A Single-Center Retrospective Study on Early Treatment for COVID-19 in Solid Organ Transplant Recipients During the Omicron Era: Outcomes and SARS-CoV-2 Viral Kinetics
Abstract
1. Introduction
2. Materials and Methods
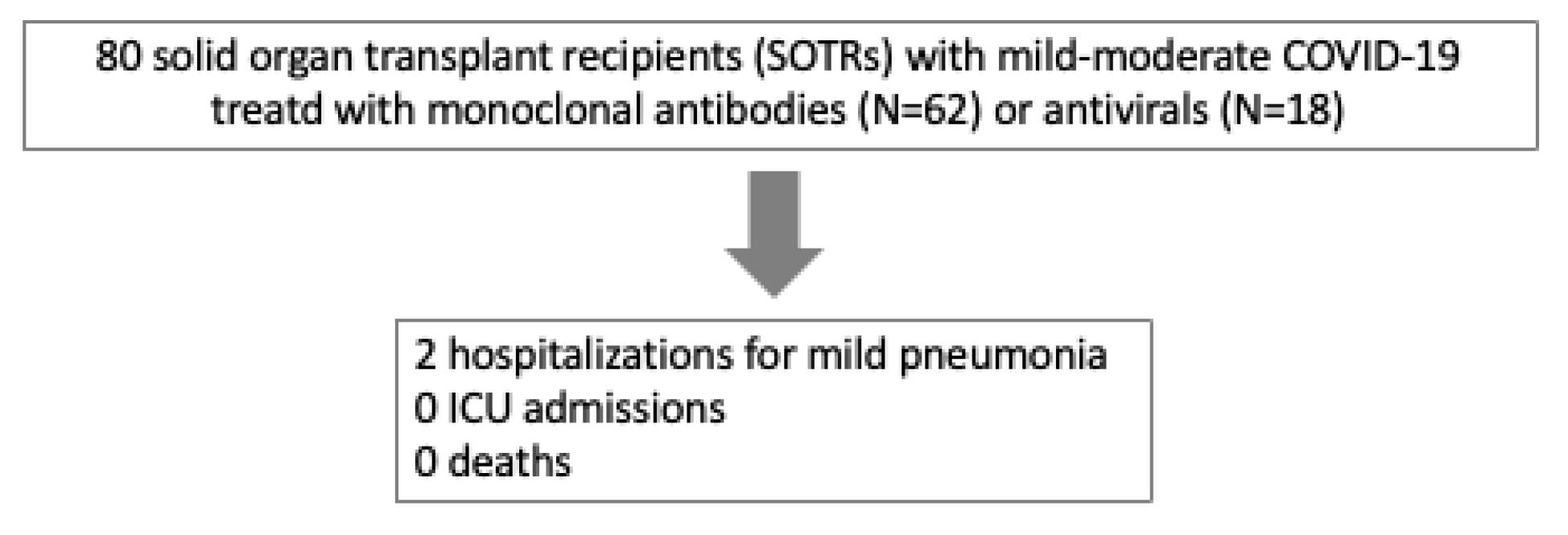
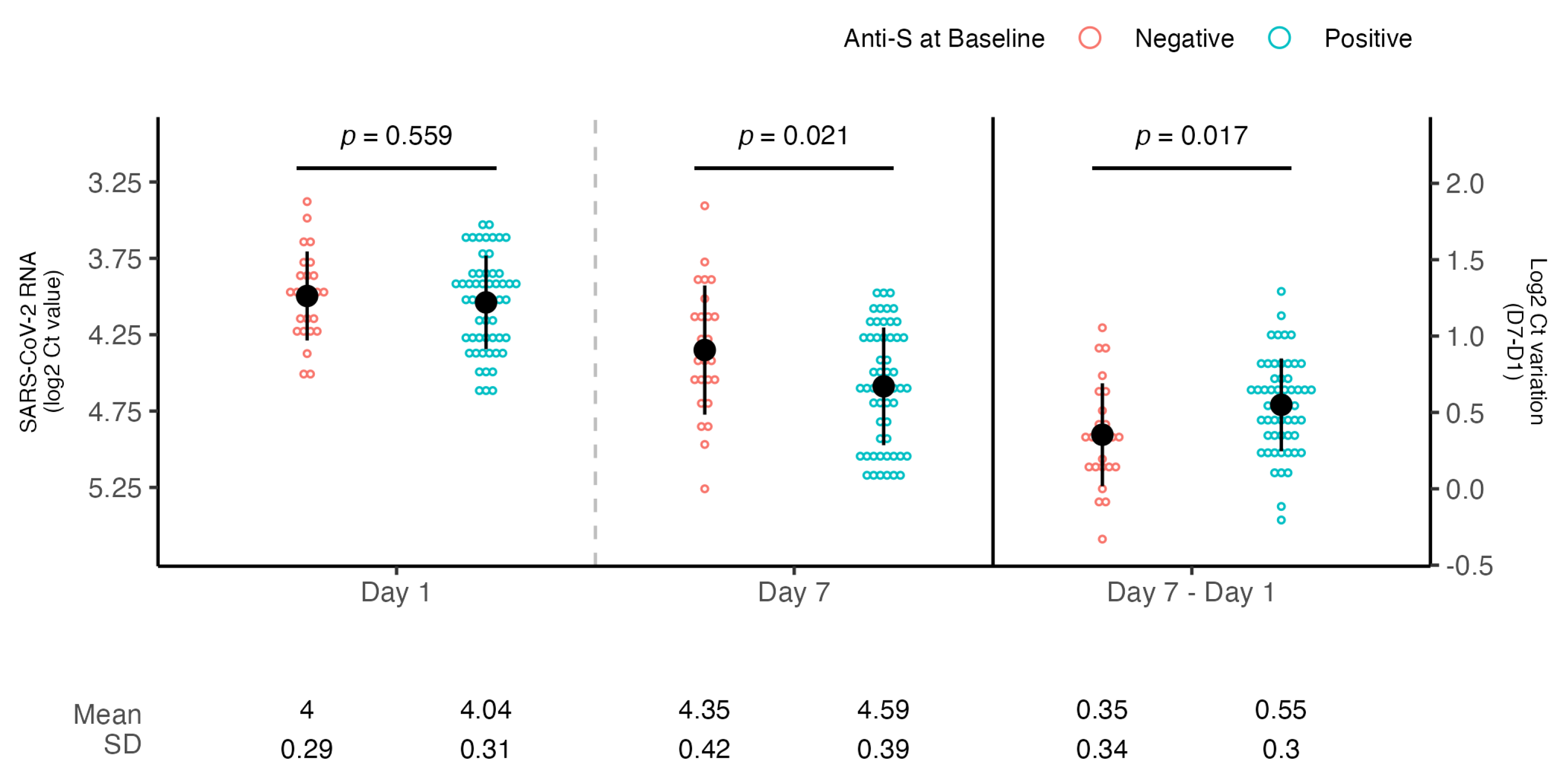
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOTRs | Solid organ transplant recipients |
| ICU | Intensive care unit |
| NPS | Naso-pharingeal swabs |
| Ct | Cycle threshold |
| mAbs | Monoclonal antibodies |
| CMIA | Chemiluminescence microparticle assay |
| N | Nucleoprotein |
| S | Spike |
| e-GFR | Estimated glomerular filtration rate |
| AST | Aspartate aminotransferase |
| ALT | Alanina aminotransferase |
References
- Kates, O.S.; Haydel, B.M.; Florman, S.S.; Rana, M.M.; Chaudhry, Z.S.; Ramesh, M.S.; Safa, K.; Kotton, C.N.; Blumberg, E.A.; Besharatian, B.D.; et al. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e4090–e4099. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.R.; Mohan, S.; Cohen, D.J.; Husain, S.A.; Dube, G.K.; Ratner, L.E.; Arcasoy, S.; Aversa, M.M.; Benvenuto, L.J.; Dadhania, D.M.; et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am. J. Transplant. 2020, 20, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, T.; Ferreira, V.H.; Ierullo, M.; Ku, T.; Lilly, L.; Kim, S.J.; Schiff, J.; Sidhu, A.; McDonald, M.; Hosseini-Moghaddam, S.M.; et al. Prospective Clinical, Virologic, and Immunologic Assessment of COVID-19 in Transplant Recipients. Transplantation 2021, 105, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Stacey, H.D.; D’Agostino, M.R.; Tugg, Y.; Marzok, A.; Miller, M.S. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat. Rev. Immunol. 2023, 23, 381–396. [Google Scholar] [CrossRef]
- Pinchera, B.; Buonomo, A.R.; Trucillo, E.; Susini, S.; D’Agostino, A.; Di Filippo, I.; Tanzillo, A.; Villari, R.; Carrano, R.; Troisi, R.I.; et al. COVID-19 in solid organ transplant recipients after 2 years of pandemic: Outcome and impact of antiviral treatments in a single-center study. Front. Transplant. 2023, 2, 1095225. [Google Scholar] [CrossRef]
- Radcliffe, C.; Palacios, C.F.; Azar, M.M.; Cohen, E.; Malinis, M. Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge. Am. J. Transplant. 2022, 22, 2458–2463. [Google Scholar] [CrossRef]
