Association of Helicobacter pylori as an Extragastric Reservoir in the Oral Cavity with Oral Diseases in Patients with and Without Gastritis—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Development and Registration of the Protocol
2.2. Research Question
2.3. PECO Criteria
2.4. Search Strategy
2.5. Selection Criteria
- -
- Inclusion criteria
- -
- Exclusion Criteria
2.6. Data Extraction and Selection
2.7. Quality Analysis
| Author | Selection (Max. 4) | Comparability (Max. 2) | Result (Max. 3) | Total Score (Max. 9) | Methodological Quality |
|---|---|---|---|---|---|
| Abdul et al. [17] | 4 | 1 | 2 | 7 | High |
| Agarwal et al. [22] | 4 | 1 | 2 | 7 | High |
| Bouziane et al. [19] | 4 | 1 | 2 | 7 | High |
| Chen et al. [18] | 4 | 1 | 2 | 7 | High |
| Chen et al. [23] | 4 | 2 | 3 | 9 | High |
| El Batawi et al. [24] | 3 | 1 | 3 | 7 | High |
| Eskandari et al. [25] | 4 | 1 | 2 | 7 | High |
| Li et al. [26] | 4 | 2 | 3 | 9 | High |
| Medina et al. [27] | 4 | 1 | 3 | 8 | High |
| Mehdipour et al. [28] | 4 | 2 | 3 | 9 | High |
| Navabi et al. [29] | 4 | 1 | 2 | 7 | High |
| Ozturk [20] | 4 | 2 | 3 | 9 | High |
| Pataro et al. [30] | 3 | 1 | 3 | 7 | High |
| Ren et al. [31] | 4 | 2 | 3 | 9 | High |
| Sghaireen et al. [32] | 3 | 1 | 2 | 6 | Moderate |
| Sruthi et al. [33] | 3 | 1 | 3 | 7 | High |
| Tsimpiris et al. [34] | 4 | 1 | 3 | 8 | High |
| Tsimpiris et al. [35] | 4 | 2 | 3 | 9 | High |
| Urrutia-Baca et al. [21] | 4 | 2 | 3 | 9 | High |
| Zaric et al. [36] | 3 | 1 | 2 | 6 | Moderate |
| Zhang et al. [37] | 4 | 2 | 3 | 9 | High |
| Zheng et al. [38] | 4 | 2 | 3 | 9 | High |
3. Results
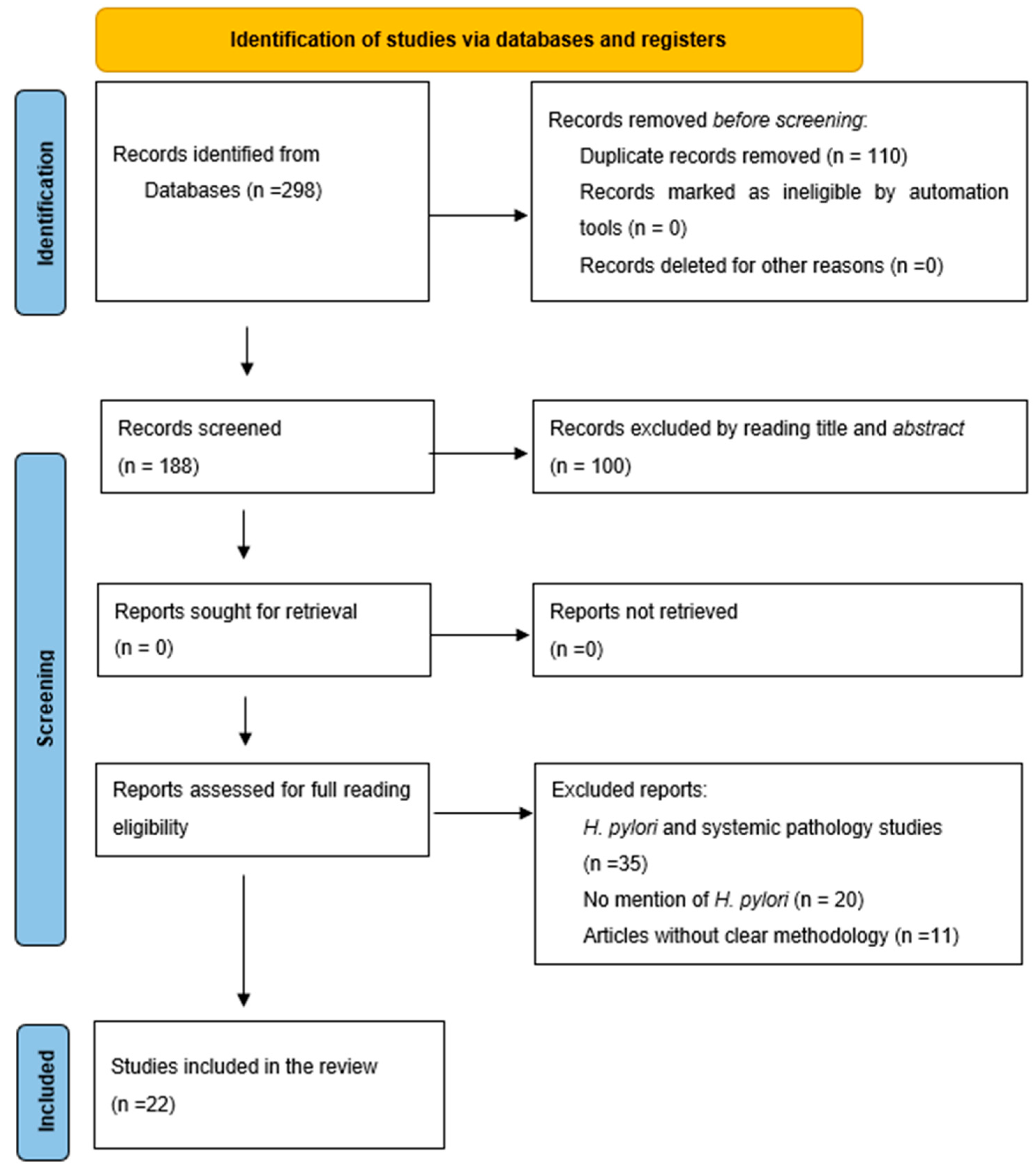
3.1. Selection Process for Articles of Interest
3.2. Study Characteristics
3.3. Summary of Results
- -
- Relationship between oral H. pylori and gastritis
- -
- Distribution of H. pylori in different oral pathologies
- -
- Influence of detection techniques on findings
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, T.; Liu, F.; Liu, L.; Zhang, Z.; Dong, W.; Bai, S.; Ma, L.; Kang, L. Effects of Helicobacter pylori Infection on the Oral Microbiota of Reflux Esophagitis Patients. Front. Cell. Infect. Microbiol. 2021, 11, 732613. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, X.; Guo, J.; Yu, D.; Xiao, Y.; Wang, H.; Li, Y. Helicobacter pylori infection alters gastric and tongue coating microbial communities. Helicobacter 2019, 24, e12567. [Google Scholar] [CrossRef]
- Kadota, T.; Hamada, M.; Nomura, R.; Ogaya, Y.; Okawa, R.; Uzawa, N.; Nakano, K. Distribution of Helicobacter pylori and Periodontopathic Bacterial Species in the Oral Cavity. Biomedicines 2020, 8, 161. [Google Scholar] [CrossRef]
- Alagl, A.S.; Abdelsalam, M.; El Tantawi, M.; Madi, M.; Aljindan, R.; Alsayyah, A.; AlHumaid, J.; Hussameddin, A.M.; Alsulaiman, R.M.; AlQurain, A. Association between Helicobacter pylori gastritis and dental diseases: A cross-sectional, hospital-based study in Eastern Saudi Arabia. J. Periodontol. 2019, 90, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.-G.; Chong, J.-Y.; Lamichhane, B.; Webberley, K.M.; Marshall, B.J.; Wise, M.J.; Tay, C.-Y. Gastric Helicobacter pylori infection perturbs human oral microbiota. PeerJ 2019, 7, e6336. [Google Scholar] [CrossRef]
