Sustainable Lipid Production with Cutaneotrichosporon oleaginosus: Insights into Metabolism, Feedstock Valorization and Bioprocess Development
Abstract
1. Introduction
2. Far from Conventional—Cutaneotrichosporon oleaginosus, an SCO Production Powerhouse
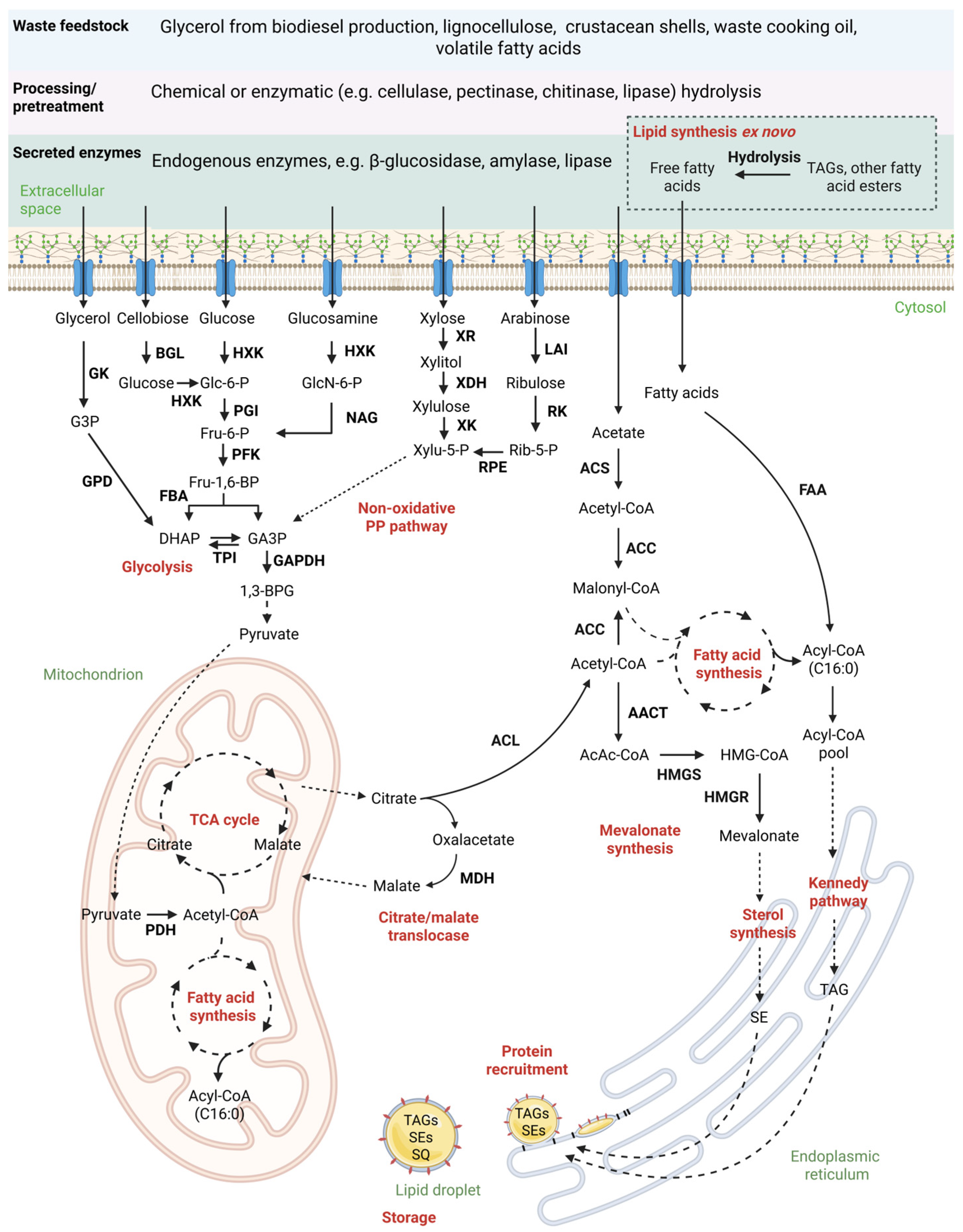
3. Understanding De Novo Lipid Formation—Key to Utilization of Biogenic Side Streams
3.1. Step 1—Production of Ac-CoA
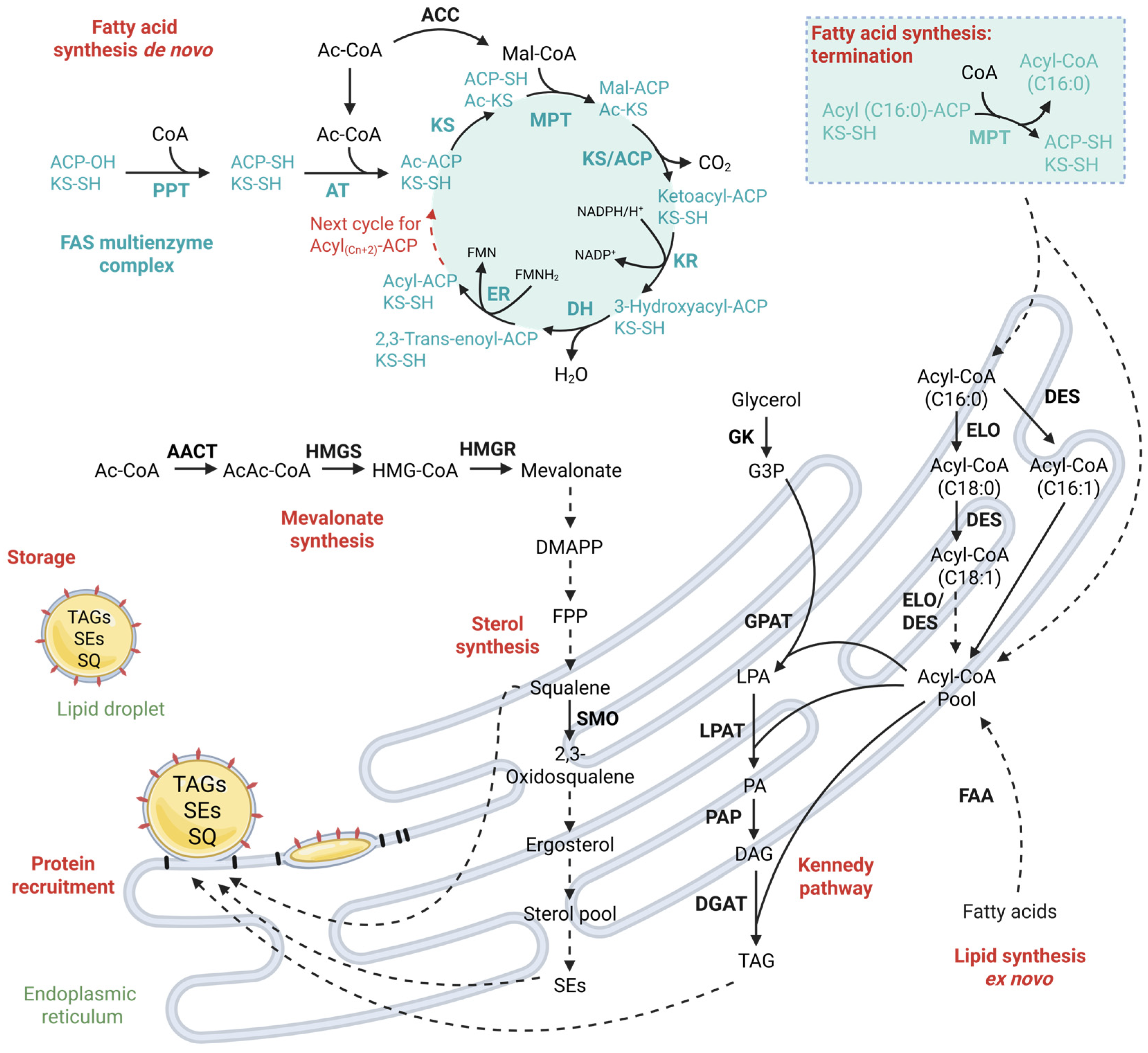
3.2. Step 2—Biosynthesis of Fatty Acyl Chains
3.3. Step 3—Elongation, Desaturation and TAG Synthesis
3.4. Step 4—Lipid Droplet Biogenesis
4. Ex Novo Lipid Synthesis—Harnessing the Potential of Oily Waste Streams
5. Bioprocessing for SCO Production
6. Enhancing C. oleaginosus SCO Bioprocessing Through Genetic Engineering Approaches
7. Industrial Waste as an Alternative Substrate for Oil Production with C. oleaginosus
7.1. Lignocellulosic Biomass
7.2. By-Products of Biodiesel Production (Crude Glycerol)
7.3. Chitin-Based By-Products
7.4. Cheese Whey Permeate
7.5. Fungal Biomass
7.6. Algal and Microalgal Biomass
8. Industrial Applications of SCO Produced by C. oleaginosus Fermentation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. World Energy Outlook 2024. Available online: https://www.iea.org/reports/world-energy-outlook-2024 (accessed on 2 June 2025).
- Cavelius, P.; Engelhart-Straub, S.; Mehlmer, N.; Lercher, J.; Awad, D.; Brück, T. The potential of biofuels from first to fourth generation. PLoS Biol. 2023, 21, e3002063. [Google Scholar] [CrossRef]
- Lynch, J.; Cain, M.; Frame, D.; Pierrehumbert, R. Agriculture’s Contribution to Climate Change and Role in Mitigation Is Distinct From Predominantly Fossil CO2-Emitting Sectors. Front. Sustain. Food Syst. 2021, 4, 518039. [Google Scholar] [CrossRef]
- Parsons, S.; Raikova, S.; Chuck, C.J. The viability and desirability of replacing palm oil. Nat. Sustain. 2020, 3, 412–418. [Google Scholar] [CrossRef]
- Madani, M.; Enshaeieh, M.; Abdoli, A. Single cell oil and its application for biodiesel production. Process Saf. Environ. Prot. 2017, 111, 747–756. [Google Scholar] [CrossRef]
- Murphy, D.J.; Goggin, K.; Paterson, R.R.M. Oil palm in the 2020s and beyond: Challenges and solutions. CABI Agric. Biosci. 2021, 2, 39. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Molina-Hernandez, J.B.; Chaves-Lopez, C.; Romanazzi, G.; Paparella, A. The Role of Fungi in the Cocoa Production Chain and the Challenge of Climate Change. J. Fungi 2021, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Bugge, M.M.; Hansen, T.; Klitkou, A. What is the bioeconomy? A review of the literature. Sustainability 2016, 8, 691. [Google Scholar] [CrossRef]
- Eversberg, D.; Holz, J.; Pungas, L. The bioeconomy and its untenable growth promises: Reality checks from research. Sustain. Sci. 2023, 18, 569–582. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.M.; Ripoll-Bosch, R.; Van Zanten, H.H.E.; Metze, T.A.P.; Termeer, C.J.A.M.; van Ittersum, M.K.; de Boer, I.J.M. Principles, drivers and opportunities of a circular bioeconomy. Nat. Food 2021, 2, 561–566, Erratum in Nat. Food 2021, 2, 742. [Google Scholar] [CrossRef]
- Wei, X.; Luo, J.; Pu, A.; Liu, Q.; Zhang, L.; Wu, S.; Long, Y.; Leng, Y.; Dong, Z.; Wan, X. From biotechnology to bioeconomy: A review of development dynamics and pathways. Sustainability 2022, 14, 10413. [Google Scholar] [CrossRef]
- Gallego-García, M.; Susmozas, A.; Negro, M.J.; Moreno, A.D. Challenges and prospects of yeast-based microbial oil production within a biorefinery concept. Microb. Cell Factories 2023, 22, 246. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mu, L.; Shi, Y.; Rova, U.; Christakopoulos, P.; Matsakas, L. Single-cell oils from oleaginous microorganisms as green bio-lubricants: Studies on their tribological performance. Energies 2021, 14, 6685. [Google Scholar] [CrossRef]
- Uğur, Ş.; Zieniuk, B.; Fabiszewska, A. Nutritional and medicinal properties of microbial oil. Appl. Sci. 2024, 14, 4232. [Google Scholar] [CrossRef]
- Kyle, D.J.; Ratledge, C. Industrial Applications of Single Cell Oils; AOCS Publishing: Champaign, IL, USA, 1992. [Google Scholar]
- Abeln, F.; Chuck, C.J. The history, state of the art and future prospects for oleaginous yeast research. Microb. Cell Factories 2021, 20, 221. [Google Scholar] [CrossRef]
- Davies, R. Scale up of yeast oil technology. In Industrial Applications of Single Cell Oils; AOCS Publishing: Champaign, IL, USA, 1992; pp. 196–218. [Google Scholar]
- Pereira, A.A.; Yaverino-Gutierrez, M.A.; Monteiro, M.C.; Souza, B.A.; Bachheti, R.K.; Chandel, A.K. Precision fermentation in the realm of microbial protein production: State-of-the-art and future insights. Food Res. Int. 2024, 200, 115527. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Kieliszek, M. Reprocessing of side-streams towards obtaining valuable bacterial metabolites. Appl. Microbiol. Biotechnol. 2023, 107, 2169–2208. [Google Scholar] [CrossRef]