- Avery, R.K. Update on COVID-19 Therapeutics for Solid Organ Transplant Recipients, Including the Omicron Surge. Transplantation 2022, 106, 1528–1537. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, Q.; Madhira, V.; Olex, A.L.; Anzalone, A.J.; Vinson, A.; Singh, J.A.; French, E.; Abraham, A.G.; Mathew, J.; et al. Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US. JAMA Intern. Med. 2022, 182, 153. [Google Scholar] [CrossRef]
- Vinson, A.J.; Anzalone, A.J.; Sun, J.; Dai, R.; Agarwal, G.; Lee, S.B.; French, E.; Olex, A.; Ison, M.G.; Mannon, R.B. The risk and consequences of breakthrough SARS-CoV-2 infection in solid organ transplant recipients relative to non-immunosuppressed controls. Am. J. Transplant. 2022, 22, 2418–2432. [Google Scholar] [CrossRef]
- Mastrorosa, I.; Lepri, A.C.; Borgo, C.D.; Rosati, S.; Rueca, M.; Sarmati, L.; Mastroianni, C.; Fantoni, M.; Maggi, F.; Nicastri, E.; et al. Incidence and predictors of clinical failure after early treatment for mild-to-moderate COVID-19 in high-risk individuals: A multicentric cohort study. J. Intern. Med. 2025, 297, 328–334. [Google Scholar] [CrossRef]
- Solera, J.T.; Árbol, B.G.; Mittal, A.; Hall, V.; Marinelli, T.; Bahinskaya, I.; Selzner, N.; McDonald, M.; Schiff, J.; Sidhu, A.; et al. Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023. Am. J. Transplant. 2024, 24, 1303–1316. [Google Scholar] [CrossRef]
- Yetmar, Z.A.; Thao, V.; Helfinstine, D.A.; Pennington, K.M.; Razonable, R.R. Comparative Effectiveness of Outpatient COVID-19 Therapies in Solid Organ Transplant Recipients. Transpl. Infect. Dis. 2025, 27, e14436. [Google Scholar] [CrossRef]
- Mazzotta, V.; Cozzi Lepri, A.; Colavita, F.; Rosati, S.; Lalle, E.; Cimaglia, C.; Paulicelli, J.; Mastrorosa, I.; Vita, S.; Fabeni, L.; et al. Viral load decrease in SARS-CoV-2 BA.1 and BA.2 Omicron sublineages infection after treatment with monoclonal antibodies and direct antiviral agents. J. Med. Virol. 2023, 95, e28186. [Google Scholar] [CrossRef] [PubMed]
- Meschi, S.; Matusali, G.; Colavita, F.; Lapa, D.; Bordi, L.; Puro, V.; Leoni, B.D.; Galli, C.; Capobianchi, M.R.; Castilletti, C. Predicting the protective humoral response to a SARS-CoV-2 mRNA vaccine. Clin. Chem. Lab. Med. (CCLM) 2021, 59, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Finks, S.W.; Van Matre, E.; Budd, W.; Lemley, E.; Ray, N.K.; Mahon, M.; Chambers, E.; Finks, A.L. Clinical Significance of Quantitative Viral Load in Patients Positive for SARS-CoV-2. Am. J. Med. Open 2023, 10, 100050. [Google Scholar] [CrossRef]
