M.L.; Filippova, E.V.; Walker-Peddakotla, A.J.; Bäsell, K.; Becher, D.; Anderson, W.F.; Antelmann, H.; Wolfe, A.J. Acetylation of the Response Regulator RcsB Controls Transcription from a Small RNA Promoter. J. Bacteriol. 2013, 195, 4174–4186. [Google Scholar] [CrossRef]
- Silversmith, R.E.; Bourret, R.B. Throwing the switch in bacterial chemotaxis. Trends Microbiol. 1999, 7, 16–22. [Google Scholar] [CrossRef]
- Singh, K.K.; Bhardwaj, N.; Sankhe, G.D.; Udaykumar, N.; Singh, R.; Malhotra, V.; Saini, D.K. Acetylation of Response Regulator Proteins, TcrX and MtrA in M. tuberculosis Tunes their Phosphotransfer Ability and Modulates Two-Component Signaling Crosstalk. J. Mol. Biol. 2019, 431, 777–793. [Google Scholar] [CrossRef]
- AbouElfetouh, A.; Kuhn, M.L.; Hu, L.I.; Scholle, M.D.; Sorensen, D.J.; Sahu, A.K.; Becher, D.; Antelmann, H.; Mrksich, M.; Anderson, W.F.; et al. The E. coli sirtuin CobB shows no preference for enzymatic and nonenzymatic lysine acetylation substrate sites. Microbiologyopen 2015, 4, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M.L.; Zemaitaitis, B.; Hu, L.I.; Sahu, A.; Sorensen, D.; Minasov, G.; Lima, B.P.; Scholle, M.; Mrksich, M.; Anderson, W.F.; et al. Structural, Kinetic and Proteomic Characterization of Acetyl Phosphate-Dependent Bacterial Protein Acetylation. PLoS ONE 2014, 9, e94816. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Schuster, M.; Bourret, R.B. Acetylation at Lys-92 enhances signaling by the chemotaxis response regulator protein CheY. Proc. Natl. Acad. Sci. USA 1998, 95, 4918–4923. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.D.; Papavinasasundaram, K.G.; Zheng, X.; Chávez-Steenbock, A.; Wang, X.; Lee, G.Q.; Av-Gay, Y. Convergence of Ser/Thr and Two-component Signaling to Coordinate Expression of the Dormancy Regulon in Mycobacterium tuberculosis. J. Biol. Chem. 2010, 285, 29239–29246. [Google Scholar] [CrossRef] [PubMed]
- Libby, E.A.; Goss, L.A.; Dworkin, J. The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in Bacillus subtilis. PLoS Genet. 2015, 11, e1005275. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-J.; Walthers, D.; Connelly, J.E.; Burnside, K.; Jewell, K.A.; Kenney, L.J.; Rajagopal, L. Threonine phosphorylation prevents promoter DNA binding of the Group B Streptococcus response regulator CovR. Mol. Microbiol. 2009, 71, 1477–1495. [Google Scholar] [CrossRef] [PubMed]
- Canova, M.J.; Baronian, G.; Brelle, S.; Cohen-Gonsaud, M.; Bischoff, M.; Molle, V. A novel mode of regulation of the Staphylococcus aureus Vancomycin-resistance-associated response regulator VraR mediated by Stk1 protein phosphorylation. Biochem. Biophys. Res. Commun. 2014, 447, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J. Ser/Thr phosphorylation as a regulatory mechanism in bacteria. Curr. Opin. Microbiol. 2015, 24, 47–52. [Google Scholar] [CrossRef]
- Silberberg, J.M.; Ketter, S.; Böhm, P.J.N.; Jordan, K.; Wittenberg, M.; Grass, J.; Hänelt, I. KdpD is a tandem serine histidine kinase that controls K+ pump KdpFABC transcriptionally and post-translationally. Nat. Commun. 2024, 15, 3223. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.R.; Saif Zaman, M.; Dubey, G.P.; Arora, A.; Narayan, A.; Jailkhani, N.; Rathore, K.; Maiti, S.; Singh, Y. Spo0B of Bacillus anthracis-a protein with pleiotropic functions. FEBS J. 2008, 275, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Charles, T.C.; Nester, E.W. A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 1993, 175, 6614–6625. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, T.; Hu, Q.; Yao, Z.; Li, L.; Huang, Q.; Zhou, R. Inhibitors targeting the autophosphorylation of serine/threonine kinase of Streptococcus suis show potent antimicrobial activity. Front. Microbiol. 2022, 13, 990091. [Google Scholar] [CrossRef]
