Abstract
Alzheimer’s disease (AD), a prevalent neurodegenerative disorder in the aging population, remains without definitive therapeutic solutions. Emerging insights into the gut microbiota (GM) and its bidirectional communication with the central nervous system(CNS) through the microbiota–gut–brain axis (MGBA) have unveiled potential correlative mechanisms that may contribute to AD pathogenesis, though causal evidence remains limited. Dysregulation of GM composition (dysbiosis) exacerbates AD progression via neuroinflammation, amyloid-β (Aβ) deposition, and tau hyperphosphorylation (p-tau), while restoring microbial homeostasis presents a promising therapeutic strategy. Fecal microbiota transplantation (FMT), a technique to reconstitute gut ecology by transferring processed fecal matter from healthy donors, has demonstrated efficacy in ameliorating cognitive deficits and neuropathology in AD animal models. Preclinical studies reveal that FMT reduces Aβ plaques, normalizes tau phosphorylation, suppresses inflammasome activation, and restores microglial homeostasis through modulation of microbial metabolites and immune pathways. Although clinical evidence remains limited to case reports and small-scale trials showing potential therapeutic effect, safety concerns regarding long-term effects and protocol standardization necessitate further investigation. This review synthesizes current knowledge on GM–AD interactions, evaluates FMT’s mechanistic potential, and discusses challenges in translating this ancient practice into a cutting-edge AD therapy. Rigorous randomized controlled trials and personalized microbiota-based interventions are imperative to advance FMT from bench to bedside.
1. Introduction
Alzheimer’s disease (AD), the most prevalent neurodegenerative disorder worldwide, currently affects over 35 million individuals and is projected to triple by 2050 due to global population aging [1]. Characterized by progressive cognitive decline and neuropathological hallmarks including amyloid-β (Aβ) plaques, neurofibrillary tangles (NFTs) of hyperphosphorylated tau, and chronic neuroinflammation, AD remains incurable with existing therapies offering only symptomatic relief [2]. Current pharmacological interventions for AD primarily comprise symptomatic treatments, such as cholinesterase inhibitors (e.g., donepezil) and memantine, which provide modest cognitive improvement but fail to halt disease progression [3,4]. Emerging disease-modifying therapies (DMTs)—notably anti-amyloid β monoclonal antibodies like lecanemab—have demonstrated efficacy in slowing cognitive decline during early AD stages; however, their long-term benefits and safety profiles remain controversial [5]. The failure of more than 200 clinical trials targeting Aβ and tau pathology in the past decade [6] has necessitated a paradigm shift toward understanding modifiable risk factors, particularly the emerging role of gut–brain axis dysregulation in AD pathogenesis.
Recent breakthroughs in microbiome research have unveiled the gut microbiota (GM) as a critical regulator of neurological health through the microbiota–gut–brain axis (MGBA) [7]. Mounting evidence indicates that GM dysbiosis precedes AD onset and correlates with disease severity [8], mediated through multiple interconnected mechanisms: (1) microbial metabolite-mediated neuroinflammation [9], (2) dysregulation of neurotransmitter systems (serotonin, GABA) [10], (3) endocrine disruption via hypothalamus–pituitary–adrenal (HPA) axis overactivation [11], and (4) compromised intestinal and blood–brain barrier (BBB) integrity [12]. Notably, the “gut-first” pathological model posits that age-related GM changes may initiate peripheral inflammation that subsequently breaches theBBB, creating a self-perpetuating cycle of neurodegeneration [13].
In this context, fecal microbiota transplantation(FMT) has emerged as a revolutionary therapeutic strategy. While traditionally used for Clostridioides difficile infection, preclinical studies demonstrate FMT’s capacity to remodel gut ecosystems, reduce Aβ burden by 40–60% in AD mouse models [14], and reverse tau hyperphosphorylation (p-tau) [15]. The GUT-PARFECT trial’s recent success in Parkinson’s disease [16] and case reports of cognitive improvement in AD patients [17] further support its translational potential. However, critical knowledge gaps persist regarding donor selection criteria, long-term safety profiles, and the precise mechanisms underlying FMT’s neuromodulatory effects.
This review offers the first comprehensive analysis of FMT dual regulatory role in AD progression. Additionally, we propose a novel “multi-hit” model that integrates neural, endocrine, metabolic, and immunological pathways. This model elucidates how GM modulation influences AD pathology and aims to bridge the translational gap between animal studies and clinical applications.
2. Gut Microbiota and Microbial–Gut–Brain Axis Mechanisms
2.1. Gut Microbiota
The human body hosts a vast array of microorganisms, collectively termed the microbiota, which reside in various niches including the urogenital tract, respiratory system, skin, oral cavity, and most abundantly, the GIT. It is estimated that 95% of these symbiotic microorganisms are found in the gut [18], comprising a diverse ecosystem known as the GM, which contains approximately 100 trillion microorganisms, including bacteria, fungi, phages, and viruses [19]. In a healthy state, the gut is dominated by the phyla Firmicutes and Bacteroidetes, which together account for more than 90% of the microbial population. Based on the interaction of these bacteria with the host, the GM can be categorized into three groups: symbiotic beneficial bacteria such as Bifidobacteria and Lactobacilli; commensal opportunistic pathogens like Escherichia and Enterococcus; and pathogenic bacteria such as Proteus and Salmonella typhi [20].
The GM is integral to human health, participating in metabolic processes, immune system constitution, nutrient absorption, detoxification, vitamin synthesis, and pathogen exclusion [21]. These microorganisms coexist in a delicate balance, with their interdependence maintaining homeostasis in healthy individuals. However, this balance shifts with age. Mitsuoka [22] observed that as age increases, the gut Bifidobacteria decrease, while Clostridium, Streptococcus, and Enterobacteriaceae become more abundant. Elderly individuals exhibit a reduced abundance of probiotics and an increase in proinflammatory opportunistic pathogens compared to younger adults [23]. A diminished diversity and altered abundance of GM lead to dysbiosis, a state that can precipitate metabolic disorders and is closely associated with the development of gastrointestinal, immune system, metabolic, and nervous system diseases [24]. Dysbiosis may serve as a disease indicator, and its intervention could be a crucial strategy for disease prevention and treatment.
Research indicates that approximately 45% of bacterial species detected in the gut originate from the oral cavity, underscoring the significant contribution of oral microbiota to intestinal microbial diversity [25]. Oral dysbiosis refers to the disruption of microbial community equilibrium in the oral niche, characterized by overgrowth of pathobionts (e.g., Porphyromonas gingivalis, Fusobacterium nucleatum) and depletion of beneficial taxa (e.g., Streptococcus salivarius, Lactobacillus spp.) [26]. Salivary microbiome analyses reveal that Porphyromonas gingivalis—a recognized periodontal pathogen—can survive gastric acidity and proliferate upon intestinal translocation, thereby driving gut dysbiosis [27]. Lipopolysaccharide (LPS) produced by P. gingivalis enters systemic circulation, triggering low-grade systemic inflammation, insulin resistance, and increased intestinal permeability [28]. Moreover, Fusobacterium nucleatum, another periodontitis-associated pathobiont, translocates to the gut, disrupting microbial composition and impairing immune and metabolic regulation [29]. Collectively, oral dysbiosis is not an isolated event; through dynamic crosstalk with the gut microbiome, it synergistically accelerates cognitive decline via immunologic, metabolic, and neural pathways.
2.2. MGBA Mechanisms of AD
2.2.1. Neural Pathways
The MGBA is a complex and dynamic communication network that encompasses the bidirectional interactions between the GM and the central nervous system (CNS). This intricate axis facilitates the influence of the GM on CNS activity through a multitude of pathways, which include neural, endocrine, metabolic, and immune signaling, as well as the modulation of the gut mucosal barrier and the BBB (Figure 1). These interactions play a pivotal role in regulating brain function, impacting the nervous system, and shaping cognitive abilities.
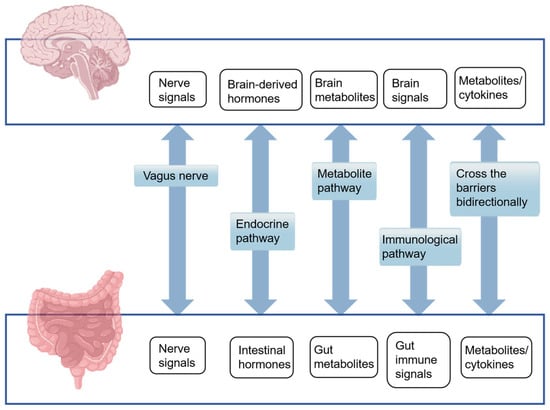
Figure 1.
Five major pathways of the human microbiota–gut–brain axis mechanism.
The human body is equipped with a vast network of enteric neurons and the vagus nerve, which are integral to the communication between the gut and the brain. The vagus nerve, a key component of the parasympathetic nervous system, is composed of approximately 80% afferent fibers and 20% efferent fibers [30]. This nerve serves as a conduit connecting the GIT to the brain and is a vital element of the MGBA. Information originating from the gut is conveyed to the brain via enteric neurons and the vagus nerve, enabling the brain to regulate and modulate gut functions.
Research by Liu et al. [31] demonstrated that the administration of Lacticaseibacillus rhamnosus to mice led to a reduction in anxiety behaviors, a decrease in HPA axis activity, and an increase in exploratory behavior. However, these effects were not observed in mice with a severed vagus nerve, thereby underscoring the essential role of the vagus nerve in mediating the communication between gut bacteria and the brain. The GM can influence the vagus nerve through two primary mechanisms. Firstly, it can directly interact with the enteric nervous system, thereby activating the associated vagus nerve. The local signals generated by this interaction are transmitted through sensory neural circuits to brain regions responsible for cognition, emotion, fear, and anxiety [32], thereby modulating brain function and potentially impacting the progression of AD. Secondly, the GM produces metabolites such as short-chain fatty acids (SCFAs) and lipopolysaccharides(LPS) that can stimulate the vagus nerve. The vagus nerve’s surface is equipped with toll-like receptors (TLRs) 2, 3, 4, and 7, which are capable of detecting signals from the GM [33,34], establishing a bidirectional communication pathway between the gut and the brain (Figure 2).
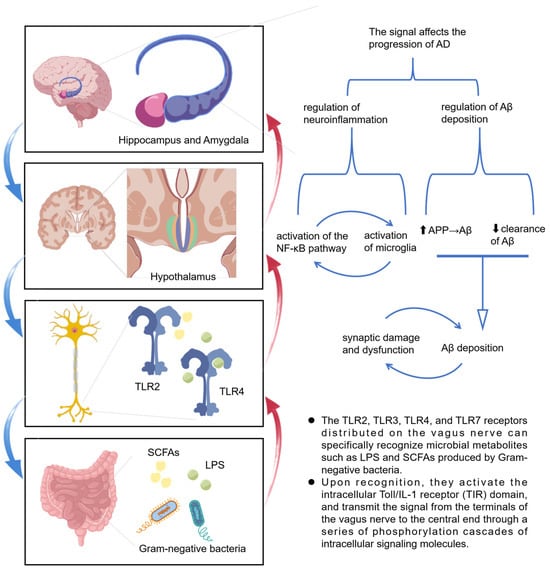
Figure 2.
Schematic diagram of vagus nerve signaling stimulated by microbial metabolites (SCFAs, LPS) in Alzheimer’s disease progression. The upward arrow “⬆” indicates that the expression of amyloid precursor protein (APP) is increased, which promotes the formation of amyloid-β (Aβ), while the downward arrow “⬇” indicates that the clearance of Aβ is decreased; these two mechanisms together lead to the accumulation of Aβ.
