Abstract
Streptococcus pyogenes (Group A Streptococcus, GAS) is a major human pathogen capable of causing infections ranging from mild pharyngitis and impetigo to severe invasive diseases such as bacteremia, necrotizing fasciitis, and streptococcal toxic shock syndrome (STSS). Historically, the incidence of GAS infections declined during the early antibiotic era but began rising again from the early 2000s, driven partly by the emergence of hyper-virulent strains such as emm1 and emm12. From 2005 onward, significant increases in GAS infections were reported globally, accompanied by rising antibiotic resistance, particularly to macrolides and tetracyclines. During the COVID-19 pandemic, widespread public health measures led to a sharp decline in GAS infections, including invasive cases, but this trend reversed dramatically in late 2022 and 2023, with surges exceeding pre-pandemic levels, notably in children. Recent data implicate factors such as “immunity debt,” viral co-infections, and the spread of virulent clones like M1UK. Looking forward, continued surveillance of GAS epidemiology, virulence factors, and resistance patterns is critical. Moreover, the emergence of GAS isolates with reduced susceptibility to beta-lactams underscores the need for vigilance despite the absence of fully resistant strains. The development of an effective vaccine remains an urgent priority to reduce GAS disease burden and prevent severe outcomes. Future research should focus on vaccine development, molecular mechanisms of virulence, and strategies to curb antimicrobial resistance.
1. Introduction
Streptococcus pyogenes, commonly referred to as Group A Streptococcus (GAS), is a highly versatile and globally significant human pathogen. It is capable of asymptomatic colonization, particularly in the pharynx, where it can be detected in up to 20% of healthy individuals [1]. Despite its potential for benign carriage, GAS is responsible for a wide spectrum of diseases that vary greatly in severity, clinical manifestations, and pathogenic mechanisms.
GAS is the leading cause of several common and generally mild infections, including acute pharyngitis, which accounts for a large proportion of sore throat cases in children and adults, and superficial skin infections such as impetigo. However, GAS can also invade sterile sites and cause relatively rare but severe invasive infections (iGAS), such as primary bacteremia, streptococcal toxic shock syndrome (STSS), necrotizing fasciitis, osteomyelitis, and pneumonia. IGAS are often associated with high morbidity and mortality, particularly in vulnerable populations including the very young, the elderly, and individuals with underlying comorbidities [2]. Notably, the clinical presentation of GAS infections may differ substantially between immunocompetent and immunocompromised individuals. In immunocompetent hosts, infections typically remain localized—manifesting as acute pharyngitis, impetigo, or scarlet fever—whereas immunocompromised individuals are at increased risk for fulminant invasive diseases such as bacteremia, necrotizing fasciitis, and streptococcal toxic shock syndrome, often with atypical or attenuated early symptoms [2]. These differences complicate diagnosis and treatment and underscore the need for heightened clinical vigilance and tailored management strategies in high-risk populations.
Beyond acute infections, GAS is also recognized for its role in the development of post-infectious immune-mediated diseases. Acute rheumatic fever (ARF), a sequela of untreated or inadequately treated GAS pharyngitis, can lead to chronic rheumatic heart disease, a significant cause of cardiovascular morbidity worldwide. Similarly, acute post-streptococcal glomerulonephritis can result in transient or, in some cases, permanent renal impairment. These immunological complications add another layer of clinical significance to GAS infections, particularly in regions with limited healthcare resources [2].
The global burden of GAS infections remains substantial. Current estimates indicate approximately 700 million GAS infections per year worldwide, encompassing both pharyngitis and skin infections. Of these, over 650,000 severe iGAS cases occur annually, with an associated 25% case fatality rate, translating into more than 160,000 deaths each year. In addition, rheumatic heart disease (RHD)—a key autoimmune complication following inadequately treated GAS pharyngitis—accounted for an estimated 319,000 deaths in 2015 [3]. This disease burden is compounded by significant economic consequences. In the United States alone, the annual combined costs associated with iGAS disease and acute upper respiratory infections—including direct medical expenses, indirect costs from lost productivity, and the long-term impact of complications—have been estimated at approximately $6.08 billion [4].
These staggering figures highlight the critical importance of prompt diagnosis, effective treatment, and robust preventive strategies in managing GAS infections. The development and implementation of rapid diagnostic techniques for the detection of GAS in clinical specimens have significantly improved the timely initiation of appropriate antimicrobial therapy, thereby reducing transmission and the risk of complications. Concurrently, considerable research efforts are focused on developing safe and effective vaccines targeting GAS, which could dramatically reduce the incidence of both primary infections and their immune-mediated sequelae [5].
In recent years, the epidemiology of GAS infections has shown notable changes, including shifts in the distribution of circulating emm types, which encode the highly variable M protein, a major virulence factor and a key target for vaccine development. Moreover, the emergence of GAS strains exhibiting increased virulence and reduced susceptibility to commonly used antibiotics, such as macrolides and clindamycin, has raised significant public health concerns. These trends underscore the potential for changes in disease incidence, severity, and treatment efficacy, necessitating ongoing surveillance and adaptation of clinical management guidelines [5].
Given these developments, it is imperative to reassess the current understanding of GAS epidemiology and its clinical impact. The primary aim of this narrative review is to examine the evolving epidemiological landscape of GAS infections, providing a comprehensive overview of recent trends in disease burden, pathogen characteristics, antimicrobial resistance patterns, and the implications for future prevention and control strategies.
2. Methods
To perform this narrative review, we conducted a literature search using PubMed, Scopus, Web A systematic literature search was performed to identify studies investigating the epidemiology, clinical characteristics, and antimicrobial resistance patterns of Streptococcus pyogenes (Group A Streptococcus, GAS) infections. The electronic databases searched included PubMed/MEDLINE, Scopus, Web of Science, and the Cochrane Library. The search strategy was developed using a combination of Medical Subject Headings (MeSH) terms and free-text keywords related to GAS infections, employing Boolean operators (AND, OR) to optimize sensitivity and specificity. Search terms encompassed various combinations of the following keywords: “group A Streptococcus,” “GAS,” “Streptococcus pyogenes,” “invasive infections,” “acute pharyngitis,” “skin infections,” “scarlet fever,” “epidemiology,” “COVID-19,” “infants,” and “children.” No language restrictions were applied. The time frame for the search spanned from database inception to April 2025, ensuring comprehensive inclusion of both historical and recent data.