- Vieira, T.F.; Martins, F.G.; Moreira, J.P.; Barbosa, T.; Sousa, S.F. In Silico Identification of Possible Inhibitors for Protein Kinase B (PknB) of Mycobacterium tuberculosis. Molecules 2021, 26, 6162. [Google Scholar] [CrossRef]
- Lim, W.A. Designing customized cell signalling circuits. Nat. Rev. Mol. Cell Biol. 2010, 11, 393–403. [Google Scholar] [CrossRef]
- Cheng, A.A.; Lu, T.K. Synthetic Biology: An Emerging Engineering Discipline. Annu. Rev. Biomed. Eng. 2012, 14, 155–178. [Google Scholar] [CrossRef] [PubMed]
- Fridman, M.; Williams, G.D.; Muzamal, U.; Hunter, H.; Siu, K.W.M.; Golemi-Kotra, D. Two unique phosphorylation-driven signaling pathways crosstalk in Staphylococcus aureus to modulate the cell-wall charge: Stk1/Stp1 meets GraSR. Biochemistry 2013, 52, 7975–7986. [Google Scholar] [CrossRef] [PubMed]
- Ulijasz, A.T.; Falk, S.P.; Weisblum, B. Phosphorylation of the RitR DNA-binding domain by a Ser–Thr phosphokinase: Implications for global gene regulation in the streptococci. Mol. Microbiol. 2009, 71, 382–390. [Google Scholar] [CrossRef]
- Meier, S.S.M.; Multamäki, E.; Ranzani, A.T.; Takala, H.; Möglich, A. Leveraging the histidine kinase-phosphatase duality to sculpt two-component signaling. Nat. Commun. 2024, 15, 4876. [Google Scholar] [CrossRef] [PubMed]
- Multamäki, E.; Nanekar, R.; Morozov, D.; Lievonen, T.; Golonka, D.; Wahlgren, W.Y.; Stucki-Buchli, B.; Rossi, J.; Hytönen, V.P.; Westenhoff, S.; et al. Comparative analysis of two paradigm bacteriophytochromes reveals opposite functionalities in two-component signaling. Nat. Commun. 2021, 12, 4394. [Google Scholar] [CrossRef]
- Jacques, D.A.; Langley, D.B.; Hynson, R.M.G.; Whitten, A.E.; Kwan, A.; Guss, J.M.; Trewhella, J. A Novel Structure of an Antikinase and its Inhibitor. J. Mol. Biol. 2011, 405, 214–226. [Google Scholar] [CrossRef]
- Rowland, S.L.; Burkholder, W.F.; Cunningham, K.A.; Maciejewski, M.W.; Grossman, A.D.; King, G.F. Structure and mechanism of action of Sda, an inhibitor of the histidine kinases that regulate initiation of sporulation in Bacillus subtilis. Mol. Cell 2004, 13, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Lippa, A.M.; Goulian, M. Feedback Inhibition in the PhoQ/PhoP Signaling System by a Membrane Peptide. PLoS Genet. 2009, 5, e1000788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Atkinson, M.R.; Srisawat, C.; Sun, Q.; Ninfa, A.J. Functional dissection of the dimerization and enzymatic activities of Escherichia coli nitrogen regulator II and their regulation by the PII protein. Biochemistry 2000, 39, 13433–13449. [Google Scholar] [CrossRef] [PubMed]
- Gerken, H.; Charlson, E.S.; Cicirelli, E.M.; Kenney, L.J.; Misra, R. MzrA: A novel modulator of the EnvZ/OmpR two-component regulon. Mol. Microbiol. 2009, 72, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Vega-Baray, B.; Domenzain, C.; Poggio, S.; Dreyfus, G.; Camarena, L. The Histidine Kinase CckA Is Directly Inhibited by a Response Regulator-like Protein in a Negative Feedback Loop. mBio 2022, 13, e01481-22. [Google Scholar] [CrossRef] [PubMed]
- Perego, M. Kinase-phosphatase competition regulates Bacillus subtilis development. Trends Microbiol. 1998, 6, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Foussard, M.; Garnerone, A.M.; Ni, F.; Soupène, E.; Boistard, P.; Batut, J. Negative autoregulation of the Rhizobium meliloti fixK gene is indirect and requires a newly identified regulator, FixT. Mol. Microbiol. 1997, 25, 27–37. [Google Scholar] [CrossRef]