2.2.2. Endocrine Pathway
The endocrine pathway is a critical component of the MGBA, through which the GM can exert significant influence on neurotransmitter balance and brain function, potentially impacting the development and progression of AD. Neurotransmitters, the chemical messengers of the nervous system, are categorized as either excitatory or inhibitory. An imbalance between these two types can lead to abnormal neural activity, which may contribute to the pathology of AD [35]. The GM plays a dual role in this context: it can directly produce precursors to neurotransmitters and can also modulate their synthesis through dietary metabolism, thereby influencing neurotransmitter levels via neuroendocrine pathways. Notable neurotransmitters in this context include 5-hydroxytryptamine (5-HT, also known as serotonin) and γ-aminobutyric acid (GABA). More than 90% of the body synthesis of 5-HT, a neurotransmitter closely associated with mood regulation, occurs in enterochromaffin cells. The GM can interact with colonic epithelial cells to regulate 5-HT levels in the colon and serum. Furthermore, it can modulate the gene expression of tryptophan hydroxylase 1, the rate-limiting enzyme in 5-HT synthesis, thereby influencing 5-HT levels and brain function [10]. 5-HT has been shown to have a positive effect on cognitive function in AD patients. Direct injection of 5-HT into the hippocampus of mice has been observed to reduce levels of Aβ in the cerebrospinal fluid [36]. The GM also plays a role in GABA synthesis. Microbiota such as Bifidobacterium and Lactobacillus can convert glutamate to GABA. With aging, the abundance of these beneficial bacteria tends to decrease, leading to reduced GABA production. This can disrupt the balance between the sympathetic and parasympathetic nervous systems, potentially leading to overactivity of the brain. Excessive excitation can damage neurons, or even lead to the loss of neurons, ultimately affecting memory formation and maintenance [37]. Additionally, the GM can modulate brain and gut function through the HPA axis [11]. The HPA axis is a crucial part of the neuroendocrine system that responds to stress. Under stress, the HPA axis can become overactivated, leading to the overproduction of corticosteroids and alterations in GM composition. These changes can influence the CNS by modulating stress hormones, resulting in elevated corticosteroid levels that can affect the structure of the hippocampus and amygdala, thereby impairing cognitive function [11,38]. In summary, the endocrine pathway within the MGBA provides a mechanism through which the GM can directly and indirectly influence neurotransmitter synthesis and brain function.
2.2.3. Microbial Metabolite
Microbial metabolites and bioactive peptides are key players in the MGBA, exerting significant influence on the progression of AD. Among these metabolites, LPS and SCFAs produced by the GM have been implicated in shaping the disease’s trajectory.
Ageing is associated with an increase in Gram-negative bacteria, particularly Bacteroides in the GM, leading to heightened endotoxin LPS production. LPS is known to trigger neuroinflammatory responses by engaging TLR4, which in turn leads to the overactivation of microglia and amplifies their cytotoxic effects. This can result in irreversible pathological changes characteristic of AD and cognitive memory impairments [39]. Furthermore, LPS can induce the transformation of astrocytes into neurotoxic astrocytes, contributing to neuronal and oligodendrocyte death [40]. Clinical studies have revealed higher LPS levels in the brains of AD patients compared to age-matched controls, underscoring the link between excessive LPS and AD pathogenesis [41].
Short-chain fatty acids (SCFAs, e.g., butyrate, propionate)—produced by GM fermenting dietary fiber—exert neuroprotection via epigenetic regulation, mitochondrial homeostasis maintenance, and immunomodulation. In 5xFAD transgenic AD mice, butyrate inhibits histone deacetylase (HDAC), upregulates brain-derived neurotrophic factor (BDNF) expression, and enhances synaptophysin (SYP) activity, improving spatial memory (reduction in Morris water maze escape latency) [42]. Propionic acid activates GPR41/GPR43 receptors to regulate neuronal mitochondrial dynamics by ① downregulating Drp1 to reduce excessive fission, and ② activating the PINK1/PARKIN pathway to clear damaged mitochondria via mitophagy, decreasing Aβ-induced neuronal apoptosis [43]. Notably, butyrate-producing bacteria (e.g., Faecalibacterium prausnifera) are depleted in AD patients and models, while exogenous butyrate restores blood–brain barrier integrity and suppresses microglial IL-6 secretion [44,45]. Research has indicated significantly reduced SCFAs levels in the intestines of AD patients [8]. A deficiency in SCFAs may, therefore, lead to impaired Aβ clearance, contributing to the pathogenesis of AD [46,47].
Secondary bile acids (e.g., deoxycholic acid, DCA) suppress neuroinflammation by activating the farnesoid X receptor (FXR). However, reduced DCA/CA (cholic acid) ratios in AD patients impair FXR signaling, resulting in an increase in microglial IL-1β secretion [48]. Trimethylamine N-oxide (TMAO)—derived from gut microbial metabolism of dietary choline—disrupts blood–brain barrier tight junctions, promotes tau hyperphosphorylation, and activates the NLRP3 inflammasome, correlating with elevated mild cognitive impairment (MCI)-to-AD conversion risk [48,49]. Furthermore, polyunsaturated fatty acid metabolites like prostaglandin E2 (PGE2) are enriched in the AD gut, exacerbating neuroinflammation and Aβ generation via the C/EBPβ/AEP pathway. Aspirin-mediated inhibition of PGE2 synthesis ameliorates cognitive deficits in 5xFAD mice [45,50].
Tryptophan metabolites exert bidirectional effects on AD pathogenesis through kynurenine and indole derivative pathways. In the kynurenine pathway, upregulated tryptophan 2,3-dioxygenase (TDO) in the AD gut leads to accumulation of neurotoxic quinolinic acid, which activates NMDA receptors and induces oxidative stress (hippocampal lipid peroxidation ↑ 48%). Quinolinic acid crosses the blood–brain barrier, triggering microglial TNF-α release and exacerbating Aβ deposition [48]. Conversely, in the indole pathway, probiotics (e.g., Lactobacillus fermentum) convert tryptophan to indole-3-propionic acid (IPA). IPA activates the aryl hydrocarbon receptor (AhR) to inhibit NF-κB signaling, reducing Aβ plaques and phosphorylated tau in APP/PS1 mice. It also preserves GABAergic synaptic function, improving cognitive impairment [42,49]. Interventions combining Qingke β-glucan with lactobacilli significantly lower the kynurenine/tryptophan ratio, reversing neurotransmitter imbalances in AD models [42].
In summary, microbial metabolites such as LPS and SCFAs are integral to the MGBA and can significantly influence AD progression.
2.2.4. Immunological Pathway
As shown in Figure 3, the immunological pathway is a critical component of the MGBA, through which the GM plays a pivotal role in modulating host immune homeostasis and influencing the pathogenesis of AD. As we age, the GM undergoes significant changes in both quantity and composition, often favoring the proliferation of pro-inflammatory bacteria while suppressing the growth of anti-inflammatory species. These microbial shifts can activate peripheral immune cells, leading to a surge in inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). A critical mediator linking these microbial shifts to amplified inflammatory responses is the NLRP3 inflammasome, a cytosolic multiprotein complex that acts as a key sensor of cellular stress. Upon activation by microbial components, aggregated Aβ, or oxidative stress—all of which are hallmarks of AD pathogenesis—the NLRP3 inflammasome triggers the cleavage of pro-caspase-1 into its active form, thereby promoting maturation and secretion of pro-inflammatory cytokines like IL-1β, a central driver of neuroinflammation in AD [51,52]. Additionally, NLRP3 activation induces pyroptosis, a pro-inflammatory form of cell death that releases intracellular contents, further exacerbating local and systemic inflammation. In the context of AD, sustained NLRP3 activation in microglia and astrocytes accelerates Aβ deposition and tau hyperphosphorylation [53], creating a vicious cycle between neuroinflammation and neurodegeneration. Notably, gut microbial metabolites such as SCFAs, whose levels decline with age-related dysbiosis, normally suppress NLRP3 activation [54], highlighting a direct link between GM dysregulation and inflammasome-mediated neuroinflammatory processes in AD. This activation can initiate a cascade of localized systemic inflammation, increase gastrointestinal permeability, and affect the CNS through the bloodstream. It can compromise the integrity of the BBB, foster neuroinflammation, and ultimately impair cognitive function [13,55]. The gut microbiome also plays a role in the selection and activity of invariant natural killer T cells (iNKT), further modulating immune responses [56]. This activation stimulates immune cells to secrete pro-inflammatory cytokines and immunoglobulins (IgM/IgA), thereby exacerbating systemic inflammation [57,58]. These pro-inflammatory cytokines can, in turn, enhance the expression of the amyloid precursor protein (APP), promoting the formation of Aβ in the hippocampus [59]. Microglia, the resident immune effector cells of the CNS, can initiate neuroinflammation in response to changes in GM metabolites, further contributing to the neuroinflammatory environment associated with AD. In summary, the immunological pathway within the MGBA is a key conduit through which the GM can influence the peripheral immune system and, consequently, the CNS. By modulating the balance of peripheral inflammatory factors, the GM can either promote or ameliorate the neuroinflammatory processes that characterize AD.
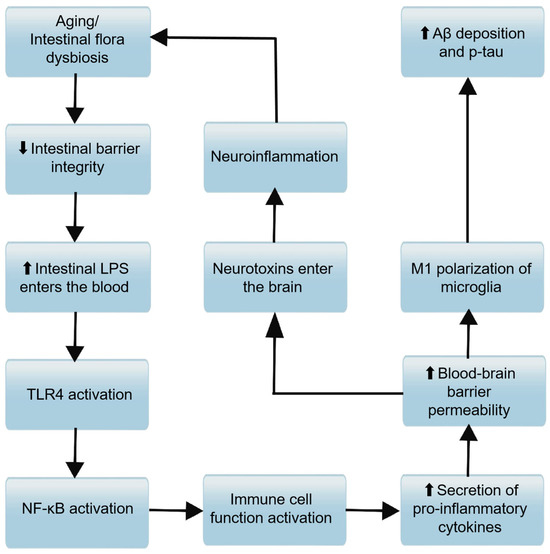
Figure 3.
Molecular mechanism associations between immune pathways and barrier functions in the progression of AD. Aging or intestinal flora dysbiosis downregulates intestinal barrier integrity (the downward arrow “⬇” denotes decreased integrity). Reduced intestinal barrier integrity allows more intestinal lipopolysaccharide (LPS) to enter the bloodstream (the upward arrow “⬆” denotes increased LPS entry). NF-κB activation promotes immune cell function activation, leading to increased secretion of pro-inflammatory cytokines.Elevated pro-inflammatory cytokines enhance blood-brain barrier permeability (the upward arrow “⬆” denotes increased permeability). Increased blood-brain barrier permeability facilitates M1 polarization of microglia; M1-polarized microglia then contribute to enhanced amyloid-β (Aβ) deposition and phosphorylation of tau (p-tau) (the upward arrow “⬆” denotes increased levels). Meanwhile, heightened blood-brain barrier permeability also enables neurotoxins to enter the brain; neurotoxins induce neuroinflammation, and neuroinflammation further reinforces “aging or intestinal flora dysbiosis,” forming a vicious cycle that drives AD progression.
2.2.5. Intestinal Wall Barrier and Blood–Brain Barrier
The integrity of the intestinal wall barrier and the BBB is integral to maintaining health and preventing disease, including AD. The MGBA encompasses these barriers, highlighting the influence of GM on their function and the subsequent impact on neurological health. Alterations in the GM’s structure can disrupt the ecological balance of the gut, leading to changes in intestinal barrier permeability. Aging is associated with increased permeability of both the intestinal mucosal barrier and the BBB, a phenomenon that can have profound implications for systemic health and neurological function. The GM is crucial for maintaining the integrity of the BBB: studies in germ-free mice have shown increased BBB permeability, possibly due to reduced expression of tight junction proteins, which are essential for maintaining the barrier’s integrity [12]. When intestinal barrier permeability is increased, pathogenic bacteria and harmful microbial metabolites, such as LPS, can translocate into the systemic circulation. LPS can directly damage endothelial cells or bind to TLR4, initiating a cascade of systemic inflammation by activating the expression of NF-κB and producing inflammatory mediators such as IL-6, TNF-α, and prostaglandin E2 [9]. This activation of the host immune defense system can lead to a state of chronic low-grade inflammation. These inflammatory mediators subsequently compromise BBB integrity, initiating a cascade of events that includes neuroinflammation, cognitive deterioration, and impaired CNS regulation of gut function. This self-perpetuating cycle creates a detrimental feedback loop that potentially accelerates AD pathogenesis and progression. Increased permeability of the BBB is indeed considered a hallmark of dysfunction in AD models [60]. In summary, the intestinal wall barrier and the BBB are critical components of the MGBA (Figure 3), with the GM playing a significant role in their maintenance. Disruptions in these barriers can lead to a cycle of inflammation and neuroinflammation, contributing to the pathogenesis of AD.