Eligible studies included peer-reviewed original research articles, randomized controlled trials, cohort studies, case–control studies, systematic reviews, meta-analyses, and official reports or guidelines published by recognized health authorities, such as the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), the European Medicines Agency (EMA), and the U.S. Food and Drug Administration (FDA). To supplement the electronic search, the reference lists of all relevant articles were manually screened to identify additional studies that might have been missed in the initial search process.
Studies were considered eligible for inclusion if they examined the epidemiology of GAS infections before and after the COVID-19 pandemic, provided data on the severity or clinical spectrum of GAS infections—including both noninvasive and invasive disease presentations—explored antimicrobial resistance patterns in GAS isolates, or analyzed real-world time-related variations in the epidemiology or severity of GAS infections across different regions or populations.
Conversely, studies were excluded if they were narrative reviews, commentaries, editorials, or opinion pieces without original data or systematic methodology. Case reports or small case series involving fewer than five patients were also excluded unless they presented novel epidemiological or antimicrobial resistance information. Additionally, studies were excluded if they lacked sufficient methodological detail to allow an assessment of the validity or quality of the findings.
The process of study selection involved two independent reviewers (MM and CC) who screened titles and abstracts to assess potential relevance. Full-text articles were then retrieved and evaluated in detail to confirm eligibility according to the predefined inclusion and exclusion criteria. Any disagreements regarding study inclusion were resolved through discussion and consensus, with input from a third reviewer (SR) if necessary. Data extracted from the included studies encompassed publication details such as authorship, year of publication, and study location, as well as study design, population characteristics, and outcomes related to GAS epidemiology, disease severity, antimicrobial resistance patterns, and the potential impact of the COVID-19 pandemic on GAS infection dynamics.
3. Group A Streptococcus (GAS) General Characteristics
GAS is a Gram-positive bacterium belonging to the genus Streptococcus. It is typically classified based on the characteristics of the emm gene, which encodes the M protein—a major virulence factor central to the bacterium’s pathogenicity. The M protein plays a critical role in bacterial adhesion, evasion of phagocytosis, and interference with the host immune response. To date, more than 220 distinct emm gene variants and their corresponding M protein types have been identified, each exhibiting differences in virulence and tissue tropism [6].
Beyond the M protein, GAS possesses a diverse arsenal of virulence factors that collectively contribute to its pathogenic potential [6,7,8,9,10,11,12,13,14]. Table 1 summarizes key virulence factors of GAS.

Table 1.
Key virulence factors of Group A Streptococcus.
The interplay between these bacterial factors and the host immune system substantially influences the severity and clinical manifestations of GAS infections [7]. GAS has mechanisms to degrade host chemokines, DNA, and immunoglobulins, thereby impairing various arms of innate and adaptive immunity. The bacterium secretes numerous extracellular products, including streptolysins S and O (SLS and SLO), which damage host cell membranes and contribute to the characteristic beta-hemolysis observed on blood agar. Additional virulence factors include streptokinase, which promotes fibrinolysis and facilitates bacterial dissemination; hyaluronidase, which breaks down connective tissue and promotes tissue invasion; and cysteine proteinases, particularly SpeB, which degrade a wide range of host proteins and modulate immune responses [8].
Among GAS-secreted proteins, the streptococcal pyrogenic exotoxins (Spes) are particularly significant due to their potent superantigen activity. These exotoxins can bind simultaneously to major histocompatibility complex (MHC) class II molecules on antigen-presenting cells and T cell receptors, triggering polyclonal T cell activation. This excessive immune activation leads to massive cytokine release, which underlies the pathophysiology of severe conditions such as streptococcal toxic shock syndrome (STSS) [9]. Several Spes have been identified, and many are encoded by lysogenic bacteriophages or putative bacteriophage elements integrated into the GAS genome. Notable examples include SpeA, SpeC, SpeG, SpeH, SpeI, SpeJ, SpeK, SpeL, and SpeM, as well as streptococcal superantigen (SSA) and streptococcal mitogenic exotoxin Z (SmeZ). Among these, SpeA and SpeC are the best-characterized superantigens and have been frequently detected in GAS strains isolated from patients with STSS [10,11,12,13].
Specific emm types are associated with differing propensities for severe disease, reflecting variations in virulence factor expression. For instance, emm1 and emm3 GAS strains have been consistently linked to an increased risk of invasive infections and STSS, whereas strains such as emm4 are generally less associated with severe disease [14].
GAS infections can occur across all age groups but are more prevalent in certain populations. The burden of GAS infections is especially significant in low-income countries, where factors such as overcrowding, poor hygiene, and limited access to healthcare facilitate the spread and severity of infections [15]. However, elevated rates of GAS infections are also observed in indigenous populations within some high-income countries, including Australia and New Zealand. In these communities, a combination of genetic predisposition [16], environmental exposures, and socioeconomic challenges contributes to disproportionately high incidence rates [17].
Seasonality also influences the epidemiology of GAS infections, with the highest incidence typically occurring during winter and early spring. Patterns of disease distribution vary by age and clinical manifestation. Acute pharyngitis and scarlet fever are most commonly diagnosed in children aged 5 to 15 years, whereas impetigo predominantly affects younger children between 2 and 5 years of age [18]. In contrast, iGAS infections are more frequently reported among infants and older adults, particularly those over the age of 65. Sex-specific differences have also been noted, with females more often affected by GAS pharyngitis, while males may exhibit slightly higher rates of iGAS infections [19].
Although noninvasive GAS infections can affect virtually anyone, iGAS infections are more likely to occur in individuals with specific risk factors, including skin injuries, chronic conditions, and immunocompromised status, as shown in several studies highlighting increased susceptibility and worse outcomes in these populations [20,21,22,23]. Viral infections, particularly influenza [24,25] and varicella [26,27,28], are well-established predisposing factors for iGAS disease in children, as these infections compromise mucosal barriers and immune defenses. Furthermore, iGAS infections may arise in the postpartum period, contributing to maternal morbidity and mortality, especially in developing countries where sepsis remains a leading cause of maternal death [29].