- Ogura, M.; Tsukahara, K. Autoregulation of the Bacillus subtilis response regulator gene degU is coupled with the proteolysis of DegU-P by ClpCP. Mol. Microbiol. 2010, 75, 1244–1259. [Google Scholar] [CrossRef]
- Song, H.; Choi, E.; Lee, E.-J. Membrane-Bound Protease FtsH Protects PhoP from the Proteolysis by Cytoplasmic ClpAP Protease in Salmonella Typhimurium. J. Microbiol. Biotechnol. 2023, 33, 1130–1140. [Google Scholar] [CrossRef]
- Yeom, J.; Wayne, K.J.; Groisman, E.A. Sequestration from Protease Adaptor Confers Differential Stability to Protease Substrate. Mol. Cell. 2017, 66, 234–246.e5. [Google Scholar] [CrossRef] [PubMed]
- Coornaert, A.; Lu, A.; Mandin, P.; Springer, M.; Gottesman, S.; Guillier, M. MicA sRNA links the PhoP regulon to cell envelope stress. Mol. Microbiol. 2010, 76, 467–479. [Google Scholar] [CrossRef]
- Coornaert, A.; Chiaruttini, C.; Springer, M.; Guillier, M. Post-transcriptional control of the Escherichia coli PhoQ-PhoP two-component system by multiple sRNAs involves a novel pairing region of GcvB. PLoS Genet. 2013, 9, e1003156. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.N.; Stewart, V. Negative control in two-component signal transduction by transmitter phosphatase activity. Mol. Microbiol. 2011, 82, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Yigit, K.; Chien, P. Proteolytic control of FixT by the Lon protease impacts FixLJ signaling in Caulobacter crescentus. J. Bacteriol. 2024, 206, e00237-24. [Google Scholar] [CrossRef]
- Rapun-Araiz, B.; Haag, A.F.; De Cesare, V.; Gil, C.; Dorado-Morales, P.; Penades, J.R.; Lasa, I.; Methe, B. Systematic Reconstruction of the Complete Two-Component Sensorial Network in Staphylococcus aureus. mSystems 2020, 5, e00511-20. [Google Scholar] [CrossRef]
- Vemparala, B.; Valiya Parambathu, A.; Saini, D.K.; Dixit, N.M. An Evolutionary Paradigm Favoring Cross Talk between Bacterial Two-Component Signaling Systems. mSystems 2022, 7, e00298-22. [Google Scholar] [CrossRef]
- Bijlsma, J.J.E.; Groisman, E.A. Making informed decisions: Regulatory interactions between two-component systems. Trends Microbiol. 2003, 11, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Skerker, J.M.; Prasol, M.S.; Perchuk, B.S.; Biondi, E.G.; Laub, M.T. Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: A system-level analysis. PLoS Biol. 2005, 3, e334. [Google Scholar] [CrossRef]
- Kenney, L.J. How Important Is the Phosphatase Activity of Sensor Kinases? Curr. Opin. Microbiol. 2010, 13, 168–176. [Google Scholar] [CrossRef]
- Wanner, B.L. Gene regulation by phosphate in enteric bacteria. J. Cell. Biochem. 1993, 51, 47–54. [Google Scholar] [CrossRef]
- Godoy, M.S.; Nikel, P.I.; Cabrera Gomez, J.G.; Pettinari, M.J. The CreC Regulator of Escherichia coli, a New Target for Metabolic Manipulations. Appl. Environ. Microbiol. 2015, 82, 244–254. [Google Scholar] [CrossRef]
