Unmasking MRSA’s Armor: Molecular Mechanisms of Resistance and Pioneering Therapeutic Countermeasures
Abstract
1. Introduction
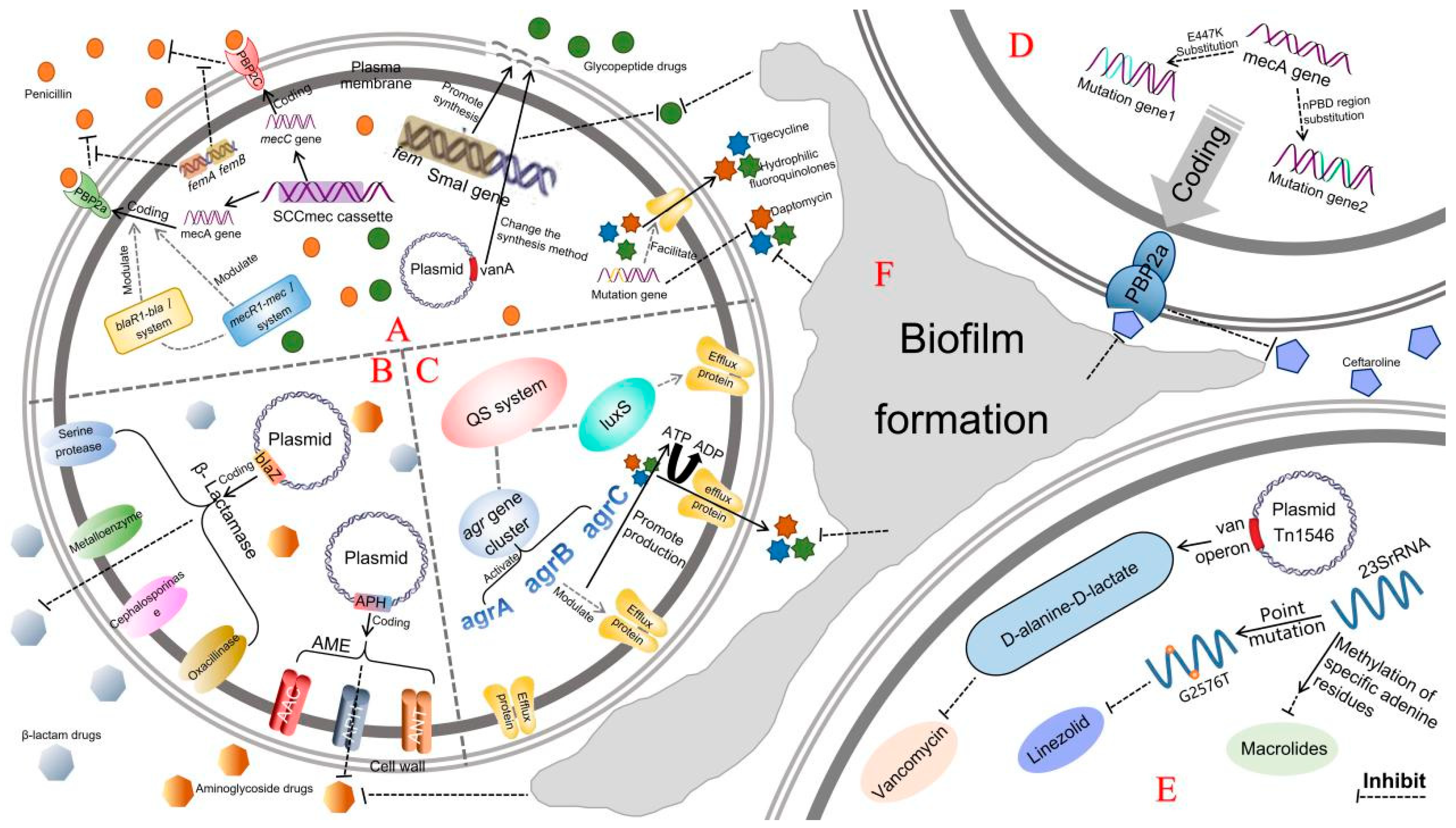
2. Drug-Resistance Mechanisms of MRSA
2.1. Gene-Mediated Drug-Resistance Mechanisms
2.1.1. mec Gene-Mediated Drug Resistance
2.1.2. fem Gene-Mediated Drug Resistance
2.1.3. vanA Gene-Mediated Drug Resistance
2.1.4. SCCmec-Mediated Drug Resistance
2.1.5. Genetic Mutation-Mediated Drug Resistance
2.2. Production of Inactivated and Modified Enzymes
2.2.1. β-Lactamases
2.2.2. Modification Enzymes
2.3. Drug Efflux Pump
2.3.1. Quorum-Sensing (QS) System-Mediated Regulation
2.3.2. Functional Proteins Responsible for Membrane Transport
2.4. Formation of Biofilms
2.4.1. Formation of MRSA Biofilm and Regulation of Related Genes
2.4.2. Resistance Mechanisms Mediated by MRSA Biofilm
2.5. The Change of Antibacterial Targets and Affinity
2.5.1. Changes in the Targets of Antibacterial Agents
Resistance Mechanisms of MRSA to Vancomycin
Resistance Mechanisms of MRSA to Linezolid
MRSA Resistance to Other Drugs Caused by Changes of Action Targets
2.5.2. Changes of Target Affinity of Antimicrobial Agents
3. Novel Treatment Strategies for MRSA
3.1. Combined Therapy
3.2. Bacteriophage Therapy
3.3. Nanobiologic Therapy
3.4. Antimicrobial Peptides (AMPs)
3.5. Live Bio-Therapeutics
3.6. Chinese Herbal Drugs Therapy
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chalmers, S.J.; Wylam, M.E. Methicillin-Resistant Staphylococcus aureus Infection and Treatment Options. In Methicillin-Resistant Staphylococcus aureus (MRSA) Protocols; Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2069, pp. 229–251. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, L.; Wei, J.; Xu, B. Progress in the Prevalence, Classification and Drug Resistance Mechanisms of Methicillin-Resistant Staphylococcus aureus. Infect. Drug Resist. 2023, 16, 3271–3292. [Google Scholar] [CrossRef]
- Tasneem, U.; Mehmood, K.; Majid, M.; Ullah, S.R.; Andleeb, S. Methicillin resistant Staphylococcus aureus: A brief review of virulence and resistance. JPMA J. Pak. Med. Assoc. 2022, 72, 509–515. [Google Scholar] [CrossRef]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, A.G.; Atcha, K.R.; Sagurthi, S.R. Cloning, Purification, and Biophysical Characterization of FemB Protein from Methicillin-Resistant Staphylococcus aureus and Inhibitors Screening. Appl. Biochem. Biotechnol. 2024, 196, 4974–4992. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.C.; Weigel, L.M.; Patel, J.B.; Tenover, F.C. Comparison of Tn1546-like elements in vancomycin-resistant Staphylococcus aureus isolates from Michigan and Pennsylvania. Antimicrob. Agents Chemother. 2005, 49, 470–472. [Google Scholar] [CrossRef]
- Groundwater, P.W.; Xu, S.; Lai, F.; Váradi, L.; Tan, J.; Perry, J.D.; Hibbs, D.E. New Delhi metallo-β-lactamase-1: Structure, inhibitors and detection of producers. Future Med. Chem. 2016, 8, 993–1012. [Google Scholar] [CrossRef]
- Liu, Q.; He, D.; Wang, L.; Wu, Y.; Liu, X.; Yang, Y.; Chen, Z.; Dong, Z.; Luo, Y.; Song, Y. Efficacy and Safety of Antibiotics in the Treatment of Methicillin-Resistant Staphylococcus aureus (MRSA) Infections: A Systematic Review and Network Meta-Analysis. Antibiotics 2024, 13, 866. [Google Scholar] [CrossRef]
- Zou, D.; Xie, K.; Wang, H.; Chen, Y.; Xie, M. Inhibitory effects of biochanin A on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA). Acta Microbiol. Sin. 2014, 54, 1204–1211. [Google Scholar]
- Hosseini, M.; Shapouri Moghaddam, A.; Derakhshan, S.; Hashemipour, S.M.A.; Hadadi-Fishani, M.; Pirouzi, A.; Khaledi, A. Correlation Between Biofilm Formation and Antibiotic Resistance in MRSA and MSSA Isolated from Clinical Samples in Iran: A Systematic Review and Meta-Analysis. Microb. Drug Resist. 2020, 26, 1071–1080. [Google Scholar] [CrossRef]