- Suzuki, N.; Beppu, R.; Yoneda, M.; Takeshita, T.; Asakawa, M.; Yamashita, Y.; Hanioka, T.; Hirofuji, T.; Shinohara, T. Effects of eradication of Helicobacter pylori on oral malodor and the oral environment: A single-center observational study. BMC Res. Notes 2020, 13, 406. [Google Scholar] [CrossRef]
- Wongsuwanlert, M.; Teanpaisan, R.; Pahumunto, N.; Kaewdech, A.; Ruangsri, P.; Sunpaweravong, S. Prevalence and virulence factors of Helicobacter pylori isolated from oral cavity of non-disease, gastritis, and gastric cancer patients. J. Dent. Sci. 2024, 19, 1036–1043. [Google Scholar] [CrossRef]
- Anbari, F.; Ashouri Moghaddam, A.; Sabeti, E.; Khodabakhshi, A. Halitosis: Helicobacter pylori or oral factors. Helicobacter 2019, 24, e12556. [Google Scholar] [CrossRef] [PubMed]
- Popovska, M.; Osmani-Jusuf, Ž.; Radojkova-Nikolovska, V.; Evrosimovska, B.; Mitić, K.; Nikolovski, B.; Spasovska, A.; Rusevska, B. The role of Helicobacter pylori in development of lesion in oral cavity. Balkan J. Dent. Med. 2020, 24, 77–83. [Google Scholar] [CrossRef]
- Almashhadany, D.A.; Zefenkey, Z.F.; Zaki, A.M. Dental risk factors associated with oral Helicobacter pylori infection: A cross-sectional study based on saliva antigen test. J. Infect. Dev. Ctries. 2022, 16, 516–521. [Google Scholar] [CrossRef]
- Iwai, K.; Watanabe, I.; Yamamoto, T.; Kuriyama, N.; Matsui, D.; Nomura, R.; Ogaya, Y.; Oseko, F.; Adachi, K.; Takizawa, S.; et al. Association between Helicobacter pylori infection and dental pulp reservoirs in Japanese adults. BMC Oral Health 2019, 19, 267. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yang, Z.; Li, J.; Li, Y.; Li, H.; Li, W.; Jia, J.; Ge, S.; Sun, Y. Helicobacter pylori infection is correlated with the incidence of erosive oral lichen planus and the alteration of the oral microbiome composition. BMC Microbiol. 2021, 21, 122. [Google Scholar] [CrossRef]
- Morales-Espinosa, R.; Fernandez-Presas, A.; Gonzalez-Valencia, G.; Flores-Hernandez, S.; Delgado-Sapien, G.; Mendez-Sanchez, J.L.; Sanchez-Quezada, E.; Muñoz-Pérez, L.; Leon-Aguilar, R.; Hernandez-Guerrero, J.; et al. Helicobacter pylori in the oral cavity is associated with gastroesophageal disease. Oral Microbiol. Immunol. 2009, 24, 464–468. [Google Scholar] [CrossRef]
- Gürbüz, A.K.; Ozel, A.M.; Yazgan, Y.; Celik, M.; Yildirim, S. Oral colonization of Helicobacter pylori: Risk factors and response to eradication therapy. South. Med. J. 2003, 96, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Valdovinos-Díaz, M.A.; Hani, A.; Defilippi-Guerra, C.; Fernando-Pineda, L.; Remes-Troche, J.M.; Riquelme, A.; Abrahão-Junior, L.J.; Aguilar-Paiz, L.; Almonte-Nuñez, C.; Burgos, H.; et al. Good clinical practice recommendations for the management of gastroesophageal reflux disease. A Latin American expert review. Rev. Gastroenterol. Mex. (Engl. Ed.) 2025, 90, 288–308. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Abdul, N.S.; Khalid Alkhelaiwi, A.; Awadh Alenazi, A.; Fehaid Alrashidi, R.; Ghaleb Salma, R. The Association of Helicobacter pylori in the Oral Cavity With Dental Caries in Patients With and Without Gastric Infection: A Systematic Review. Cureus 2023, 15, e38398. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cai, J.; Chen, Y.-M.; Wei, J.; Li, H.-B.; Lu, Y.; Zhou, Z.; Chen, X.-L. A meta-analysis of the association between the presence of Helicobacter pylori and periodontal diseases. Medicine 2019, 98, e15922. [Google Scholar] [CrossRef]
- Bouziane, A.; Ahid, S.; Abouqal, R.; Ennibi, O. Effect of periodontal therapy on prevention of gastric Helicobacter pylori recurrence: A systematic review and meta-analysis. J. Clin. Periodontol. 2012, 39, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A. Periodontal Treatment Is Associated With Improvement in Gastric Helicobacter pylori Eradication: An Updated Meta-analysis of Clinical Trials. Int. Dent. J. 2021, 71, 188–196. [Google Scholar] [CrossRef]
- Urrutia-Baca, V.H.; Paz-Michel, B.A.; Calderon-Porras, A.N.; Valle, J.A.J.-D.; Alvarez-Fernández, W.J.; Mervitch-Sigal, N.; Rodríguez-León, M.A.; De La Garza-Ramos, M.A. Oral Hygiene With Neutral Electrolyzed Water and Systemic Therapy Increases Gastric Helicobacter pylori Eradication and Reduces Recurrence. Clin. Exp. Dent. Res. 2024, 10, e927. [Google Scholar] [CrossRef]
- Agarwal, S.; Jithendra, K.D. Presence of Helicobacter pylori in subgingival plaque of periodontitis patients with and without dyspepsia, detected by polymerase chain reaction and culture. J. Indian. Soc. Periodontol. 2012, 16, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, G.; Liu, S.-G.; Wei, F.-Z.; Lin, S.-Z. Influence of Different Periodontal Therapies on Eradication and Recurrence of Helicobacter pylori Infection. J. Int. Dent. Med. Res. 2023, 16, 204–208. [Google Scholar]
- El Batawi, H.Y.; Venkatachalam, T.; Francis, A.; Abujabal, R.; Shehadat, S.A. Dental Caries–A Hiding Niche for Helicobacter pylori in Children. J. Clin. Pediatr. Dent. 2020, 44, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, A.; Mahmoudpour, A.; Abolfazli, N.; Lafzi, A. Detection of Helicobacter pylori using PCR in dental plaque of patients with and without gastritis. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e28–e31. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Liang, D.; Hu, N.; Dai, X.; He, J.; Zhuang, H.; Zhao, W. Helicobacter pylori inhibited cell proliferation in human periodontal ligament fibroblasts through the Cdc25C/CDK1/cyclinB1 signaling cascade. J. Periodontal Implant. Sci. 2019, 49, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.-L.; Medina, M.-G.; Martín, G.-T.; Picón, S.-O.; Bancalari, A.; Merino, L.-A. Molecular detection of Helicobacter pylori in oral samples from patients suffering digestive pathologies. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e38–e42. [Google Scholar] [CrossRef]
