Climate Change Adaptation in Winemaking: Combined Use of Non-Saccharomyces Yeasts to Improve the Quality of Pedro Ximénez Wines
Abstract
1. Introduction
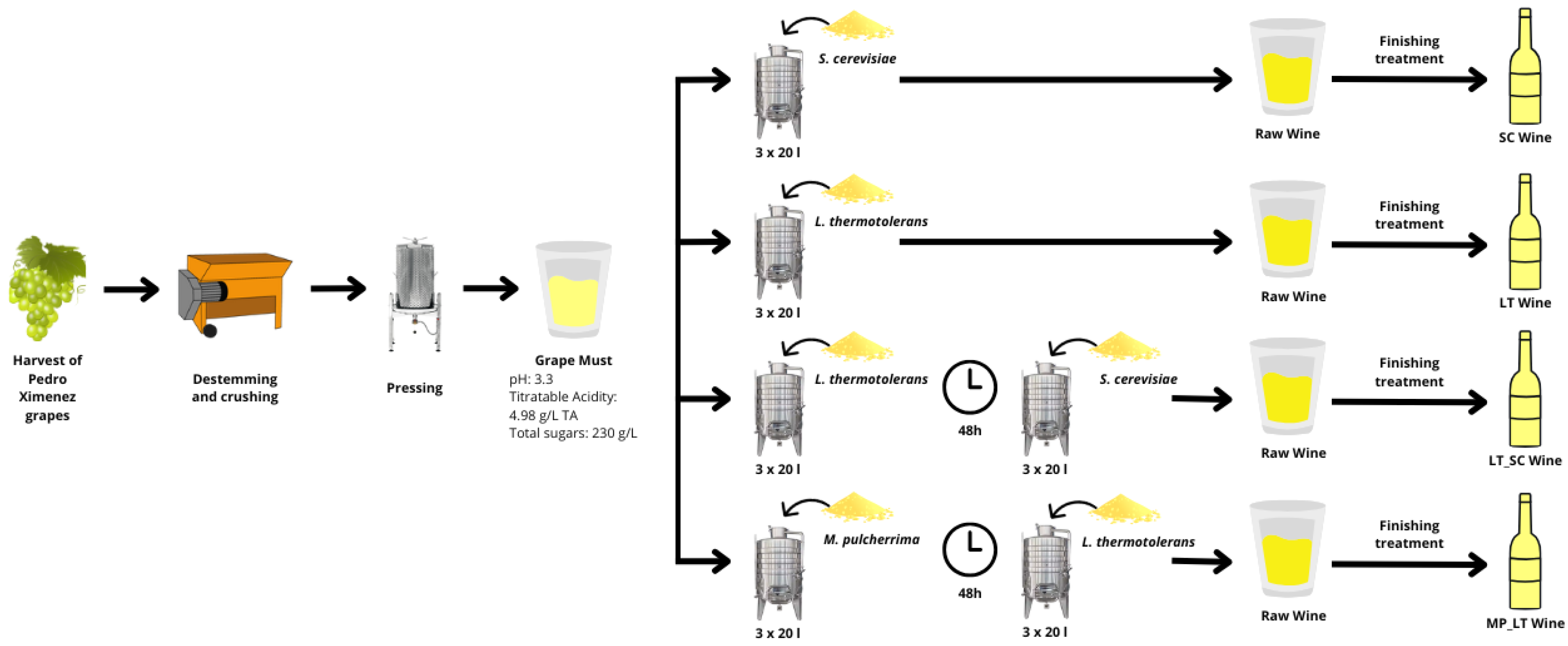
2. Materials and Methods
2.1. Fermentation Conditions
2.2. Oenological Parameters
2.3. Analysis of Volatile Compounds
2.3.1. Major Volatile Compounds and Polyols
2.3.2. Minor Volatile Compounds
2.3.3. Calculation of Aromatic Series
2.4. Organoleptic Characterisation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Oenological Parameters
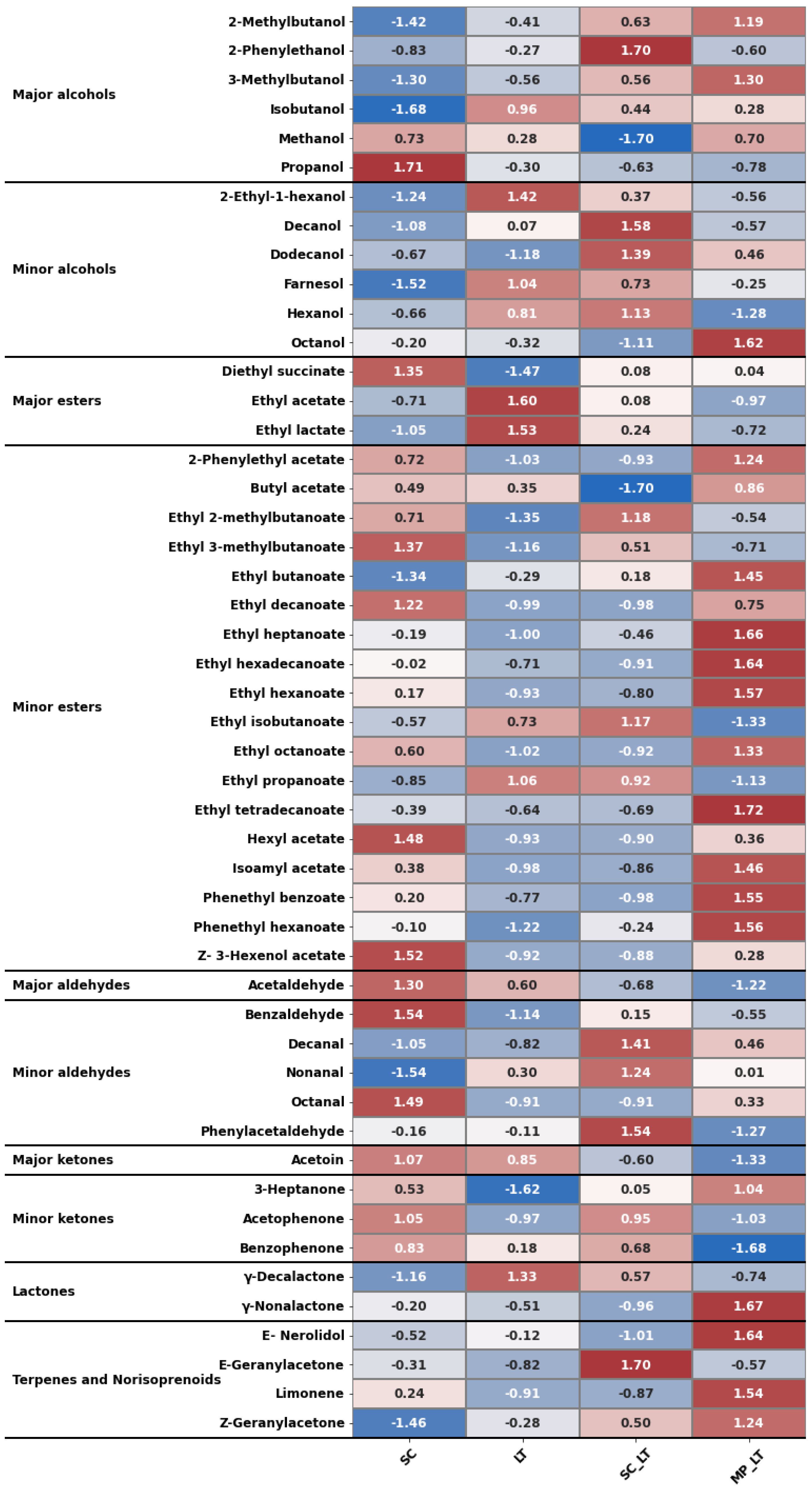
3.2. Volatile Aroma Compounds
3.3. Odor Activity Values and Aromatic Series
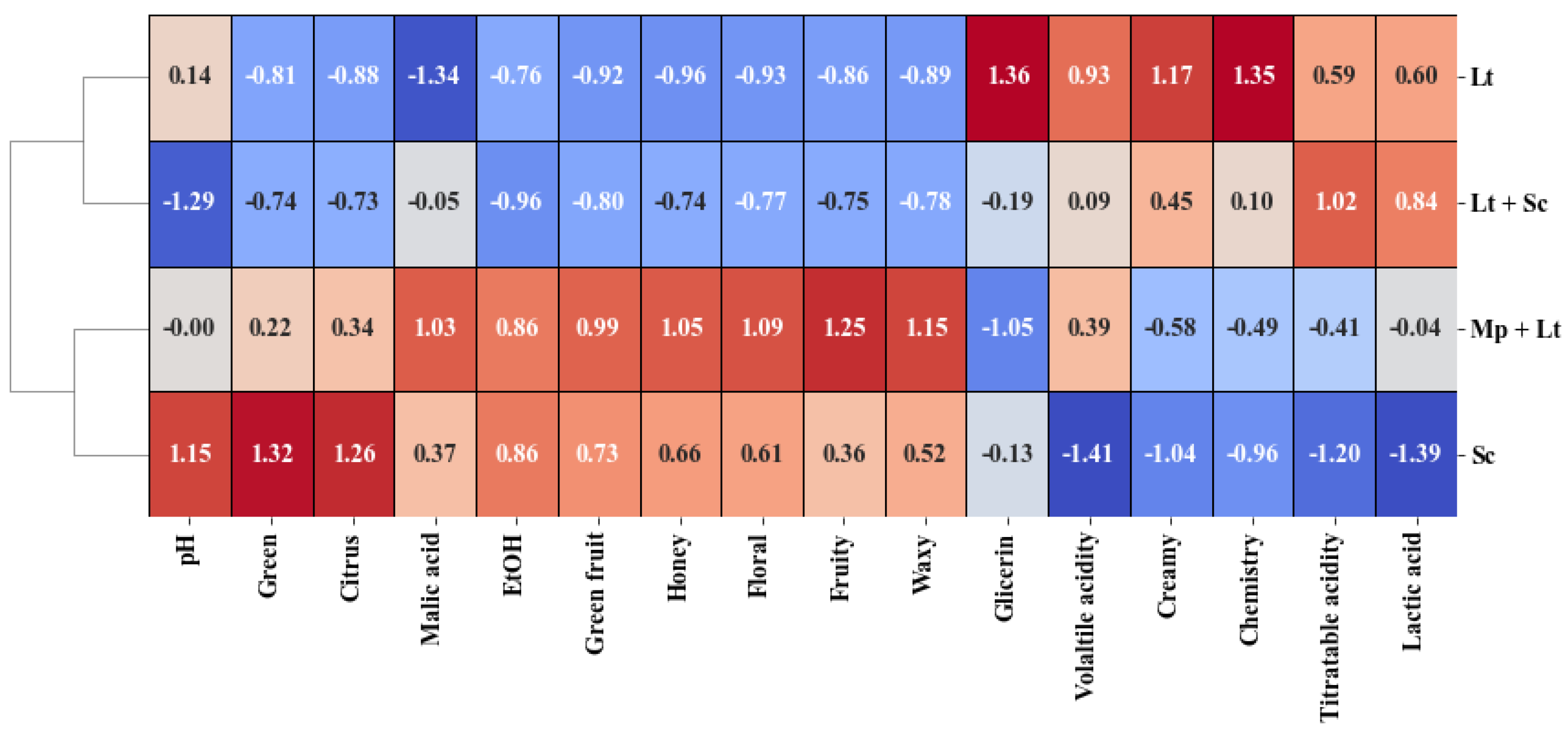
3.4. Multivariate Analysis, Cluster Analysis, and Heat Map
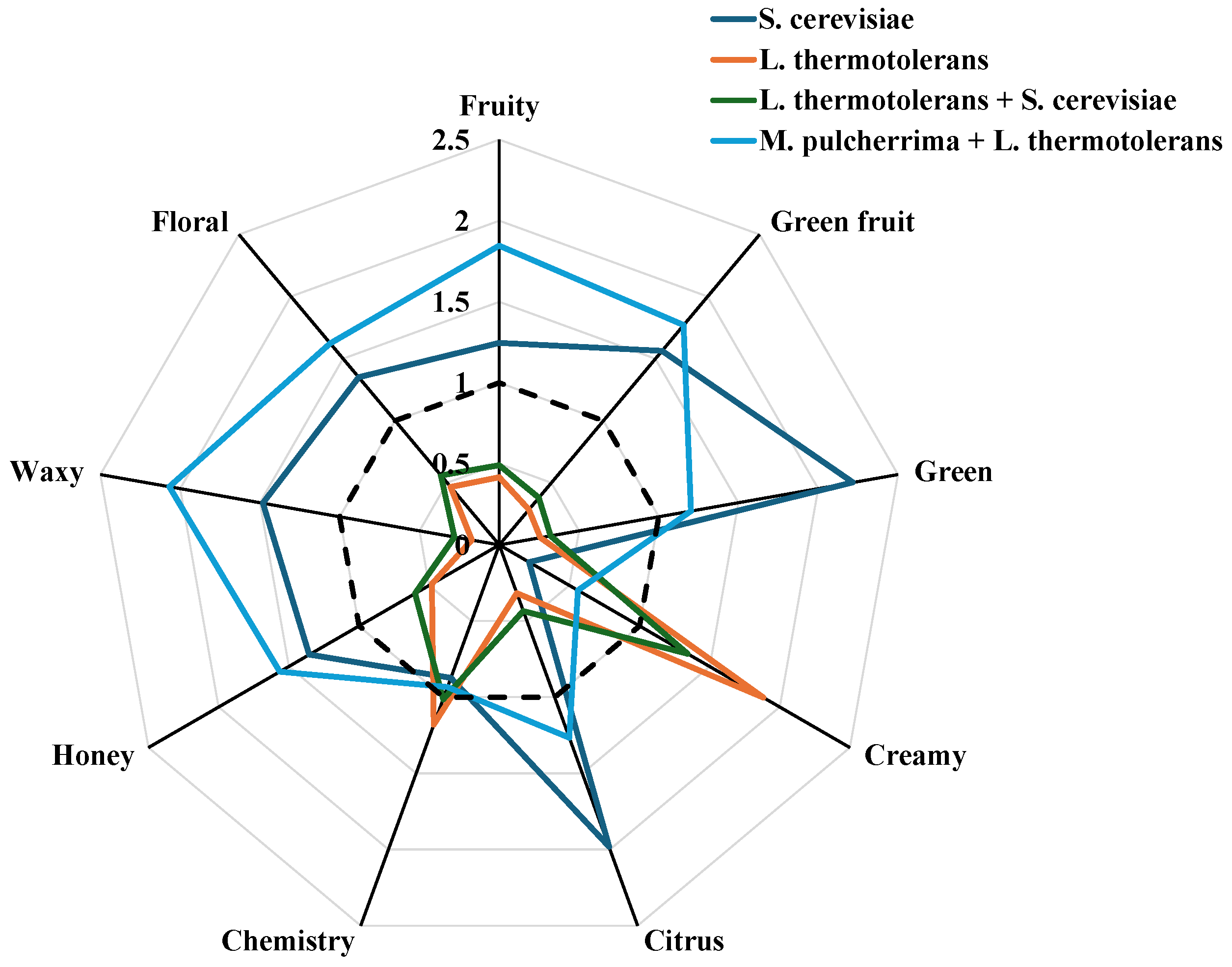
3.5. Organoleptic Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC-FID | Gas chromatography flame ionisation detector |
| GC-MS | Gas chromatography mass spectrum detector |
| PX | Pedro Ximenez grape variety |
References
- Van Leeuwen, C.; Sgubin, G.; Bois, B.; Ollat, N.; Swingedouw, D.; Zito, S.; Gambetta, G.A. Climate Change Impacts and Adaptations of Wine Production. Nat. Rev. Earth Environ. 2024, 5, 258–275. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate Change Associated Effects on Grape and Wine Quality and Production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario—A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef] [PubMed]
- Campos-Arguedas, F.; Sarrailhé, G.; Nicolle, P.; Dorais, M.; Brereton, N.J.B.; Pitre, F.E.; Pedneault, K. Different Temperature and UV Patterns Modulate Berry Maturation and Volatile Compounds Accumulation in Vitis Sp. Front. Plant Sci. 2022, 13, 862259. [Google Scholar] [CrossRef]
- Arrizabalaga, M.; Morales, F.; Oyarzun, M.; Delrot, S.; Gomès, E.; Irigoyen, J.J.; Hilbert, G.; Pascual, I. Tempranillo Clones Differ in the Response of Berry Sugar and Anthocyanin Accumulation to Elevated Temperature. Plant Sci. 2018, 267, 74–83. [Google Scholar] [CrossRef]
- Morata, A.; Bañuelos, M.A.; Vaquero, C.; Loira, I.; Cuerda, R.; Palomero, F.; González, C.; Suárez-Lepe, J.A.; Wang, J.; Han, S.; et al. Lachancea Thermotolerans as a Tool to Improve PH in Red Wines from Warm Regions. Eur. Food Res. Technol. 2019, 245, 885–894. [Google Scholar] [CrossRef]