- Tschierske, M.; Mori, C.; Rohrer, S.; Ehlert, K.; Shaw, K.J.; Berger-Bächi, B. Identification of three additional femAB-like open reading frames in Staphylococcus aureus. FEMS Microbiol. Lett. 1999, 171, 97–102. [Google Scholar] [CrossRef]
- International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC). Classification of staphylococcal cassette chromosome mec (SCCmec): Guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 2009, 53, 4961–4967. [Google Scholar] [CrossRef]
- Kim, C.; Milheiriço, C.; Gardete, S.; Holmes, M.A.; Holden, M.T.; de Lencastre, H.; Tomasz, A. Properties of a novel PBP2A protein homolog from Staphylococcus aureus strain LGA251 and its contribution to the β-lactam-resistant phenotype. J. Biol. Chem. 2012, 287, 36854–36863. [Google Scholar] [CrossRef]
- Liu, M.J.; Zhang, Y.L.; Wan, X.Y. Research progress on methicillin-resistant Staphylococcus aureus biofilm. Zhonghua Nei Ke Za Zhi 2020, 59, 473–476. [Google Scholar] [CrossRef]
- Hamad, P.A. Phenotypic and Molecular Detection of Biofilm Formation in Methicillin-Resistant Staphylococcus Aureus Isolated from Different Clinical Sources in Erbil City. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023016. [Google Scholar] [CrossRef] [PubMed]
- Dermota, U.; Turm, A.C.; Triglav, T.; Smrdel, K.S.; Velimirovi, I. Whole genome sequencing and molecular epidemiology of methicillin-resistant Staphylococcus aureus isolated from patients with bacteraemia in Slovenia. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 969–977. [Google Scholar] [CrossRef]
- Mckinney, T.K.; Sharma, V.K.; Craig, W.A.; Archer, G.L. Transcription of the Gene Mediating Methicillin Resistance in Staphylococcus aureus (mecA) Is Corepressed but Not Coinduced by Cognate mecA and -Lactamase Regulators. J. Bacteriol. 2001, 183, 6862–6868. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, L.; Holden, M.T.G.; Lindsay, H.; Webb, C.R.; Holmes, M.A. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. Lancet Infect. Dis. 2011, 11, 595–603. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Ma, L. Research progress of methicillin-resistant Staphylococcus aureus. J. Chin. Pract. Diagn. Ther. 2015, 29, 1145–1147. [Google Scholar]
- El-Baghdady, K.Z.; El-Borhamy, M.I.; Abd El-Ghafar, H.A. Prevalence of resistance and toxin genes in community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus clinical isolates. Iran. J. Basic Med. Sci. 2020, 23, 1251–1260. [Google Scholar] [CrossRef]
- Appelbaum, P.C. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin. Infect. 2007, 45 (Suppl. 3), S165–S170. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ono, D.; Sato, A. Staphylococcal Cassette Chromosome mec (SCCmec) Analysis of MRSA. Methods Mol. Biol. 2020, 2069, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Arakere, G.; Nadig, S.; Ito, T.; Ma, X.X.; Hiramatsu, K. A novel type-III staphylococcal cassette chromosome mec (SCCmec) variant among Indian isolates of methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 2009, 292, 141–148. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, C.J.; Su, L.H.; Hu, S.; Yu, J.; Chiu, C.H. Evolution and pathogenesis of Staphylococcus aureus: Lessons learned from genotyping and comparative genomics. FEMS Microbiol. Rev. 2008, 32, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Handzlik, J.; Matys, A.; Kieć-Kononowicz, K. Recent Advances in Multi-Drug Resistance (MDR) Efflux Pump Inhibitors of Gram-Positive Bacteria S. aureus. Antibiotics 2013, 2, 28–45. [Google Scholar] [CrossRef]
- Dabul, A.N.G.; Avaca-Crusca, J.S.; Van Tyne, D.; Gilmore, M.S.; Camargo, I.L.B.C. Resistance in In Vitro Selected Tigecycline-Resistant Methicillin-Resistant Staphylococcus aureus Sequence Type 5 Is Driven by Mutations in mepR and mepA Genes. Microb. Drug Resist. 2018, 24, 519–526. [Google Scholar] [CrossRef]