4. Epidemiology of Group A Streptococcus (GAS) Infections
4.1. In the First Years of the Antibiotic Era Until 2005
Over the past seventy years, the incidence of GAS infections has undergone significant fluctuations, largely influenced by identifiable factors. During the early decades of the antibiotic era, extending until the late 1980s, the global incidence of GAS infections progressively declined, particularly in industrialized nations, owing to improvements in living conditions and the widespread availability and use of effective antibiotics. From that point until around 2005, GAS infection rates remained relatively stable. However, certain countries reported a modest resurgence in the frequency and severity of GAS infections, including iGAS disease. These unexpected increases were hypothesized to be linked to the circulation of more virulent GAS strains. This hypothesis was supported by evidence indicating that surges in severe infections were frequently associated with specific GAS strains, notably emm1 GAS [30,31,32].
4.2. From 2005 to the Beginning of the COVID-19 Pandemic
Beginning in 2005, reports indicated a steady global rise in GAS infections. In 2011, Hong Kong experienced a significant outbreak of scarlet fever, with case numbers far exceeding the baseline of the previous two decades [33]. Other countries similarly observed gradual increases in GAS infections, which eventually reached levels substantially higher than those reported in earlier periods. Notably, although overall GAS infections increased, the most pronounced rise was seen in severe cases and iGAS infections. In Hong Kong, the 2011 outbreak included a higher proportion of severe scarlet fever cases, some complicated by STSS. Between 2012 and 2015, Ireland recorded iGAS incidence rates approximately two to three times higher than those observed during the preceding eight years [34]. In the United States, the incidence of iGAS increased from 1.04 per 100,000 people in 2008 to 4.76 per 100,000 in 2019 [35]. Similarly, in Australia, iGAS incidence rose from 4.1 per 100,000 in 2008/2009 to 8.3 per 100,000 in 2017/2018 [36].
Microbiological investigations during this period confirmed that the rising burden of iGAS was strongly associated with increased circulation of GAS strains exhibiting enhanced virulence. Isolates recovered from patients with iGAS infections frequently produced superantigens and displayed resistance to commonly used antibiotics. For instance, a study from Hong Kong [37] revealed that most scarlet fever cases were caused by emm12 GAS strains exhibiting resistance to macrolides and tetracyclines and carrying the SSA and SpeC superantigens. In Ireland, iGAS infections were primarily associated with the emm1, emm3, emm28, emm12, and emm89 strains, which produced various superantigens, including SpeA, SpeG, and SpeJ [38]. In the United Kingdom, the rise in iGAS incidence coincided with the emergence of a novel lineage of the emm1 strain, designated M1UK, distinguished by its elevated expression of the scarlet fever toxin SpeA [39].
During this period, the increasing prevalence of antibiotic resistance among GAS isolates was recognized as a significant driver of the spread of virulent strains. Although resistance rates varied by region, substantial increases in GAS resistance to macrolides, lincosamides, tetracyclines, fluoroquinolones, and sulfonamides were documented worldwide. Notably, resistance often clustered in GAS strains linked to severe disease and iGAS. In Taiwan, macrolide resistance in GAS rose from 18.1% to 19.3% between 2000 and 2009, escalating further to 58.4–61.6% in the subsequent decade, with most resistant isolates identified as emm12 GAS [40]. A study from China [41] documented a striking increase in the prevalence of emm1 GAS among scarlet fever cases, rising from 3.8% in 2011 to 48.6% in 2014. Nearly all isolates from patients (n = 1451) and carriers (n = 96) demonstrated resistance to erythromycin (97.5%), clindamycin (97.3%), and tetracycline (95.7%). Tetracycline resistance varied significantly by geography, ranging from about 10% in Australia to over 80% in China. While resistance to fluoroquinolones remained generally lower, clinically significant levels were reported in Hungary (10%) and Japan (14%) [42].
4.3. The Period of the COVID-19 Pandemic
The epidemiology of GAS infections underwent marked changes during and following the COVID-19 pandemic. Contrary to the upward trend noted before the pandemic, GAS infections—including iGAS—decreased sharply in the early years of SARS-CoV-2 circulation worldwide. According to data from the Centers for Disease Control and Prevention (CDC), the incidence of iGAS in the United States fell 52%, 74%, and 27% below expected levels in 2020, 2021, and 2022, respectively. Declines were observed across all high-risk populations, with incidence reductions of 73% among children and 33% among adults aged 65 and older [43]. Similarly, data from an Italian tertiary center (Policlinico Agostino Gemelli, Rome) demonstrated a 50% reduction in diagnoses of streptococcal pharyngitis and other GAS infections in 2020, followed by an additional 30% decrease during 2021–2022 compared to pre-pandemic years [44].
This declining trend reversed dramatically during the latter months of 2022 and into early 2023, when a sharp resurgence in GAS infections was documented worldwide. Incidence levels during this period surpassed those observed in pre-pandemic years, with the increase being particularly pronounced for iGAS infections in younger children, though older individuals continued to experience the highest recorded overall incidence rates. A growing proportion of iGAS cases required hospitalization and intensive care unit admission. In France, a study documented a significant rise in severe iGAS infections during the later pandemic period, with longer hospital stays (9.4 vs. 6.4 days; p = 0.0007), higher rates of pleural empyema (15% vs. 4%), bone and joint infections (14% vs. 7%; p = 0.008), and more complicated ENT infections (22% vs. 7%; p = 0.003) [45]. In the UK, by June 2023, the incidence of scarlet fever and iGAS infections exceeded the five-year pre-pandemic average [46]. In the United States, the observed incidence of iGAS during November and December 2022 was 117% higher than expected [45]. At the previously mentioned tertiary center in Rome, the proportion of positive pharyngeal swabs for GAS rose to 13% in 2023, compared to just 2% in 2022 [44].
In the latter part of 2023 and into early 2024, provisional data suggested that GAS incidence had declined again but remained modestly above typical seasonal expectations. In the UK, reports indicated that scarlet fever notifications followed typical seasonal patterns, albeit at the higher end of historical ranges. Meanwhile, iGAS incidence returned to expected levels, predominantly affecting older adults rather than children, in contrast to the spike seen in the immediate post-pandemic period [46].
