The Clinical Profile of Pediatric M. pneumoniae Infections in the Context of a New Post-Pandemic Wave
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Type and Area
2.2. Study Population and Design
2.3. Statistical Analysis
3. Results
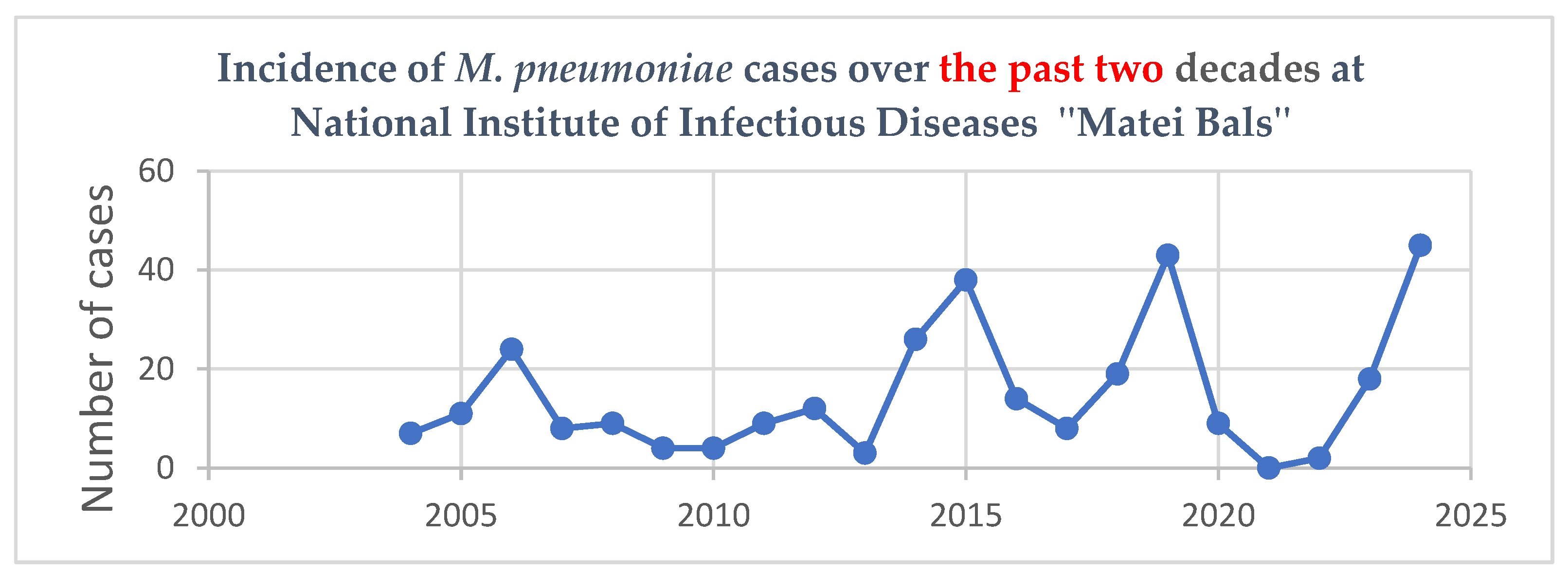
3.1. Epidemiological and Demographic Data
3.2. Symptomatology and Paraclinical Aspects
3.3. Extrapulmonary Complications
3.4. Coinfections
3.5. Hospitalization Days
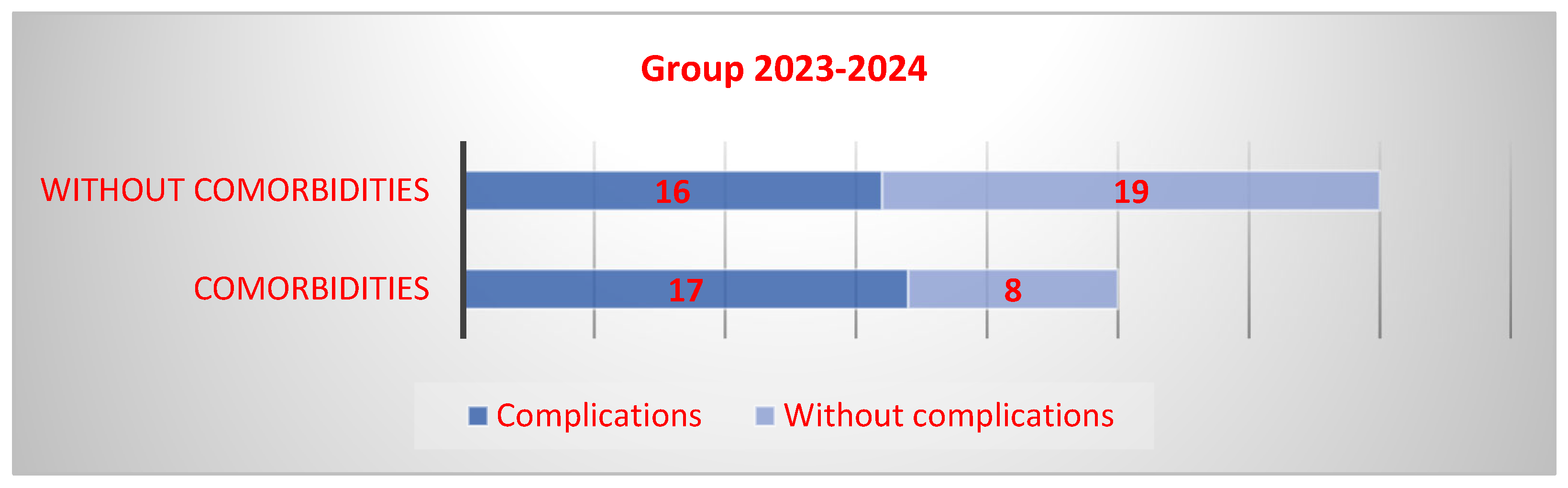
3.6. Comorbidities
3.7. Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dungua, K.H.S.; Holm, M.; Hartling, U.; Jensen, L.H.; Nielsen, A.B.; Schmidt, L.S. Mycoplasma pneumoniae incidence, phenotype, and severity in children and adolescents in Denmark before, during, and after the COVID-19 pandemic: A nationwide multicentre population-based cohort study. Lancet Reg. Health Eur. 2024, 47, 101103. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Xiao, L.; Liu, Y.; Balish, M.F.; Atkinson, T.P. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin. Microbiol. Rev. 2017, 30, 747–809. [Google Scholar] [CrossRef] [PubMed]
- Sharland, M. Manual of Childhood Infections—The Blue Book; Oxford University Press: Oxford, UK, 2011; Available online: https://www.euro-libris.ro/carte/manual-of-childhood-infections-blue-book-oxford-specialist-handbooks-in-paediatrics--i175411 (accessed on 2 February 2025).
- Sauteur, P.M.M.; Beeton, M.L. Mycoplasma pneumoniae: Delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe. 2024, 5, e100–e101. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Balish, M.F.; Atkinson, T.P. New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol. 2008, 3, 635–648. [Google Scholar] [CrossRef]