- Meschi, S.; Colavita, F.; Bordi, L.; Matusali, G.; Lapa, D.; Amendola, A.; Vairo, F.; Ippolito, G.; Capobianchi, M.R.; Castilletti, C. Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test. J. Clin. Virol. 2020, 129, 104539. [Google Scholar] [CrossRef]
- D’Offizi, G.; Agrati, C.; Visco-Comandini, U.; Castilletti, C.; Puro, V.; Piccolo, P.; Montalbano, M.; Meschi, S.; Tartaglia, E.; Sorace, C.; et al. Coordinated cellular and humoral immune responses after two-dose SARS-CoV2 mRNA vaccination in liver transplant recipients. Liver Int. 2022, 42, 180–186. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Van Breukelen, G.J.P. ANCOVA Versus CHANGE From Baseline in Nonrandomized Studies: The Difference. Multivar. Behav. Res. 2013, 48, 895–922. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Pinto-Álvarez, M.; Fernández-Niño, J.A.; Arregocés-Castillo, L.; Rojas-Botero, M.L.; Palacios, A.F.; Galvis-Pedraza, M.; Ruiz-Gomez, F. Real-world Evidence of COVID-19 Vaccines Effectiveness in Solid-organ Transplant Recipient Population in Colombia: A Study Nested in the Esperanza Cohort. Transplantation 2023, 107, 216–224. [Google Scholar] [CrossRef]
- Hamm, S.R.; Rezahosseini, O.; Møller, D.L.; Loft, J.A.; Poulsen, J.R.; Knudsen, J.D.; Pedersen, M.S.; Schønning, K.; Harboe, Z.B.; Rasmussen, A.; et al. Incidence and severity of SARS-CoV-2 infections in liver and kidney transplant recipients in the post-vaccination era: Real-life data from Denmark. Am. J. Transplant. 2022, 22, 2637–2650. [Google Scholar] [CrossRef]
- Solera, J.T.; Árbol, B.G.; Alshahrani, A.; Bahinskaya, I.; Marks, N.; Humar, A.; Kumar, D. Impact of Vaccination and Early Monoclonal Antibody Therapy on Coronavirus Disease 2019 Outcomes in Organ Transplant Recipients During the Omicron Wave. Clin. Infect. Dis. 2022, 75, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.L.; Knoll, G.A.; Smith, G.; McArthur, E.; Kwong, J.C.; Dixon, S.N.; Treleaven, D.; Kim, S.J. Effectiveness of a Fourth COVID-19 mRNA Vaccine Dose Against the Omicron Variant in Solid Organ Transplant Recipients. Transplantation 2024, 108, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Shin, E.C.; Jung, M.K. SARS-CoV-2 vaccine-elicited immune responses in solid organ transplant recipients. Clin. Transplant. Res. 2024, 38, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.L.; Azzi, J.R.; Eghtesad, B.; Priddy, F.; Stolman, D.; Siangphoe, U.; Leony Lasso, I.; De Windt, E.; Girard, B.; Zhou, H.; et al. Safety and Immunogenicity of the mRNA-1273 Coronavirus Disease 2019 Vaccine in Solid Organ Transplant Recipients. J. Infect. Dis. 2024, 230, e591–e600. [Google Scholar] [CrossRef]