- Mehdipour, A.; Chaboki, P.; Rasouli Asl, F.; Aghaali, M.; Sharifinejad, N.; Shams, S. Comparing the prevalence of Helicobacter pylori and virulence factors cagA, vacA, and dupA in supra-gingival dental plaques of children with and without dental caries: A case-control study. BMC Oral Health 2022, 22, 170. [Google Scholar] [CrossRef]
- Navabi, N.; Aramon, M.; Mirzazadeh, A. Does the presence of the Helicobacter pylori in the dental plaque associate with its gastric infection? A meta-analysis and systematic review. Dent. Res. J. 2011, 8, 178–182. [Google Scholar] [CrossRef]
- Pataro, A.L.; Cortelli, S.C.; Abreu, M.H.N.G.; Cortelli, J.R.; Franco, G.C.N.; Aquino, D.R.; Cota, L.O.M.; Costa, F.O. Frequency of periodontal pathogens and Helicobacter pylori in the mouths and stomachs of obese individuals submitted to bariatric surgery: A cross-sectional study. J. Appl. Oral Sci. 2016, 24, 229–238. [Google Scholar] [CrossRef]
- Ren, Q.; Yan, X.; Zhou, Y.; Li, W.X. Periodontal therapy as adjunctive treatment for gastric Helicobacter pylori infection. Cochrane Database Syst. Rev. 2016, 2, CD009477. [Google Scholar] [CrossRef] [PubMed]
- Sghaireen, M.G.; Alkhatib, A.; Alswilem, R.; Toriya, J.; Mizohata, A.; Alrowili, M.; Patil, S.; Osuga, N.; Alam, M.K. Relationship between Cotinine and Helicobactor Pylori with Caries among Saudi Adults. J. Hard Tissue Biol. 2017, 26, 293–296. [Google Scholar] [CrossRef]
- Sruthi, M.A.; Mani, G.; Ramakrishnan, M.; Selvaraj, J. Dental caries as a source of Helicobacter pylori infection in children: An RT-PCR study. Int. J. Paediatr. Dent. 2023, 33, 82–88. [Google Scholar] [CrossRef]
- Tsimpiris, A.; Grigoriadis, A.; Tsolianos, I.; Moschos, I.; Goulis, D.G.; Kouklakis, G. Periodontitis and Helicobacter pylori Infection: Eradication and Periodontal Therapy Combination. Eur. J. Dent. 2022, 16, 145–152. [Google Scholar] [CrossRef]
- Tsimpiris, A.; Tsolianos, I.; Grigoriadis, A.; Moschos, I.; Goulis, D.G.; Kouklakis, G. Association of Chronic Periodontitis with Helicobacter pylori Infection in Stomach or Mouth: A Systematic Review and Meta-Analysis. Eur. J. Dent. 2023, 17, 270–282. [Google Scholar] [CrossRef]
- Zaric, S.; Bojic, B.; Popovic, B.; Milasin, J. Eradication of gastric Helicobacter pylori ameliorates halitosis and tongue coating. J. Contemp. Dent. Pract. 2015, 16, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Deng, X.; Zhou, X.; Hao, Y.; Li, Y. Influence of Helicobacter pylori culture supernatant on the ecological balance of a dual-species oral biofilm. J. Appl. Oral Sci. 2018, 26, e20170113. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, M.; Shu, H.; Chen, Z.; Liu, G.; Zhang, Y. Relationship between oral problems and Helicobacter pylori infection. Arch. Oral Biol. 2014, 59, 938–943. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.A.; Kheur, S.; Raj, A.T.; Mahajan, P. Association of Helicobacter pylori with oral potentially malignant disorders and oral squamous cell carcinoma-a systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Inchingolo, A.D.; Fatone, M.C.; Ferrante, L.; Casamassima, L.; Trilli, I.; Inchingolo, F.; Palermo, A.; Dipalma, G. The effect of periodontal treatment on Helicobacter pylori-infection: A systematic review. Periodontal Implant Res. 2025, 9, 3. [Google Scholar] [CrossRef]