- Abdulhadi, B.; Kiel, J. Mycoplasma Pneumonia. Nort. Healthc. Med. J. 2024, 1, 66–75. [Google Scholar]
- Sagar, T.; Rathi, A.; EV, V.; Verma, N.; Kabra, M. Atypical Presentation of an Atypical Bacteria—Mycoplasma pneumoniae. Indian J. Med. Microbiol. 2025, 55, 100830. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Chen, H.; Hao, Y.; Zhang, J.; Zha, S.; Zhou, B.; Yi, Y.; Xiao, R. Comparison of the clinical characteristics in parents and their children in a series of family clustered Mycoplasma pneumoniae infections. BMC Pulm. Med. 2024, 24, 107. [Google Scholar] [CrossRef]
- Yang, S.; Lu, S.; Guo, Y.; Luan, W.; Liu, J.; Wang, L. A comparative study of general and severe Mycoplasma pneumoniae pneumonia in children. BMC Infect. Dis. 2024, 24, 449. [Google Scholar] [CrossRef]
- Chevalier, K.; Holub, M.; Palich, R.; Blanckaert, K.; Terriou, L.; Arnould, B.; Deshayes, S.; Merindol, J.; Rolland, S.; Valentin, S.; et al. Cold Autoimmune Hemolytic Anemia Secondary to Mycoplasma pneumoniae Infection in Adults: Results from a Large French Observational Study (MYCOLD study). Blood 2024, 144, 3840. [Google Scholar] [CrossRef]
- Radiopaedia—Mycoplasma pneumoniae. Available online: https://radiopaedia.org/articles/mycoplasma-pneumonia?lang=us (accessed on 30 March 2025).
- Bono, M.J. Mycoplasmal Pneumonia Medication. Available online: https://emedicine.medscape.com/article/1941994-medication (accessed on 23 March 2025).