3. FMT for the Treatment of AD
3.1. FMT
3.1.1. The History and Development of FMT
FMT is a therapeutic procedure with a rich historical backdrop, dating back to ancient China. During the Eastern Jin Dynasty, the physician Ge Hong detailed the use of fecal suspensions to treat conditions like food poisoning and diarrhea in his seminal work Zhou Hou Bei Ji Fang [61]. In the 16th century, the renowned Chinese physician Li Shizhen further chronicled the effectiveness of FMT in treating severe vomiting, abdominal pain, diarrhea, constipation, and other digestive ailments in Bencao Gangmu [62].
FMT gained significant recognition in 2013 when Zhang et al. included it in clinical guidelines for the treatment of recurrent Clostridioides difficile infection (CDI) in the United States [63]. The expanding field of GM research has revealed increasingly promising therapeutic applications for FMT. The scope of FMT has evolved substantially beyond its initial focus on gastrointestinal disorders to encompass a broader spectrum of conditions, including neurological and metabolic diseases. Emerging experimental evidence demonstrates that FMT can effectively modulate the GM composition and regulate both neurotransmitter systems and microbial metabolites, potentially enhancing cognitive function in AD patients [13,64]. These findings provide novel therapeutic perspectives and mechanistic insights for AD intervention strategies.
3.1.2. Selection of FMT Donors
Optimal donor selection for FMT requires comprehensive multidimensional evaluation to ensure safety and efficacy. Donors must undergo rigorous health screening to exclude active infections, inflammatory bowel diseases, metabolic disorders, and malignancies. Blood and stool testing should eliminate pathogens including Clostridioides difficile, multidrug-resistant organisms, and SARS-CoV-2. Psychological evaluation via the Symptom Checklist-90 (SCL-90) excludes mental health disorders (e.g., anxiety, depression), while behavioral screening disqualifies individuals with high-risk sexual behaviors or recent infectious disease exposure [65]. Antibiotic usage within 12 months prior to donation—particularly broad-spectrum antibiotics—significantly reduces FMT success rates and must be strictly restricted [66]. Microbial diversity and functional integrity are paramount: donors should exhibit high α-diversity (e.g., Shannon index > 5), stable core microbiota (e.g., phyla Bacteroidetes and Firmicutes), and abundant SCFAs-producing bacteria (e.g., Faecalibacterium prausnitzii) [65,67]. Metagenomic sequencing must assess functional gene profiles related to carbohydrate metabolism and anti-inflammatory pathways [68]. Younger donors (aged 18–30 years) demonstrate superior clinical outcomes [69].
A subset of donors demonstrating consistently higher remission rates across multiple recipients are termed “super-donors” [70]. China’s 2022 Expert Consensus on Donor Screening and Management for FMT establishes a “five-step six-dimensional” framework integrating physiological, psychological, lifestyle, and microbiological assessments to standardize donor quality [65]. International guidelines further emphasize multiplex screening for multidrug-resistant organisms to mitigate transmission risks [71].
3.1.3. Fecal Microbiota Preparation
The clinical implementation of FMT encompasses a rigorous and time-sensitive protocol. The procedure initiates with the procurement of fresh fecal material from thoroughly screened donors, followed by immediate anaerobic transport to designated FMT facilities. The microbiota preparation process utilizes sophisticated automated isolation systems (e.g., GenFMTer), employing sequential microfiltration and centrifugation techniques to achieve optimal microbial enrichment and purification, culminating in a standardized fecal suspension. This preparation is administered promptly, although cryopreservation with glycerol at −80 °C is feasible when necessary. The entire procedural workflow is optimized to comply with the “one-hour FMT protocol” [72].
3.1.4. Antibiotic Pretreatment
Recipient preparation typically involves a standardized protocol of antibiotic preconditioning and bowel preparation to establish an optimal environment for microbial engraftment [73]. Substantial evidence confirms that antibiotic preconditioning enhances FMT efficacy by minimizing microbial biomass and diversity in the gut, thereby creating a reduced niche for subsequent engraftment [74,75,76]. A recent real-world cohort study of 1170 CDI patients receiving FMT demonstrated a positive correlation between antibiotic pretreatment duration and clinical success: cure rates were 45% with 1–5 days versus 65% with >30 days [77]. This association was particularly pronounced in antibiotic-refractory CDI cases. The standard antibiotic regimen for patients with CDI is vancomycin (0.5 g, twice daily for 3 days), which enhances the colonization efficiency of donor microbiota by eliminating residual pathogens and reducing microbial load [78].
Although FMT is established as an effective therapy for recurrent Clostridioides difficile infection (rCDI), approximately 30% of patients fail to respond to initial FMT in clinical practice [77]. Intestinal biofilms are three-dimensional structures formed by microorganisms (bacteria, fungi, viruses) and their secreted extracellular polymeric substances (EPS, e.g., polysaccharides, proteins, DNA), adhering to the intestinal mucosa or luminal content [79]. These structurally complex and functionally diverse biofilms impair FMT efficacy through multiple mechanisms including antibiotic resistance shielding, engraftment interference, and preconditioning recalcitrance [80,81]. The combination of antibiotics and bowel cleansing enhances overall FMT efficacy [78]. Phage therapy may also target specific strains to disrupt biofilms and potentiate FMT outcomes [82]. Current FMT preconditioning remains dependent on conventional antibiotics such as vancomycin or antibiotic cocktail regimens. However, biofilm eradication necessitates prolonged high-dose therapies that rarely achieve satisfactory resolution and may elevate safety risks [79].
3.1.5. Administration Routes of FMT
Administration routes are categorized into upper and lower gastrointestinal approaches [83]. Upper gastrointestinal delivery modalities include oral capsule administration and nasogastric tube placement, while lower gastrointestinal approaches encompass endoscopic procedures and enema administration. Colonoscopy-guided FMT enables direct delivery of microbial suspensions to the colonic mucosa, ensuring high-dose microbiota engraftment and demonstrating superior efficacy for ulcerative colitis or severe (CDI [84]. However, this invasive approach carries a risk of perforation (albeit rare) and requires bowel preparation, which reduces patient acceptance. Oral capsules offer non-invasive, outpatient-administrable convenience, yet gastric acidity and digestive enzymes may compromise microbial viability, potentially attenuating therapeutic effects [85]. Nasogastric/nasojejunal tubes bypass gastric degradation but pose aspiration risks, particularly in sedated or neurologically impaired patients. Enemas provide low-cost, technically simple administration but are restricted to the distal colon, failing to reach proximal regions where microbiota–gut interactions are critical for conditions like irritable bowel syndrome [86]. Gastroscope-mediated gastroduodenal delivery shares limitations similar to enteric tubes, including procedural discomfort and limited proximal engraftment. Collectively, no single route demonstrates absolute superiority; optimal FMT administration necessitates weighing efficacy, safety, and disease-specific pathophysiological mechanisms.
The therapeutic applications of FMT demonstrate significant potential beyond traditional gastrointestinal interventions. Contemporary research has revealed promising therapeutic implications for neurological disorders, notably Parkinson’s disease. The GUT-PARFECT clinical trial demonstrated significant amelioration of motor symptoms in early-stage Parkinson’s disease patients, as quantified by MDS-UPDRS metrics, concurrent with improvement in associated gastrointestinal symptomatology [16]. Moreover, preclinical studies in AD mouse models have demonstrated FMT’s capacity to enhance cognitive function, attenuate β-amyloid deposition, and suppress tau hyperphosphorylation, collectively contributing to reduced neuroinflammation. These findings suggest the potential therapeutic utility of FMT in AD intervention strategies [14]. FMT represents a therapeutic paradigm that bridges historical medical practices with contemporary scientific advancement. Its emerging applications in AD and other neurological disorders exemplify the convergence of microbiome science and clinical medicine, representing a promising frontier in therapeutic innovation.
3.2. Role of FMT in Inducing and Alleviating AD in Mice
Animal studies have preliminarily shown that GM is closely linked to the onset and progression of AD. FMT from AD donors and health donors can deteriorate and improve symptoms and pathological manifestations in animal models, respectively. However, research is still confined to the animal experimental stage, and further investigation and validation are needed before clinical application.
3.2.1. FMT from AD Donors Promotes Disease Progression
Table 1 [87,88,89,90,91,92,93,94,95,96,97] summarizes some FMT animal experiments using AD donors. Transplanting microbiota from AD mice or patients into recipient animals has accelerated AD progression, primarily manifested by Aβ accumulation, hyperphosphorylation of tau protein, enhanced neuroinflammation, abnormalities in glial cells, and impairment of the intestinal wall barrier and the BBB. These pathological changes are interconnected, influencing each other through the MGBA, and collectively exacerbating cognitive impairment (Figure 4).

Table 1.
FMT animal experiments with AD donors.
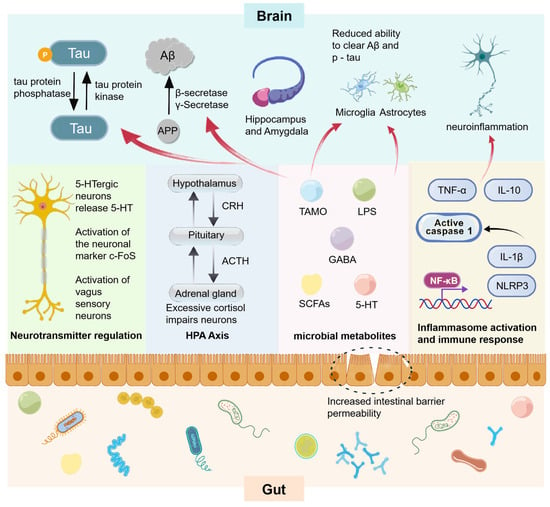
Figure 4.
Pathological processes and associations in animal experiments with fecal microbiota transplants associated with AD. Intestinal flora affects related enzymes and immune cells through multiple pathways including neural pathways, endocrine pathways, metabolite pathways, and immune pathways, and promotes the following AD pathological changes in brain structures dominated by the hippocampus and amygdala: (1) Tau protein is phosphorylated under the action of tau protein kinase, resulting in an increase in phosphorylated tau protein (p-tau); (2) The process of amyloid precursor protein (APP) generating amyloid-β protein (Aβ) through the action of β-secretase and γ-secretase is enhanced; (3) The ability of microglia and astrocytes to clear Aβ and p-tau is reduced; (4) The aforementioned processes further induce neuroinflammation. NF-κB activates the transcription of genes such as NLRP3. The NLRP3 inflammasome then activates caspase 1, which processes pro-IL-1β into active IL-1β. Cytokines like TNF-and IL-10 also participate. This drives inflammasome activation and immune response, contributing to neuroinflammation in AD.