- Yuksel Sert, S.; Ozturk, A.; Bektas, A.; Cengiz, M.I. Periodontal treatment is more effective in gastric Helicobacter pylori eradication in those patients who maintain good oral hygiene. Int. Dent. J. 2019, 69, 392–399. [Google Scholar] [CrossRef] [PubMed]
| N° | Author/Year | Type of Study | Country | Sample Size | Average Age | Samples in the Oral Cavity | With Gastric Infection | H. pylori Detection Method | Main Results |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abdul et al., 2023 [17] | Systematic review | Saudi Arabia | 15 included studies (ranging in size from 10 to 1050 subjects) | Variable according to study (between 3 and 74 years) | Miscellaneous (dental plaque, saliva, oral specimens) | Includes studies with and without gastric infection | Miscellaneous: mainly PCR, plus endoscopy, antigen, and checkerboard DNA-DNA | Associated oral H. pylori with increased caries, even without gastric infection. |
| 2 | Agarwal et al., 2012 [22] | Clinical study (cases and controls) | India | 50 patients (30 cases and 20 controls) | Cases: 46.2 ± 11.44 years; controls: 44.5 ± 11.36 years | Subgingival plaque | Cases: with infection. Controls: no symptoms. | PCR (16S rRNA) and culture | H. pylori was found in the subgingival plaque, related to periodontitis and dyspepsia. |
| 3 | Bouziane et al., 2012 [19] | Meta-analysis and systematic review | Morocco | 298 patients | Unspecified | Unspecified | Yes | Urea 13C breath test and PCR | Periodontal therapy reduced recurrence of gastric H. pylori by 63%. |
| 4 | Chen et al., 2019 [18] | Meta-analysis | China | 6200 patients | Unspecified | Unspecified | It includes both patients with gastric disease and the general population. | RUT, PCR, and ELISA (according to study) | Oral H. pylori increases the risk of periodontal disease (OR 2.31), especially in gastric patients (OR 3.50). |
| 5 | Chen et al., 2023 [23] | Randomized clinical trial | China | 160 | Unspecified | Not applicable | Yes | Urea 13C breath test | Periodontal therapy improves H. pylori eradication (87% vs. 75%) and reduces recurrence. |
| 6 | El Batawi et al., 2020 [24] | Cross-sectional study | United Arab Emirates | 48 children | Approximately 5.2 years (range 4–7) | Dentin samples from cavitated carious lesions | No (a non-gastric niche is studied) | PCR | H. pylori in childhood caries (30%) suggests oral reservoir. |
| 7 | Eskandari et al., 2010 [25] | Observational research study | Iran | 67 patients | 42.3 ± 12.52 years | Biofilm (supra- and subgingival) | 23 patients with gastritis; 44 without gastritis | PCR | H. pylori in dental plaque (5.9%) associated with gastritis (p = 0.012), a possible source of reinfection. |