- Jones, T.; Yeaman, M.R.; Sakoulas, G.; Yang, S.J.; Proctor, R.A.; Sahl, H.G.; Schrenzel, J.; Xiong, Y.Q.; Bayer, A.S. Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 2008, 52, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, M.; Chen, L.; Li, H. The development of New Delhi metallo-β-lactamase-1 inhibitors since 2018. Microbiol. Res. 2022, 261, 127079. [Google Scholar] [CrossRef]
- Lima, L.M.; Silva, B.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef]
- Hashizume, H.; Takahashi, Y.; Masuda, T.; Ohba, S.I.; Ohishi, T.; Kawada, M.; Igarashi, M.J. In vivo efficacy of β-lactam/tripropeptin C in a mouse septicemia model and the mechanism of reverse β-lactam resistance in methicillin-resistant Staphylococcus aureus mediated by tripropeptin C. J. Antibiot. 2018, 71, 79–85. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Perumal, N.; Murugesan, S.; Krishnan, P. Distribution of genes encoding aminoglycoside-modifying enzymes among clinical isolates of methicillin-resistant staphylococci. Indian J. Med. Microbiol. 2016, 34, 350–352. [Google Scholar] [CrossRef]
- Khosravi, A.D.; Jenabi, A.; Montazeri, E.A. Distribution of genes encoding resistance to aminoglycoside modifying enzymes in methicillin-resistant Staphylococcus aureus (MRSA) strains. Kaohsiung J. Med. Sci. 2017, 33, 587–593. [Google Scholar] [CrossRef]
- Lin, D.L.; Tran, T.; Alam, J.Y.; Herron, S.R.; Ramirez, M.S.; Tolmasky, M.E. Inhibition of aminoglycoside 6′-N-acetyltransferase type Ib by zinc: Reversal of amikacin resistance in Acinetobacter baumannii and Escherichia coli by a zinc ionophore. Antimicrob. Agents Chemother. 2014, 58, 4238–4241. [Google Scholar] [CrossRef]
- Miller, M.; Bassler, B. Quorum sensing in bacteria Annu. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Purchase, D.; Govarthanan, M.; Chandra, R.; Yadav, S. Regulatory and innovative mechanisms of bacterial quorum sensing-mediated pathogenicity: A review. Environ. Monit. Assess. 2022, 195, 75. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Smith, K.M. Molecular mechanisms of bacterial quorum sensing as a new drug target. Curr. Opin. Chem. Biol. 2003, 7, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Buroni, S.; Pasca, M.R.; Flannagan, R.S.; Bazzini, S.; Milano, A.; Bertani, I.; Venturi, V.; Valvano, M.A.; Riccardi, G. Assessment of three Resistance-Nodulation-Cell Division drug efflux transporters of Burkholderia cenocepacia in intrinsic antibiotic resistance. BMC Microbiol. 2009, 9, 200. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Ganjloo, S.; Pourmand, M.R.; Mashhadi, R.; Ghazvini, K. Epidemiology of efflux pumps genes mediating resistance among Staphylococcus aureus; a systematic review. Microb. Pathog. 2019, 139, 103850. [Google Scholar] [CrossRef]
- Deng, X.; Sun, F.; Ji, Q.; Liang, H.; Missiakas, D.; Lan, L.; He, C. Expression of Multidrug Resistance Efflux Pump Gene norA Is Iron Responsive in Staphylococcus aureus. J. Bacteriol. 2012, 194, 1753–1762. [Google Scholar] [CrossRef]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef]
- Thong, K.; Hanifah, Y.; Lim, K.; Yusof, M. ermA, ermC, tetM and tetK are essential for erythromycin and tetracycline resistance among methicillin-resistant Staphylococcus aureus strains isolated from a tertiary hospital in Malaysia. Indian J. Med. Microbiol. 2012, 30, 203–207. [Google Scholar]
- Olson, M.E.; Garvin, K.L.; Fey, P.D.; Rupp, M.E. Adherence of Staphylococcus epidermidis to biomaterials is augmented by PIA. Clin. Orthop. Relat. Res. 2006, 451, 21–24. [Google Scholar] [CrossRef]