Increment of Brain Aβ Levels
The Aβ hypothesis is a predominant theory for AD etiology. Both Aβ40 and Aβ42 are metabolites of APP, with Aβ42 exhibiting stronger neurotoxicity and a greater propensity to form plaques [98]. The hypothesis posits that increased Aβ production and/or decreased clearance leads to excessive accumulation, particularly of Aβ42, gradually forming oligomers and amyloid plaques [99]. This triggers pathological cascades, including tau hyperphosphorylation, NFTs, synaptic dysfunction, neuronal damage, and ultimately cognitive impairment [100]
Studies have shown that after receiving FMT from APP/PS1 transgenic mice, young recipient mice exhibited increased brain levels of Aβ38, Aβ40, and Aβ42, along with GM alterations [87]. It was also found that in the fecal microbiota of APP/PS1 mice, a notable reduction was observed in the phyla Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, while there was an increase in Bacteroides and Verrucomicrobia. At the genus level, Allobaculum and Akkermansia were found to decrease, whereas Rikenellaceae and the unclassified genus S24-7 were observed to increase. Furthermore, the relative abundance of Akkermansia exhibited a negative correlation with brain Aβ42 levels, whereas several bacterial genera showed a positive correlation with brain Aβ42 levels. In a study by Wang et al. [91], 3-month-old APPSWE/PS1ΔE9 mice that received fecal matter from 16-month-old APPSWE/PS1ΔE9 mice also demonstrated a significant increase in Aβ plaques, accompanied by inhibited activation of astrocytes surrounding the plaques. These findings suggest that the colonization of GM from AD mice in recipient mice can alter the GM structure and abundance, thereby promoting the progression of AD through increased Aβ pathology in the brain.
Increment of Harmful Microbial Metabolites
Trimethylamine N-oxide (TMAO), a metabolite derived from choline, betaine, and carnitine by the GM, has been implicated in promoting neuronal aging, enhancing mitochondrial damage, and increasing superoxide production. It is also associated with increased synaptic damage and a reduction in the expression levels of proteins related to synaptic plasticity by inhibiting the mTOR signaling pathway [101]. Additionally, TMAO has been linked to p-tau and the p-tau/Aβ42 ratio, indicating a potential close relationship with amyloid deposition and axonal injury in neurons [102]. Elevated TMAO levels disrupt blood–brain barrier tight junctions, promote tau hyperphosphorylation (p-Tau Ser396), and activate the NLRP3 inflammasome, exhibiting a positive correlation with mild cognitive impairment (MCI) to AD conversion risk [48].
The transplantation of fecal microbiota from APP/PS1 mice and AD patients into 6-month-old male C57BL/6J mice led to elevated plasma levels of TMAO and significant endoplasmic reticulum (ER) stress in the brain cortex [94]. The GM of the recipient mice became dysbiotic, with a decrease in Bacteroidetes and S24-7, and an increase in Firmicutes, Spirochaetaceae, and Desulfovibrionaceae. Elevated phosphorylation levels of cortical proteins and eIF2α indicated excessive ER stress. Another study also found increased levels of histidine and aminohexanoic acid in the recipient mice post-transplantation [93]. These experiments suggest that FMT from the GM of AD patients can increase harmful intestinal microbial metabolites in recipient mice, thereby affecting the gut–brain axis and promoting AD progression.
Impact on Neurotransmitter Endocrinology
5-HT signaling, associated with Aβ deposition and APP expression, mediates AD pathology through neuroendocrine pathways [103,104]. Muscarinic acetylcholine receptors (mAChRs), crucial in neurotransmitter regulation, play a significant role in neuronal excitability, synaptic transmission, and neurotransmitter release. A study transplanted feces from Tg2576 mice into AiDM-ICR mice, observing a decrease in 5-HT levels in the mid-colon of the recipients, along with reduced levels of mAChR M2, M3, and Gα proteins [96]. Similarly, a decrease in GABA levels in the intestines of germ-free C57BL/6N mice following FMT from an 82-year-old male AD patient was observed [88]. GABA deficiency can lead to hyperactive prefrontal and hippocampal neurons, compromising cognitive functions such as attention and memory [105]. As neurotransmitters influenced by GM, 5-HT and GABA regulate neural function and affect the CNS. FMT from AD donors can alter the gut ecology of the host, reducing neurotransmitter levels and weakening their regulatory effects on neural function within the MGBA through neuroendocrine pathways, ultimately leading to cognitive impairment.
Activation of Inflammatory Vesicles
The NLRP3 inflammasome, a key component of the innate immune system, plays a critical role in resisting pathogen infection and danger signal stimulation. Its activation is closely linked to AD. Transplanting the GM from AD patients into APP/PS1 double-transgenic mice resulted in increased NLRP3 expression in the intestines and elevated levels of inflammatory factors in peripheral blood. Mice receiving the transplant exhibited more severe cognitive impairments compared to controls that did not receive FMT from AD patients [89].
The NLRP3 inflammasome can be activated by various pathogens and stimuli, such as TLR4 or TNF signaling. This activation initiates the transcription of inflammasome components like NLRP3 and IL-1β via the NF-kB signaling pathway. The active aggregates of these components mediate the activation of caspase-1 and the maturation and release of downstream inflammatory factors, as well as apoptosis [106]. Kim et al. reported increased levels of TNF-α and IL-1β in both the colon and plasma of recipient mice that received FMT from 5xFAD mice [90]. The elevated levels of IL-1β, IL-10, and NLRP3 were also observed in mice after receiving FMT from AD patients [93]. Excessive inflammatory cytokines can lead to a persistent inflammatory response, excessive immunity, and neuronal apoptosis, which can also reach the CNS through blood circulation, deepening neuroinflammation and promoting cognitive decline and AD progression.
Abnormal Activation of Microglia
Microglia, which originate from the erythromyeloid progenitors in the yolk sac, are specialized macrophages that perform a variety of critical functions within the CNS. They regulate programmed cell death in neurons, eliminate superfluous synapses during neural development, and foster the formation of new synaptic connections. These cells are essential for preserving tissue homeostasis, coordinating responses to injury, and defending against infections [107]. Microglia engage with neurons and astrocytes to offer nutritional support, react to cytokines and metabolic cues from the neural milieu, and facilitate the optimization of functional neuronal networks [108]. When stimulated, microglia can transition from a quiescent state to either an M1 pro-inflammatory or an M2 anti-inflammatory phenotype, enabling them to engulf and clear away damaged cells, as well as surplus neurons and synapses [109].
In the research conducted by Jin et al. [97], WT mice that received microbiota transplants from 12-month-old APP/PS1 mice showed elevated levels of Iba1 and iNOS in the cortex and hippocampus of FMT-AD mice. These markers are typically indicative of microglial activation, suggesting an augmentation in the activation state of the recipient microglia. Kim et al. [90] reported that oral administration of fecal matter from 5xFAD mice to C57BL/6 mice resulted in heightened microglial activation. Transplantation of GM from AD donors modifies the recipient’s existing microbial ecosystem, leading to aberrant microglial activation and the release of numerous inflammatory mediators and neurotoxic agents [110]. This process mediates chronic neuroinflammation in the brain, which can damage neurons and synapses, ultimately resulting in cognitive deficits and accelerating the progression of AD [111].
3.2.2. Healthy Donor FMT Relieves Disease Progression
It has been observed that FMT from healthy mice or human volunteers can exert therapeutic effects on AD (Table 2 [14,15,89,112,113,114,115,116]). The levels of Aβ and hyperphosphorylated Tau protein in the recipients’ brains are observed to decline. Additionally, neuroinflammation is mitigated, the secretion of inflammatory cytokines decreases, microglial cells preserve their homeostatic state, and both the gastrointestinal and BBB function properly. As illustrated in Figure 4, the proliferation of beneficial gut bacteria and their metabolites alleviates the progression of AD by modulating various pathways.

Table 2.
FMT animal experiments with healthy donors.
Reduction Brain Aβ Level
The prevailing approach to AD treatment relies on the Aβ hypothesis [100]. A multitude of drugs and vaccines aimed at Aβ have been developed by researchers. However, the majority have failed to demonstrate the anticipated efficacy during Phase II or III clinical trials [6,117]. In light of the challenges faced by traditional drug development, FMT may present a novel therapeutic avenue for eliminating Aβ and preventing its aggregation. Elangovan et al. [114] performed a 7-day oral gavage FMT using feces from healthy B6SJL WT donor mice on 16 elderly 5xFAD recipient mice. The findings indicated that the FMT mice experienced a decrease in cortical amyloid protein load and quantifiable plaque counts, along with enhancements in cognitive function, new object recognition ability, and spatial memory. Additionally, the reduction in Aβ plaque load was inversely correlated with the trend of cognitive improvement. FMT from healthy donors may have impacted the mechanisms of Aβ clearance or renewal in the amygdala, hippocampus, and other brain structures. Animal studies suggest that FMT from healthy mice can effectively lower brain Aβ levels and successfully ameliorate cognitive deficits, underscoring the considerable potential of FMT in the treatment of AD.
Reduces Brain Tau Protein Phosphorylation Level
The hyperphosphorylation of tau protein, which contributes to the formation of NFTs, is a primary pathological hallmark of AD. Research suggests that the chronic inflammatory response in glial cells and neurons is a significant contributor to the hyperphosphorylation of tau protein, and this hyperphosphorylation subsequently amplifies the inflammatory response, further exacerbating AD pathology [118]. The serine/threonine protein phosphatase 2A (PP2A) is the most active phosphatase known for dephosphorylating hyperphosphorylated tau protein back to its normal state [119]. Following dephosphorylation, tau protein can resume its normal function. Experiments have employed carnosic acid (CA) to modulate PP2A and suppress the hyperphosphorylation of tau protein in the brains of APP/PS1 mice [120]. Recent research has introduced a dephosphorylation-targeting chimera (DEPTACs) strategy, designed to specifically recruit phosphatases to tau to mitigate its hyperphosphorylation as a therapeutic approach for AD. Despite the initiation of numerous clinical trials targeting tau protein, several challenges persist, and many potential drug trials have been halted due to toxicity and/or lack of efficacy [121]. FMT experiments revealed that tau phosphorylation at the threonine 231 site was notably reduced in mice treated with FMT, and cognitive function was enhanced, indicating that FMT can inhibit tau hyperphosphorylation in Tg mice [14]. Additionally, some studies propose that phosphorylated tau at serine 396 and 404 (p-tau396, 404) may act as a therapeutic target for AD, with p-tau396, 404 being more likely than Aβ plaques to induce neuronal damage [122,123,124]. These experiments confirm that FMT from healthy donors can ameliorate cognitive impairment by reducing the abnormal phosphorylation of tau protein at various brain sites.
Regulation of Abnormal GM and Its Metabolites
The GM and its metabolites, including TMAO, GABA, SCFA, and endotoxins, play a significant role in the progression of AD through various mechanisms. Dysbiosis of the gut microbiome results in alterations of metabolites that can exacerbate cognitive deficits. Introducing a healthy gut microbiome to restore normal levels of microorganisms and metabolites may alleviate symptoms of AD.
Transferring the GM from WT mice to AD mice reversed the increases of Firmicutes and Prevotella and the decreases of Bacteroides and Akkermansia [115]. Furthermore, the abundance of Vibrio, Odoribacter, and AF12 increased. Following FMT, the AD mice exhibited significant improvements in short-term memory and cognitive abilities, along with a reduction in inflammatory factors in the plasma and a decrease in Aβ plaque load in the hippocampus and cortex. Sun et al. [14] administered a fresh fecal solution from WT mice to APPswe/PS1dE9 transgenic mice, resulting in a reversal of the AD-related changes in GM and SCFAs levels in the recipient mice, with an increase in SCFAs and a decrease in Aβ40 and Aβ42. Meanwhile, cognitive function also improved. FMT from healthy donors regulated the abnormal composition and abundance of GM in AD mice, reversed the AD-related changes in the gut environment, reduced Aβ plaques, and improved cognitive dysfunction, demonstrating promising therapeutic effects for AD.
Reducing the Inflammatory Response
Glycolysis and lipid metabolism are pivotal in the activation of the NLRP3 inflammasome [125]. Curbing aberrant glucose metabolism can attenuate the activation of the NLRP3 inflammasome [126]. Furthermore, certain immune metabolites, including kynurenine and β-hydroxybutyrate (BHB), have been shown to inhibit the activation of the NLRP3 inflammasome [127,128,129]. Studies have suggested that suppressing the activation and expression of the NLRP3 inflammasome can diminish the polarization of microglia towards the M1 phenotype, reduce Aβ deposition and NFT formation, and alleviate neuroinflammation, positioning it as a crucial target for the prevention and treatment of early cognitive dysfunction [130].