- Sánchez-Suárez, F.; Peinado, R.A. Use of Non-Saccharomyces Yeast to Enhance the Acidity of Wines Produced in a Warm Climate Region: Effect on Wine Composition. Fermentation 2023, 10, 17. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Heras, J.M.; Carrau, F.; González, C.; Morata, A. Biocompatibility in Ternary Fermentations With Lachancea Thermotolerans, Other Non-Saccharomyces and Saccharomyces Cerevisiae to Control PH and Improve the Sensory Profile of Wines from Warm Areas. Front. Microbiol. 2021, 12, 656262. [Google Scholar] [CrossRef]
- Vicente, J.; Navascués, E.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. An Integrative View of the Role of Lachancea Thermotolerans in Wine Technology. Foods 2021, 10, 2878. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Bañuelos, M.A.; Heras, J.M.; Cuerda, R.; Morata, A. Industrial Performance of Several Lachancea Thermotolerans Strains for PH Control in White Wines from Warm Areas. Microorganisms 2020, 8, 830. [Google Scholar] [CrossRef]
- Benito, S. The Impacts of Lachancea Thermotolerans Yeast Strains on Winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.P.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea Thermotolerans on Chemical Composition and Sensory Profiles of Merlot Wines. Food Chem. 2021, 349, 129015. [Google Scholar] [CrossRef]
- Vaquero, C.; Escott, C.; Heras, J.M.; Carrau, F.; Morata, A. Co-Inoculations of Lachancea Thermotolerans with Different Hanseniaspora Spp.: Acidification, Aroma, Biocompatibility, and Effects of Nutrients in Wine. Food Res. Int. 2022, 161, 111891. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Vaquero, C.; Loira, I.; López, C.; González, C.; Morata, A. Synergetic Effect of Metschnikowia Pulcherrima and Lachancea Thermotolerans in Acidification and Aroma Compounds in Airén Wines. Foods 2022, 11, 3734. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castells, R.; Moreno, J.; García-Martínez, T.; Mauricio, J.C.; Moreno-García, J. Chemometric Differentiation of White Wines from a Low-Aromatic Grape Obtained by Spontaneous Fermentation, Enriched with Non-Saccharomyces, or with a High-Glutathione-Producing Saccharomyces Yeast. Fermentation 2023, 9, 1023. [Google Scholar] [CrossRef]
- Boban, A.; Vrhovsek, U.; Carlin, S.; Milanović, V.; Gajdoš Kljusurić, J.; Jurun, Z.; Mucalo, A.; Budić-Leto, I. Impact of Indigenous Non-Saccharomyces Yeasts on Early Volatile Fermentation Metabolites of White Maraština Grape Must. Eur. Food Res. Technol. 2025. [Google Scholar] [CrossRef]