- Perrier, Q.; Lupo, J.; Gerster, T.; Augier, C.; Falque, L.; Rostaing, L.; Pelletier, L.; Bedouch, P.; Blanc, M.; Saint-Raymond, C.; et al. SARS-CoV-2 anti-spike antibodies after a fourth dose of COVID-19 vaccine in adult solid-organ transplant recipients. Vaccine 2022, 40, 6404–6411. [Google Scholar] [CrossRef]
- Davidov, Y.; Indenbaum, V.; Tsaraf, K.; Cohen-Ezra, O.; Likhter, M.; Ben Yakov, G.; Halperin, R.; Levy, I.; Mor, O.; Agmon-Levin, N.; et al. A third dose of the BNT162b2 mRNA vaccine significantly improves immune responses among liver transplant recipients. J. Hepatol. 2022, 77, 702–709. [Google Scholar] [CrossRef]
- Petr, V.; Zahradka, I.; Modos, I.; Roder, M.; Fialova, M.; Machkova, J.; Kabrtova, K.; Hruba, P.; Magicova, M.; Slavcev, A.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA Vaccine Booster Doses in Kidney Transplant Recipients: Results of a 12-mo Follow-up From a Prospective Observational Study. Transplant. Direct 2024, 10, e1645. [Google Scholar] [CrossRef]
- Mendoza, M.A.; Razonable, R.R. Coronavirus Disease 2019 Management Strategies in Solid Organ Transplant Recipients. Infect. Dis. Clin. N. Am. 2023, 37, 475–493. [Google Scholar] [CrossRef]
- Vasishta, S.; Aberg, J.; Patel, G.; Gownivaripally, P.A.; Rana, M. Clinical outcomes in immunocompromised adults with COVID-19, based on anti-spike IgG serostatus and monoclonal antibody therapy: A retrospective cohort study in the Omicron period. Ther. Adv. Infect. Dis. 2025, 12, 20499361251320711. [Google Scholar] [CrossRef]
- Bang, L.L.; Madsen, L.W.; Pedersen, R.M.; Nilsson, A.C.; Johansen, I.S.; Andersen, T.E. Sotrovimab lost neutralization efficacy against SARS-CoV-2 subvariants but remained clinically effective: Were monoclonal antibodies against COVID-19 rejected too early? J. Infect. Public Health 2024, 17, 102512. [Google Scholar] [CrossRef]
- Iketani, S.; Liu, L.; Guo, Y.; Liu, L.; Chan, J.F.W.; Huang, Y.; Wang, M.; Luo, Y.; Yu, J.; Chu, H.; et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022, 604, 553–556. [Google Scholar] [CrossRef]
- FDA. FDA Updates Sotrovimab Emergency Use Authorization. 2022. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization (accessed on 12 May 2025).