- Matsubara, M.; Kitaoka, S.I.; Takeda, S.I.; Mizuno, T. Tuning of the porin expression under anaerobic growth conditions by his-to-Asp cross-phosphorelay through both the EnvZ-osmosensor and ArcB-anaerosensor in Escherichia coli. Genes Cells. 2000, 5, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Salvail, H.; Groisman, E.A. The phosphorelay BarA/SirA activates the non-cognate regulator RcsB in Salmonella enterica. PLoS Genet. 2020, 16, e1008722. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.L.; Jiang, W.; Wanner, B.L.; Walsh, C.T. Cross-talk between the histidine protein kinase VanS and the response regulator PhoB. Characterization and identification of a VanS domain that inhibits activation of PhoB. J. Biol. Chem. 1995, 270, 23143–23149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Shao, W.; Perego, M.; Hoch, J.A. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 2000, 38, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.L.; Kojetin, D.; Bassler, B.L.; Cavanagh, J.; Loria, J.P. Solution Structure and Dynamics of LuxU from Vibrio harveyi, a Phosphotransferase Protein Involved in Bacterial Quorum Sensing. J. Mol. Biol. 2005, 347, 297–307. [Google Scholar] [CrossRef]
- Freeman, J.A.; Bassler, B.L. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 1999, 31, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.; Studdert, C.A.; Reiser, R.H.; Parkinson, J.S. Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA 2002, 99, 7060–7065. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.I.; Delgado, A.; Gunsalus, R.P. Signal-dependent phosphorylation of the membrane-bound NarX two-component sensor-transmitter protein of Escherichia coli: Nitrate elicits a superior anion ligand response compared to nitrite. J. Bacteriol. 1999, 181, 5309–5316. [Google Scholar] [CrossRef]
- Feng, X.; Oropeza, R.; Kenney, L.J. Dual regulation by phospho-OmpR of ssrA/B gene expression in Salmonella pathogenicity island 2. Mol. Microbiol. 2003, 48, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Groisman, E.A. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 2004, 18, 2302–2313. [Google Scholar] [CrossRef] [PubMed]
- Birkey, S.M.; Liu, W.; Zhang, X.; Duggan, M.F.; Hulett, F.M. Pho signal transduction network reveals direct transcriptional regulation of one two-component system by another two-component regulator: Bacillus subtilis PhoP directly regulates production of ResD. Mol. Microbiol. 1998, 30, 943–953. [Google Scholar] [CrossRef]
- Hulett, F.M. The signal-transduction network for Pho regulation in Bacillus subtilis. Mol. Microbiol. 1996, 19, 933–939. [Google Scholar] [CrossRef]
- Elsen, S.; Simon, V.; Attrée, I. Cross-regulation and cross-talk of conserved and accessory two-component regulatory systems orchestrate Pseudomonas copper resistance. PLoS Genet. 2024, 20, e1011325. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.P.; Huang, J.; Yindeeyoungyeon, W.; Denny, T.P.; Schell, M.A. Multicomponent transcriptional regulation at the complex promoter of the exopolysaccharide I biosynthetic operon of Ralstonia solanacearum. J. Bacteriol. 2000, 182, 6659–6666. [Google Scholar] [CrossRef] [PubMed]
- Mouslim, C.; Groisman, E.A. Control of the Salmonella ugd gene by three two-component regulatory systems. Mol. Microbiol. 2003, 47, 335–344. [Google Scholar] [CrossRef]
- Oshima, T.; Aiba, H.; Masuda, Y.; Kanaya, S.; Sugiura, M.; Wanner, B.L.; Mori, H.; Mizuno, T. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol Microbiol. 2002, 46, 281–291. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ogura, M.; Yamaguchi, H.; Yoshida, K.; Ogasawara, N.; Tanaka, T.; Fujita, Y. Comprehensive DNA microarray analysis of Bacillus subtilis two-component regulatory systems. J. Bacteriol. 2001, 183, 7365–7370. [Google Scholar] [CrossRef] [PubMed]
- Wanner, B.L.; Chang, B.D. The phoBR operon in Escherichia coli K-12. J. Bacteriol. 1987, 169, 5569–5574. [Google Scholar] [CrossRef]