- Shen, D.; He, X.; Weng, P.; Liu, Y.; Wu, Z. A review of yeast: High cell-density culture, molecular mechanisms of stress response and tolerance during fermentation. FEMS Yeast Res. 2022, 22, foac050. [Google Scholar] [CrossRef]
- Glencross, B.D.; Huyben, D.; Schrama, J.W. The application of single-cell ingredients in aquaculture feeds—A review. Fishes 2020, 5, 22. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.-f.; Xiong, L.; Ma, L.-l.; Chen, Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnol. Adv. 2013, 31, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Engelhart-Straub, S.; Cavelius, P.; Hölzl, F.; Haack, M.; Awad, D.; Brueck, T.; Mehlmer, N. Effects of Light on Growth and Metabolism of Rhodococcus erythropolis. Microorganisms 2022, 10, 1680. [Google Scholar] [CrossRef] [PubMed]
- Engelhart-Straub, S.; Haack, M.; Awad, D.; Brueck, T.; Mehlmer, N. Optimization of Rhodococcus erythropolis JCM3201T Nutrient Media to Improve Biomass, Lipid, and Carotenoid Yield Using Response Surface Methodology. Microorganisms 2023, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Kassab, E.; Mehlmer, N.; Brueck, T. GFP Scaffold-Based Engineering for the Production of Unbranched Very Long Chain Fatty Acids in Escherichia coli With Oleic Acid and Cerulenin Supplementation. Front. Bioeng. Biotechnol. 2019, 7, 408. [Google Scholar] [CrossRef]
- Masri, M.A.; Garbe, D.; Mehlmer, N.; Brück, T.B. A sustainable, high-performance process for the economic production of waste-free microbial oils that can replace plant-based equivalents. Energy Environ. Sci. 2019, 12, 2717–2732. [Google Scholar] [CrossRef]
- Koruyucu, A.; Blums, K.; Peest, T.; Schmack-Rauscher, L.; Brück, T.; Weuster-Botz, D. High-Cell-Density Yeast Oil Production with Diluted Substrates Imitating Microalgae Hydrolysate Using a Membrane Bioreactor. Energies 2023, 16, 1757. [Google Scholar] [CrossRef]
- Beopoulos, A.; Chardot, T.; Nicaud, J.-M. Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 2009, 91, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Nicaud, J.-M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog. Lipid Res. 2016, 61, 40–50. [Google Scholar] [CrossRef]
- Takaku, H.; Matsuzawa, T.; Yaoi, K.; Yamazaki, H. Lipid metabolism of the oleaginous yeast Lipomyces starkeyi. Appl. Microbiol. Biotechnol. 2020, 104, 6141–6148. [Google Scholar] [CrossRef]
- Jacob, A.; Mathew, J. Recent Advances in using Lipomyces starkeyi for the Production of Single-Cell Oil. J. Pure Appl. Microbiol. 2023, 17, 693–704. [Google Scholar] [CrossRef]
- Ye, Z.; Sun, T.; Hao, H.; He, Y.; Liu, X.; Guo, M.; Chen, G. Optimising nutrients in the culture medium of Rhodosporidium toruloides enhances lipids production. AMB Express 2021, 11, 149. [Google Scholar] [CrossRef]
- Dai, C.-c.; Tao, J.; Xie, F.; Dai, Y.-j.; Zhao, M. Biodiesel generation from oleaginous yeast Rhodotorula glutinis with xylose assimilating capacity. Afr. J. Biotechnol. 2007, 6, 2130–2134. [Google Scholar] [CrossRef]
- Sun, L.; Shao, S.; Bao, J. Microbial lipid fermentation of Trichosporon cutaneum in high saline water. Bioresour. Bioprocess. 2021, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zong, M.; Wu, H. Efficient lipid production with Trichosporonfermentans and its use for biodiesel preparation. Bioresour. Technol. 2008, 99, 7881–7885. [Google Scholar] [CrossRef]
- Shaigani, P.; Awad, D.; Redai, V.; Fuchs, M.; Haack, M.; Mehlmer, N.; Brueck, T. Oleaginous yeasts-substrate preference and lipid productivity: A view on the performance of microbial lipid producers. Microb. Cell Factories 2021, 20, 220. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Shabbir Hussain, M.; Gambill, L.; Blenner, M. Alternative Substrate Metabolism in Yarrowia lipolytica. Front. Microbiol. 2018, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Z.; Wang, Q.-M.; Göker, M.; Groenewald, M.; Kachalkin, A.; Lumbsch, H.T.; Millanes, A.; Wedin, M.; Yurkov, A.; Boekhout, T. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [CrossRef]
- Fuchs, T.; Melcher, F.; Rerop, Z.S.; Lorenzen, J.; Shaigani, P.; Awad, D.; Haack, M.; Prem, S.A.; Masri, M.; Mehlmer, N.; et al. Identifying carbohydrate-active enzymes of Cutaneotrichosporon oleaginosus using systems biology. Microb. Cell Factories 2021, 20, 205. [Google Scholar] [CrossRef]
- Awad, D.; Bohnen, F.; Mehlmer, N.; Brueck, T. Multi-factorial-guided media optimization for enhanced biomass and lipid formation by the oleaginous yeast Cutaneotrichosporon oleaginosus. Front. Bioeng. Biotechnol. 2019, 7, 54. [Google Scholar] [CrossRef]