- Vuong, C.; Kidder, J.B.; Jacobson, E.R.; Otto, M.; Proctor, R.A.; Somerville, G.A. Staphylococcus epidermidis Polysaccharide Intercellular Adhesin Production Significantly Increases during Tricarboxylic Acid Cycle Stress. J. Bacteriol. 2005, 187, 2967. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, J.; Jeong, S.-I.; Jahng, K.Y.; Yu, K.-Y. Antimicrobial Effects and Resistant Regulation of Magnolol and Honokiol on Methicillin-Resistant Staphylococcus aureus. BioMed Res. Int. 2015, 2015, 283630. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant material. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zeng, C. Research progress on related gene regulation of MRSA biofilm formation. Chin. J. Hosp. Pharm. 2017, 37, 482–485. [Google Scholar]
- Anderl, J.N.; Zahller, J.; Roe, F.; Stewart, P.S. Role of Nutrient Limitation and Stationary-Phase Existence in Klebsiella pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2003, 47, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- de Kievit, T.R.; Iglewski, B.H. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 2000, 68, 4839–4849. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Deng, W.; Huang, X. Efficacy and adverse reactions of teicoplanin and vancomycin for pneumonia infected by methicillin-resistant strains of Staphylococcus aureus. Chin. J. Clin. Electron. Ed. 2013, 7, 8711–8714. [Google Scholar]
- Unni, S.; Siddiqui, T.J.; Bidaisee, S. Reduced Susceptibility and Resistance to Vancomycin of Staphylococcus aureus: A Review of Global Incidence Patterns and Related Genetic Mechanisms. Cureus 2021, 13, e18925. [Google Scholar] [CrossRef]
- Rose, W.E.; Fallon, M.; Moran, J.J.M.; Vanderloo, J.P. Vancomycin Tolerance in Methicillin-Resistant Staphylococcus aureus: Influence of Vancomycin, Daptomycin, and Telavancin on Differential Resistance Gene Expression. Antimicrob. Agents Chemother. 2012, 56, 4422–4427. [Google Scholar] [CrossRef] [PubMed]
- Wessam, A.; Liang, C.; Bayer, A.S.; Kati, S.; Yeaman, M.R.; Kreiswirth, B.N.; Xiong, Y.Q. Early agr activation correlates with vancomycin treatment failure in multi-clonotype MRSA endovascular infections. J. Antimicrob. Chemother. 2015, 70, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, T.J.; Leonard, S.N.; Vilay, A.M.; Mercier, R.-C. Vancomycin and Piperacillin-Tazobactam Against Methicillin-Resistant Staphylococcus aureus and Vancomycin-Intermediate Staphylococcus aureus in an In Vitro Pharmacokinetic/Pharmacodynamic Model. Clin. Ther. 2014, 36, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Deshpande, L.M.; Farrell, D.J.; Teresa, S.; Giovanni, F.; Jones, R.N. Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J. Antimicrob. Chemother. 2010, 65, 2329–2335. [Google Scholar] [CrossRef]
- Baos, E.; Candel, F.J.; Merino, P.; Pena, I.; Picazo, J.J. Characterization and monitoring of linezolid-resistant clinical isolates of Staphylococcus epidermidis in an intensive care unit 4 years after an outbreak of infection by cfr-mediated linezolid-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2013, 76, 325–329. [Google Scholar] [CrossRef]
- Cafini, F.; Nguyen, L.T.T.; Higashide, M.; Román, F.; Prieto, J.; Morikawa, K. Horizontal gene transmission of the cfr gene to MRSA and Enterococcus: Role of Staphylococcus epidermidis as a reservoir and alternative pathway for the spread of linezolid resistance. J. Antimicrob. Chemother. 2016, 71, 587–592. [Google Scholar] [CrossRef]
- Han, D.; Liu, Y.; Li, J.; Liu, C.; Gao, Y.; Feng, J.; Lu, H.; Yang, G. Twenty-seven-nucleotide repeat insertion in the rplV gene confers specific resistance to macrolide antibiotics in Staphylococcus aureus. Oncotarget 2018, 9, 26086–26095. [Google Scholar] [CrossRef]
- Yao, J.; Shao, L.; Liu, P.Y.; Chen, D.J.; Zhang, Y.B. Macrolide-resistant phenotypes and genotypes of Staphylococcus aureus isolated from clinical samples. Pharm. Biotechnol. 2016, 23, 291–295. [Google Scholar]
- Matsuoka, M.; Inoue, M.; Endo, Y.; Nakajima, Y. Characteristic expression of three genes, msr(A), mph(C) and erm(Y), that confer resistance to macrolide antibiotics on Staphylococcus aureus. FEMS Microbiol. Lett. 2010, 220, 287–293. [Google Scholar] [CrossRef]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of quinolone action and resistance: Where do we stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef]