A healthy GM can mitigate inflammatory responses by modulating digestive metabolic processes. When healthy human GM was transplanted into APP/PS1 mice that had previously been colonized with GM from AD patients, a downregulation of NLRP3 expression in the gut was observed, indicating enhancements in cognitive abilities, and a reduction in neuroinflammatory factors [89]. In addition, APP/PS1 transgenic mice that received FMT from healthy C57BL/6 J mice experienced regulation of their GM composition and abundance, which led to decreased levels of inflammatory factors such as IL-1β and IL-6, as well as reduced Aβ, resulting in improved cognitive function [116]. These animal experiments illustrate that the introduction of GM from healthy donors into mice can suppress the activation of the NLRP3 inflammasome, leading to decreased neuroinflammation and neuronal damage. This, in turn, enhances cognitive function and yields therapeutic benefits against AD.
Restoration of Microglia Homeostasis
Microglia play a pivotal role in the pathological progression of AD. Typically, they are responsible for eliminating excess neurons and preserving the equilibrium of the CNS. Nevertheless, they may transition into an aberrant activation state upon stimulation, secreting neurotoxic cytokines that inflict neuronal harm and result in cognitive deficits. Research focusing on microglia has put forth two strategies to ameliorate cognitive dysfunction. One involves restoring microglial equilibrium [131,132], while the other aims at selectively eradicating microglia [133,134]. A multitude of animal model studies suggest that modulating the function of dysfunctional microglia to reestablish normalcy could be a potent approach for eliminating Aβ plaques and preventing AD pathology [135,136]. Healthy human GM was transplanted into APP/PS1 mice that had previously been given GM from AD patients. The results showed that the activation of central hippocampal microglia was suppressed, and the cognitive abilities of the mice were enhanced [89]. Additionally, the reactivity of microglia in recipient mice was diminished, and a reduction in Aβ plaques and NFTs was observed, accompanied by improvements in cognitive impairment [15]. These animal experiments corroborate that following the recipient’s receipt of FMT from healthy donors, the abnormal activation of microglia can be mitigated, progressively reinstating healthy homeostasis and neuroprotective functions, thereby diminishing neuronal damage and exerting therapeutic effects against AD.
3.3. The Role of FMT in Clinical Remission of AD
The application of FMT in the treatment of AD has yielded encouraging outcomes in animal trials, prompting researchers to delve into its potential through clinical studies. To date, there have been several documented cases of FMT being utilized for AD treatment.
Hazan presented the first case study involving an 82-year-old male patient with AD, who also suffered from pneumonia and recurrent CDI in 2020 [17]. This patient underwent FMT from his 85-year-old wife. Remarkably, two months following the procedure, the patient exhibited enhanced mental acuity and emotional well-being, as evidenced by a rise in his MMSE score from 20 to 26. By the fourth month post-FMT, the patient’s memory had continued to improve, and his symptoms had not worsened. By the six-month mark post-procedure, the patient demonstrated substantial emotional progress, improved social interaction, and increased expressiveness, with his MMSE score further improving to 29.
A patient diagnosed with diabetes mellitus, hypertension, chronic kidney disease, and CDI underwent FMT from a healthy 29-year-old male donor. One month subsequent to the initial FMT, her cognitive abilities exhibited a modest enhancement, with her MMSE score increasing from 15 to 18 and MoCA score from 11 to 12. Following one week after the second FMT, MMSE and MoCA scores further improved to 20 and 17, respectively. The patient also reported substantial enhancements in mood and daily functioning, demonstrating a greater range of emotional expression. The report suggested that post-FMT, the patient’s GM displayed elevated levels of certain bacterial genera, such as Lactobacillus, Bacteroides, Tannerella, and Actinobacteria, which rectified the microbial imbalance in the recipient and modified the abundance of microbes impacting the MGBA [137].
In a clinical case-control study conducted [138], ten dementia patients with severe CDI who underwent FMT were compared with ten dementia patients with severe CDI treated with antibiotics. The FMT group showed significant improvements in clinical symptoms and cognitive function compared to those treated with antibiotics, accompanied by changes in gut microbiome composition, including increased abundance of Proteobacteria and Bacteroides, as well as enhancements in the metabolism of alanine, aspartate, and glutamate. This study preliminarily suggests the potential of FMT to alleviate and slow down cognitive decline in dementia patients.
Post-acute coronavirus disease 2019 syndrome (PACS) is a multisystem disorder characterized by a constellation of debilitating symptoms persisting beyond severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or coronavirus disease 2019 (COVID-19) [139]. PACS typically manifests with long-term debilitating symptoms affecting multiple organs and systems, and is frequently associated with gut microbiome dysbiosis [140]. An in vivo study demonstrated that FMT from PACS patients into germ-free mice—without SARS-CoV-2 exposure—induced pulmonary inflammation, exacerbated pathological lung alterations following multidrug-resistant Klebsiella pneumoniae infection, and triggered cognitive impairment [141]. Furthermore, a prospective interventional study involving 60 PACS patients with insomnia revealed that FMT effectively and safely alleviated post-COVID insomnia and anxiety, improved sleep quality, and reduced daytime hypersomnolence [142].
Dale E. Bredesen and Kenneth Sharlin outlined a cohort study of 100 patients with AD, mild cognitive impairment (MCI), and subjective cognitive impairment (SCI) who achieved cognitive improvement through the personalized precision medicine protocol ReCODE. This approach emphasizes identifying and targeting underlying etiological factors—including pathogen infections, intestinal permeability, insulin resistance, nutritional deficiencies, hormonal deficits, and toxin exposure—with tailored interventions such as dietary modification, gut barrier repair, insulin sensitivity enhancement, hormone replacement, and GM modulation. The study validated the efficacy of multifactorial targeted therapy [143]. These findings underscore the necessity for individualized treatment due to the heterogeneous combination of pathological drivers in each patient’s cognitive decline, thus providing a framework for integrating FMT with other therapeutic modalities to modify AD progression.
In summary, existing clinical studies have shown that FMT can effectively regulate the gut microbiome of patients with AD, and improvements in patients’ clinical symptoms and cognitive functions have been observed in the studies. However, the current scope of clinical trials is limited, mainly focusing on case reports and case-control studies with small sample sizes. To further confirm the clinical efficacy of FMT, more large-scale randomized controlled trials are needed.
4. Current Limitations and Perspectives of FMT in the Treatment of AD
Despite promising results in AD treatment, FMT faces several notable limitations and challenges that warrant careful consideration. The primary concern relates to adverse events (AE), ranging from mild gastrointestinal symptoms to severe complications. Common mild adverse events include diarrhea, bloating, and abdominal pain [144]. A case report documented an AD patient experiencing severe gastrointestinal distress following initial FMT, though symptoms improved after subsequent treatment [137]. Of particular concern are immunocompromised individuals and those with inflammatory bowel disease, who have been identified as high-risk populations for FMT-related complications [145]. This was tragically highlighted when the FDA reported fatality in an immunocompromised patient due to multi-drug resistant bacterial infection post-FMT [146]. Research indicates that FMT-related adverse events predominantly occur in patients with compromised mucosal barriers, with the upper gastrointestinal delivery route associated with higher adverse event incidence compared to lower gastrointestinal administration [147].
Long-term safety concerns persist regarding microbiome modification, including potential risks of obesity, metabolic syndrome, autoimmune diseases, and colon cancer [148]. Post-FMT weight gain has been documented in both preclinical and clinical studies [149,150]. While rare, the risk of pathogen transmission remains relevant, with reported cases of post-FMT Norovirus gastroenteritis [151]. To address these safety concerns, both the American Gastroenterology Association’s national FMT registry and China’s FMT platform have initiated comprehensive follow-up studies [152,153]. Methodological challenges in current FMT research include the persistence of indigenous microbiota despite broad-spectrum antibiotic pre-treatment, potentially confounding experimental results [63]. Furthermore, standardization issues persist regarding treatment protocols, dosing regimens, and intervention timing and frequency.
Recent research has revealed promising directions for FMT optimization. Studies demonstrate superior cognitive outcomes and reduced cortical amyloid levels with young versus elderly donors [114], suggesting donor age as a crucial variable for treatment efficacy. Raber’s team discovered that germ-free mice receiving FMT from AD model mice exhibited sex-specific differences in cognitive changes, which were further modulated by specific genotypes (e.g., AppNL-G-F/E4). For instance, male AppNL-G-F recipients showed impaired novel object recognition, whereas females did not. Among male recipients, those receiving AppNL-G-F/E4 microbiota demonstrated a trend toward a higher percentage of entries into the novel arm of the Y-maze compared to WT recipients. In contrast, donor genotype had no significant effect on the percentage of novel arm entries in female recipients [154]. A 2025 review highlighted that in FMT studies targeting cognitive improvement in hepatic encephalopathy or Parkinson’s disease, several trials reported sex-dependent efficacy (e.g., greater enhancement in executive function among male patients) [155]. However, no randomized controlled trials (RCTs) specifically investigating sex-specific responses to FMT in AD patients currently exist. Existing clinical data primarily derive from small exploratory studies that did not control for gender as a confounding variable. Additionally, targeted microbiota transplantation successfully restored GM composition and improved AD-related pathology using eight dominant species [116]. Future research directions should prioritize the identification and isolation of specific beneficial GM species for more targeted interventions, potentially leading to more precise and effective treatments. Investigation of preventive applications at different AD stages could expand our understanding of optimal intervention timing and potentially establish FMT as a preventive measure against severe AD progression. Integrated multi-omics analyses incorporatingGM, metabolome (e.g., SCFAs, TMAO), proteome (e.g., p-tau, NFL), and miRNA profiles (e.g., hsa-miR-4455) would enable the construction of high-specificity predictive models for AD during its prodromal phase or mild cognitive impairment (MCI) stage. A research project found that hsa-miR-4455 is the best biomarker in all omics analyses. The diagnostic index taking a ratio of hsa-miR-4455 to hsa-let-7b-3p predicted aMCI patients against healthy subjects with 97% overall accuracy [156]. One-hundred-and-sixty-two adults with asymptomatic cardiovascular disease but without diagnoses of cognitive impairment or dementia were evaluated. Phosphorylated tau217 accurately differentiated cognitive impairment in patients with cardiovascular disease [157]. Under the criteria based on relevant biomarkers, FMT can be implemented during the prodromal stage of AD or in patients with mild cognitive impairment (MCI) to better mitigate the progression of AD.
Additionally, optimization of donor selection criteria, particularly regarding donor age, warrants thorough investigation given the demonstrated superiority of young donors in preclinical studies. The development of standardized treatment protocols and delivery methods is crucial for improving reproducibility and clinical outcomes. Furthermore, establishment of long-term safety and efficacy profiles through rigorous clinical trials remains paramount.
5. Conclusions
Emerging evidence substantiates the pivotal role of GM dysbiosis in AD pathogenesis through multi-dimensional interactions along the MGBA. Our synthesis demonstrates that FMT exerts therapeutic effects by modulating Aβ metabolism and tau phosphorylation, restoring neuroendocrine equilibrium, attenuating neuroinflammation, enhancing intestinal and blood–brain barrier integrity. While preclinical studies demonstrate FMT’s remarkable potential in alleviating AD pathology, clinical translation requires methodologically rigorous randomized controlled trials to establish efficacy and safety profiles. Critical challenges include donor–recipient compatibility optimization, administration protocol standardization, and long-term outcome monitoring. Future research should focus on mechanistic exploration, technological innovation, personalized strategies, and preventive applications. This evolving therapeutic paradigm bridges ancient medical wisdom with modern systems biology, offering a transformative approach to combat neurodegenerative diseases through ecological restoration of the human microbiome.