- Porter, T.J.; Divol, B.; Setati, M.E. Investigating the Biochemical and Fermentation Attributes of Lachancea Species and Strains: Deciphering the Potential Contribution to Wine Chemical Composition. Int. J. Food Microbiol. 2019, 290, 273–287. [Google Scholar] [CrossRef]
- Tzamourani, A.; Paramithiotis, S.; Favier, M.; Coulon, J.; Moine, V.; Paraskevopoulos, I.; Dimopoulou, M. New Insights into the Production of Assyrtiko Wines from the Volcanic Terroir of Santorini Island Using Lachancea Thermotolerans. Microorganisms 2024, 12, 786. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.-D.; Rauhut, D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.P.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea Thermotolerans on Chemical Composition and Sensory Profiles of Viognier Wines. J. Fungi 2022, 8, 474. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The Combined Use of Schizosaccharomyces Pombe and Lachancea Thermotolerans—Effect on the Anthocyanin Wine Composition. Molecules 2017, 22, 739. [Google Scholar] [CrossRef]
- Ivić, S.; Jeromel, A.; Kozina, B.; Prusina, T.; Budić-Leto, I.; Boban, A.; Vasilj, V.; Jagatić Korenika, A.-M. Sequential Fermentation in Red Wine Cv. Babić Production: The Influence of Torulaspora Delbrueckii and Lachancea Thermotolerans Yeasts on the Aromatic and Sensory Profile. Foods 2024, 13, 2000. [Google Scholar] [CrossRef]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and Sensory Profiling of Shiraz Wines Co-Fermented with Commercial Non- Saccharomyces Inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airen Wines Fermented by Sequential Inoculation of Lachancea Thermotolerans and Saccharomyces Cerevisiae. Food Technol Biotechnol. 2016, 54. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.L.; Fierro-Risco, J.; Ríos-Reina, R.; Ubeda, C.; Paneque, P. Influence of Saccharomyces Cerevisiae and Lachancea Thermotolerans Co-Inoculation on Volatile Profile in Fermentations of a Must with a High Sugar Content. Food Chem. 2019, 276, 427–435. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2023; ISBN 9782850380686. [Google Scholar]
- Peinado, R.A.; Moreno, J.A.; Muñoz, D.; Medina, M.; Moreno, J. Gas Chromatographic Quantification of Major Volatile Compounds and Polyols in Wine by Direct Injection. J. Agric. Food Chem. 2004, 52, 6389–6393. [Google Scholar] [CrossRef]
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of Two Yeast Strains in Free, Bioimmobilized or Immobilized with Alginate Forms on the Aromatic Profile of Long Aged Sparkling Wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef]