- Gao, C.; Fan, Y.L.; Zhao, F.; Ren, Q.C.; Wu, X.; Chang, L.; Gao, F. Quinolone derivatives and their activities against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2018, 157, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Barrenechea, V.; Vargas-Reyes, M.; Quiliano, M.; Milón, P. A Complementary Mechanism of Bacterial mRNA Translation Inhibition by Tetracyclines. Front. Microbiol. 2021, 12, 682682. [Google Scholar] [CrossRef]
- Nøhr-Meldgaard, K.; Struve, C.; Ingmer, H.; Agersø, Y. The Tetracycline Resistance Gene, tet(W) in Bifidobacterium animalis subsp. lactis Follows Phylogeny and Differs From tet(W) in Other Species. Front. Microbiol. 2021, 12, 658943. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Stein, G.E.; Johnson, L.B. Ceftaroline: A novel cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, 1156–1163. [Google Scholar] [CrossRef]
- Schaumburg, F.; Peters, G.; Alabi, A.; Becker, K.; Idelevich, E.A. Missense mutations of PBP2a are associated with reduced susceptibility to ceftaroline and ceftobiprole in African MRSA. J. Antimicrob. Chemother. 2016, 71, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, D.; Cuénod, A.; Hinic, V.; Morgenstern, M.; Khanna, N.; Egli, A.; Kuehl, R. Genomic characterization of inpatient evolution of MRSA resistant to daptomycin, vancomycin and ceftaroline. J. Antimicrob. Chemother. 2019, 74, 1452–1454. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Bi, H.; Li, X.; Lv, X.; Liu, Y. Inhibition of S. aureus biofilm formation by linezolid alleviates sepsis-induced lung injury caused by S. aureus infection through direct inhibition of icaA activity. New Microbiol. 2023, 46, 285–295. [Google Scholar]
- Siala, W.; Rodriguez-Villalobos, H.; Fernandes, P.; Tulkens, P.M.; Van Bambeke, F. Activities of Combinations of Antistaphylococcal Antibiotics with Fusidic Acid against Staphylococcal Biofilms in In Vitro Static and Dynamic Models. Antimicrob. Agents Chemother. 2018, 62, e00598-18. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Zhou, Z.; Yang, J.; Liu, W.; Zhang, Y.; Zhang, P.; Guo, T.; Li, G. Baicalein Resensitizes Multidrug-Resistant Gram-Negative Pathogens to Doxycycline. Microbiol. Spectr. 2023, 11, e0470222. [Google Scholar] [CrossRef]
- Huff, W.E.; Huff, G.R.; Rath, N.C.; Balog, J.M.; Donoghue, A.M. Therapeutic efficacy of bacteriophage and Baytril (enrofloxacin) individually and in combination to treat colibacillosis in broilers. Poult. Sci. 2004, 83, 1944–1947. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Lye, D.C.; Yahav, D.; Sud, A.; Robinson, J.O.; Nelson, J.; Archuleta, S.; Roberts, M.A.; Cass, A.; Paterson, D.L.; et al. Effect of Vancomycin or Daptomycin With vs Without an Antistaphylococcal β-Lactam on Mortality, Bacteremia, Relapse, or Treatment Failure in Patients With MRSA Bacteremia: A Randomized Clinical Trial. JAMA 2020, 323, 527–537. [Google Scholar] [CrossRef]
- Gao, L.; Chen, J.; Chen, Y.; Xu, T.; Chen, X.; Yang, Y. Study on mutant selection window of cefquinome combined with 3 antibiotics against Staphylococcus aureus. Heilongjiang Anim. Sci. Vet. Med. 2019, 131–134+138. [Google Scholar] [CrossRef]
- Gonzales, P.R.; Pesesky, M.W.; Bouley, R.; Ballard, A.; Biddy, B.A.; Suckow, M.A.; Wolter, W.R.; Schroeder, V.A.; Burnham, C.A.; Mobashery, S.; et al. Synergistic, collaterally sensitive β-lactam combinations suppress resistance in MRSA. Nat. Chem. Biol. 2015, 11, 855–861. [Google Scholar] [CrossRef]
- Sharma, A.D.; Gutheil, W.G. Synergistic Combinations of FDA-Approved Drugs with Ceftobiprole against Methicillin-Resistant Staphylococcus aureus. Microbiol. Spectr. 2023, 11, e0372622. [Google Scholar] [CrossRef]