Author Contributions
Conception and design: C.T. and J.R. conceived and designed the study. Data collection: J.R. performed data collection and experiments. Data analysis and interpretation: J.R. analyzed the data and interpreted the results. Manuscript writing: J.R. wrote the first draft of the manuscript. Manuscript revision: J.R. and C.T. revised the manuscript. Funding acquisition: H.H. and C.T. provided funding, materials, or technical support. Supervision: Q.W. supervised the project and provided critical feedback. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by One health Interdisciplinary Research Project, Ningbo University (HY202411), National 111 Project of China (D16013), and the Health Fund of Translational Biomedicine (H2024000276).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This review was supported by Health BioMed Co., Ltd., Ningbo, Zhejiang, China and The Health Fund of Translational Biomedicine. We would like to express our gratitude for their support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AD | Alzheimer’s disease |
| GM | gut microbiota |
| MGBA | microbiota–gut–brain axis |
| FMT | fecal microbiota transplantation |
| CNS | central nervous system |
| Aβ | amyloid-β |
| NFTs | neurofibrillary tangles |
| HPA | hypothalamus–pituitary–adrenal |
| BBB | blood–brain barrier |
| SCFAs | short-chain fatty acids |
| BDNF | brain-derived neurotrophic factor |
| GIT | gastrointestinal tract |
| LPS | lipopolysaccharides |
| TLRs | toll-like receptors |
| 5-HT | 5-hydroxytryptamine |
| GABA | γ-aminobutyric acid |
| IL-1 | interleukin-1 |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-alpha |
| iNKT | invariant natural killer T cells |
| APP | amyloid precursor protein |
| CDI | Clostridioides difficile infection |
| TMAO | Trimethylamine N-oxide |
| p-tau | tau hyperphosphorylation |
| ER | endoplasmic reticulum |
| mAChRs | Muscarinic acetylcholine receptors |
| PP2A | protein phosphatase 2A |
| CA | carnosic acid |
| DEPTACs | dephosphorylation-targeting chimera |
| BHB | β-hydroxybutyrate |
References
- Patterson, C. World alzheimer report 2018—The state of the art of dementia research: New frontiers. In NEW FRONTIERS; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Jagust, W. Imaging the evolution and pathophysiology of alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, CD003154. [Google Scholar] [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 2018, CD001190. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The microbiota–gut–brain axis and alzheimer’s disease: Neuroinflammation is to blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut microbiota is altered in patients with alzheimer’s disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lukiw, W.J. Bacteroidetes neurotoxins and inflammatory neurodegeneration. Mol. Neurobiol. 2018, 55, 9100–9107. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276, Erratum in Cell 2015, 163, 258. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramírez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From probiotics to psychobiotics: Live beneficial bacteria which act on the brain-gut axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Varesi, A.; Pierella, E.; Romeo, M.; Piccini, G.B.; Alfano, C.; Bjørklund, G.; Oppong, A.; Ricevuti, G.; Esposito, C.; Chirumbolo, S.; et al. The potential role of gut microbiota in alzheimer’s disease: From diagnosis to treatment. Nutrients 2022, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal microbiota transplantation alleviated alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.-W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, A.; Vandendriessche, C.; Hamerlinck, H.; De Looze, D.; Tate, D.J.; Vuylsteke, M.; De Commer, L.; Devolder, L.; Raes, J.; Verhasselt, B.; et al. Safety and efficacy of faecal microbiota transplantation in patients with mild to moderate Parkinson’s disease (GUT-PARFECT): A double-blind, placebo-controlled, randomised, phase 2 trial. eClinicalMedicine 2024, 71, 102563. [Google Scholar] [CrossRef]
- Hazan, S. Rapid improvement in alzheimer’s disease symptoms following fecal microbiota transplantation: A case report. J. Int. Med. Res. 2020, 48, 300060520925930. [Google Scholar] [CrossRef]
- de Jesus Rodrigues De-Paula, V.; Forlenza, A.S.; Forlenza, O.V. Relevance of gutmicrobiota in cognition, behaviour and alzheimer’s disease. Pharmacol. Res. 2018, 136, 29–34. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Haque, S.Z.; Haque, M. The ecological community of commensal, symbiotic, and pathogenic gastrointestinal microorganisms—An appraisal. Clin. Exp. Gastroenterol. 2017, 10, 91–103. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.-J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef]
- Mitsuoka, T. Intestinal Flora and Aging. Nutr. Rev. 2009, 50, 438–446. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and healthy aging: A mini-review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Lau, H.C.H.; Sung, J.J.-Y.; Yu, J. Gut microbiota: Impacts on gastrointestinal cancer immunotherapy. Gut Microbes 2021, 13, 1869504. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Adil, N.A.; Omo-Erigbe, C.; Yadav, H.; Jain, S. The oral–gut microbiome–brain axis in cognition. Microorganisms 2025, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Medel, M.; Pinto, M.P.; Goralsky, L.; Cáceres, M.; Villarroel-Espíndola, F.; Manque, P.; Pinto, A.; Garcia-Bloj, B.; de Mayo, T.; Godoy, J.A.; et al. Porphyromonas gingivalis, a bridge between oral health and immune evasion in gastric cancer. Front. Oncol. 2024, 14, 1403089. [Google Scholar] [CrossRef]
- Jia, S.; Li, X.; Du, Q. Host insulin resistance caused by Porphyromonas gingivalis-review of recent progresses. Front. Cell Infect. Microbiol. 2023, 13, 1209381. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. The vagus nerve in the neuro-immune axis: Implications in the pathology of the gastrointestinal tract. Front. Immunol. 2017, 8, 1452. [Google Scholar] [CrossRef]
- Liu, Y.; Sanderson, D.; Mian, M.F.; Neufeld, K.-A.M.; Forsythe, P. Loss of vagal integrity disrupts immune components of the microbiota-gut-brain axis and inhibits the effect of Lactobacillus rhamnosus on behavior and the corticosterone stress response. Neuropharmacology 2021, 195, 108682. [Google Scholar] [CrossRef]
- Yu, C.D.; Xu, Q.J.; Chang, R.B. Vagal sensory neurons and gut-brain signaling. Curr. Opin. Neurobiol. 2020, 62, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Gaykema, R.P.A.; Nguyen, K.T.; Lee, J.E.; Tilders, F.J.H.; Maier, S.F.; Watkins, L.R. Interleukin-1β in immune cells of the abdominal vagus nerve: A link between the immune and nervous systems? J. Neurosci. 1999, 19, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Kirkup, A.J.; Brunsden, A.M.; Thompson, D.G.; Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G907–G915. [Google Scholar] [CrossRef] [PubMed]
- Maestú, F.; de Haan, W.; Busche, M.A.; DeFelipe, J. Neuronal excitation/inhibition imbalance: Core element of a translational perspective on Alzheimer pathophysiology. Ageing Res. Rev. 2021, 69, 101372. [Google Scholar] [CrossRef]
- Strac, D.Š.; Pivac, N.; Mück-Šeler, D. The serotonergic system and cognitive function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar] [CrossRef]
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the extra (synaptic) mile: Excitotoxicity as the road toward neurodegenerative diseases. Front. Cell Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef]
- van Bodegom, M.; Homberg, J.R.; Henckens, M.J.A.G. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front. Cell Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef]
- Aziz, Q.; Doré, J.; Emmanuel, A.; Guarner, F.; Quigley, E.M.M. Gut microbiota and gastrointestinal health: Current concepts and future directions. Neurogastroenterol. Motil. 2013, 25, 4–15. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Zhan, X.; Stamova, B.; Jin, L.-W.; DeCarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef]
- Wu, Y.; Shao, Y.; Shao, X.; Yu, H.; Wang, M.; Wang, J.; She, Y.; Liu, J.; Zhang, T.; Li, Z.; et al. Qingke β-glucan and lactobacillus mitigate neuroinflammation and enhance cognitive function in an alzheimer’s disease mouse model. Int. J. Biol. Macromol. 2025, 319, 145427. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Yuan, B.; Liu, L.; Zhang, H.; Zhu, M.; Chai, H.; Peng, J.; Huang, Y.; Zhou, S.; et al. Akkermansia muciniphila and its metabolite propionic acid maintains neuronal mitochondrial division and autophagy homeostasis during alzheimer’s disease pathologic process via GPR41 and GPR43. Microbiome 2025, 13, 16. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, B.; Chen, K.; Kong, Y.; Fang, N.; Gong, T.; Wang, F.; Ling, Z.; Liu, J. Effect of Clostridium butyricum against microglia—Mediated neuroinflammation in alzheimer’s disease via regulating gut microbiota and metabolites butyrate. Mol. Nutr. Food Res. 2020, 64, e1900636. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liao, J.; Xia, Y.; Liu, X.; Jones, R.; Haran, J.; McCormick, B.; Sampson, T.R.; Alam, A.; Ye, K. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut 2022, 71, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.-R.; Kuang, Q.; Zhang, F.; Chen, B.; Zhong, Z.-G. Functional roles of the microbiota-gut-brain axis in alzheimer’s disease: Implications of gut microbiota-targeted therapy. Transl. Neurosci. 2021, 12, 581–600. [Google Scholar] [CrossRef] [PubMed]
- Doens, D.; Fernández, P.L. Microglia receptors and their implications in the response to amyloid β for alzheimer’s disease pathogenesis. J. Neuroinflamm. 2014, 11, 48. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef]
- Kostiuchenko, O.; Lushnikova, I.; Skibo, G. The role of gut microbiota metabolites in the regeneration and protection of nervous tissue: A narrative review. Regen. Med. Rep. 2024, 1, 12–30. [Google Scholar] [CrossRef]
- Xia, Y.; Xiao, Y.; Wang, Z.-H.; Liu, X.; Alam, A.M.; Haran, J.P.; McCormick, B.A.; Shu, X.; Wang, X.; Ye, K. Bacteroides fragilis in the gut microbiomes of alzheimer’s disease activates microglia and triggers pathogenesis in neuronal C/EBPβ transgenic mice. Nat. Commun. 2023, 14, 5471. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Tagé, B.S.S.; Gonzatti, M.B.; Vieira, R.P.; Keller, A.C.; Bortoluci, K.R.; Aimbire, F. Three main SCFAs mitigate lung inflammation and tissue remodeling Nlrp3-Dependent in murine HDM-induced neutrophilic asthma. Inflammation 2024, 47, 1386–1402. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Cui, Y.; Wan, Q. NKT Cells in neurological diseases. Front. Cell Neurosci. 2019, 13, 245. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef]
- Kumari, S.; Dhapola, R.; Sharma, P.; Singh, S.K.; Reddy, D.H. Implicative role of cytokines in neuroinflammation mediated AD and associated signaling pathways: Current progress in molecular signaling and therapeutics. Ageing Res. Rev. 2023, 92, 102098. [Google Scholar] [CrossRef]