- Shim, J.Y.; Stamm, D.R.; Stankewicz, H.A. Current Perspectives on Atypical Pneumonia in Children. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532239/ (accessed on 23 March 2025).
- Sauteur, P.M.M.; Beeton, M.L.; on behalf of the ESGMAC MAPS Study Group. Mycoplasma pneumoniae: Gone Forever? Available online: https://www.escmid.org/esgmac/ (accessed on 26 March 2025).
- Increase in Respiratory Infections Caused by Mycoplasma pneumoniae in EU/EEA Countries. Available online: https://insp.gov.ro/download/informare-privind-cresterea-numarului-de-cazuri-de-infectii-respiratorii-cauzate-de-mycoplasma-pneumoniae-in-tarile-eu_eea/ (accessed on 1 April 2025).
- Principi, N.; Autore, G.; Ramundo, G.; Esposito, S. Epidemiology of Respiratory Infections during the COVID-19 Pandemic. Viruses 2023, 15, 1160. [Google Scholar] [CrossRef]
- Lorenz, N.; James, A.; Van Rooyen, T.; Paterson, A.; Ramiah, C.; Carlton, L.H.; Sharma, P.; Baker, M.G.; Charlewood, R.; McGregor, R.; et al. Decline of Antibodies to Major Viral and Bacterial Respiratory Pathogens During the COVID-19 Pandemic. J. Infect. Dis. 2025, 231, e77–e81. [Google Scholar] [CrossRef]
- Meng, M.; Wei, R.; Wu, Y.; Zeng, R.; Luo, D.; Ma, Y.; Zhang, L.; Huang, W.; Zeng, H.; Leung, F.W.; et al. Long-term risks of respiratory diseases in patients infected with SARS-CoV-2: A longitudinal, population-based cohort study. EClinicalMedicine 2024, 69, 102500. [Google Scholar] [CrossRef] [PubMed]
- Edouard, S.; Boughammoura, H.; Colson, P.; La Scola, B.; Fournier, P.-E.; Fenollar, F. Large-Scale Outbreak of Mycoplasma pneumoniae Infection, Marseille, France, 2023–2024. Emerg. Infect. Dis. 2024, 30, 1481–1484. [Google Scholar] [CrossRef]
- Stoicescu, S.M.; Mohora, R.; Luminos, M.; Merisescu, M.M.; Jugulete, G.; Nastase, L. PRESEPSIN—New Marker of SEPSIS Romanian Neonatal Intensive Care Unit Experience. Rev. Chim. 2019, 70, 3008–3013. [Google Scholar] [CrossRef]
- Jugulete, G.; Merișescu, M.; Bastian, A.E.; Luminos, M. Clinical aspects and medico-legal implications of purpura fulminans in children. Rom. J. Leg. Med. 2017, 25, 364–368. [Google Scholar]
- Birrell, J.M.; Woolnough, E.; Spence, E. Mycoplasma pneumoniae with para-infectious cerebral complications. Respir. Med. Case Rep. 2019, 27, 100859. [Google Scholar] [CrossRef] [PubMed]
- Inchley, C.S.; Berg, A.S.; Benam, A.V.; Kvissel, A.K.; Leegaard, T.M.; Nakstad, B. Mycoplasma pneumoniae: A Cross-sectional Population-based Comparison of Disease Severity in Preschool and School-age Children. Pediatr. Infect. Dis. J. 2017, 36, 930–936. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuo, J.; Zhuang, X. The Prevalence of Mycoplasma pneumoniae in Children in Shandong, China Before, During, and After COVID-19. Available online: https://www.scienceopen.com/document?vid=fedf1912-0652-4b00-acee-4e7ad8b5b89e (accessed on 1 April 2025).
- Meyer Sauteur, P.M.; Unger, W.W.J.; van Rossum, A.M.C.; Berger, C. The Art and Science of Diagnosing Mycoplasma pneumoniae Infection. Available online: https://www.zora.uzh.ch/id/eprint/160899/1/document(1).pdf (accessed on 1 April 2025).