- Topka-Bielecka, G.; Dydecka, A.; Necel, A.; Bloch, S.; Nejman-Faleńczyk, B.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-Derived Depolymerases against Bacterial Biofilm. Antibiotics 2021, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Johnson, C.N.; Hill, C.; Ross, R.P. Efficacy of Phage- and Bacteriocin-Based Therapies in Combatting Nosocomial MRSA Infections. Front. Mol. Biosci. 2021, 8, 654038. [Google Scholar] [CrossRef] [PubMed]
- Shizuya, H. Methods for Identifying an Essential Gene in a Prokaryotic Microorganism. U.S. Patent No. 6,991,900, 31 January 2006. [Google Scholar]
- Rahimzadeh, G.; Gill, P.; Rezai, M.S. Characterization of Methicillin-Resistant Staphylococcus aureus (MRSA) Phages From Sewage at a Tertiary Pediatric Hospital. Arch. Pediatr. Infect. Dis. 2016, 5, e39615. [Google Scholar] [CrossRef]
- Mohammed-Ali, M.N.; Jamalludeen, N.M. Isolation and Characterization of Bacteriophage against Methicillin Resistant Staphylococcus aureus. J. Med. Microbiol. Diagn. 2015, 5, 213. [Google Scholar] [CrossRef]
- Kebriaei, R.; Lev, K.L.; Stamper, K.C.; Lehman, S.M.; Rybak, M.J. Bacteriophage AB-SA01 Cocktail in Combination with Antibiotics against MRSA-VISA Strain: In vitro Pharmacokinetic/Pharmacodynamic Model. Antimicrob. Agents Chemother. 2020, 65, e01863-20. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef]
- Wu, D.; Walsh, T.R.; Wu, Y.J. World Antimicrobial Awareness Week 2021-Spread Awareness, Stop Resistance. China CDC Wkly. 2021, 3, 987–993. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef]
- Moo, C.L.; Yang, S.K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Lim, S.H.; Lai, K.S. Mechanisms of Antimicrobial Resistance (AMR) and Alternative Approaches to Overcome AMR. Curr. Drug Discov. Technol. 2020, 17, 430–447. [Google Scholar] [CrossRef]
- Laganà, A.; Visalli, G.; Corpina, F.; Ferlazzo, M.; Di Pietro, A.; Facciolà, A. Antibacterial activity of nanoparticles and nanomaterials: A possible weapon in the fight against healthcare-associated infections. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3645–3663. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Hung, A.; Li, R.; Barlow, A.; Singleton, W.; Matthyssen, T.; Sani, M.A.; Hossain, M.A.; Wade, J.D.; O’Brien-Simpson, N.M.; et al. Systematic comparison of activity and mechanism of antimicrobial peptides against nosocomial pathogens. Eur. J. Med. Chem. 2022, 231, 114135. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Datta, M.; Rajeev, A.; Chattopadhyay, I. Application of antimicrobial peptides as next-generation therapeutics in the biomedical world. Biotechnol. Genet. Eng. Rev. 2023, 40, 2458–2496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767. [Google Scholar] [CrossRef] [PubMed]
- Pahar, B.; Madonna, S.; Das, A.; Albanesi, C.; Girolomoni, G. Immunomodulatory Role of the Antimicrobial LL-37 Peptide in Autoimmune Diseases and Viral Infections. Vaccines 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Patrulea, V.; Borchard, G.; Jordan, O. An Update on Antimicrobial Peptides (AMPs) and Their Delivery Strategies for Wound Infections. Pharmaceutics 2020, 12, 840. [Google Scholar] [CrossRef]
- Rizzetto, G.; Gambini, D.; Maurizi, A.; Candelora, M.; Molinelli, E.; Cirioni, O.; Brescini, L.; Giacometti, A.; Offidani, A.; Simonetti, O. Our Experience over 20 Years: Antimicrobial Peptides against Gram Positives, Gram Negatives, and Fungi. Pharmaceutics 2022, 15, 40. [Google Scholar] [CrossRef]
- Kang, S.J.; Nam, S.H.; Lee, B.J. Engineering Approaches for the Development of Antimicrobial Peptide-Based Antibiotics. Antibiotics 2022, 11, 1338. [Google Scholar] [CrossRef]
- Linares, D.M.; Ross, P.; Stanton, C. Beneficial Microbes: The pharmacy in the gut. Bioengineered 2016, 7, 11–20. [Google Scholar] [CrossRef]
- Rueda-Robles, A.; Rodríguez-Lara, A.; Meyers, M.S.; Sáez-Lara, M.J.; Álvarez-Mercado, A.I. Effect of Probiotics on Host-Microbiota in Bacterial Infections. Pathogens 2022, 11, 986. [Google Scholar] [CrossRef]