- Walker, K.A.; Gottesman, R.F.; Wu, A.; Knopman, D.S.; Gross, A.L.; Mosley, T.H.; Selvin, E.; Windham, B.G. Systemic inflammation during midlife and cognitive change over 20 years. Neurology 2019, 92, E1256–E1267. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, W.; Shi, Y.; Fan, Z.; Ji, G. Should we standardize the 1700-year-old fecal microbiota transplantation? Am. J. Gastroenterol. 2012, 107, 1755. [Google Scholar] [CrossRef]
- Li, S. Bencao Gangmu; Huaxia Publishing House: Jinan, China, 2011; Volume 52. [Google Scholar]
- Zhang, T.; Gao, G.; Kwok, L.Y.; Sun, Z. Gut microbiome-targeted therapies for alzheimer’s disease. Gut Microbes 2023, 15, 2271613. [Google Scholar] [CrossRef]
- Nandwana, V.; Debbarma, S. Fecal microbiota transplantation: A microbiome modulation technique for alzheimer’s disease. Cureus 2021, 13, e16503. [Google Scholar] [CrossRef]
- Society of Parenteral and Enteral Nutrition, Chinese Medical Association; Microecology Professional Committee of Shanghai Preventive Medicine Association. Chinese expert consensus on screening and management of fecal microbiota transplantation donors (2022 edition). Zhonghua Wei Chang Wai Ke Za Zhi 2022, 25, 757–765. [Google Scholar] [CrossRef]
- Saha, S.; Mara, K.; Pardi, D.S.; Khanna, S. Durability of response to fecal microbiota transplantation after exposure to risk factors for recurrence in patients with Clostridioides difficile Infection. Clin. Infect. Dis. 2020, 73, e1706–e1712. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Rossen, N.G.; van der Spek, M.J.; Hartman, J.H.A.; Huuskonen, L.; Korpela, K.; Salojärvi, J.; Aalvink, S.; de Vos, W.M.; D’hAens, G.R.; et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017, 11, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Cochrane, K.; Schroeter, K.; Daigneault, M.C.; Yen, S.; Verdu, E.F.; Allen-Vercoe, E.; Dorrestein, P.C. Effects of antibiotic pretreatment of an ulcerative colitis-derived fecal microbial community on the integration of therapeutic bacteria in vitro. mSystems 2020, 5, e00404-19. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Song, Y.; Garg, S.; Girotra, M.; Sinha, A.; Sivaraman, A.; Phillips, L.; Dutta, S.K. Effect of aging on the composition of fecal microbiota in donors for FMT and its impact on clinical outcomes. Dig. Dis. Sci. 2017, 62, 1002–1008. [Google Scholar] [CrossRef]
- Wilson, B.C.; Vatanen, T.; Cutfield, W.S.; O’SUllivan, J.M. The super-donor phenomenon in fecal microbiota transplantation. Front. Cell Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Kelly, C.R.; Mullish, B.H.; Allegretti, J.R.; Kassam, Z.; Putignani, L.; Fischer, M.; Keller, J.J.; Costello, S.P.; et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019, 68, 2111–2121. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Millan, B.; Madsen, K.L. Pretreatment with antibiotics may enhance the efficacy of fecal microbiota transplantation in ulcerative colitis: A meta-analysis. Mucosal Immunol. 2017, 10, 565–566. [Google Scholar] [CrossRef]
- Ji, S.K.; Yan, H.; Jiang, T.; Guo, C.Y.; Liu, J.J.; Dong, S.Z.; Yang, K.L.; Wang, Y.J.; Cao, Z.J.; Li, S.L. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Front. Microbiol. 2017, 8, 1208. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Dozier, E.A.; Glover, M.S.; Novick, S.; Ford, M.; Morehouse, C.; Warrener, P.; Caceres, C.; Hess, S.; Sellman, B.R.; et al. Engraftment of bacteria after fecal microbiota transplantation is dependent on both frequency of dosing and duration of preparative antibiotic regimen. Microorganisms 2021, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, V.; Rajaruban, S.; Dang, J.; Kung, J.Y.; Deehan, E.C.; Madsen, K.L. Repeated fecal microbial transplantations and antibiotic pre-treatment are linked to improved clinical response and remission in inflammatory bowel disease: A Systematic Review and Pooled Proportion Meta-Analysis. J. Clin. Med. 2021, 10, 959. [Google Scholar] [CrossRef] [PubMed]
- Paaske, S.E.; Baunwall, S.M.D.; Rubak, T.; Rågård, N.; Kelsen, J.; Hansen, M.M.; Lødrup, A.B.; Erikstrup, L.T.; Mikkelsen, S.; Erikstrup, C.; et al. Clinical management of Clostridioides difficile infection with faecal microbiota transplantation: A real-world cohort study. eClinicalMedicine 2025, 85, 103302. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tian, H.L.; Yang, B.; Lin, Z.L.; Zhao, D.; Ye, C.; Zhang, X.Y.; Qin, H.L.; Li, N. Effect of intestinal preparation on the efficacy and safety of fecal microbiota transplantation treatment. Zhonghua Wei Chang Wai Ke Za Zhi 2020, 23, 48–55. [Google Scholar] [CrossRef]
- Jandl, B.; Dighe, S.; Gasche, C.; Makristathis, A.; Muttenthaler, M.; Staley, C. Intestinal biofilms: Pathophysiological relevance, host defense, and therapeutic opportunities. Clin. Microbiol. Rev. 2024, 37, e0013323. [Google Scholar] [CrossRef]
- Prudent, V.; Demarre, G.; Vazeille, E.; Wery, M.; Quenech’dU, N.; Ravet, A.; Dauverd-Girault, J.; van Dijk, E.; Bringer, M.-A.; Descrimes, M.; et al. The crohn’s disease-related bacterial strain LF82 assembles biofilm-like communities to protect itself from phagolysosomal attack. Commun. Biol. 2021, 4, 627. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med. Microbiol. 2017, 307, 481–489. [Google Scholar] [CrossRef]
- Faccin, I.D.; de Almeida de Souza, G.H.; Vicente, J.C.P.; da Silva Damaceno, N.; de Oliveira Perez, E.V.; Martins, W.; Gales, A.C.; Simionatto, S. The potential of bacteriophages in treating multidrug-resistant ESKAPE pathogen infections. Expert Opin. Ther. Pat. 2025, 1–17. [Google Scholar] [CrossRef]
- Bokoliya, S.C.; Dorsett, Y.; Panier, H.; Zhou, Y. Procedures for fecal microbiota transplantation in murine microbiome studies. Front. Cell Infect. Microbiol. 2021, 11, 711055. [Google Scholar] [CrossRef]
- Ramai, D.; Zakhia, K.; Fields, P.J.; Ofosu, A.; Patel, G.; Shahnazarian, V.; Lai, J.K.; Dhaliwal, A.; Reddy, M.; Chang, S. Fecal microbiota transplantation (FMT) with colonoscopy is superior to enema and nasogastric tube while comparable to capsule for the treatment of recurrent clostridioides difficile infection: A systematic review and meta-analysis. Dig. Dis. Sci. 2021, 66, 369–380. [Google Scholar] [CrossRef]
- Kamath, S.; Bryant, R.V.; Costello, S.P.; Day, A.S.; Forbes, B.; Haifer, C.; Hold, G.; Kelly, C.R.; Li, A.; Pakuwal, E.; et al. Translational strategies for oral delivery of faecal microbiota transplantation. Gut 2025, gutjnl-2025-335077. [Google Scholar] [CrossRef]
- Gulati, M.; Singh, S.K.; Corrie, L.; Kaur, I.P.; Chandwani, L. Delivery routes for faecal microbiota transplants: Available, anticipated and aspired. Pharmacol. Res. 2020, 159, 104954. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.B.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Nguyen, T.T.T.; Fujimura, Y.; Kameya, N.; Nakamura, S.; Arakawa, K.; Morita, H. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with alzheimer’s disease. Biosci. Biotechnol. Biochem. 2019, 83, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Guan, Q.; Zhang, X.; Yuan, C.; Tan, Z.; Zhai, L.; Hao, Y.; Gu, Y.; Han, C. New mechanism of neuroinflammation in Alzheimer’s disease: The activation of NLRP3 inflammasome mediated by gut microbiota. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 100, 109884. [Google Scholar] [CrossRef]
- Kim, N.; Jeon, S.H.; Ju, I.G.; Gee, M.S.; Do, J.; Oh, M.S.; Lee, J.K. Transplantation of gut microbiota derived from alzheimer’s disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain Behav. Immun. 2021, 98, 357–365. [Google Scholar] [CrossRef]
- Wang, M.; Cao, J.; Gong, C.; Amakye, W.K.; Yao, M.; Ren, J. Exploring the microbiota—Alzheimer’s disease linkage using short-term antibiotic treatment followed by fecal microbiota transplantation. Brain Behav. Immun. 2021, 96, 227–238. [Google Scholar] [CrossRef]
- Valeri, F.; dos Santos Guilherme, M.; He, F.; Stoye, N.M.; Schwiertz, A.; Endres, K. Impact of the age of cecal material transfer donors on alzheimer’s disease pathology in 5xFAD Mice. Microorganisms 2021, 9, 2548. [Google Scholar] [CrossRef]
- Grabrucker, S.; Marizzoni, M.; Silajdžić, E.; Lopizzo, N.; Mombelli, E.; Nicolas, S.; Dohm-Hansen, S.; Scassellati, C.; Moretti, D.V.; Rosa, M.; et al. Faecal microbiota transplantation from alzheimer’s participants induces impairments in neurogenesis and cognitive behaviours in rats. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Y.; Xu, C.; Du, K.; Zhao, C.; Zhao, Y.; Liu, X. Transplantation of fecal microbiota from APP/PS1 mice and alzheimer’s disease patients enhanced endoplasmic reticulum stress in the cerebral cortex of wild-type mice. Front. Aging Neurosci. 2022, 14, 858130. [Google Scholar] [CrossRef] [PubMed]
- Soriano, S.; Curry, K.; Wang, Q.; Chow, E.; Treangen, T.J.; Villapol, S. Fecal microbiota transplantation derived from alzheimer’s disease mice worsens brain trauma outcomes in wild-type controls. Int. J. Mol. Sci. 2022, 23, 4476. [Google Scholar] [CrossRef]
- Kim, J.-E.; Roh, Y.-J.; Choi, Y.-J.; Lee, S.-J.; Jin, Y.-J.; Song, H.-J.; Seol, A.-Y.; Son, H.-J.; Hong, J.-T.; Hwang, D.-Y. Dysbiosis of fecal microbiota in Tg2576 mice for alzheimer’s disease during pathological constipation. Int. J. Mol. Sci. 2022, 23, 14928. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xu, Z.; Zhang, L.; Zhang, C.; Zhao, X.; Mao, Y.; Zhang, H.; Liang, X.; Wu, J.; Yang, Y.; et al. Gut-derived β-amyloid: Likely a centerpiece of the gut–brain axis contributing to alzheimer’s pathogenesis. Gut Microbes 2023, 15, 2167172. [Google Scholar] [CrossRef]
- Ling, Y.; Morgan, K.; Kalsheker, N. Amyloid precursor protein (APP) and the biology of proteolytic processing: Relevance to Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2003, 35, 1505–1535. [Google Scholar] [CrossRef]
- Viola, K.L.; Velasco, P.T.; Klein, W.L. Why alzheimer’s is a disease of memory: The attack on synapses by Aß oligomers (ADDLs). J. Nutr. Health Aging 2008, 12, S51–S57. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef]
- Gründer, G.; Cumming, P. Serotonin and amyloid deposition: A link between depression and alzheimer’s disease? An editorial highlight on pimavanserin, a 5HT2A receptor inverse agonist, rapidly suppresses aβ production and related pathology in a mouse model of alzheimer’s disease on page 658. J. Neurochem. 2021, 156, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Wallace, C.E.; Yan, P.; Davis, T.A.; Gardiner, W.D.; Doherty, B.M.; King, D.; Yuede, C.M.; Lee, J.-M.; Sheline, Y.I. Effect of escitalopram on Aβ levels and plaque load in an alzheimer mouse model. Neurology 2020, 95, e2666–e2674. [Google Scholar] [CrossRef] [PubMed]
- Bast, T.; Pezze, M.; McGarrity, S. Cognitive deficits caused by prefrontal cortical and hippocampal neural disinhibition. Br. J. Pharmacol. 2017, 174, 3211–3225. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.; Hu, Y.; Liu, X.; Fan, G.; Zhao, S. The protease caspase-1: Activation pathways and functions. Biochem. Biophys. Res. Commun. 2024, 717, 149978. [Google Scholar] [CrossRef]
- Borst, K.; Dumas, A.A.; Prinz, M. Microglia: Immune and non-immune functions. Immunity 2021, 54, 2194–2208. [Google Scholar] [CrossRef]