- Søndergaard, M.J.; Friis, M.B.; Hansen, D.S.; Jørgensen, I.M. Clinical manifestations in infants and children with Mycoplasma pneumoniae infection. PLoS ONE 2018, 13, e0195288. [Google Scholar] [CrossRef]
- Vlad, R.M.; Dobritoiu, R.; Turenschi, A.; Pacurar, D. Where Reality and Fantasy Collide—Prolonged Fever to Munchausen Syndrome by Proxy. Children 2024, 11, 1482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kashyap, S.; Sarkar, M. Mycoplasma pneumonia: Clinical features and management. Lung 2010, 27, 75–85. [Google Scholar] [CrossRef]
- Canavan, T.N.; Mathes, E.F.; Frieden, I.; Shinkai, K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: A systematic review. J. Am. Acad. Dermatol. 2015, 72, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Echevarría, A.; Calle-Miguel, L.; Miralbés, S.; Barreiro-Pérez, S.; Afonso-Rodriguez, O.; Soler-Simón, J.A.; Espeleta, A.; Jiménez-Jiménez, A.B.; Méndez-Sánchez, A.; Rementeria-Radigales, J.I.; et al. Increased Severity of Mycoplasma pneumoniae Infections in Spanish Children. Pediatr. Infect. Dis. J. 2024; online ahead of print. [Google Scholar]
- Wu, Q.; Pan, X.; Han, D.; Ma, Z.; Zhang, H. New Insights into the Epidemiological Characteristics of Mycoplasma pneumoniae Infection before and after the COVID-19 Pandemic. Microorganisms 2024, 12, 2019. [Google Scholar] [CrossRef]
- Zhang, X.-B.; He, W.; Gui, Y.-H.; Lu, Q.; Yin, Y.; Zhang, J.-H.; Dong, X.-Y.; Wang, Y.-W.; Ye, Y.-Z.; Xu, H.; et al. Current Mycoplasma pneumoniae epidemic among children in Shanghai: Unusual pneumonia caused by usual pathogen. World J. Pediatr. 2024, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Dekyi; Xiao, Y.; Wang, X.; Feng, S.; Wang, Y.; Liao, L.; Wang, S.; Deng, Y.; Zheng, J.; Zhao, D. Predominance of A2063G mutant strains in the Mycoplasma pneumoniae epidemic in children: A clinical and epidemiological study in 2023 in Wuhan, China. Int. J. Infect. Dis. 2024, 145, 107074. [Google Scholar] [CrossRef]
- Raghuram, A.; Furmanek, S.; Chandler, T.; Rashid, S.; Mattingly, W.; Ramirez, J. Description of a Current Outbreak of Mycoplasma pneumoniae in the United States. Pathogens 2025, 14, 60. [Google Scholar] [CrossRef]
- Yu, A.; Ran, L.; Sun, X.; Feng, T. Significance of respiratory virus coinfection in children with Mycoplasma pneumoniae pneumonia. BMC Pulm. Med. 2024, 24, 585. [Google Scholar] [CrossRef]