- Bang, W.Y.; Kim, H.; Chae, S.A.; Yang, S.Y.; Ban, O.H.; Kim, T.Y.; Kwon, H.S.; Jung, Y.H.; Yang, J. A Quadruple Coating of Probiotics for Enhancing Intestinal Adhesion and Competitive Exclusion of Salmonella Typhimurium. J. Med. Food 2022, 25, 213–218. [Google Scholar] [CrossRef]
- Blackwood, B.P.; Yuan, C.Y.; Wood, D.R.; Nicolas, J.D.; Grothaus, J.S.; Hunter, C.J. Probiotic Lactobacillus Species Strengthen Intestinal Barrier Function and Tight Junction Integrity in Experimental Necrotizing Enterocolitis. J. Probiotics Health 2017, 5, 1000159. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Zhaoxin, L.; Jianhao, Z.; Abid, M.; Khan, K.R.; Korma, S.A.; Alghamdi, M.A.; El-Saadony, M.T.; Abd El-Hack, M.E.; et al. Probiotic-Based Bacteriocin: Immunity Supplementation Against Viruses. An Updated Review. Front. Microbiol. 2022, 13, 876058. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, Z.; Hu, H.W.; Howell, K.; Fang, Z.; Zhang, P. Food substances alter gut resistome: Mechanisms, health impacts, and food components. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70143. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Forssten, S.; Hibberd, A.A.; Lyra, A.; Stahl, B. Probiotic approach to prevent antibiotic resistance. Ann. Med. 2016, 48, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, B.A.; Kebede, B. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon 2022, 8, e09725. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections with Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef]
- Silva, D.R.; Sardi, J.D.C.O.; de Souza Pitangui, N.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Caselli, E.; Arnoldo, L.; Rognoni, C.; D’Accolti, M.; Soffritti, I.; Lanzoni, L.; Bisi, M.; Volta, A.; Tarricone, R.; Brusaferro, S.; et al. Impact of a probiotic-based hospital sanitation on antimicrobial resistance and HAI-associated antimicrobial consumption and costs: A multicenter study. Infect. Drug Resist. 2019, 12, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Lu, L.; Li, Z.; Yu, Q.; Zhang, X. Antibacterial Effects of 31 Kinds of Traditional Chinese Medicine Monomers on MRSA in Vitro. China Pharm. 2014, 23, 20–22. [Google Scholar]
- Huang, H.; Lou, Q.; Li, X.; Jin, X.; Zhu, J.; Zhang, H.; Ma, Y. The study of antibacterial effect and related mechanism of garlic oil on MRSA strain. J. Chin. J. Microecol. 2011, 23, 429–431. [Google Scholar]
- Ren, S.; Cao, D.; Yang, J.; Yang, S.; Song, Y. Bacteriostatic Activity of Combined Application of Galla chinensis, Scutellaria baicalensis and Rhizoma coptidis Against MRSA in Vitro. China Pharm. 2010, 21, 198–199. [Google Scholar]
- Ren, S.; Yang, J.; Wang, C. The study of the antibacterial activity of combined application of Rhizoma coptidis, Rhubarb and Sophora flavescens ait on MRSA in vitro. Hebei Med. J. 2011, 33, 2253–2254. [Google Scholar]
- Fu, R.; Yu, Q.; Meng, D.; Lu, L.; Zhang, X. Antibacterial Effects of 21 Extracts of Chinese Herbal Medicine on MRSA. China Pharm. 2011, 22, 4056–4058. [Google Scholar]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Li, W.; Lin, L. Research on antibacterial effect of eucalyptus extracts combined with oxacillin against MRSA. Food Mach. 2015, 31, 123–126. [Google Scholar]
- Wu, M.; Wang, J.; Liu, G.; Huang, H. Bactericidal effect of drug combination of Semen cassiae torae and Benzylpenicillin on clinical strains of MRSA. Qingdao Med. J. 2014, 46, 340–341. [Google Scholar]
- Elakkia, S.A.; Venkatesalu, V. Anti-MRSA activity of different extracts of selected Cassia species. Int. J. Pharm. Clin. Sci. 2014, 4, 11–17. [Google Scholar]
- Zuo, G.Y.; Zhang, X.J.; Yang, C.X.; Han, J.; Wang, G.C.; Bian, Z.Q. Evaluation of traditional Chinese medicinal plants for anti-MRSA activity with reference to the treatment record of infectious diseases. Molecules 2012, 17, 2955–2967. [Google Scholar] [CrossRef]