- Cowan, M.; Petri, W.A. Microglia: Immune regulators of neurodevelopment. Front. Immunol. 2018, 9, 2576. [Google Scholar] [CrossRef]
- Füger, P.; Hefendehl, J.K.; Veeraraghavalu, K.; Wendeln, A.-C.; Schlosser, C.; Obermüller, U.; Wegenast-Braun, B.M.; Neher, J.J.; Martus, P.; Kohsaka, S.; et al. Microglia turnover with aging and in an alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 2017, 20, 1371–1376. [Google Scholar] [CrossRef]
- Vandenbark, A.A.; Offner, H.; Matejuk, S.; Matejuk, A. Microglia and astrocyte involvement in neurodegeneration and brain cancer. J. Neuroinflam. 2021, 18, 298. [Google Scholar] [CrossRef]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef]
- Zhan, G.; Yang, N.; Li, S.; Huang, N.; Fang, X.; Zhang, J.; Zhu, B.; Yang, L.; Yang, C.; Luo, A. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging 2018, 10, 1257–1267. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Kuntz, T.; Shaik, S.M.; Baufeld, C.; Leibowitz, J.; Zhang, X.; Gottel, N.; Zhang, X.; Butovsky, O.; Gilbert, J.A.; et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 2019, 216, 1542–1560. [Google Scholar] [CrossRef]
- Elangovan, S.; Borody, T.J.; Holsinger, R.M.D. Fecal microbiota transplantation reduces pathology and improves cognition in a mouse model of alzheimer’s disease. Cells 2022, 12, 119. [Google Scholar] [CrossRef]
- Hang, Z.; Cai, S.; Lei, T.; Xiao, Z.; Wang, D.; Li, Y.; Bi, W.; Yang, Y.; Deng, S.; Wang, L.; et al. Transfer of tumor-bearing mice intestinal flora can ameliorate cognition in alzheimer’s disease mice. J. Alzheimer’s Dis. 2022, 86, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, H.; Shao, C.; Liu, Y.; Wen, S.; Tang, L. Application of dominant gut microbiota promises to replace fecal microbiota transplantation as a new treatment for alzheimer’s disease. Microorganisms 2023, 11, 2854. [Google Scholar] [CrossRef] [PubMed]
- Yadollahikhales, G.; Rojas, J.C. Anti-amyloid immunotherapies for alzheimer’s disease: A 2023 clinical update. Neurotherapeutics 2023, 20, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Y. Tau and neuroinflammation in Alzheimer’s disease: Interplay mechanisms and clinical translation. J. Neuroinflammation 2023, 20, 914–931. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Xia, Y.-Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal hyperphosphorylation of tau: Sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimer’s Dis. 2013, 33 (Suppl. S1), S123–S139. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Li, X.; Yang, B.; Zhang, X.; Kang, L.; Ling, Y.; Sui, P.; Tian, S.; Wang, Y. Carnosic acid inhibits tau hyperphosphorylation via PP2A signaling in an Alzheimer’s disease transgenic mouse model. Prog. Anat. Sci. 2024, 1–7. [Google Scholar]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef]
- Torres, A.K.; Jara, C.; Olesen, M.A.; Tapia-Rojas, C. Pathologically phosphorylated tau at S396/404 (PHF-1) is accumulated inside of hippocampal synaptic mitochondria of aged Wild-type mice. Sci. Rep. 2021, 11, 4448. [Google Scholar] [CrossRef]
- Evans, D.B.; Rank, K.B.; Bhattacharya, K.; Thomsen, D.R.; Gurney, M.E.; Sharma, S.K. Tau phosphorylation at serine 396 and serine 404 by human recombinant tau protein kinase II inhibits tau’s ability to promote microtubule assembly. J. Biol. Chem. 2000, 275, 24977–24983. [Google Scholar] [CrossRef]
- Gu, J.; Congdon, E.E.; Sigurdsson, E.M. Two Novel Tau Antibodies Targeting the 396/404 Region Are Primarily Taken Up by Neurons and Reduce Tau Protein Pathology. J. Biol. Chem. 2013, 288, 33081–33095. [Google Scholar] [CrossRef]
- Olona, A.; Leishman, S.; Anand, P.K. The NLRP3 inflammasome: Regulation by metabolic signals. Trends Immunol. 2022, 43, 978–989. [Google Scholar] [CrossRef]
- Vinaik, R.; Barayan, D.; Auger, C.; Abdullahi, A.; Jeschke, M.G. Regulation of glycolysis and the warburg effect in wound healing. J. Clin. Investig. 2020, 5, e138949. [Google Scholar] [CrossRef] [PubMed]
- Hooftman, A.; Angiari, S.; Hester, S.; Corcoran, S.E.; Runtsch, M.C.; Ling, C.; Ruzek, M.C.; Slivka, P.F.; McGettrick, A.F.; Banahan, K.; et al. The immunomodulatory metabolite itaconate modifies NLRP3 and inhibits inflammasome activation. Cell Metab. 2020, 32, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.-G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Chen, W.; Guo, C.; Huang, S.; Jia, Z.; Wang, J.; Zhong, J.; Ge, H.; Yuan, J.; Chen, T.; Liu, X.; et al. MitoQ attenuates brain damage by polarizing microglia towards the M2 phenotype through inhibition of the NLRP3 inflammasome after ICH. Pharmacol. Res. 2020, 161, 105122. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Li, D.; Zhang, N.; Liu, R.; Han, B.; Wei, C.; Liu, H.; Xu, X.; Hao, J. Everolimus (RAD001) ameliorates vascular cognitive impairment by regulating microglial function via the mTORC1 signaling pathway. J. Neuroimmunol. 2016, 299, 164–171. [Google Scholar] [CrossRef]
- Kettenmann, H.; Kirchhoff, F.; Verkhratsky, A. Microglia: New roles for the synaptic stripper. Neuron 2013, 77, 10–18. [Google Scholar] [CrossRef]
- Han, J.; Harris, R.A.; Zhang, X.-M. An updated assessment of microglia depletion: Current concepts and future directions. Mol. Brain 2017, 10, 25. [Google Scholar] [CrossRef]
- Rice, R.A.; Pham, J.; Lee, R.J.; Najafi, A.R.; West, B.L.; Green, K.N. Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia 2017, 65, 931–944. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, T.; Chen, Q.; Li, C.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; Chen, H.; Zhou, Z.; et al. Biomimetic dendrimer–peptide conjugates for early multi-target therapy of alzheimer’s disease by inflammatory microenvironment modulation. Adv. Mater. 2021, 33, 2100746. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.D.; Daggett, A.; Gu, X.; Jiang, L.-L.; Langfelder, P.; Li, X.; Wang, N.; Zhao, Y.; Park, C.S.; Cooper, Y.; et al. Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in alzheimer’s disease models. Neuron 2018, 97, 1032–1048.e5. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lee, J.H.; Shin, J.; Kim, J.-S.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; Choi, S.H. Cognitive function improvement after fecal microbiota transplantation in Alzheimer’s dementia patient: A case report. Curr. Med. Res. Opin. 2021, 37, 1739–1744. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, J.-H.; Kim, J.-S.; Kim, T.J.; Shin, J.; Im, J.H.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; et al. Fecal microbiota transplantation can improve cognition in patients with cognitive decline and Clostridioides difficile infection. Aging 2022, 14, 6449–6466. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.I.; Su, Q.; Ng, S.C. Long COVID and gut microbiome: Insights into pathogenesis and therapeutics. Gut Microbes 2025, 17, 2457495. [Google Scholar] [CrossRef]
- de Almeida, V.M.; Engel, D.F.; Ricci, M.F.; Cruz, C.S.; Lopes, Í.S.; Alves, D.A.; Auriol, M.D.; Magalhães, J.; Machado, E.C.; Rocha, V.M.; et al. Gut microbiota from patients with COVID-19 cause alterations in mice that resemble post-COVID symptoms. Gut Microbes 2023, 15, 2249146. [Google Scholar] [CrossRef]
- Lau, R.I.; Su, Q.; Ching, J.Y.; Lui, R.N.; Chan, T.T.; Wong, M.T.; Lau, L.H.; Wing, Y.K.; Chan, R.N.; Kwok, H.Y.; et al. Fecal Microbiota Transplantation for Sleep Disturbance in Post-acute COVID-19 Syndrome. Clin. Gastroenterol. Hepatol. 2024, 22, 2487–2496.e6. [Google Scholar] [CrossRef]
- Bredesen, D.E.; Sharlin, K.; Jenkins, D.; Okuno, M.; Youngberg, W.; Cohen, S.H.; Stefani, A.; Brown, R.; Conger, S.; Tanio, C.; et al. Reversal of cognitive decline: 100 patients. J. Alzheimer’s Dis. Parkinsonism. 2018, 8, 2161-0460. [Google Scholar] [CrossRef]
- Baxter, M.; Colville, A. Adverse events in faecal microbiota transplant: A review of the literature. J. Hosp. Infect. 2016, 92, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Dailey, F.E.; Turse, E.P.; Daglilar, E.; Tahan, V. The dirty aspects of fecal microbiota transplantation: A review of its adverse effects and complications. Curr. Opin. Pharmacol. 2019, 49, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Lavergne, V.; Skinner, A.M.; Gonzales-Luna, A.J.; Garey, K.W.; Kelly, C.P.; Wilcox, M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides difficile Infection in Adults. Clin. Infect. Dis. 2021, 73, e1029–e1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, M.; Wang, W.; Cao, X.; Piao, M.; Khan, S.; Yan, F.; Cao, H.; Wang, B.; Grivennikov, S. Systematic review: Adverse events of fecal microbiota transplantation. PLoS ONE 2016, 11, e0161174. [Google Scholar] [CrossRef]
- Holleran, G.; Scaldaferri, F.; Ianiro, G.; Lopetuso, L.; Mc Namara, D.; Mele, M.; Gasbarrini, A.; Cammarota, G. Fecal microbiota transplantation for the treatment of patients with ulcerative colitis and other gastrointestinal conditions beyond Clostridium difficile infection: An update. Drugs Today 2018, 54, 123–136. [Google Scholar] [CrossRef]
- Alang, N.; Kelly, C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2015, 2, ofv004. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Schwartz, M.; Gluck, M.; Koon, S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am. J. Gastroenterol. 2013, 108, 1367. [Google Scholar] [CrossRef]
- Kelly, C.R.; Yen, E.F.; Grinspan, A.M.; Kahn, S.A.; Atreja, A.; Lewis, J.D.; Moore, T.A.; Rubin, D.T.; Kim, A.M.; Serra, S.; et al. Fecal microbiota transplantation is highly effective in real-world practice: Initial results from the FMT national registry. Gastroenterology 2021, 160, 183–192. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, S.; Qin, H.; Li, N.; Chen, Q. Long-term safety of faecal microbiota transplantation for gastrointestinal diseases in China. Lancet Gastroenterol. Hepatol. 2022, 7, 702–703. [Google Scholar] [CrossRef]
- Kundu, P.; Stagaman, K.; Kasschau, K.; Holden, S.; Shulzhenko, N.; Sharpton, T.J.; Raber, J. Fecal implants from AppNL–G–F and AppNL–G–F/E4 donor mice sufficient to induce behavioral phenotypes in germ-free mice. Front. Behav. Neurosci. 2022, 16, 791128. [Google Scholar] [CrossRef]
- Alaeddin, S.; Chatterjee, A.; Roberts, T.L.; Steiner-Lim, G.Z.; Jensen, S.O.; Gyengesi, E.; Muench, G.; Ho, V. Exploring the effects of faecal microbiota transplantation on cognitive function: A review of clinical trials. Brain Behav. Immun. Health 2025, 48, 101049. [Google Scholar] [CrossRef]
- Tatara, Y.; Yamazaki, H.; Katsuoka, F.; Chiba, M.; Saigusa, D.; Kasai, S.; Nakamura, T.; Inoue, J.; Aoki, Y.; Shoji, M.; et al. Multiomics and artificial intelligence enabled peripheral blood-based prediction of amnestic mild cognitive impairment. Curr. Res. Transl. Med. 2023, 71, 103367. [Google Scholar] [CrossRef]
- French, S.R.; Arias, J.C.; Zahra, S.; Ally, M.; Escareno, C.; Heitkamp, E.; Vazquez, F.; Hillis, M.; Wiskoski, H.; Ainapurapu, K.; et al. Cognitive impairment and p-tau217 are high in a vascular patient cohort. Alzheimer’s Dement. 2025, 21, e70565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).