- Yaguchi, A.; Franaszek, N.; O’Neill, K.; Lee, S.; Sitepu, I.; Boundy-Mills, K.; Blenner, M. Identification of oleaginous yeasts that metabolize aromatic compounds. J. Ind. Microbiol. Biotechnol. 2020, 47, 801–813. [Google Scholar] [CrossRef]
- Banwell, M.G.; Pollard, B.; Liu, X.; Connal, L.A. Exploiting Nature’s Most Abundant Polymers: Developing New Pathways for the Conversion of Cellulose, Hemicellulose, Lignin and Chitin into Platform Molecules (and Beyond). Chem. Asian J. 2021, 16, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Rerop, Z.S.; Stellner, N.I.; Graban, P.; Haack, M.; Mehlmer, N.; Masri, M.; Brück, T.B. Bioconversion of a lignocellulosic hydrolysate to single cell oil for biofuel production in a cost-efficient fermentation process. Fermentation 2023, 9, 189. [Google Scholar] [CrossRef]
- Garay, L.A.; Boundy-Mills, K.L.; German, J.B. Accumulation of high-value lipids in single-cell microorganisms: A mechanistic approach and future perspectives. J. Agric. Food Chem. 2014, 62, 2709–2727. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Regulation of lipid accumulation in oleaginous micro-organisms. Biochem. Soc. Trans. 2002, 30, 1047–1050. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Mitra, M.; Maiti, M.K. Recent advances in lipid metabolic engineering of oleaginous yeasts. Biotechnol. Adv. 2021, 53, 107722. [Google Scholar] [CrossRef]
- Pham, N.; Reijnders, M.; Suarez-Diez, M.; Nijsse, B.; Springer, J.; Eggink, G.; Schaap, P.J. Genome-scale metabolic modeling underscores the potential of Cutaneotrichosporon oleaginosus ATCC 20509 as a cell factory for biofuel production. Biotechnol. Biofuels 2021, 14, 2. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, H. Metabolic engineering of oleaginous yeasts for production of fuels and chemicals. Front. Microbiol. 2017, 8, 2185. [Google Scholar] [CrossRef] [PubMed]
- Sreeharsha, R.V.; Mohan, S.V. Obscure yet promising oleaginous yeasts for fuel and chemical production. Trends Biotechnol. 2020, 38, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Stellner, N.I. An Unconventional Yeast for Exceptional Products: Generation of High-Value Compounds with Cutaneotrichosporon oleaginosus. Ph.D. Thesis, Technical University of Munich, München, Germany, 2025. [Google Scholar]
- De Vicente, M.; Gonzalez-Fernández, C.; Nicaud, J.M.; Tomás-Pejó, E. Turning residues into valuable compounds: Organic waste conversion into odd-chain fatty acids via the carboxylate platform by recombinant oleaginous yeast. Microb. Cell Factories 2025, 24, 32. [Google Scholar] [CrossRef]
- Huang, C.; Luo, M.-T.; Chen, X.-F.; Qi, G.-X.; Xiong, L.; Lin, X.-Q.; Wang, C.; Li, H.-L.; Chen, X.-D. Combined “de novo” and “ex novo” lipid fermentation in a mix-medium of corncob acid hydrolysate and soybean oil by Trichosporon dermatis. Biotechnol. Biofuels 2017, 10, 147. [Google Scholar] [CrossRef]
- Mota, M.N.; Múgica, P.; Sá-Correia, I. Exploring yeast diversity to produce lipid-based biofuels from agro-forestry and industrial organic residues. J. Fungi 2022, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Matsakas, L. A comparative study on de novo and ex novo lipid fermentation by oleaginous yeast using glucose and sonicated waste cooking oil. Ultrason. Sonochem. 2019, 52, 364–374. [Google Scholar] [CrossRef]
- Evans, C.T.; Scragg, A.H.; Ratledge, C. A comparative study of citrate efflux from mitochondria of oleaginous and non-oleaginous yeasts. Eur. J. Biochem. 1983, 130, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Shen, H.; Zhou, W.; Wang, Y.; Yang, X.; Zhao, Z.K. Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2015, 8, 189. [Google Scholar] [CrossRef]
- Lian, J.; Garcia-Perez, M.; Coates, R.; Wu, H.; Chen, S. Yeast fermentation of carboxylic acids obtained from pyrolytic aqueous phases for lipid production. Bioresour. Technol. 2012, 118, 177–186. [Google Scholar] [CrossRef]
- Tehlivets, O.; Scheuringer, K.; Kohlwein, S.D. Fatty acid synthesis and elongation in yeast. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 255–270. [Google Scholar] [CrossRef]
- Schweizer, M.; Roberts, L.M.; Höltke, H.-J.; Takabayashi, K.; Höllerer, E.; Hoffmann, B.; Müller, G.; Köttig, H.; Schweizer, E. The pentafunctional FAS1 gene of yeast: Its nucleotide sequence and order of the catalytic domains. Mol. Gen. Genet. MGG 1986, 203, 479–486. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Chirala, S.S.; Mody, N.H.; Huang, W.-Y.; Wakil, S. Primary structure of the multifunctional alpha subunit protein of yeast fatty acid synthase derived from FAS2 gene sequence. J. Biol. Chem. 1988, 263, 12315–12325. [Google Scholar] [CrossRef]