- Choo, S.; Lee, Y.Y.; Lee, E. Clinical significance of respiratory virus coinfection in children with Mycoplasma pneumoniae pneumonia. BMC Pulm. Med. 2022, 22, 212. [Google Scholar] [CrossRef] [PubMed]
- Merișescu, M.-M.; Luminos, M.L.; Pavelescu, C.; Jugulete, G. Clinical Features and Outcomes of the Association of Co-Infections in Children with Laboratory-Confirmed Influenza during the 2022–2023 Season: A Romanian Perspective. Viruses 2023, 15, 2035. [Google Scholar] [CrossRef]
- Jugulete, G.; Merișescu, M.M.; Bastian, A.E.; Zurac, S.; Stoicescu, S.M.; Luminos, M.L. Severe form of AIHI influenza in a children- case presentation. Rom. J. Leg. Med. 2018, 26, 387–391. [Google Scholar]
- McIntosh, K. Community-acquired pneumonia in children. N. Engl. J. Med. 2002, 346, 429. [Google Scholar] [CrossRef]

| Group 1 (2018–2019) (N = 61) | Group 2 (2023–2024) (N = 60) | p Value | |
|---|---|---|---|
| Gender, n (%) | p = 1 | ||
| Female | 30 (49%) | 30 (50%) | |
| Male | 31 (51%) | 30 (50%) | |
| Age | |||
| <1 year old | 0 | 1 (2%) | p = 0.496 |
| 1–5 years old | 29 (48%) | 23 (38%) | p = 0.360 |
| 6–12 years old | 27 (44%) | 30 (50%) | p = 0.587 |
| 13–18 years old | 5 (8%) | 6 (10%) | p = 0.762 |
| Residence | p = 0.360 | ||
| Urban | 38 | 32 | |
| Rural | 23 | 28 |
| Group 1, 2018–2019 (N = 61) | Group 2, 2023–2024 (N = 60) | p Value | |
|---|---|---|---|
| Symptoms | |||
| Fever | 54 (89%) | 50 (83%) | p > 0.05 |
| Cough | 43 (70%) | 53 (88%) | p = 0.023 |
| Dyspnea | 0 | 7 (11.6%) | p = 0.006 |
| Abdominal pain, vomiting, diarrhea | 25 (41%) | 10 (16.6%) | p = 0.004 |
| Neurological impairment (paresthesia, headache) | 6 (10%) | 7 (12%) | p > 0.05 |
| Skin rash and mucositis | 10 (16%) | 7 (12%) | p > 0.05 |
| Paraclinical investigations | |||
| Normal leukocytes | 42 (69%) | 43 (72%) | p > 0.05 |
| Leukocytosis | 17 (28%) | 15 (25%) | p > 0.05 |
| Leucopenia | 2 (3%) | 2 (3%) | p > 0.05 |
| C-reactive protein (mg/L), mean | 28 (IQR 4–30.7) | 33 (IQR 6–48) | |
| Fibrinogen, mean | 381 (IQR 303–443) | 380 (IQR 310–434) | |
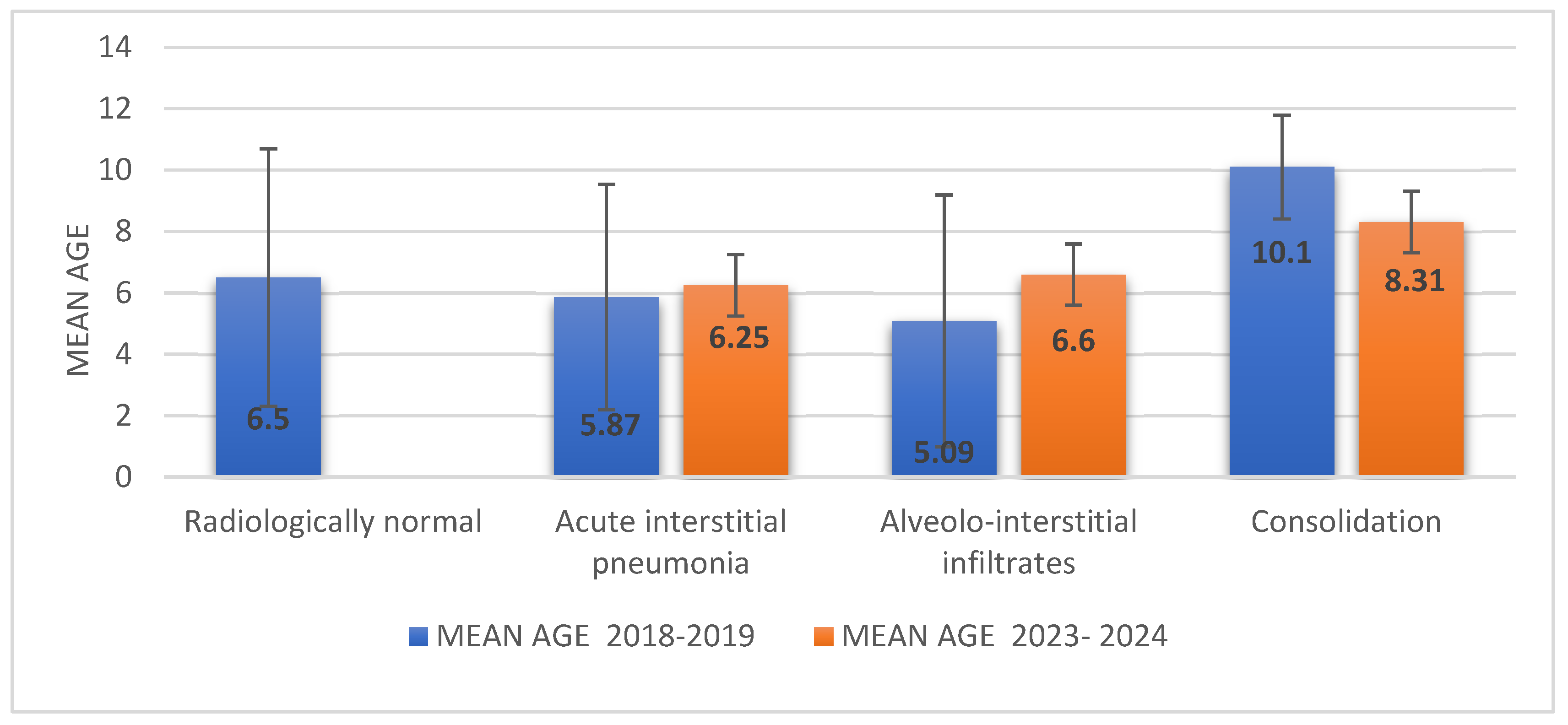
| Radiographic findings | |||
| Radiologically normal | 8 (13%) | 1 (2%) | p = 0.040 |
| Acute interstitial pneumonia | 24 (39%) | 22 (37%) | p > 0.05 |
| Alveolar–interstitial infiltrates | 20 (31%) | 18 (33%) | p > 0.05 |
| Consolidation | 9 (15%) | 19 (32%) | p = 0.016 |
| Pleural reaction | 1 | 6 (10%) | p > 0.05 |
| Extrapulmonary Complications | Group 1 | Group 2 | p |
|---|---|---|---|