| 8 | Li et al., 2019 [26] | In vitro experimental study | China | Not applicable (hPDLF culture) | Range 14–25 years | Periodontal ligament | Not applicable | Observation of invasion by transmission electron microscopy (TEM) | H. pylori inhibits the proliferation of periodontal fibroblasts via Cdc25C/CDK1/cyclin B1, causing G2 arrest. |
| 9 | Medina et al., 2010 [27] | Case–control study | Argentina | 98 patients (43 cases and 55 controls) | 43.7 years | Saliva and dental plaque | Yes (cases with digestive symptoms; 43 patients with positive biopsy) | PCR in oral specimens; histology (Giemsa and H&E) in gastric biopsies | H. pylori in the oral (18.4%) and gastric (88.4%) cavities correlates with periodontal disease (p < 0.05), risk of reinfection. |
| 10 | Mehdipour et al., 2022 [28] | Case–control study | Iran | 72 (36 cases and 36 controls) | 7.97 ± 1.83 years | Dental plaque, lower molars | No | PCR (16S rRNA gene detection and virulence analysis: vacAm1, vacAs1, dupA) | H. pylori in dental plaque in children (20.8%), not related to caries. |
| 11 | Navabi et al., [29] | Meta-analysis and systematic review | Iran | 1861 patients (745 men and 790 women) | 42.8 ± 7.4 years | Dental plaque | Coinfection in plaque and stomach: 49.7% | Several (PCR, RUT, culture, serology, histology) depending on each study. | 50% with oral–gastric coinfection, but doubtful role in reinfection. |
| 12 | Ozturk, 2021 [20] | Meta-analysis | Türkiye | 1450 participants | Unspecified | Not assessed (meta-analysis study) | Yes (patients undergoing eradication therapy) | Breath test with 13C urea and other methods reported in clinical trials | Periodontal treatment improves gastric eradication (OR = 4.11) and reduces recurrence (OR = 5.36). |
| 13 | Pataro et al., 2016 [30] | Cross-sectional study | Brazil | 154 participants | Adults (18–65 years old) | Saliva and tongue scraping | H. pylori detected in 83.3% of gastric biopsies | PCR | Obese people have a high presence of periodontal pathogens and H. pylori in the mouth/stomach. Bariatric surgery reduces bacteria in the stomach but increases them in the mouth. |
| 14 | Ren et al., 2016 [31] | Systematic review and meta-analysis | China | 691 participants | 17–78 years | Saliva, dental plaque, periodontal pockets | Yes | Urea breath test (13C or 14C), RUT, histology, PCR | Periodontal therapy improves gastric H. pylori eradication and reduces recurrence, but more studies are needed. |
| 15 | Sghaireen et al., 2017 [32] | Cross-sectional study | Saudi Arabia | 120 male students | 22.37 ± 1.50 years | Dental plaque (PCR) | Yes (93 students, 77.5%) | Stool antigen test (Helicobacter Antigen Quick, GA Inc., Dresden, Germany) | Significant association between H. pylori, cotinine (tobacco), and dental caries. |
| 16 | Sruthi et al., 2023 [33] | Cross-sectional study | India | 20 children | 4.85 years | Deep carious lesions (dentin) | No (only children without gastric diseases) | RT-PCR (reverse transcription and polymerase chain reaction) | H. pylori in 70% of children with severe caries, related to an increase in FMDD. |