- AIFA. Modifica Registro—Anticorpi Monoclonali COVID-19. 2024. Available online: https://www.aifa.gov.it/-/modifica-registro-anticorpi-monoclonali-COVID-19-2 (accessed on 12 May 2025).
- Drysdale, M.; Tibble, H.; Patel, V.; Gibbons, D.C.; Lloyd, E.J.; Kerr, W.; Macdonald, C.; Birch, H.J.; Sheikh, A. Characteristics and outcomes of patients with COVID-19 at high risk of disease progression receiving sotrovimab, oral antivirals, or no treatment: A retrospective cohort study. BMC Infect. Dis. 2024, 24, 670. [Google Scholar] [CrossRef]

| N = 80 1 | |
|---|---|
| Age, years | 57.0 (48.0, 65.5) |
| Sex, male | 48 (60.0%) |
| BMI, Kg/m2 | 24.5 (21.8, 27.3) |
| COVID-19 mRNA vaccine | |
| 2 doses | 7 (8.7%) |
| 3 doses | 50 (62.5%) |
| 4 doses | 23 (28.8%) |
| Days since symptoms onset | 3.0 (2.0, 4.0) |
| eGFR, mL/min/1.73 m2 | 52.0 (38.6, 71.1) |
| ALT, U/L | 19.0 (12.0, 35.0) |
| AST, U/L | 26.0 (20.0, 34.0) |
| Comorbidities | |
| Number of comorbidities | 1.0 (0.0, 2.0) |
| Autoimmune disease | 2 (2.5%) |
| Cardiovascular disesase | 10 (12.5%) |
| Cerebrovascular disease | 2 (2.5%) |
| Diabetes | 12 (15.0%) |
| Ematological disease | 1 (1.3%) |
| HIV | 0 (0.0%) |
| Chronic kidney disease | 17 (21.3%) |
| Oncological disease | 4 (5.0%) |
| Neurological disease | 1 (1.3%) |
| Respiratory disease | 2 (2.5%) |
| Transplant | |
| Kidney | 50 (62.5%) |
| Kidney/liver | 2 (2.5%) |
| Liver | 20 (25.0%) |
| Heart | 7 (8.8%) |
| Heart/kidney | 1 (1.3%) |
| Years since transplant | 5.0 (3.0, 10.5) |
| Immunosuppressive drugs | |
| Everolimus | 10 (12.5%) |
| Tacrolimus | 59 (73.8%) |
| Mycophenolate mofetil | 46 (57.5%) |
| Cyclosporine | 7 (8.8%) |
| Corticosteroids | 45 (56.3%) |
| Early Treatment | N = 80 1 |
|---|---|
| Sotrovimab | 52 (65.0%) |
| Tixagevimab + Cilgavimib | 3 (3.8%) |
| Molnupiravir | 13 (16.3%) |
| Nirmaltrelvir + Ritonavir | 1 (1.3%) |
| Remdesivir | 4 (5.0%) |
| Casirivimab + Imdevimab | 2 (2.5%) |
| Bamlanivimab + Etesevimab | 5 (6.3%) |
| Univariable Regression | Multivariable Regression | |||||
|---|---|---|---|---|---|---|
| Characteristic | Beta | 95% CI | p-Value | Beta | 95% CI | p-Value |
| Age, years | −0.004 | −0.009, 0.002 | 0.2 | 0.000 | −0.006, 0.006 | >0.9 |
| Sex | ||||||
| M | — | — | — | — | ||
| F | −0.072 | −0.220, 0.076 | 0.3 | −0.008 | −0.163, 0.146 | >0.9 |
| Type of transplant | ||||||
| Kidney | — | — | ||||
| Kidney/Liver | −0.108 | −0.579, 0.362 | 0.6 | |||
| Liver | −0.118 | −0.291, 0.055 | 0.2 | |||
| Heart | −0.071 | −0.334, 0.193 | 0.6 | |||
| Heart/Kidney | 0.274 | −0.385, 0.934 | 0.4 | |||
| Type of early treatment | ||||||
| Antivirals | — | — | — | — | ||
| Monoclonals | −0.024 | −0.198, 0.151 | 0.8 | −0.044 | −0.223, 0.136 | 0.6 |