- Wuichet, K.; Cantwell, B.J.; Zhulin, I.B. Evolution and phyletic distribution of two-component signal transduction systems. Curr. Opin. Microbiol. 2010, 13, 219–225. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, P.; Singla-Pareek, S.L.; Siddique, K.H.M.; Pareek, A. The Journey from Two-Step to Multi-Step Phosphorelay Signaling Systems. Curr. Genomics. 2021, 22, 59–74. [Google Scholar] [CrossRef]
- Appleby, J.L.; Parkinson, J.S.; Bourret, R.B. Signal transduction via the multi-step phosphorelay: Not necessarily a road less traveled. Cell 1996, 86, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Hoch, J.A. Two-component and phosphorelay signal transduction. Curr. Opin. Microbiol. 2000, 3, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Burbulys, D.; Trach, K.A.; Hoch, J.A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 1991, 64, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Defeu Soufo, H.J. A Novel Cell Type Enables B. subtilis to Escape from Unsuccessful Sporulation in Minimal Medium. Front. Microbiol. 2016, 7, 1810. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J. The Rcs phosphorelay: More than just a two-component pathway. Futur. Microbiol. 2010, 5, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Mizuno, T.; Shimizu, T.; Hakoshima, T. Insights into Multistep Phosphorelay from the Crystal Structure of the C-Terminal HPt Domain of ArcB. Cell 1997, 88, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Teran-Melo, J.L.; Peña-Sandoval, G.R.; Silva-Jimenez, H.; Rodriguez, C.; Alvarez, A.F.; Georgellis, D. Routes of phosphoryl group transfer during signal transmission and signal decay in the dimeric sensor histidine kinase ArcB. J. Biol. Chem. 2018, 293, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Zan, J.; Heindl, J.E.; Liu, Y.; Fuqua, C.; Hill, R.T. The CckA-ChpT-CtrA phosphorelay system is regulated by quorum sensing and controls flagellar motility in the marine sponge symbiont Ruegeria sp. KLH11. PLoS ONE 2013, 8, e66346. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.A.; Binns, A.N. The Receiver of the Agrobacterium tumefaciens VirA Histidine Kinase Forms a Stable Interaction with VirG to Activate Virulence Gene Expression. Front. Microbiol. 2015, 6, 1546. [Google Scholar] [CrossRef]
- Ortet, P.; Fochesato, S.; Bitbol, A.-F.; Whitworth, D.E.; Lalaouna, D.; Santaella, C.; Heulin, T.; Achouak, W.; Barakat, M. Evolutionary history expands the range of signaling interactions in hybrid multikinase networks. Sci. Rep. 2021, 11, 11763. [Google Scholar] [CrossRef]
- Capra, E.J.; Laub, M.T. Evolution of two-component signal transduction systems. Annu. Rev. Microbiol. 2012, 66, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, L. Distribution and evolution of multiple-step phosphorelay in prokaryotes: Lateral domain recruitment involved in the formation of hybrid-type histidine kinases. Microbiology 2005, 151, 2159–2173. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Whitworth, D.E. Evolution of Prokaryotic Two-Component System Signaling Pathways: Gene Fusions and Fissions. Mol. Biol. Evol. 2007, 24, 2355–2357. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Han, Z.-J.; He, C. Two-component signal transduction systems of Xanthomonas spp.: A lesson from genomics. Mol. Plant Microbe Interact. 2008, 21, 151–161. [Google Scholar] [CrossRef]
- Wachter, S.; Larson, C.L.; Virtaneva, K.; Kanakabandi, K.; Darwitz, B.; Crews, B.; Storrud, K.; Heinzen, R.A.; Beare, P.A.; O’TOole, G. A Survey of Two-Component Systems in Coxiella burnetii Reveals Redundant Regulatory Schemes and a Requirement for an Atypical PhoBR System in Mammalian Cell Infection. J. Bacteriol. 2023, 205, e0041622. [Google Scholar] [CrossRef]