- OIV. Available online: https://www.oiv.int/es (accessed on 12 May 2025).
- Battjes, J.; Melkonian, C.; Mendoza, S.N.; Haver, A.; Al-Nakeeb, K.; Koza, A.; Schrubbers, L.; Wagner, M.; Zeidan, A.A.; Molenaar, D.; et al. Ethanol-Lactate Transition of Lachancea Thermotolerans Is Linked to Nitrogen Metabolism. Food Microbiol. 2023, 110, 104167. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Iorizzo, M.; Di Renzo, M.; Coppola, R.; Succi, M. Preliminary Characterisation of Metschnikowia Pulcherrima to Be Used as a Starter Culture in Red Winemaking. Beverages 2024, 10, 88. [Google Scholar] [CrossRef]
- Snyder, E.C.; Jiranek, V.; Hranilovic, A. Impact of Lachancea Thermotolerans Strain and Lactic Acid Concentration on Oenococcus Oeni and Malolactic Fermentation in Wine. OENO One 2021, 55, 365–380. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; Wiley: Hoboken, NJ, USA, 2016; ISBN 9781118627808. [Google Scholar]
- Zhang, B.; Hu, J.; Cheng, C.; Xu, Y.; Duan, C.; Yan, G. Effects of Native Lachancea Thermotolerans Combined with Saccharomyces Cerevisiae on Wine Volatile and Phenolic Profiles in Pilot and Industrial Scale. Food Chem. Adv. 2023, 2, 100258. [Google Scholar] [CrossRef]
- Li, J.; Hong, M. Impact of Metschnikowia Pulcherrima during Fermentation on Aromatic Profile of Vidal Blanc Icewine. In IVES Conference Series, OENO Macrowine 2023; IVES-International Viticulture and Enology Society: Villenave d’Ornon, France, 2023. [Google Scholar]
- Coppola, F.; Testa, B.; Cozzolino, R.; Karaulli, J.; Pannella, G.; Di Renzo, M.; Matarazzo, C.; Succi, M.; Iorizzo, M. Effects of Inoculation Timing and Mixed Fermentation with Metschnikowia Pulcherrima and Saccharomyces Cerevisiae on the Aroma and Sensory Properties of Falanghina Wine. Eur. Food Res. Technol. 2025, 251, 1699–1717. [Google Scholar] [CrossRef]
- Wanikawa, A.; Hosoi, K.; Kato, T. Conversion of Unsaturated Fatty Acids to Precursors of γ-Lactones by Lactic Acid Bacteria during the Production of Malt Whisky. J. Am. Soc. Brew. Chem. 2000, 58, 51–56. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; del Fresno, J.M.; Malfeito-Ferreira, M.; Mena-Morales, A.; García-Romero, E.; Heras, J.M.; Loira, I.; González, C.; Morata, A. Wine Bioacidification: Fermenting Airén Grape Juices with Lachancea Thermotolerans and Metschnikovia Pulcherrima Followed by Sequential Saccharomyces Cerevisiae Inoculation. Int. J. Food Microbiol. 2025, 427, 110977. [Google Scholar] [CrossRef]