- Schweizer, E.; Hofmann, J. Microbial type I fatty acid synthases (FAS): Major players in a network of cellular FAS systems. Microbiol. Mol. Biol. Rev. 2004, 68, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; Oh, C.-S.; Jiang, Y. Regulation of long chain unsaturated fatty acid synthesis in yeast. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 271–285. [Google Scholar] [CrossRef]
- Sorger, D.; Daum, G. Triacylglycerol biosynthesis in yeast. Appl. Microbiol. Biotechnol. 2003, 61, 289–299. [Google Scholar] [CrossRef]
- Kory, N.; Farese, R.V.; Walther, T.C. Targeting fat: Mechanisms of protein localization to lipid droplets. Trends Cell Biol. 2016, 26, 535–546. [Google Scholar] [CrossRef]
- Long, A.P.; Manneschmidt, A.K.; VerBrugge, B.; Dortch, M.R.; Minkin, S.C.; Prater, K.E.; Biggerstaff, J.P.; Dunlap, J.R.; Dalhaimer, P. Lipid droplet de novo formation and fission are linked to the cell cycle in fission yeast. Traffic 2012, 13, 705–714. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Bhutada, G.; Kavšček, M.; Hofer, F.; Gogg-Fassolter, G.; Schweiger, M.; Darnhofer, B.; Kordiš, D.; Birner-Gruenberger, R.; Natter, K. Characterization of a lipid droplet protein from Yarrowia lipolytica that is required for its oleaginous phenotype. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Spanova, M.; Zweytick, D.; Lohner, K.; Klug, L.; Leitner, E.; Hermetter, A.; Daum, G. Influence of squalene on lipid particle/droplet and membrane organization in the yeast Saccharomyces cerevisiae. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 647–653. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Yarrowia lipolytica: A model microorganism used for the production of tailor-made lipids. Eur. J. Lipid Sci. Technol. 2010, 112, 639–654. [Google Scholar] [CrossRef]
- Kordi, M.; Salami, R.; Bolouri, P.; Delangiz, N.; Asgari Lajayer, B.; van Hullebusch, E.D. White biotechnology and the production of bio-products. Syst. Microbiol. Biomanuf. 2022, 2, 413–429. [Google Scholar] [CrossRef]
- Villadsen, J. Innovative technology to meet the demands of the white biotechnology revolution of chemical production. Chem. Eng. Sci. 2007, 62, 6957–6968. [Google Scholar] [CrossRef]
- Rowe, G.E.; Margaritis, A. Bioprocess Design and Economic Analysis for the Commercial Production of Environmentally Friendly Bioinsecticides. Biotechnol. Bioeng. 2004, 86, 377–388. [Google Scholar] [CrossRef]
- Ykema, A.; Verbree, E.C.; Kater, M.M.; Smit, H. Optimization of lipid production in the oleaginous yeast Apiotrichum curvatum in wheypermeate. Appl. Microbiol. Biotechnol. 1988, 29, 211–218. [Google Scholar] [CrossRef]
- Meo, A.; Priebe, X.L.; Weuster-Botz, D. Lipid production with Trichosporon oleaginosus in a membrane bioreactor using microalgae hydrolysate. J. Biotechnol. 2017, 241, 1–10. [Google Scholar] [CrossRef]
- Bracharz, F.; Redai, V.; Bach, K.; Qoura, F.; Brück, T. The effects of TORC signal interference on lipogenesis in the oleaginous yeast Trichosporon oleaginosus. BMC Biotechnol. 2017, 17, 27. [Google Scholar] [CrossRef]
- Mersmann, A.; Schneider, G.; Voit, H.; Wenzig, E. Selection and design of aerobic bioreactors. Chem. Eng. Technol. 1990, 13, 357–370. [Google Scholar] [CrossRef]
- Benz, G.T. Bioreactor design for chemical engineers. Chem. Eng. Prog. 2011, 107, 21–26. [Google Scholar]
- Storhas, W. Bioreaktoren und Periphere Einrichtungen: Ein Leitfaden für die Hochschulausbildung, für Hersteller und Anwender; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar]
- Ykema, A.; Verbree, E.C.; Nijkamp, H.J.J.; Smit, H. Isolation and characterization of fatty acid auxotrophs from the oleaginous yeast Apiotrichum curvatum. Appl. Microbiol. Biotechnol. 1989, 32, 76–84. [Google Scholar] [CrossRef]
- Bracharz, F.; Beukhout, T.; Mehlmer, N.; Brück, T. Opportunities and challenges in the development of Cutaneotrichosporon oleaginosus ATCC 20509 as a new cell factory for custom tailored microbial oils. Microb. Cell Factories 2017, 16, 178. [Google Scholar] [CrossRef]
- Stellner, N.I.; Rerop, Z.S.; Mehlmer, N.; Masri, M.; Ringel, M.; Brück, T.B. Expanding the genetic toolbox for Cutaneotrichosporon oleaginosus employing newly identified promoters and a novel antibiotic resistance marker. BMC Biotechnol. 2023, 23, 40. [Google Scholar] [CrossRef]
- Shaigani, P.; Fuchs, T.; Graban, P.; Prem, S.; Haack, M.; Masri, M.; Mehlmer, N.; Brueck, T. Mastering targeted genome engineering of GC-rich oleaginous yeast for tailored plant oil alternatives for the food and chemical sector. Microb. Cell Factories 2023, 22, 25. [Google Scholar] [CrossRef]