- Schramm, A.; Lee, B.; Higgs, P.I. Intra- and interprotein phosphorylation between two-hybrid histidine kinases controls Myxococcus xanthus developmental progression. J. Biol. Chem. 2012, 287, 25060–25072. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, H.; Hui, K.; Barraud, N.; Filloux, A. The pathogenicity island encoded PvrSR/RcsCB regulatory network controls biofilm formation and dispersal in Pseudomonas aeruginosa PA14. Mol. Microbiol. 2013, 89, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, Y. Sequences, Domain Architectures, and Biological Functions of the Serine/Threonine and Histidine Kinases in Synechocystis sp. PCC 6803. Appl. Biochem. Biotechnol. 2019, 188, 1022–1065. [Google Scholar] [CrossRef] [PubMed]
- Perraud, A.L.; Kimmel, B.; Weiss, V.; Gross, R. Specificity of the BvgAS and EvgAS phosphorelay is mediated by the C-terminal HPt domains of the sensor proteins. Mol. Microbiol. 1998, 27, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Zhu, J.; Winans, S.C. Pleiotropic phenotypes caused by genetic ablation of the receiver module of the Agrobacterium tumefaciens VirA protein. J. Bacteriol. 1996, 178, 4710–4716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogino, T.; Matsubara, M.; Kato, N.; Nakamura, Y.; Mizuno, T. An Escherichia coli protein that exhibits phosphohistidine phosphatase activity towards the HPt domain of the ArcB sensor involved in the multistep His-Asp phosphorelay. Mol. Microbiol. 1998, 27, 573–585. [Google Scholar] [CrossRef]
- Urao, T.; Miyata, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Possible His to Asp phosphorelay signaling in an Arabidopsis two-component system. FEBS Lett. 2000, 478, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Georgellis, D.; Kwon, O.; Lin, E.C.; Wong, S.M.; Akerley, B.J. Redox signal transduction by the ArcB sensor kinase of Haemophilus influenzae lacking the PAS domain. J. Bacteriol. 2001, 183, 7206–7212. [Google Scholar] [CrossRef]
- Gora, K.G.; Cantin, A.; Wohlever, M.; Joshi, K.K.; Perchuk, B.S.; Chien, P.; Laub, M.T. Regulated proteolysis of a transcription factor complex is critical to cell cycle progression in Caulobacter crescentus. Mol. Microbiol. 2013, 87, 1277–1289. [Google Scholar] [CrossRef]
- Mann, T.H.; Seth Childers, W.; Blair, J.A.; Eckart, M.R.; Shapiro, L. A cell cycle kinase with tandem sensory PAS domains integrates cell fate cues. Nat. Commun. 2016, 7, 11454. [Google Scholar] [CrossRef] [PubMed]
- Perego, M.; Brannigan, J.A. Pentapeptide regulation of aspartyl-phosphate phosphatases. Peptides 2001, 22, 1541–1547. [Google Scholar] [CrossRef]
- Domínguez-Bernal, G.; Pucciarelli, M.G.; Ramos-Morales, F.; García-Quintanilla, M.; Cano, D.A.; Casadesús, J.; Portillo, F.G. Repression of the RcsC-YojN-RcsB phosphorelay by the IgaA protein is a requisite for Salmonella virulence. Mol. Microbiol. 2004, 53, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Orué Lucana, D.; Groves, M.R. The three-component signalling system HbpS-SenS-SenR as an example of a redox sensing pathway in bacteria. Amino Acids 2009, 37, 479–486. [Google Scholar] [CrossRef]
- Zschiedrich, C.P.; Keidel, V.; Szurmant, H. Molecular mechanisms of two-component signal transduction. J. Mol. Biol. 2016, 428, 3752–3775. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, S.R.; Ekness, F.; Sofjan, K.; Daeffler, K.N.-M.; Brink, K.R.; Landry, B.P.; Gerhardt, K.P.; Dyulgyarov, N.; Sheth, R.U.; Tabor, J.J. Rewiring bacterial two-component systems by modular DNA-binding domain swapping. Nat. Chem. Biol. 2019, 15, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, S.R.; Sheth, R.U.; Wu, A.; Tabor, J.J. Refactoring and Optimization of Light-Switchable Escherichia coli Two-Component Systems. ACS Synth. Biol. 2014, 3, 820–831. [Google Scholar] [CrossRef]