| Dermatological | 15 | 12 | p > 0.05 |
| Neurologic | 6 | 2 | p > 0.05 |
| Gastrointestinal | 37 | 17 | p < 0.001 |
| Hemolytic anemia | 2 | 0 | p > 0.05 |
| Otitis | 5 | 2 | p > 0.05 |
| Parotitis | 2 | 0 | p > 0.05 |
| Group 1 (2018–2019) (N = 61) | Group 2 (2023–2024) (N = 60) | p Value | |
|---|---|---|---|
| Coinfections | 22 (36.07%) | 28(46.67%) | 0.271 |
| Coinfections with one pathogen | 19 (31.15%) | 18 (30%) | 1 |
| Multi-organism coinfections | 3 (4.92%) | 10 (16.67%) | 0.044 |
| Hospitalization Days | Group 1 (2018–2019) | Group 2 (2023–2024) | p Value |
|---|---|---|---|
| All patients | n = 61 (Median = 7, IQR 5–9) | n = 60 (Median = 6, IQR 5–7) | p < 0.001 |
| With coinfections | n = 23 (Median = 7, IQR 5–8.5) | n = 28 (Median = 6, IQR 5–7) | p < 0.001 |
| Without coinfections | n = 38 (Median = 7.5, IQR 6–9) | n = 32 (Median = 5, IQR 4.75–7) | p < 0.001 |
| With corticosteroid therapy | n = 28 (Median = 8, IQR 6–10) | n = 21 (Median = 6, IQR 5–7) | p < 0.001 |
| Without corticosteroid therapy | n = 33 (Median = 6, IQR 5–8) | n = 39 (Median = 5, IQR 4.5–7) | p < 0.001 |
| With comorbidities | n = 12 (Median = 7.5, IQR 6–8.5) | n = 25 (Median = 6, IQR 5–9) | p = 0.447 |
| Without comorbidities | n = 49 (Median = 7, IQR 5–9) | n = 35 Median = 6 (IQR 5–7) | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merișescu, M.M.; Jugulete, G.; Dijmărescu, I.; Dragomirescu, A.O.; Răduț, L.M. The Clinical Profile of Pediatric M. pneumoniae Infections in the Context of a New Post-Pandemic Wave. Microorganisms 2025, 13, 1152. https://doi.org/10.3390/microorganisms13051152
Merișescu MM, Jugulete G, Dijmărescu I, Dragomirescu AO, Răduț LM. The Clinical Profile of Pediatric M. pneumoniae Infections in the Context of a New Post-Pandemic Wave. Microorganisms. 2025; 13(5):1152. https://doi.org/10.3390/microorganisms13051152
Chicago/Turabian StyleMerișescu, Mădălina Maria, Gheorghiță Jugulete, Irina Dijmărescu, Anca Oana Dragomirescu, and Larisa Mirela Răduț. 2025. "The Clinical Profile of Pediatric M. pneumoniae Infections in the Context of a New Post-Pandemic Wave" Microorganisms 13, no. 5: 1152. https://doi.org/10.3390/microorganisms13051152
APA StyleMerișescu, M. M., Jugulete, G., Dijmărescu, I., Dragomirescu, A. O., & Răduț, L. M. (2025). The Clinical Profile of Pediatric M. pneumoniae Infections in the Context of a New Post-Pandemic Wave. Microorganisms, 13(5), 1152. https://doi.org/10.3390/microorganisms13051152