- Duman-Özdamar, Z.E.; Julsing, M.K.; Verbokkem, J.A.; Wolbert, E.; Dos Santos, V.A.M.; Hugenholtz, J.; Suarez-Diez, M. Model-driven engineering of Cutaneotrichosporon oleaginosus ATCC 20509 for improved microbial oil production. Bioresour. Technol. 2025, 421, 132142. [Google Scholar] [CrossRef] [PubMed]
- Koivuranta, K.; Castillo, S.; Jouhten, P.; Ruohonen, L.; Penttilä, M.; Wiebe, M.G. Enhanced triacylglycerol production with genetically modified Trichosporon oleaginosus. Front. Microbiol. 2018, 9, 1337. [Google Scholar] [CrossRef] [PubMed]
- Donzella, S.; Compagno, C. Heterologous Expression of CFL1 Confers Flocculating Ability to Cutaneotrichosporon oleaginosus Lipid-Rich Cells. J. Fungi 2022, 8, 1293. [Google Scholar] [CrossRef] [PubMed]
- Görner, C.; Redai, V.; Bracharz, F.; Schrepfer, P.; Garbe, D.; Brück, T. Genetic engineering and production of modified fatty acids by the non-conventional oleaginous yeast Trichosporon oleaginosus ATCC 20509. Green Chem. 2016, 18, 2037–2046. [Google Scholar] [CrossRef]
- Diwan, B.; Parkhey, P.; Gupta, P. From agro-industrial wastes to single cell oils: A step towards prospective biorefinery. Folia Microbiol. 2018, 63, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Dourou, M.; Aggeli, D.; Papanikolaou, S.; Aggelis, G. Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 2509–2523. [Google Scholar] [CrossRef]
- Di Fidio, N.; Minonne, F.; Antonetti, C.; Raspolli Galletti, A.M. Cutaneotrichosporon oleaginosus: A versatile whole-cell biocatalyst for the production of single-cell oil from agro-industrial wastes. Catalysts 2021, 11, 1291. [Google Scholar] [CrossRef]
- Younes, S.; Bracharz, F.; Awad, D.; Qoura, F.; Mehlmer, N.; Brueck, T. Microbial lipid production by oleaginous yeasts grown on Scenedesmus obtusiusculus microalgae biomass hydrolysate. Bioprocess Biosyst. Eng. 2020, 43, 1629–1638. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Tang, M.; Zhao, M.; Yang, X.; Li, L.; Liu, Y.; Zhou, W.; Gong, Z. Combined utilization of the blend of low-cost substrates facilitates mutual achievement for overproducing biodiesel feedstock by the oleaginous yeast Cutaneotrichosporon oleaginosum. Ind. Crops Prod. 2022, 189, 115759. [Google Scholar] [CrossRef]
- Caporusso, A.; De Bari, I.; Valerio, V.; Albergo, R.; Liuzzi, F. Conversion of cardoon crop residues into single cell oils by Lipomyces tetrasporus and Cutaneotrichosporon curvatus: Process optimizations to overcome the microbial inhibition of lignocellulosic hydrolysates. Ind. Crops Prod. 2021, 159, 113030. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; He, Q.; Liu, Y.; Zhao, M.; Liu, Y.; Zhou, W.; Gong, Z. Highly efficient fed-batch modes for enzymatic hydrolysis and microbial lipogenesis from alkaline organosolv pretreated corn stover for biodiesel production. Renew. Energy 2022, 197, 1133–1143. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Tan, D.; Liu, Y.; Li, L.; Zhou, W.; Gong, Z. Efficient lipid production from herbal extraction residue hydrolysate by the oleaginous yeast Cutaneotrichosporon oleaginosum for biodiesel production. Biomass Convers. Biorefin. 2022, 14, 8681–8692. [Google Scholar] [CrossRef]
- Tang, M.; Wang, Y.; Zhou, W.; Yang, M.; Liu, Y.; Gong, Z. Efficient conversion of chitin-derived carbon sources into microbial lipid by the oleaginous yeast Cutaneotrichosporon oleaginosum. Bioresour. Technol. 2020, 315, 123897. [Google Scholar] [CrossRef]
- Donzella, S.; Serra, I.; Fumagalli, A.; Pellegrino, L.; Mosconi, G.; Lo Scalzo, R.; Compagno, C. Recycling industrial food wastes for lipid production by oleaginous yeasts Rhodosporidiobolus azoricus and Cutaneotrichosporon oleaginosum. Biotechnol. Biofuels Bioprod. 2022, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Juanssilfero, A.B.; Kahar, P.; Amza, R.L.; Miyamoto, N.; Otsuka, H.; Matsumoto, H.; Kihira, C.; Thontowi, A.; Ogino, C.; Prasetya, B. Selection of oleaginous yeasts capable of high lipid accumulation during challenges from inhibitory chemical compounds. Biochem. Eng. J. 2018, 137, 182–191. [Google Scholar] [CrossRef]
- Di Fidio, N.; Liuzzi, F.; Mastrolitti, S.; Albergo, R.; De Bari, I. Single cell oil production from undetoxified Arundo donax L. hydrolysate by Cutaneotrichosporon curvatus. J. Microbiol. Biotechnol. 2019, 29, 256–267. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Antczak, A.; Marchwicka, M.; Szadkowski, J.; Drożdżek, M.; Gawron, J.; Radomski, A.; Zawadzki, J. Sugars yield obtained after acid and enzymatic hydrolysis of fast-growing poplar wood species. BioResources 2018, 13, 8629–8645. [Google Scholar] [CrossRef]