Both the sharp reduction in GAS infections during the early COVID-19 pandemic and the subsequent resurgence are primarily attributed to public health measures aimed at controlling SARS-CoV-2 transmission. Nonpharmaceutical interventions (NPIs)—including travel restrictions, event cancelations, social distancing, curfews, and lockdowns—not only curtailed the spread of COVID-19 but also significantly reduced the transmission of other respiratory pathogens. Consequently, there was a marked decline in respiratory infections and related healthcare utilization. The effect was particularly profound for nonenveloped viruses such as influenza and respiratory syncytial virus (RSV), whereas enveloped viruses like rhinovirus, enterovirus, human bocavirus, and human adenovirus were less affected due to differences in environmental stability and susceptibility to disinfection [47]. Similar trends were documented for other bacterial pathogens; alongside GAS, there was a substantial decrease in Streptococcus pneumoniae infections. Multiple studies reported dramatic declines in invasive pneumococcal diseases (IPDs), including bacteremic pneumonia, during the early pandemic period, particularly among children under five years of age [48,49,50,51,52,53,54].
As NPIs were gradually relaxed in the later stages of the pandemic, previously suppressed pathogens resumed circulation, albeit at varying speeds. Some pathogens quickly returned to pre-pandemic levels, while others showed delayed resurgence, sometimes only reaching baseline levels after three years [47]. Notably, RSV infections exhibited an off-season resurgence [55], occasionally accompanied by shifts in age distribution toward older children [56].
Several factors likely contributed to the increased incidence of respiratory infections during the late pandemic period. Reduced exposure to common pathogens led to gaps in population-level immunity, particularly among infants born during the pandemic and women of childbearing age, resulting in greater susceptibility once pathogen circulation resumed—a phenomenon sometimes described as “immunity debt” [57]. Some studies have hypothesized that children with prior SARS-CoV-2 infections may experience immune dysregulation, which could increase susceptibility to other infections, including invasive disease caused by virulent strains of GAS; however, further research is needed to clarify this potential link [58,59,60]. Concurrent viral infections may also have played a role. Influenza and varicella are well-established risk factors for subsequent GAS infections, including severe invasive cases, likely due to the immunosuppressive effects of viral infections and damage to mucosal barriers, which facilitate bacterial invasion [58].
Supporting this link, a study in Denmark during the 2022–2023 season found that 22% of pediatric iGAS cases were preceded by varicella infection, and 47% followed an upper respiratory viral infection [59]. In England, data from October to November 2022 identified RSV and human metapneumovirus (hMPV) as the most common co-infecting viruses in pediatric GAS cases [60]. Similarly, a Portuguese study conducted between September 2022 and May 2023 observed that varicella and upper respiratory infections preceded iGAS in 24.6% of cases [61]. Further evidence from the Netherlands indicated that between January 2022 and March 2023, 34% of GAS skin and soft tissue infections (SSTIs) in children were attributable to varicella. Additionally, among pediatric and adult iGAS pneumonia or sepsis cases in the same period, 34% (95% CI: 20–49) and 25% (95% CI: 18–32), respectively, were linked to preceding respiratory virus infections, with influenza A contributing the largest share at 17% [62].
While the concepts of immunity debt and increased viral infections undoubtedly contributed to the overall rise in GAS infections, it is highly probable that the resurgence of iGAS was primarily driven by the increased circulation of highly virulent GAS strains already present prior to the pandemic. By late 2022, in the Netherlands, the proportion of emm1 among iGAS isolates rose from 32% in November to 68% in December, maintaining levels above 50% until March 2023 [63]. Moreover, in this same period, emm1 strains were largely replaced by the M1UK variant [63], which had undergone further evolution, acquiring new virulence determinants [64].
Table 2 shows results from main studies with global incidence data for iGAS, whereas Table 3 summarizes studies on iGAS antimicrobial resistance patterns.

Table 2.
Main studies with global incidence data for invasive GAS infections.

Table 3.
Antimicrobial resistance patterns in GAS (2000–2024).
Figure 1 synthesizes major epidemiological milestones, changes in dominant GAS phenotypes, and shifts in incidence patterns, including the impact of the COVID-19 pandemic.
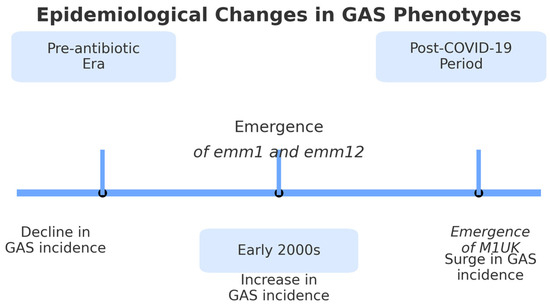
Figure 1.
Timeline of epidemiological changes in Group A Streptococcus (GAS) phenotypes from the pre-antibiotic era to the post-COVID-19 period.
5. Future Outlook for Group A Streptococcus Infections
Although data collected in 2024 remain provisional, current trends suggest that, barring extraordinary events such as the COVID-19 pandemic, the epidemiology of GAS infections will likely return to patterns observed prior to the pandemic. The overall number of GAS infections is expected to stabilize at levels similar to, or somewhat higher than, those seen before the pandemic, with a modest yet persistent rise in severe cases. This increase may be driven by the continued circulation of highly virulent GAS strains. The situation is further complicated by growing concerns over reduced susceptibility of certain strains to commonly used antibiotics, which can promote the selection and broader dissemination of resistant bacteria [65].
To mitigate the future burden of GAS infections, several critical measures are essential. Foremost among these is the sustained surveillance of GAS epidemiology and ongoing characterization of circulating strains to assess virulence factors and antimicrobial susceptibility profiles. Understanding which strains most frequently cause severe disease is crucial for informing public health interventions and for guiding the development of an effective vaccine. Despite numerous attempts, no vaccine is currently available for GAS, leaving a significant gap in preventive strategies.
Additionally, rigorous monitoring of antimicrobial resistance trends is imperative to ensure appropriate therapeutic choices and to preserve the efficacy of existing treatments. Special attention should be directed toward the emergence of GAS isolates with reduced susceptibility to beta-lactam antibiotics. Although no naturally occurring beta-lactam-resistant GAS strains have been definitively documented, beta-lactams remain the first-line therapy for all GAS infections. Recent reports of isolates with decreased susceptibility to penicillin have raised legitimate concerns regarding the potential evolution of true beta-lactam resistance. Reduced susceptibility has been linked to specific mutations in the penicillin-binding protein 2X (PBP2X), which alter its structure and diminish its affinity for beta-lactam antibiotics, leading to elevated minimal inhibitory concentrations in vitro [66,67].