- Moreno, J.J.; Peinado, R.A. Enological Chemistry; Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Perpetuini, G.; Rossetti, A.P.; Quadrani, L.; Arfelli, G.; Piva, A.; Suzzi, G.; Tofalo, R. Sequential Inoculation of Metschnikowia Pulcherrima and Saccharomyces Cerevisiae as a Biotechnological Tool to Increase the Terpenes Content of Pecorino White Wines. Fermentation 2023, 9, 785. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining Wine Aroma from Compositional Data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Dumitriu (Gabur), G.-D.; Peinado, R.A.; Cotea, V.V.; López de Lerma, N. Volatilome Fingerprint of Red Wines Aged with Chips or Staves: Influence of the Aging Time and Toasting Degree. Food Chem. 2020, 310, 125801. [Google Scholar] [CrossRef]
- Palenzuela, M.d.V.; López de Lerma, N.; Sánchez-Suárez, F.; Martínez-García, R.; Peinado, R.A.; Rosal, A. Aroma Composition of Wines Produced from Grapes Treated with Organic Amendments. Appl. Sci. 2023, 13, 8001. [Google Scholar] [CrossRef]
- Hernandez-Orte, P.; Bely, M.; Cacho, J.; Ferreira, V. Impact of Ammonium Additions on Volatile Acidity, Ethanol, and Aromatic Compound Production by Different Saccharomyces Cerevisiae Strains during Fermentation in Controlled Synthetic Media. Aust. J. Grape Wine Res. 2006, 12, 150–160. [Google Scholar] [CrossRef]
- García-Martínez, T.; Moreno, J.; Mauricio, J.C.; Peinado, R. Natural Sweet Wine Production by Repeated Use of Yeast Cells Immobilized on Penicillium Chrysogenum. LWT—Food Sci. Technol. 2015, 61, 503–509. [Google Scholar] [CrossRef]
- Pérez, D.; Denat, M.; Heras, J.M.; Guillamón, J.M.; Ferreira, V.; Querol, A. Effect of Non-Wine Saccharomyces Yeasts and Bottle Aging on the Release and Generation of Aromas in Semi-Synthetic Tempranillo Wines. Int. J. Food Microbiol. 2022, 365, 109554. [Google Scholar] [CrossRef]
- Seguinot, P.; Rollero, S.; Sanchez, I.; Sablayrolles, J.-M.; Ortiz-Julien, A.; Camarasa, C.; Mouret, J.-R. Impact of the Timing and the Nature of Nitrogen Additions on the Production Kinetics of Fermentative Aromas by Saccharomyces Cerevisiae during Winemaking Fermentation in Synthetic Media. Food Microbiol. 2018, 76, 29–39. [Google Scholar] [CrossRef]