- Samantaray, B.; Mohapatra, S.; Mishra, R.R.; Behera, B.C.; Thatoi, H. Bioethanol production from agro-wastes: A comprehensive review with a focus on pretreatment, enzymatic hydrolysis, and fermentation. Int. J. Green Energy 2024, 21, 1398–1424. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and specialty industrial applications of lignocellulosic biomass. Waste Biomass Valorization 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of lignocellulosic biomass. In Lignocellulosic Biomass to Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar]
- Kormelink, F.; Voragen, A. Degradation of different [(glucurono) arabino] xylans by a combination of purified xylan-degrading enzymes. Appl. Microbiol. Biotechnol. 1993, 38, 688–695. [Google Scholar] [CrossRef]
- Sugiarto, S.; Pong, R.; Tan, Y.; Leow, Y.; Sathasivam, T.; Zhu, Q.; Loh, X.; Kai, D. Advances in sustainable polymeric materials from lignocellulosic biomass. Mater. Today Chem. 2022, 26, 101022. [Google Scholar] [CrossRef]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production strategies and applications of microbial single cell oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Fernandes, T.; Bos, G.K.; Zeeman, G.; Sanders, J.; Van Lier, J. Effects of thermo-chemical pre-treatment on anaerobic biodegradability and hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 2575–2579. [Google Scholar] [CrossRef]
- Uthandi, S.; Kaliyaperumal, A.; Srinivasan, N.; Thangavelu, K.; Muniraj, I.K.; Zhan, X.; Gathergood, N.; Gupta, V.K. Microbial biodiesel production from lignocellulosic biomass: New insights and future challenges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2197–2225. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Wang, H.; Peng, X.; Zhang, H.; Yang, S.; Li, H. Microorganisms-promoted biodiesel production from biomass: A review. Energy Convers. Manag. X 2021, 12, 100137. [Google Scholar] [CrossRef]
- McNutt, J.; Yang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Chol, C.G.; Dhabhai, R.; Dalai, A.K.; Reaney, M. Purification of crude glycerol derived from biodiesel production process: Experimental studies and techno-economic analyses. Fuel Process. Technol. 2018, 178, 78–87. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Yaguchi, A.; Blenner, M. Oleaginous yeast for biofuel and oleochemical production. Curr. Opin. Biotechnol. 2019, 57, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, J.; Edwards, T.A. Innovation from waste with biomass-derived chitin and chitosan as green and sustainable polymer: A review. Energy Nexus 2022, 8, 100149. [Google Scholar] [CrossRef]
- Azelee, N.I.W.; Digvijay, D.; Ayothiraman, S.; Noor, N.M.; Abd Rasid, Z.I.; Ramli, A.N.M.; Ravindran, B.; Iwuchukwu, F.U.; Selvasembian, R. Sustainable valorization approaches on crustacean wastes for the extraction of chitin, bioactive compounds and their applications—A review. Int. J. Biol. Macromol. 2023, 253, 126492. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. J. Clean. Prod. 2018, 172, 4140–4151. [Google Scholar] [CrossRef]
- Menchik, P.; Zuber, T.; Zuber, A.; Moraru, C.I. Composition of coproduct streams from dairy processing: Acid whey and milk permeate. J. Dairy Sci. 2019, 102, 3978–3984. [Google Scholar] [CrossRef]
- Lakstina, J.; Aboltina, I.; Vanaga, L.; Ciprovica, I.; Jonkus, D.; Zagorska, J.; Cinkmanis, I. The novel solution for acid whey permeate application in animal feeding. Rural Sustain. Res. 2020, 44, 1–7. [Google Scholar] [CrossRef]
- Sampaio, F.C.; de Faria, J.T.; da Silva, M.F.; de Souza Oliveira, R.P.; Converti, A. Cheese whey permeate fermentation by Kluyveromyces lactis: A combined approach to wastewater treatment and bioethanol production. Environ. Technol. 2020, 41, 3210–3218. [Google Scholar] [CrossRef] [PubMed]
- Mbabazi, J.; Pesu, H.; Mutumba, R.; Filteau, S.; Lewis, J.I.; Wells, J.C.; Olsen, M.F.; Briend, A.; Michaelsen, K.F.; Mølgaard, C. Effect of milk protein and whey permeate in large quantity lipid-based nutrient supplement on linear growth and body composition among stunted children: A randomized 2× 2 factorial trial in Uganda. PLoS Med. 2023, 20, e1004227. [Google Scholar] [CrossRef]
- Diwan, B.; Gupta, P. A deuteromycete isolate Geotrichum candidum as oleaginous cell factory for medium-chain fatty acid-rich oils. Curr. Microbiol. 2020, 77, 3738–3749. [Google Scholar] [CrossRef]
- Altun, R.; Esim, N.; Aykutoglu, G.; Baltaci, M.O.; Adiguzel, A.; Taskin, M. Production of linoleic acid-rich lipids in molasses-based medium by oleaginous fungus Galactomyces geotrichum TS61. J. Food Process. Preserv. 2020, 44, e14518. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Zhang, G.; Yang, Q.; Lu, J.; Xia, T.; Peng, L.; Wang, Y. Cascading of engineered bioenergy plants and fungi sustainable for low-cost bioethanol and high-value biomaterials under green-like biomass processing. Renew. Sustain. Energy Rev. 2021, 137, 110586. [Google Scholar] [CrossRef]