Looking ahead, two areas of particular importance are vaccine development and the advancement of novel antimicrobial strategies—especially in the context of rising antimicrobial resistance and the clinical challenges posed by rapidly progressing infections such as necrotizing soft tissue infections. Despite decades of effort, no licensed vaccine for GAS is currently available. However, recent progress has renewed optimism, with several candidates under preclinical and early clinical investigation. These include multivalent M protein-based vaccines targeting the most prevalent emm types globally, as well as vaccines utilizing conserved antigens such as streptolysin O, C5a peptidase, and Group A Carbohydrate (GAC), which aim to overcome the issue of strain variability and ensure broader protection [68,69]. Additionally, the use of mRNA and nanoparticle platforms is under early-stage evaluation for GAS vaccines [70].
On the therapeutic front, novel antimicrobial approaches are gaining attention as adjuncts or alternatives to conventional antibiotics. These include bacteriophage-derived lysins with specific lytic activity against GAS, engineered antimicrobial peptides with broad-spectrum efficacy, and anti-virulence compounds targeting toxin production or quorum sensing. Such strategies are particularly relevant for managing life-threatening infections like necrotizing fasciitis or streptococcal toxic shock syndrome, where rapid disease progression and emerging resistance can compromise the effectiveness of β-lactams and clindamycin [71,72,73]. Continued research and investment in these innovative avenues will be critical to expanding our clinical armamentarium and improving outcomes in high-risk patients.
6. Conclusions
In summary, GAS remains a clinically significant pathogen with the capacity to cause both widespread, mild infections and severe, life-threatening invasive diseases. The shifting epidemiology observed over recent decades underscores the importance of vigilant surveillance to detect emerging virulent strains and track evolving antimicrobial resistance patterns, including potential threats to beta-lactam efficacy. The development of a safe and effective GAS vaccine represents a critical unmet need that could transform the landscape of GAS prevention, particularly in high-risk populations and regions with high disease burden. Future research should prioritize the identification of conserved vaccine targets across diverse emm types, the mechanisms underlying increased virulence and invasiveness, and strategies to mitigate the rise in antimicrobial resistance. Addressing these gaps will be essential to reduce the global burden of GAS disease and to safeguard the effectiveness of current treatment options for years to come.
Author Contributions
S.E. and N.P. co-wrote the manuscript; M.M., C.C., N.C., S.R. and A.F. performed the literature review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “EU funding within the NextGenerationEU-MUR M4C 2.I.1.3PNRR Extended Partnership initiative on Emerging Infectious Diseases (PE00000007, INF-ACT) “One Health Basic and Translational Research Actions addressing Unmet Needs on Emerging Infectious Diseases” through the INF-ACT Cascade Open Call 2023 (COC-1-2023-ISS-01)—CUP I83C22001810007”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martin, J. The Streptococcus pyogenes Carrier State. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK374206/ (accessed on 10 July 2024).
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C.; Infectious Diseases Society of America (2012). Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012, 55, e86–e102. [Google Scholar] [CrossRef]
- Sanyahumbi, A.S.; Colquhoun, S.; Wyber, R.; Carapetis, J.R. Global Disease Burden of Group A Streptococcus. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Andrejko, K.; Whittles, L.K.; Lewnard, J.A. Health-Economic Value of Vaccination Against Group A Streptococcus in the United States. Clin. Infect. Dis. 2022, 74, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.B.; Fischetti, V.A.; Carapetis, J.R.; Steer, A.C.; Sow, S.; Kumar, R.; Mayosi, B.M.; Rubin, F.A.; Mulholland, K.; Hombach, J.M.; et al. Group A streptococcal vaccines: Paving a path for accelerated development. Vaccine 2013, 31 (Suppl. 2), B216–B222. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, S.; Rivera-Hernandez, T.; Curren, B.F.; Harbison-Price, N.; De Oliveira, D.M.P.; Jespersen, M.G.; Davies, M.R.; Walker, M.J. Pathogenesis, epidemiology and control of Group A Streptococcus infection. Nat. Rev. Microbiol. 2023, 21, 431–447. [Google Scholar] [CrossRef]
- Golińska, E.; van der Linden, M.; Więcek, G.; Mikołajczyk, D.; Machul, A.; Samet, A.; Piórkowska, A.; Dorycka, M.; Heczko, P.B.; Strus, M. Virulence factors of Streptococcus pyogenes strains from women in peri-labor with invasive infections. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 747–754. [Google Scholar] [CrossRef]
- Shannon, B.A.; McCormick, J.K.; Schlievert, P.M. Toxins and Superantigens of Group A Streptococci. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Flamant, A.; Demirjian, A.; Lamagni, T.; Toubiana, J.; Smeesters, P.R.; Cohen, J.F. Invasive group A streptococcal infections: Lessons learned from the 2022–23 upsurge. Lancet Infect. Dis. 2025, S1473-3099(25)00343-3. [Google Scholar] [CrossRef]
- Musser, J.M.; Hauser, A.R.; Kim, M.H.; Schlievert, P.M.; Nelson, K.; Selander, R.K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: Clonal diversity and pyrogenic exotoxin expression. Proc. Natl. Acad. Sci. USA 1991, 88, 2668–2672. [Google Scholar] [CrossRef]
- Cockerill, F.R., III.; MacDonald, K.L.; Thompson, R.L.; Roberson, F.; Kohner, P.C.; Besser-Wiek, J.; Manahan, J.M.; Musser, J.M.; Schlievert, P.M.; Talbot, J.; et al. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA 1997, 277, 38–43. [Google Scholar] [CrossRef]
- Demers, B.; Simor, A.E.; Vellend, H.; Schlievert, P.M.; Byrne, S.; Jamieson, F.; Walmsley, S.; Low, D.E. Severe invasive group A streptococcal infections in Ontario, Canada: 1987–1991. Clin. Infect. Dis. 1993, 16, 792–800. [Google Scholar] [CrossRef]