- Vicente, J.; Vladic, L.; Navascués, E.; Brezina, S.; Santos, A.; Calderón, F.; Tesfaye, W.; Marquina, D.; Rauhut, D.; Benito, S. A Comparative Study of Lachancea Thermotolerans Fermentative Performance under Standardized Wine Production Conditions. Food Chem. X 2024, 21, 101214. [Google Scholar] [CrossRef]
- Chambers, J. Graphical Methods for Data Analysis; Wadsworth & Brooks: Belmont, CA, USA, 1983. [Google Scholar]
- Gutiérrez, R.; Gonzlez, A.; Torres, F.; Gallardo, J.A. Técnicas de Anélisis de Datos Multivariable. Tratamiento Computacional; Universidad de Granada: Granada, Spain, 1994. [Google Scholar]

| SC | LT | LT → SC | MP → LT | ||
|---|---|---|---|---|---|
| pH | 3.29 ± 0.01 a | 3.15 ± 0.01 b | 3.02 ± 0.04 c | 3.18± 0.01 b | |
| Titratable Acidity | g/L TH2 | 4.56 ± 0.04 d | 6.38 ± 0.05 b | 6.83 ± 0.05 a | 5.4 ± 0.1 c |
| Ethanol | % v/v | 13.47 ± 0.06 a | 13.07 ± 0.06 b | 13.02 ± 0.03 b | 13.47 ± 0.06 a |
| Volatile Acidity | g/L AcH | 0.35 ± 0.02 c | 0.61 ± 0.02 a | 0.52 ± 0.02 b | 0.55 ± 0.02 b |
| Lactic Acid | g/L | N.D. | 1.8 ± 0.1 a | 2.0 ± 0.1 a | 1.24 ± 0.06 b |
| Malic Acid | g/L | 0.61 ± 0.06 ab | 0.55 ± 0.03 b | 0.55 ± 0.06 b | 0.71 ± 0.06 a |
| Glycerol | g/L | 4.41 ± 0.16 ab | 4.82 ± 0.36 a | 4.39 ± 0.23 ab | 4.15 ± 0.05 c |
| SC | LT | LT → SC | MP → LT | |
|---|---|---|---|---|
| Alcohols | ||||
| Major alcohols (mg/L) | 503.7 ± 0.3 a | 469 ± 2 a | 461 ± 3 a | 470.9 ± 0.9 a |
| Methanol | 50 ± 1 a | 47.98 ± 0.09 a | 40 ± 9 a | 50 ± 2 a |
| Propanol | 149.8 ± 0.6 a | 61.8 ± 0.7 b | 47 ± 9 c | 40.62 ± 0.09 c |
| Isobutanol | 26.21 ± 0.09 c | 66 ± 1 a | 58 ± 4 b | 55.7 ± 0.6 b |
| 2-methylbutanol | 27.5 ± 0.2 d | 34.2 ± 0.2 c | 41 ± 2 b | 44.9 ± 0.3 a |
| 3-methylbutanol | 227.42 ± 0.09 d | 236 ± 4 c | 248 ± 2 b | 257 ± 2 a |
| 2-phenylethanol | 23 ± 0.5 a | 23.5 ± 0.3 a | 25 ± 2 a | 23.2 ± 0.2 a |
| Minor alcohols (µg/L) | 1022 ± 79 b | 1562 ± 57 a | 1666 ± 105 a | 837 ± 88 b |
| Hexanol | 964 ± 82 b | 1499 ± 57 a | 1616 ± 103 a | 739 ± 85 c |
| 2-ethyl-1-hexanol | 22 ± 2 | 24 ± 1 | 23.1 ± 0.9 | 22 ± 2 |
| Octanol | 29 ± 4 b | 26 ± 2 b | 10 ± 1 c | 65 ± 4 a |
| Decanol | 5 ± 2 a | 6 ± 0.4 a | 7.6 ± 0.8 a | 5.3 ± 0.7 a |
| Dodecanol | 1.2 ± 0.1 c | 0.84 ± 0.08 d | 2.7 ± 0.2 a | 2 ± 0.06 b |
| Farnesol | 1.7 ± 0.3 c | 6.6 ± 0.6 a | 6 ± 0.4 a | 4.2 ± 0.5 b |
| Esters | ||||
| Major esters (mg/L) | 97.1 ± 0.4 a | 167.6 ± 0.8 a | 128 ± 10 a | 94 ± 0.7 a |
| Ethyl acetate | 70 ± 1 b | 118 ± 2 a | 87 ± 24 ab | 65 ± 1 b |
| Ethyl lactate | 18.24 ± 0.09 d | 42.4 ± 0.9 a | 34 ± 5 b | 21.3 ± 0.09 c |