- Landry, B.P.; Palanki, R.; Dyulgyarov, N.; Hartsough, L.A.; Tabor, J.J. Phosphatase activity tunes two-component system sensor detection threshold. Nat. Commun. 2018, 9, 1433. [Google Scholar] [CrossRef]
- Barrett, J.F.; Hoch, J.A. Two-Component Signal Transduction as a Target for Microbial Anti-Infective Therapy. Antimicrob. Agents Chemother. 1998, 42, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Lazar, J.T.; Tabor, J.J. Bacterial two-component systems as sensors for synthetic biology applications. Curr. Opin. Syst. Biol. 2021, 28, 100398. [Google Scholar] [CrossRef] [PubMed]
- Bem, A.E.; Velikova, N.; Pellicer, M.T.; Baarlen Pvan Marina, A.; Wells, J.M. Bacterial histidine kinases as novel antibacterial drug targets. ACS Chem. Biol. 2015, 10, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Majdi, C.; Meffre, P.; Benfodda, Z. Recent advances in the development of bacterial response regulators inhibitors as antibacterial and/or antibiotic adjuvant agent: A new approach to combat bacterial resistance. Bioorg. Chem. 2024, 150, 107606. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ciruelos, B.; Albanese, M.; Adhav, A.; Solomin, V.; Ritchie-Martinez, A.; Taverne, F.; Velikova, N.; Jirgensons, A.; Marina, A.; Finn, P.W.; et al. Repurposing Hsp90 inhibitors as antimicrobials targeting two-component systems identifies compounds leading to loss of bacterial membrane integrity. Microbiol. Spectr. 2024, 12, e0014624. [Google Scholar] [CrossRef] [PubMed]
- Worthington, R.J.; Blackledge, M.S.; Melander, C. Small-molecule inhibition of bacterial two-component systems to combat antibiotic resistance and virulence. Futur. Med. Chem. 2013, 5, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.; Hess, M.; Weimer, B.C. Two-component systems regulate bacterial virulence in response to the host gastrointestinal environment and metabolic cues. Virulence 2022, 13, 1666–1680. [Google Scholar] [CrossRef]
- Kwiecinski, J.M.; Jelani, D.A.; Fuentes, E.J.; Horswill, A.R. Therapeutic Inhibition of Staphylococcus aureus ArlRS Two-Component Regulatory System Blocks Virulence. Antimicrob. Agents Chemother. 2022, 66, e00187-22. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, G.V.; Lentini, G.; Famà, A.; Coppolino, F.; Beninati, C. Antimicrobial Resistance: Two-Component Regulatory Systems and Multidrug Efflux Pumps. Antibiotics 2023, 12, 965. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wadach, P.; Jakimowicz, D.; Gongerowska-Jac, M. Untangling the Complexity of Two-Component Signal Transduction in Bacteria. Microorganisms 2025, 13, 2013. https://doi.org/10.3390/microorganisms13092013
Wadach P, Jakimowicz D, Gongerowska-Jac M. Untangling the Complexity of Two-Component Signal Transduction in Bacteria. Microorganisms. 2025; 13(9):2013. https://doi.org/10.3390/microorganisms13092013
Chicago/Turabian StyleWadach, Patrycja, Dagmara Jakimowicz, and Martyna Gongerowska-Jac. 2025. "Untangling the Complexity of Two-Component Signal Transduction in Bacteria" Microorganisms 13, no. 9: 2013. https://doi.org/10.3390/microorganisms13092013
APA StyleWadach, P., Jakimowicz, D., & Gongerowska-Jac, M. (2025). Untangling the Complexity of Two-Component Signal Transduction in Bacteria. Microorganisms, 13(9), 2013. https://doi.org/10.3390/microorganisms13092013