| MMF | ||||||
| No | — | — | ||||
| Yes | −0.046 | −0.193, 0.101 | 0.5 | |||
| Baseline anti-S positive | ||||||
| No | — | — | — | — | ||
| Yes | 0.196 | 0.045, 0.347 | 0.012 | 0.191 | 0.031, 0.350 | 0.020 |
| Baseline anti-N positive | ||||||
| No | — | — | ||||
| Yes | 0.002 | −0.335, 0.340 | >0.9 | |||
| ALT/GPT, U/L | −0.001 | −0.003, 0.000 | 0.12 | |||
| Total bilirubine, mg/dL | −0.002 | −0.016, 0.012 | 0.8 | |||
| eGFR, ml/min/1.73 m2 | 0.003 | 0.000, 0.006 | 0.062 | |||
| PCR | −0.021 | −0.060, 0.017 | 0.3 | |||
| Lymphopenia | ||||||
| No | — | — | ||||
| Yes | −0.058 | −0.205, 0.089 | 0.4 | |||
| BMI, Kg/m2 | −0.017 | −0.038, 0.005 | 0.13 | |||
| Number of comorbidities | −0.055 | −0.106, −0.004 | 0.035 | −0.058 | −0.110, −0.005 | 0.032 |
| Number of immunosuppressive drugs | 0.039 | −0.045, 0.122 | 0.4 | |||
| Years since transplant | −0.006 | −0.018, 0.007 | 0.4 | |||
| Days since symptoms onset | 0.048 | 0.003, 0.093 | 0.035 | 0.050 | 0.006, 0.095 | 0.027 |
| Days since last vaccination | 0.000 | −0.001, 0.001 | 0.7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milozzi, E.; Biliotti, E.; Caioli, A.; Mazzotta, V.; Loiacono, L.; Meschi, S.; Rianda, A.; Antinori, A.; Maggi, F.; D’Offizi, G. A Single-Center Retrospective Study on Early Treatment for COVID-19 in Solid Organ Transplant Recipients During the Omicron Era: Outcomes and SARS-CoV-2 Viral Kinetics. Microorganisms 2025, 13, 1872. https://doi.org/10.3390/microorganisms13081872
Milozzi E, Biliotti E, Caioli A, Mazzotta V, Loiacono L, Meschi S, Rianda A, Antinori A, Maggi F, D’Offizi G. A Single-Center Retrospective Study on Early Treatment for COVID-19 in Solid Organ Transplant Recipients During the Omicron Era: Outcomes and SARS-CoV-2 Viral Kinetics. Microorganisms. 2025; 13(8):1872. https://doi.org/10.3390/microorganisms13081872
Chicago/Turabian StyleMilozzi, Eugenia, Elisa Biliotti, Alessandro Caioli, Valentina Mazzotta, Laura Loiacono, Silvia Meschi, Alessia Rianda, Andrea Antinori, Fabrizio Maggi, and Gianpiero D’Offizi. 2025. "A Single-Center Retrospective Study on Early Treatment for COVID-19 in Solid Organ Transplant Recipients During the Omicron Era: Outcomes and SARS-CoV-2 Viral Kinetics" Microorganisms 13, no. 8: 1872. https://doi.org/10.3390/microorganisms13081872
APA StyleMilozzi, E., Biliotti, E., Caioli, A., Mazzotta, V., Loiacono, L., Meschi, S., Rianda, A., Antinori, A., Maggi, F., & D’Offizi, G. (2025). A Single-Center Retrospective Study on Early Treatment for COVID-19 in Solid Organ Transplant Recipients During the Omicron Era: Outcomes and SARS-CoV-2 Viral Kinetics. Microorganisms, 13(8), 1872. https://doi.org/10.3390/microorganisms13081872