| Diethyl succinate | 8.7 ± 0.3 a | 6.99 ± 0.05 b | 7.91 ± 0.06 c | 7.89 ± 0.01 b |
| Minor esters (µg/L) | 9289 ± 629 b | 2660 ± 155 c | 3126 ± 54 c | 12,497 ± 394 a |
| Ethyl propanoate | 66 ± 7 b | 121 ± 10 a | 117.2 ± 0.9 a | 58 ± 3 b |
| Ethyl isobutanoate | 30 ± 1 c | 62 ± 7 b | 73 ± 2 a | 10.8 ± 0.3 d |
| Ethyl butanoate | 142 ± 8 c | 203 ± 17 b | 230 ± 6 b | 303 ± 9 a |
| Butyl acetate | 0.33 ± 0.04 a | 0.32 ± 0.04 a | N.D. | 0.4 ± 0.1 a |
| Ethyl 2-methylbutanoate | 3 ± 0.2 b | 0.96 ± 0.09 d | 3.5 ± 0.1 a | 1.8 ± 0.2 c |
| Ethyl 3-methylbutanoate | 7.2 ± 0.5 a | 2.2 ± 0.2 b | 5.5 ± 0.3 c | 3.1 ± 0.2 c |
| Isoamyl acetate | 4611 ± 327 b | 1722 ± 106 c | 1978 ± 38 c | 6910 ± 237 a |
| Ethyl hexanoate | 486 ± 26 b | 162 ± 15 c | 200 ± 5 c | 894 ± 55 a |
| Z-3- hexenol acetate | 473 ± 26 a | 15 ± 1 c | 21 ± 0.6 c | 239 ± 11 b |
| Hexyl acetate | 193 ± 18 a | 6.4 ± 0.5 c | 8.9 ± 0.8 c | 106 ± 5 b |
| Ethyl heptanoate | 0.27 ± 0.02 b | 0.12 ± 0.01 c | 0.22 ± 0.01 b | 0.61 ± 0.04 a |
| Ethyl octanoate | 600 ± 42 b | 48 ± 5 c | 82 ± 5 c | 849 ± 41 a |
| 2-phenylethanol acetate | 1815 ± 192 b | 159 ± 4 c | 253 ± 6 c | 2302 ± 82 a |
| Ethyl decanoate | 775 ± 44 a | 98 ± 4 c | 100.96 ± 0.04 c | 632 ± 35 b |
| Phenethyl hexanoate | 0.42 ± 0.01 b | 0.31 ± 0.01 c | 0.41 ± 0.01 b | 0.59 ± 0.04 a |
| Ethyl tetradecanoate | 21 ± 2 b | 15.8 ± 0.5 b | 14.8 ± 0.2 b | 66 ± 6 a |
| Phenethyl benzoate | 1.11 ± 0.07 ab | 1.04 ± 0.03 b | 1.02 ± 0.03 b | 1.21 ± 0.05 a |
| Ethyl hexadecanoate | 65 ± 4 b | 42.5 ± 0.8 c | 36 ± 1 c | 119 ± 11 a |
| Aldehydes | ||||
| Major aldehydes (mg/L) | 70 ± 2 a | 64 ± 4 a | 51 ± 3 b | 45.7 ± 0.8 b |
| Acetaldehyde | 71 ± 2 a | 64 ± 4 a | 51 ± 3 b | 46.1 ± 0.8 b |
| Minor aldehydes (µg/L) | 131 ± 7 a | 27 ± 2 c | 34 ± 3 c | 82 ± 2 b |
| Benzaldehyde | 3.8 ± 0.1 a | 2.5 ± 0.3 b | 3.1 ± 0.3 b | 2.8 ± 0.3 b |
| Octanal | 105 ± 5 a | 1.8 ± 0.2 c | 2 ± 0.1 c | 55 ± 2 b |
| Nonanal | 3.4 ± 0.2 b | 4.7 ± 0.5 a | 5.3 ± 0.6 a | 4.5 ± 0.4 ab |
| Decanal | 5.6 ± 0.6 c | 5.9 ± 0.6 bc | 9 ± 1 a | 7.7 ± 0.7 ab |
| Phenylacetaldehyde | 12 ± 1 ab | 12 ± 0.8 ab | 14 ± 1 b | 10.5 ± 0.5 a |
| Ketones | ||||
| Major Ketones (mg/L) | 15.2 ± 0.4 a | 14.9 ± 0.3 a | 12 ± 1 b | 11.2 ± 0.5 b |
| Acetoin | 15.2 ± 0.4 a | 14.9 ± 0.3 a | 12 ± 1 b | 11.2 ± 0.5 b |
| Minor Ketones (µg/L) | 13.9 ± 0.7 a | 5.5 ± 0.3 a | 13 ± 1 b | 6.5 ± 0.5 b |
| Benzophenone | 0.54 ± 0.08 a | 0.4 ± 0.02 b | 0.51 ± 0.02 a | N.D. |