- Hauser, A.R.; Stevens, D.L.; Kaplan, E.L.; Schlievert, P.M. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J. Clin. Microbiol. 1991, 29, 1562–1567. [Google Scholar] [CrossRef]
- Miyoshi-Akiyama, T.; Zhao, J.; Kikuchi, K.; Kato, H.; Suzuki, R.; Endoh, M.; Uchiyama, T. Quantitative and qualitative comparison of virulence traits, including murine lethality, among different M types of group A Streptococci. J. Infect. Dis. 2003, 187, 1876–1887. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avire, N.J.; Whiley, H.; Ross, K. A Review of Streptococcus pyogenes: Public Health Risk Factors, Prevention and Control. Pathogens 2021, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Smeesters, P.R.; de Crombrugghe, G.; Tsoi, S.K.; Leclercq, C.; Baker, C.; Osowicki, J.; Verhoeven, C.; Botteaux, A.; Steer, A.C. Global Streptococcus pyogenes strain diversity, disease associations, and implications for vaccine development: A systematic review. Lancet Microbe 2024, 5, e181–e193. [Google Scholar] [CrossRef]
- Nasir, B.F.; Vinayagam, R.; Rae, K. “It’s what makes us unique”: Indigenous Australian perspectives on genetics research to improve comorbid mental and chronic disease outcomes. Curr. Med. Res. Opin. 2022, 38, 1219–1228. [Google Scholar] [CrossRef]
- Mponponsuo, K.; Church, D.L.; Lu, S.J.; Viczko, J.; Naugler, C.; McDonald, T.; Dickinson, J.; Somayaji, R. Age and sex-specific incidence rates of group A streptococcal pharyngitis between 2010 and 2018: A population-based study. Future Microbiol. 2021, 16, 1053–1062. [Google Scholar] [CrossRef]
- Nelson, G.E.; Pondo, T.; Toews, K.A.; Farley, M.M.; Lindegren, M.L.; Lynfield, R.; Aragon, D.; Zansky, S.M.; Watt, J.P.; Cieslak, P.R.; et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005–2012. Clin. Infect. Dis. 2016, 63, 478–486. [Google Scholar] [CrossRef]
- Musumeci, S.; MacPhail, A.; Weisser Rohacek, M.; Erba, A.; Goldenberger, D.; Lang, C.; Colin-Benoit, E.; Leib, S.L.; Sendi, P.; Chuard, C.; et al. Role of viral coinfection in post-pandemic invasive Group A streptococcal infections in adults, a nation-wide cohort study (iGASWISS). Eur. J. Clin. Microbiol. Infect. Dis. 2025. [Google Scholar] [CrossRef]
- Ben-Abraham, R.; Keller, N.; Vered, R.; Harel, R.; Barzilay, Z.; Paret, G. Invasive group A streptococcal infections in a large tertiary center: Epidemiology, characteristics and outcome. Infection 2002, 30, 81–85. [Google Scholar] [CrossRef]
- Davies, H.D.; McGeer, A.; Schwartz, B.; Green, K.; Cann, D.; Simor, A.E.; Low, D.E. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N. Engl. J. Med. 1996, 335, 547–554. [Google Scholar] [CrossRef]
- Factor, S.H. Invasive group A streptococcal disease: Risk factors for adults. Emerg. Infect. Dis. 2003, 9, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: Implications for pandemic influenza preparedness. J. Infect. Dis. 2008, 198, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Tasher, D.; Stein, M.; Simões, E.A.; Shohat, T.; Bromberg, M.; Somekh, E. Invasive bacterial infections in relation to influenza outbreaks, 2006–2010. Clin. Infect. Dis. 2011, 53, 1199–1207. [Google Scholar] [CrossRef]
- Vugia, D.J.; Peterson, C.L.; Meyers, H.B.; Kim, K.S.; Arrieta, A.; Schlievert, P.M.; Kaplan, E.L.; Werner, S.B. Invasive group A streptococcal infections in children with varicella in Southern California. Pediatr. Infect. Dis. J. 1996, 15, 146–150. [Google Scholar] [CrossRef]
- Brogan, T.V.; Nizet, V.; Waldhausen, J.H.; Rubens, C.E.; Clarke, W.R. Group A streptococcal necrotizing fasciitis complicating primary varicella: A series of fourteen patients. Pediatr. Infect. Dis. J. 1995, 14, 588–594. [Google Scholar] [CrossRef]
- Wilson, G.J.; Talkington, D.F.; Gruber, W.; Edwards, K.; Dermody, T.S. Group A streptococcal necrotizing fasciitis following varicella in children: Case reports and review. Clin. Infect. Dis. 1995, 20, 1333–1338. [Google Scholar] [CrossRef]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Cleary, P.P.; Kaplan, E.L.; Handley, J.P.; Wlazlo, A.; Kim, M.H.; Hauser, A.R.; Schlievert, P.M. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 1992, 339, 518–521. [Google Scholar] [CrossRef]
- Musser, J.M.; Kapur, V.; Szeto, J.; Pan, X.; Swanson, D.S.; Martin, D.R. Genetic diversity and relationships among Streptococcus pyogenes strains expressing serotype M1 protein: Recent intercontinental spread of a subclone causing episodes of invasive disease. Infect Immun. 1995, 63, 994–1003. [Google Scholar] [CrossRef]
- Steer, A.C.; Lamagni, T.; Curtis, N.; Carapetis, J.R. Invasive group a streptococcal disease: Epidemiology, pathogenesis and management. Drugs 2012, 72, 1213–1227. [Google Scholar] [CrossRef]
- Lau, E.H.; Nishiura, H.; Cowling, B.J.; Ip, D.K.; Wu, J.T. Scarlet fever outbreak, Hong Kong, 2011. Emerg. Infect. Dis. 2012, 18, 1700–1702. [Google Scholar] [CrossRef]
- Meehan, M.; Murchan, S.; Gavin, P.J.; Drew, R.J.; Cunney, R. Epidemiology of an upsurge of invasive group A streptococcal infections in Ireland, 2012–2015. J. Infect. 2018, 77, 183–190. [Google Scholar] [CrossRef]
- Dunne, E.M.; Hutton, S.; Peterson, E.; Blackstock, A.J.; Hahn, C.G.; Turner, K.; Carter, K.K. Increasing Incidence of Invasive Group A Streptococcus Disease, Idaho, USA, 2008–2019. Emerg. Infect. Dis. 2022, 28, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.W.; Jack, S.; Wu, Y.; Zhang, J.; Baker, M.G.; Geelhoed, E.; Fraser, J.; Carapetis, J.R. An economic case for a vaccine to prevent group A Streptococcus skin infections. Vaccine 2018, 33, 6968–6978. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.R.; Holden, M.T.; Coupland, P.; Chen, J.H.; Venturini, C.; Barnett, T.C.; Zakour, N.L.; Tse, H.; Dougan, G.; Yuen, K.Y.; et al. Emergence of scarlet fever Streptococcus pyogenes emm12 clones in Hong Kong is associated with toxin acquisition and multidrug resistance. Nat. Genet. 2015, 47, 84–87. [Google Scholar] [CrossRef]
- Irish Meningitis and Sepsis Reference Laboratory. Annual Report 2019. Available online: https://media.childrenshealthireland.ie/documents/IMSRL-annual-Report-2019-final.pdf#:~:text=The%20main%20invasive%20types%20in%20the%20Northern,species%20confirmation%20including%20isolates%20collected%20in%202018 (accessed on 1 July 2025).