- Gmoser, R.; Sintca, C.; Taherzadeh, M.J.; Lennartsson, P.R. Combining submerged and solid state fermentation to convert waste bread into protein and pigment using the edible filamentous fungus N. intermedia. Waste Manag. 2019, 97, 63–70. [Google Scholar] [CrossRef]
- Karimi, S.; Mahboobi Soofiani, N.; Mahboubi, A.; Ferreira, J.A.; Lundh, T.; Kiessling, A.; Taherzadeh, M.J. Evaluation of nutritional composition of pure filamentous fungal biomass as a novel ingredient for fish feed. Fermentation 2021, 7, 152. [Google Scholar] [CrossRef]
- Isaza-Pérez, F.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Potential of residual fungal biomass: A review. Environ. Sci. Pollut. Res. 2020, 27, 13019–13031. [Google Scholar] [CrossRef]
- Economou, C.N.; Aggelis, G.; Pavlou, S.; Vayenas, D.V. Single cell oil production from rice hulls hydrolysate. Bioresour. Technol. 2011, 102, 9737–9742. [Google Scholar] [CrossRef]
- Thomas, N.M.; Sathasivam, V.; Thirunavukarasu, M.; Muthukrishnan, A.; Muthukrishnan, S.; Rajkumar, V.; Velusamy, G.; Packiaraj, G. Influence of Borassus flabellifer Endocarps Hydrolysate on Fungal Biomass and Fatty Acids Production by the Marine Fungus Aspergillus sp. Appl. Biochem. Biotechnol. 2023, 196, 923–948. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Hassan, N.; Ali, H.; Niazi, M.B.K.; Jahan, Z.; Ghuman, I.L.; Hassan, F.; Saqib, A. An overview of key industrial product citric acid production by Aspergillus niger and its application. J. Ind. Microbiol. Biotechnol. 2025, 52, kuaf007. [Google Scholar] [CrossRef]
- Chuppa-Tostain, G.; Hoarau, J.; Watson, M.; Adelard, L.; Shum Cheong Sing, A.; Caro, Y.; Grondin, I.; Bourven, I.; Francois, J.-M.; Girbal-Neuhauser, E. Production of Aspergillus niger biomass on sugarcane distillery wastewater: Physiological aspects and potential for biodiesel production. Fungal Biol. Biotechnol. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601. [Google Scholar] [CrossRef]
- Ŝpanêlová, M.; Machoviĉ, V.; Březina, M. Characterization and sorption properties of Aspergillus niger waste biomass. Open Chem. 2003, 1, 192–200. [Google Scholar] [CrossRef]
- Llimós Turet, J. Lignocellulolytic Enzymes Production via Solid-State Fermentation of Agroindustrial Residues: Process Optimization and Application. Ph.D. Thesis, Universitat de Vic—Universitat Central de Catalunya, Vic, Barcelona, Spain, 2022. [Google Scholar]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Correa, D.F.; Beyer, H.L.; Possingham, H.P.; Thomas-Hall, S.R.; Schenk, P.M. Global mapping of cost-effective microalgal biofuel production areas with minimal environmental impact. GCB Bioenergy 2019, 11, 914–929. [Google Scholar] [CrossRef]
- Slocombe, S.P.; Zhang, Q.; Ross, M.; Anderson, A.; Thomas, N.J.; Lapresa, Á.; Rad-Menéndez, C.; Campbell, C.N.; Black, K.D.; Stanley, M.S. Unlocking nature’s treasure-chest: Screening for oleaginous algae. Sci. Rep. 2015, 5, 9844. [Google Scholar] [CrossRef]
- Zhu, L. Microalgal culture strategies for biofuel production: A review. Biofuels Bioprod. Biorefin. 2015, 9, 801–814. [Google Scholar] [CrossRef]
- Posten, C.; Walter, C. Microalgal Biotechnology: Integration and Economy; Walter de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Rögner, M. Photosynthesis: Biotechnological Applications with Microalgae; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2021. [Google Scholar]
- Vasconcelos, B.; Teixeira, J.C.; Dragone, G.; Teixeira, J.A. Oleaginous yeasts for sustainable lipid production—From biodiesel to surf boards, a wide range of “green” applications. Appl. Microbiol. Biotechnol. 2019, 103, 3651–3667. [Google Scholar] [CrossRef]
- Christophe, G.; Kumar, V.; Nouaille, R.; Gaudet, G.; Fontanille, P.; Pandey, A.; Soccol, C.R.; Larroche, C. Recent developments in microbial oils production: A possible alternative to vegetable oils for biodiesel without competition with human food? Braz. Arch. Biol. Technol. 2012, 55, 29–46. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Zhang, Z.; Doherty, W.O.; O’Hara, I.M. The prospect of microbial oil production and applications from oil palm biomass. Biochem. Eng. J. 2019, 143, 9–23. [Google Scholar] [CrossRef]
- Stellner, N.I.; Rerop, Z.S.; Kyselka, J.; Alishevich, K.; Benes, R.; Filip, V.; Celik, G.; Haack, M.; Ringel, M.; Masri, M.; et al. Value-Added Squalene in Single-Cell Oil Produced with Cutaneotrichosporon oleaginosus for Food Applications. J. Agric. Food Chem. 2023, 71, 8540–8550. [Google Scholar] [CrossRef] [PubMed]
- Ates, A.M.; Bukowski, M. Oil Crops Outlook: September 2022. Available online: https://ers.usda.gov/sites/default/files/_laserfiche/outlooks/104714/OCS-22i.pdf?v=40726 (accessed on 21 August 2025).