| 17 | Tsimpiris et al., 2022 [34] | Clinical Study | Greece | 65 (33 with periodontitis and 32 controls) | 55.5 years (average) | Saliva (rtPCR) | 6 patients with periodontitis and 7 controls | Saliva: rtPCR (real-time PCR) | No association was found between chronic periodontitis and oral/gastric H. pylori. |
| 18 | Tsimpiris et al., 2023 [35] | Systematic review and meta-analysis | Greece | 818 (total subjects in meta-analysis) | Unspecified | Subgingival plaque | Not specified (varies between included studies) | RUT (rapid urease test) and PCR (polymerase chain reaction) | There is no H. pylori in saliva, but periodontal improvement after gastric eradication. |
| 19 | Urrutia-Baca et al., 2024 [21] | Randomized clinical trial | Mexico | 100 participants | 43.34 years (average) | Dental plaque (qPCR) | 100 patients (all with gastric infection) | Dental plaque: qPCR (quantitative PCR) | New systemic therapy achieved gastric eradication in 84–96% and fewer recurrences. |
| 20 | Zaric et al., 2015 [36] | Clinical intervention study (double-blind, case–control) | Serbia | 98 (66 with gastric infection) | Range: 19–78 years old | Saliva (nested PCR) | 66 patients (with gastric infection) | Saliva: nested PCR | Gastric eradication significantly reduced halitosis and the lining of the tongue. |
| 21 | Zhang et al., 2018 [37] | In vitro experimental study | China | Unspecified | Unspecified | Dental plaque, saliva | Yes (H. pylori-positive) | Culture and PCR | H. pylori alters the oral balance, favoring Streptococcus mutans and promoting caries. |
| 22 | Zheng et al., 2014 [38] | Cross-sectional study (population analysis) | China | 54,036 | 46.3 years | Dental plaque, saliva | Yes (46.97% positive) | 13C-labeled urea breath test | H. pylori is associated with dental calculus and tooth mobility, as well as factors such as smoking and obesity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuba, E.; Sánchez, M.C.; Ciudad, M.J.; Collado, L. Association of Helicobacter pylori as an Extragastric Reservoir in the Oral Cavity with Oral Diseases in Patients with and Without Gastritis—A Systematic Review. Microorganisms 2025, 13, 1955. https://doi.org/10.3390/microorganisms13081955
Cuba E, Sánchez MC, Ciudad MJ, Collado L. Association of Helicobacter pylori as an Extragastric Reservoir in the Oral Cavity with Oral Diseases in Patients with and Without Gastritis—A Systematic Review. Microorganisms. 2025; 13(8):1955. https://doi.org/10.3390/microorganisms13081955
Chicago/Turabian StyleCuba, Eber, María C. Sánchez, María J. Ciudad, and Luis Collado. 2025. "Association of Helicobacter pylori as an Extragastric Reservoir in the Oral Cavity with Oral Diseases in Patients with and Without Gastritis—A Systematic Review" Microorganisms 13, no. 8: 1955. https://doi.org/10.3390/microorganisms13081955
APA StyleCuba, E., Sánchez, M. C., Ciudad, M. J., & Collado, L. (2025). Association of Helicobacter pylori as an Extragastric Reservoir in the Oral Cavity with Oral Diseases in Patients with and Without Gastritis—A Systematic Review. Microorganisms, 13(8), 1955. https://doi.org/10.3390/microorganisms13081955