- Vieira, A.; Wan, Y.; Ryan, Y.; Li, H.K.; Guy, R.L.; Papangeli, M.; Huse, K.K.; Reeves, L.C.; Soo, V.W.C.; Daniel, R.; et al. Rapid expansion and international spread of M1UK in the post-pandemic UK upsurge of Streptococcus pyogenes. Nat. Commun. 2024, 15, 3916. [Google Scholar] [CrossRef]
- Tsai, W.C.; Shen, C.F.; Lin, Y.L.; Shen, F.C.; Tsai, P.J.; Wang, S.Y.; Lin, Y.S.; Wu, J.J.; Chi, C.Y.; Liu, C.C. Emergence of macrolide-resistant Streptococcus pyogenes emm12 in southern Taiwan from 2000 to 2019. J. Microbiol. Immunol. Infect. 2021, 54, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cai, J.; Davies, M.R.; Li, Y.; Zhang, C.; Yao, W.; Kong, D.; Pan, H.; Zhang, X.; Zeng, M.; et al. Increase of emm1 isolates among group A Streptococcus strains causing scarlet fever in Shanghai, China. Int. J. Infect. Dis. 2020, 98, 305–314. [Google Scholar] [CrossRef]
- Sutcliffe, C.G.; Close, R.; Brown, L.B.; Parker, D.; Patel, J.; Romancito, E.; Weatherholtz, R.; McAuley, J.; Hammitt, L.L. Group A Streptococcus among American Indian Persons, White Mountain Apache Tribal Lands, United States, 2016-20191. Emerg Infect Dis. 2025, 31, 1580–1588. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Impact of the COVID-19 Pandemic on Pediatric Invasive Group A Streptococcus Infections in the United States. Available online: https://www.cdc.gov/eis-conference/php/media-resources/group-a-Streptococcus-infections.html#:~:text=During%202004%2D2022%2C%20in%20total,2021%2C%20and%202022%2C%20respectively (accessed on 29 June 2025).
- Massese, M.; La Sorda, M.; De Maio, F.; Gatto, A.; Rosato, R.; Pansini, V.; Caroselli, A.; Fiori, B.; Sanguinetti, M.; Chiaretti, A.; et al. Epidemiology of group A streptococcal infection: Are we ready for a new scenario? Lancet Microbe 2024, 5, 620–621. [Google Scholar] [CrossRef]
- Flamant, A.; Faury, H.; Luscan, R.; Couloigner, V.; Pouletty, M.; Bustarret, O.; Chappuy, H.; Gaume, M.; Drummond, D.; Pinhas, Y.; et al. Severe Group A Streptococcus Infections in Children During and After the COVID-19 Pandemic: An Interrupted Time-series Analysis in Paris, France, 2018–2023. Pediatr. Infect. Dis. J. 2025. [Google Scholar] [CrossRef]
- UK Group A Streptococcal Infections: Third Update on Seasonal Activity in England, 2023 to 2024. Available online: https://www.gov.uk/government/publications/group-a-streptococcal-infections-report-on-seasonal-activity-in-england-2023-to-2024/group-a-streptococcal-infections-third-update-on-seasonal-activity-in-england-2023-to-2024 (accessed on 1 July 2025).
- Principi, N.; Autore, G.; Ramundo, G.; Esposito, S. Epidemiology of Respiratory Infections during the COVID-19 Pandemic. Viruses 2023, 15, 1160. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.H.; Chow, A.; Ho, H.J. Decline in pneumococcal disease incidence in the time of COVID-19 in Singapore. J. Infect. 2020, 81, e19–e21. [Google Scholar] [CrossRef] [PubMed]
- Juan, H.C.; Chao, C.M.; Lai, C.C.; Tang, H.J. Decline in invasive pneumococcal disease during COVID-19 pandemic in Taiwan. J. Infect. 2020, 82, 282–327. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.L.L.; Fok, K.M.N.; Lin, K.P.K.; Chan, E.; Ma, Y.; Lau, S.K.P.; Woo, P.C.Y. Substantial decline in invasive pneumococcal disease during Coronavirus disease 2019 pandemic in Hong Kong. Clin. Infect. Dis. 2022, 74, 335–338. [Google Scholar] [CrossRef]
- Brueggemann, A.B. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: A prospective analysis of surveillance data. Lancet Digit Health 2021, 3, e360–e370. [Google Scholar]
- Van Groningen, K.M.; Dao, B.L.; Gounder, P. Declines in invasive pneumococcal disease (IPD) during the COVID-19 pandemic in Los Angeles county. J. Infect. 2022, 85, 174–211. [Google Scholar] [CrossRef]
- Amin-Chowdhury, Z.; Aiano, F.; Mensah, A.; Sheppard, C.L.; Litt, D.; Fry, N.K.; Andrews, N.; Ramsay, M.E.; Ladhani, S.N. Impact of the Coronavirus disease 2019 (COVID-19) Pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Prospective National Cohort Study, England. Clin. Infect. Dis. 2021, 72, e65–e75. [Google Scholar] [CrossRef]
- Yasir, M.; Al-Sharif, H.A.; Al-Subhi, T.; Sindi, A.A.; Bokhary, D.H.; El-Daly, M.M.; Alosaimi, B.; Hamed, M.E.; Karim, A.M.; Hassan, A.M.; et al. Analysis of the nasopharyngeal microbiome and respiratory pathogens in COVID-19 patients from Saudi Arabia. J. Infect. Public. Health. 2023, 16, 680–688. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Lin, K.P.; Wang, L.A.; Yeh, T.K.; Liu, P.Y. The Impact of the COVID-19 Pandemic on Respiratory Syncytial Virus Infection: A Narrative Review. Infect. Drug Resist. 2023, 16, 661–675. [Google Scholar] [CrossRef]
- Britton, P.N.; Hu, N.; Saravanos, G.; Shrapnel, J.; Davis, J.; Snelling, T.; Dalby-Payne, J.; Kesson, A.M.; Wood, N.; Macartney, K.; et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc. Health. 2020, 4, e42–e43. [Google Scholar] [CrossRef]