| 3-Heptanone | 3.9 ± 0.4 a | 2.6 ± 0.2 a | 3.6 ± 0.2 b | 4.2 ± 0.4 a |
| Acetophenone | 9.4 ± 0.6 a | 2.48 ± 0.09 a | 9 ± 1 b | 2.2 ± 0.2 b |
| Lactones (µg/L) | 10 ± 1 d | 172 ± 3 a | 121 ± 5 b | 43 ± 3 c |
| G-Nonalactone | 8 ± 1 b | 7 ± 0.9 bc | 5.7 ± 0.3 c | 13.6 ± 0.5 a |
| G-Decalactone | 1.6 ± 0.1 d | 165 ± 3 a | 115 ± 6 b | 29 ± 3 c |
| Terpenoids (µg/L) | 12.6 ± 0.4 b | 10 ± 0.4 c | 12.4 ± 0.6 b | 15.9 ± 0.7 a |
| Limonene | 7.5 ± 0.5 b | 5.1 ± 0.5 c | 5.1 ± 0.3 c | 10.3 ± 0.7 a |
| E-nerolidol | 2.64 ± 0.04 b | 2.65 ± 0.02 ab | 2.62 ± 0.02 b | 2.71 ± 0.02 a |
| E-geranyl acetone | 1 ± 0.1 b | 0.57 ± 0.06 b | 2.7 ± 0.3 a | 0.78 ± 0.08 b |
| Z-geranyl acetone | 1.5 ± 0.1 b | 1.8 ± 0.2 ab | 1.9 ± 0.2 a | 2.1 ± 0.2 a |
| SC | LT | LT → SC | MP → LT | |
|---|---|---|---|---|
| Fruity | 332 ± 22 b | 111 ± 7 c | 131 ± 3 c | 492 ± 20 a |
| Green fruit | 74 ± 4 b | 14 ± 1 c | 18 ± 0 c | 84 ± 5 a |
| Green | 40 ± 2 a | 5 ± 0 c | 6 ± 0 c | 22 ± 1 b |
| Creamy | 1 ± 0.1 d | 5 ± 0.2 a | 3.3 ± 0.2 b | 1.4 ± 0.1 c |
| Citrus | 49 ± 3 a | 8 ± 1 c | 11 ± 1 c | 31 ± 1 b |
| Chemistry | 21 ± 1 b | 29 ± 2 a | 25 ± 3 ab | 23 ± 1 ab |
| Honey | 10 ± 1 b | 4 ± 0 c | 4.5 ± 0.3 c | 12 ± 0 a |
| Waxy | 128 ± 9 b | 15 ± 1 c | 24 ± 2 c | 179 ± 8 a |
| Floral | 10 ± 1 b | 3 ± 0.2 c | 4 ± 0.2 c | 12.8 ± 0.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Suárez, F.; Martínez-García, R.; Peinado, R.A. Climate Change Adaptation in Winemaking: Combined Use of Non-Saccharomyces Yeasts to Improve the Quality of Pedro Ximénez Wines. Microorganisms 2025, 13, 1908. https://doi.org/10.3390/microorganisms13081908
Sánchez-Suárez F, Martínez-García R, Peinado RA. Climate Change Adaptation in Winemaking: Combined Use of Non-Saccharomyces Yeasts to Improve the Quality of Pedro Ximénez Wines. Microorganisms. 2025; 13(8):1908. https://doi.org/10.3390/microorganisms13081908
Chicago/Turabian StyleSánchez-Suárez, Fernando, Rafael Martínez-García, and Rafael A. Peinado. 2025. "Climate Change Adaptation in Winemaking: Combined Use of Non-Saccharomyces Yeasts to Improve the Quality of Pedro Ximénez Wines" Microorganisms 13, no. 8: 1908. https://doi.org/10.3390/microorganisms13081908
APA StyleSánchez-Suárez, F., Martínez-García, R., & Peinado, R. A. (2025). Climate Change Adaptation in Winemaking: Combined Use of Non-Saccharomyces Yeasts to Improve the Quality of Pedro Ximénez Wines. Microorganisms, 13(8), 1908. https://doi.org/10.3390/microorganisms13081908