- Whiffin, F.; Santomauro, F.; Chuck, C.J. Toward a microbial palm oil substitute: Oleaginous yeasts cultured on lignocellulose. Biofuels Bioprod. Biorefin. 2016, 10, 316–334. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Parsons, S.; McManus, M.C.; Chuck, C.J. Using techno-economic modelling to determine the minimum cost possible for a microbial palm oil substitute. Biotechnol. Biofuels 2021, 14, 57. [Google Scholar] [CrossRef]
- Konzock, O.; Matsushita, Y.; Zaghen, S.; Sako, A.; Norbeck, J. Altering the fatty acid profile of Yarrowia lipolytica to mimic cocoa butter by genetic engineering of desaturases. Microb. Cell Factories 2022, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, Y.; Ji, B.; Nielsen, J. Advances in metabolic engineering of Saccharomyces cerevisiae for cocoa butter equivalent production. Front. Bioeng. Biotechnol. 2020, 8, 594081. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.F.; Marino, N.; Oliviero Rossi, C.; Seta, L.; Caputo, P.; De Luca, G. Triacylglycerol composition and chemical-physical properties of cocoa butter and its derivatives: NMR, DSC, X-ray, rheological investigation. Int. J. Mol. Sci. 2023, 24, 2090. [Google Scholar] [CrossRef] [PubMed]
- Anschau, A. Lipids from oleaginous yeasts: Production and encapsulation. In Nutrient Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 749–794. [Google Scholar]
- Jahurul, M.; Zaidul, I.; Norulaini, N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K.; Omar, A.M. Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J. Food Eng. 2013, 117, 467–476. [Google Scholar] [CrossRef]

| Microorganism | Typical Lipid Content (% DCW) | Notable Advantages | Reference |
|---|---|---|---|
| Cutaneotrichosporon oleaginosus | 50–85% | Broad substrate tolerance; high lipid yield | Masri et al. (2019) [27], Koruyucu et al. (2023) [28] |
| Yarrowia lipolytica | 20–40% | GRAS status; advanced genetic tools | Beopoulos et al. (2009) [29], Ledesma-Amaro et al. (2016) [30] |
| Lipomyces starkey | >70% | Tolerant to high sugar concentrations; high lipid yield | Takaku et al. (2020) [31], Jacob et al. (2023) [32] |
| Rhodosporidium toruloides | >65% | Co-production of carotenoids | Ye et al. (2021) [33] |
| Rhodosporidium glutinis | 50–60% | Co-production of carotenoids | Dai et al. (2007) [34] |
| Trichosporon fermentans | >60% | Can tolerate and grow under a variety of stress conditions | Sun et al. (2021) [35], Zhu et al. (2008) [36] |
| Goal | Genes of Interest/Resistance Genes | Transformation Method | Reference |
|---|---|---|---|
| Overexpression; flocculation of cells and facilitated harvesting | CFL1 (Cryptococcus neoformans) | Electroporation-assisted random plasmid integration | Donzella et al. (2022) [87] |
| HPH (optimized synthetic gene) | |||
| Overexpression; increased TAG production capacity under non-limiting conditions | PDC1 (Saccharomyces cerevisiae), ALD6 (Saccharomyces cerevisiae), ACS2 (Saccharomyces cerevisiae), PDAT (Rhizopus oryzae), all codon-optimized (Ustilago maydis) | Electroporation-assisted random plasmid integration | Koivuranta et al. (2018) [86] |
| HPH (Escherichia coli), PDR4 (Saccharomyces cerevisiae), APH (Escherichia coli) | |||
| Overexpression; increased PUFA content in SCO | D9ELO (Isochrysis galbana), D12FAD (Fusarium moniliforme), LAI (Propionibacterium acnes), all codon-optimized (C. oleaginosus) | Agrobacteria-mediated random plasmid integration | Görner et al. (2016) [88] |
| HPH (Streptomyces hygroscopicus) | |||
| Overexpression, gene knockout, promoter replacement: variation in fatty acid saturation in the SCO | D9FAD, D12FAD (C. oleaginosus) | Electroporation-assisted, CRISPR-Cas-mediated targeted integration of repair dsDNA in spheroblasts | Shaigani et al. (2023) [84] |
| URA5 (C. oleaginosus) | |||
| Overexpression; increased TAG production capacity under non-limiting conditions | ACL1, ACC, TS, HMGS (C. oleaginosus) | Electroporation-assisted random integration of plasmids (gene of interest and resistance separate) in electrocompetent cells (wild type, Δ9 and Δ12) | Duman-Özdamar et al. (2025) [85] |
| NAT | |||
| Overexpression; screening of different resistances and endogenous promoters | - | Agrobacteria-mediated random plasmid integration | Stellner et al. (2023) [83] |
| HPH (Streptomyces hygroscopicus), NAT (Streptomyces noursei), APH (bacterial transposon) |
| Alternative Substrate | Carbon Sources | Lipid Content (% Lipid per Biomass) | Lipid Titre (g/L) | Fermentation Scale (L) | Fermentation Mode | Reference |
|---|---|---|---|---|---|---|
| Cardoon-stalk hydrolysate | Glucose, xylose, arabinose, galactose and lignin | 48.8 | 7.1 | 2 | Batch and fed-batch | Caporusso et al. (2021) [94] |
| Lignocellulosic hydrolysate | Xylose, glucose, mannose and acetic acid | 75.5 | 42.1 | 0.25 | Batch and fed-batch | Rerop et al. (2023) [44] |
| Corn-stover hydrolysate | Glucose, xylose and lignin | 64.6 | 43.2 | 1 | Fed-batch | Wang et al. (2022) [95] |
| Hydrolyzed herbal extract residues | Glucose, xylose, arabinose, galactose, mannose and acetic acid | 40.7 | 8.5 | 0.25 | Batch | Zhang et al. (2022) [96] |
| Microalgae biomass hydrolysate | Glucose, mannose, galactose, rhamnose, fucose and ribose | 35.0 | 3.6 | 0.25 | Batch | Younes et al. (2020) [92] |
| By-products of biodiesel production process | Glycerol | 32.0 | 16 | 0.1 | Batch | Pham et al. (2021) [49] |
| Hydrolyzed chitin biomass | N-acetylglucosamine, glucosamine and acetic acid | 25.0 | 10.1 | 0.25 | Batch | Tang et al. (2020) [97] |
| Wheat straw hydrolysate | Glucose, xylose, mannitol and N-acetylglucosamine | 65.0 | 7.5 | 0.1 | Batch | Shaigani et al. (2021) [37] |
| Cheese whey permeate | Lactose | 68.0 | 38 | 2 | Fed-batch | Donzella et al. (2022) [98] |
| Fatty Acid | Fatty Acid Content Range (%) |
|---|---|
| C18:1 | 43–57 |
| C16:0 | 16–33 |
| C18:0 | 10–14 |
| C18:2 | 5–9 |
| C14:0 | <1 |
| C16:1 | <1 |
| C22:6 | <1 |
| C18:3 | <1 |
| C22:1 | <1 |
| C20:1 | <1 |
| C24:0 | <0.1 |
| C24:1 | <0.1 |
| C20:0 | <0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringel, M.; Paper, M.; Willing, M.; Schneider, M.; Melcher, F.; Stellner, N.I.; Brück, T. Sustainable Lipid Production with Cutaneotrichosporon oleaginosus: Insights into Metabolism, Feedstock Valorization and Bioprocess Development. Microorganisms 2025, 13, 1988. https://doi.org/10.3390/microorganisms13091988
Ringel M, Paper M, Willing M, Schneider M, Melcher F, Stellner NI, Brück T. Sustainable Lipid Production with Cutaneotrichosporon oleaginosus: Insights into Metabolism, Feedstock Valorization and Bioprocess Development. Microorganisms. 2025; 13(9):1988. https://doi.org/10.3390/microorganisms13091988
Chicago/Turabian StyleRingel, Marion, Michael Paper, Marieke Willing, Max Schneider, Felix Melcher, Nikolaus I. Stellner, and Thomas Brück. 2025. "Sustainable Lipid Production with Cutaneotrichosporon oleaginosus: Insights into Metabolism, Feedstock Valorization and Bioprocess Development" Microorganisms 13, no. 9: 1988. https://doi.org/10.3390/microorganisms13091988
APA StyleRingel, M., Paper, M., Willing, M., Schneider, M., Melcher, F., Stellner, N. I., & Brück, T. (2025). Sustainable Lipid Production with Cutaneotrichosporon oleaginosus: Insights into Metabolism, Feedstock Valorization and Bioprocess Development. Microorganisms, 13(9), 1988. https://doi.org/10.3390/microorganisms13091988