| Strategies | Substantive Contents | Action Mechanisms Against MRSA | Precautions |
|---|---|---|---|
| Combined therapy | The concurrent use of two or more antibiotics to enhance therapeutic efficacy and reduce the emergence of drug resistance. | The synergistic action among different antibiotics can more effectively kill MRSA while reducing the chances of bacteria developing resistance. | When using antibiotics with different action mechanisms in combination, it is essential to carefully assess the potential drug interactions to avoid reduced efficacy or increased toxicity. |
| Bacteriophage therapy | Utilizing specific viruses (bacteriophages) to infect and kill bacteria. | Phages are able to specifically recognize and infect MRSA and cause bacterial death by releasing enzymes and other factors that destroy bacterial cell walls. | The safety of bacteriophages needs to be rigorously assessed to ensure they do not adversely affect the host microbiota or elicit immune responses. |
| Nanobiologic therapy | Involving the use of drugs or materials prepared using nanotechnology to accurately target and treat infections. | NPs are designed to specifically recognize MRSA and treat infections by releasing drugs or directly destroying bacterial cells. | Nanoparticles, when used as drug carriers, require careful design of their size, surface properties, and release kinetics to optimize therapeutic efficacy. |
| AMPs | A class of naturally occurring peptide molecules with broad-spectrum antimicrobial activity. | By disrupting the membrane integrity of MRSA, AMPs leads to intracellular material leakage and bacterial death | AMPs have poor stability in the body and are easily degraded by proteases, thus requiring chemical modifications to enhance their stability. |
| Live bio-therapeutics | Using live microorganisms (such as probiotics) or their metabolic products to treat diseases caused by MRSA. | Controlling infections by competing for nutrients or producing substances that inhibit the growth of MRSA. | Treatment with live bacteria or their derivatives may cause immune responses and therefore needs to be monitored and managed. |
| Chinese herbal drugs therapy | Using natural plant extracts or other natural components to treat diseases. | The active components in traditional Chinese medicine may affect MRSA through multiple pathways, such as inhibiting its growth, disrupting its biofilms, or enhancing the host immune response. | The composition of traditional Chinese medicinal herbs is complex, necessitating the assurance of consistency in the source, extraction processes, and quality standards of the herbs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lu, H.; Hu, G.; Liu, J.; Lian, S.; Pang, S.; Zhu, G.; Ding, X. Unmasking MRSA’s Armor: Molecular Mechanisms of Resistance and Pioneering Therapeutic Countermeasures. Microorganisms 2025, 13, 1928. https://doi.org/10.3390/microorganisms13081928
Liu Y, Lu H, Hu G, Liu J, Lian S, Pang S, Zhu G, Ding X. Unmasking MRSA’s Armor: Molecular Mechanisms of Resistance and Pioneering Therapeutic Countermeasures. Microorganisms. 2025; 13(8):1928. https://doi.org/10.3390/microorganisms13081928
Chicago/Turabian StyleLiu, Yichen, Hao Lu, Gaowei Hu, Jiaqi Liu, Siqi Lian, Shengmei Pang, Guoqiang Zhu, and Xueyan Ding. 2025. "Unmasking MRSA’s Armor: Molecular Mechanisms of Resistance and Pioneering Therapeutic Countermeasures" Microorganisms 13, no. 8: 1928. https://doi.org/10.3390/microorganisms13081928
APA StyleLiu, Y., Lu, H., Hu, G., Liu, J., Lian, S., Pang, S., Zhu, G., & Ding, X. (2025). Unmasking MRSA’s Armor: Molecular Mechanisms of Resistance and Pioneering Therapeutic Countermeasures. Microorganisms, 13(8), 1928. https://doi.org/10.3390/microorganisms13081928