- Cohen, R.; Ashman, M.; Taha, M.K.; Varon, E.; Angoulvant, F.; Levy, C.; Rybak, A.; Ouldali, N.; Guiso, N.; Grimprel, E. Pediatric infectious disease group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now 2021, 51, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.J.; Vu, C.; Zhu, Z.; MacLachlan, J.H.; Thomson, T.N.; Campbell, P.T.; Gibney, K.B. The associations between invasive group A streptococcal disease and infection with influenza, varicella, or hepatitis C viruses: A data linkage study, Victoria, Australia. Int. J. Infect. Dis. 2024, 141, 106969. [Google Scholar] [CrossRef]
- Nygaard, U.; Hartling, U.B.; Munkstrup, C.; Nielsen, A.B.; Dungu, K.H.S.; Schmidt, L.S.; Glenthøj, J.; Matthesen, A.T.; Rytter, M.J.H.; Holm, M. Invasive group A streptococcal infections in children and adolescents in Denmark during 2022–23 compared with 2016–17 to 2021–22: A nationwide, multicentre, population-based cohort study. Lancet Child Adolesc. Health 2024, 8, 112–121. [Google Scholar] [CrossRef]
- Guy, R.; Henderson, K.L.; Coelho, J.; Hughes, H.; Mason, E.L.; Gerver, S.M.; Demirjian, A.; Watson, C.; Sharp, A.; Brown, C.S.; et al. Increase in invasive group A streptococcal infection notifications, England, 2022. Euro Surveill 2023, 28, 2200942. [Google Scholar] [CrossRef]
- Gouveia, C.; Bajanca-Lavado, M.P.; Mamede, R.; Araujo Carvalho, A.; Rodrigues, F.; Melo-Cristino, J.; Ramirez, M.; Friães, A.; Portuguese Group for the Study of Streptococcal Infections; Portuguese Study Group of Pediatric Invasive Streptococcal Disease. Sustained increase of paediatric invasive Streptococcus pyogenes infections dominated by M1(UK) and diverse emm12 isolates, Portugal, September 2022 to May 2023. Euro Surveill 2023, 28, 2300427. [Google Scholar] [CrossRef]
- de Gier, B.; van de Kassteele, J.; van Asten, L.; Schoffelen, A.F.; ISIS-AR Study Group; Hooiveld, M.; Te Wierik, M.J.; van Sorge, N.M.; de Melker, H.E.; Members of the ISIS-AR Study Group. Attribution of invasive group A streptococcal infections (iGAS) to predisposing viral infections, the Netherlands, 2010 to 2023. Euro Surveill 2024, 29, 2300739. [Google Scholar] [CrossRef] [PubMed]
- Rümke, L.W.; Davies, M.A.; Vestjens, S.M.T.; van der Putten, B.C.L.; Bril-Keijzers, W.C.M.; van Houten, M.A.; Rots, N.Y.; Wijmenga-Monsuur, A.J.; van der Ende, A.; de Gier, B.; et al. Nationwide upsurge in invasive disease in the context of longitudinal surveillance of carriage and invasive Streptococcus pyogenes 2009–2023, the Netherlands: A molecular epidemiological study. J. Clin. Microbiol. 2024, 62, e0076624. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.R.; Keller, N.; Brouwer, S.; Jespersen, M.G.; Cork, A.J.; Hayes, A.J.; Pitt, M.E.; De Oliveira, D.M.P.; Harbi-son-Price, N.; Bertolla, O.M.; et al. Detection of Streptococcus pyogenes M1UK in Australia and characterization of the mutation driving enhanced expression of superantigen SpeA. Nat. Commun. 2023, 14, 1051. [Google Scholar] [CrossRef]
- Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health. Antibiotics 2024, 13, 360. [Google Scholar] [CrossRef]
- Yu, D.; Guo, D.; Zheng, Y.; Yang, Y. A review of penicillin binding protein and group A Streptococcus with reduced-β-lactam susceptibility. Front. Cell. Infect. Microbiol. 2023, 13, 1117160. [Google Scholar] [CrossRef] [PubMed]
- ESCMID Sore Throat Guideline Group; Pelucchi, C.; Grigoryan, L.; Galeone, C.; Esposito, S.; Huovinen, P.; Little, P.; Verheij, T. Guideline for the management of acute sore throat. Clin. Microbiol. Infect. 2012, 18 (Suppl. 1), 1–28. [Google Scholar]
- Batzloff, M.R.; Davies, M.R.; Zeng, W.; Hartas, J.; Hall, M.; Dee, T.; Neve, S.; La Vincente, S.; Maxwell, T.; Frost, H.R. Vaccine development against group A Streptococcus: Progress and challenges. Curr. Opin. Infect. Dis. 2020, 33, 244–250. [Google Scholar]
- Rivera-Hernandez, T.; Carnathan, D.G.; Jones, S.; Zhao, L.; Wu, Y.; Dey, A.; Clutterbuck, E.A.; Clark, B.E.; Sivananthan, V.; Brummelman, J. A bacterial capsule is a major barrier to host immune recognition of Streptococcus pyogenes. Nat. Microbiol. 2021, 6, 574–586. [Google Scholar]
- Manish, M.; Kumar, A.; Rahi, A.; Kaur, H.; Bansal, A.; Kaur, I. mRNA vaccines and nanotechnology: A new frontier in vaccine development against emerging infectious diseases. Front. Immunol. 2022, 13, 932872. [Google Scholar]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef]
- Chopra, S.; Matsuyama, K.; Tran, T.; Maloney, E.; Brown, R.; Campagnari, A.A. Novel synthetic antimicrobial peptides targeting the M protein of Streptococcus pyogenes. Antimicrob. Agents Chemother. 2016, 60, 4559–4566. [Google Scholar]
- Melvin, J.A.; Scheller, E.V.; Miller, J.F.; Weiss, A.A. Bordetella pertussis pathogenesis: Current and future challenges. Nat. Rev. Microbiol. 2014, 12, 274–288. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).