Widespread Presence of SPX and Its Potential Role as a Phosphorus Nutrient Regulator in Dinoflagellates
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Further Analysis of dino-SPXc
2.2. Global Expression Profiling of dino-SPXc Based on TARA Oceans Metatranscriptome Data
2.3. Expression Analysis of SPXc Genes in Prorocentrum Shikokuense and Symbiodiniaceae
3. Results
3.1. Taxonomic and Ecotypic Distribution of dino-SPXc from Genomic and Transcriptomic Databases
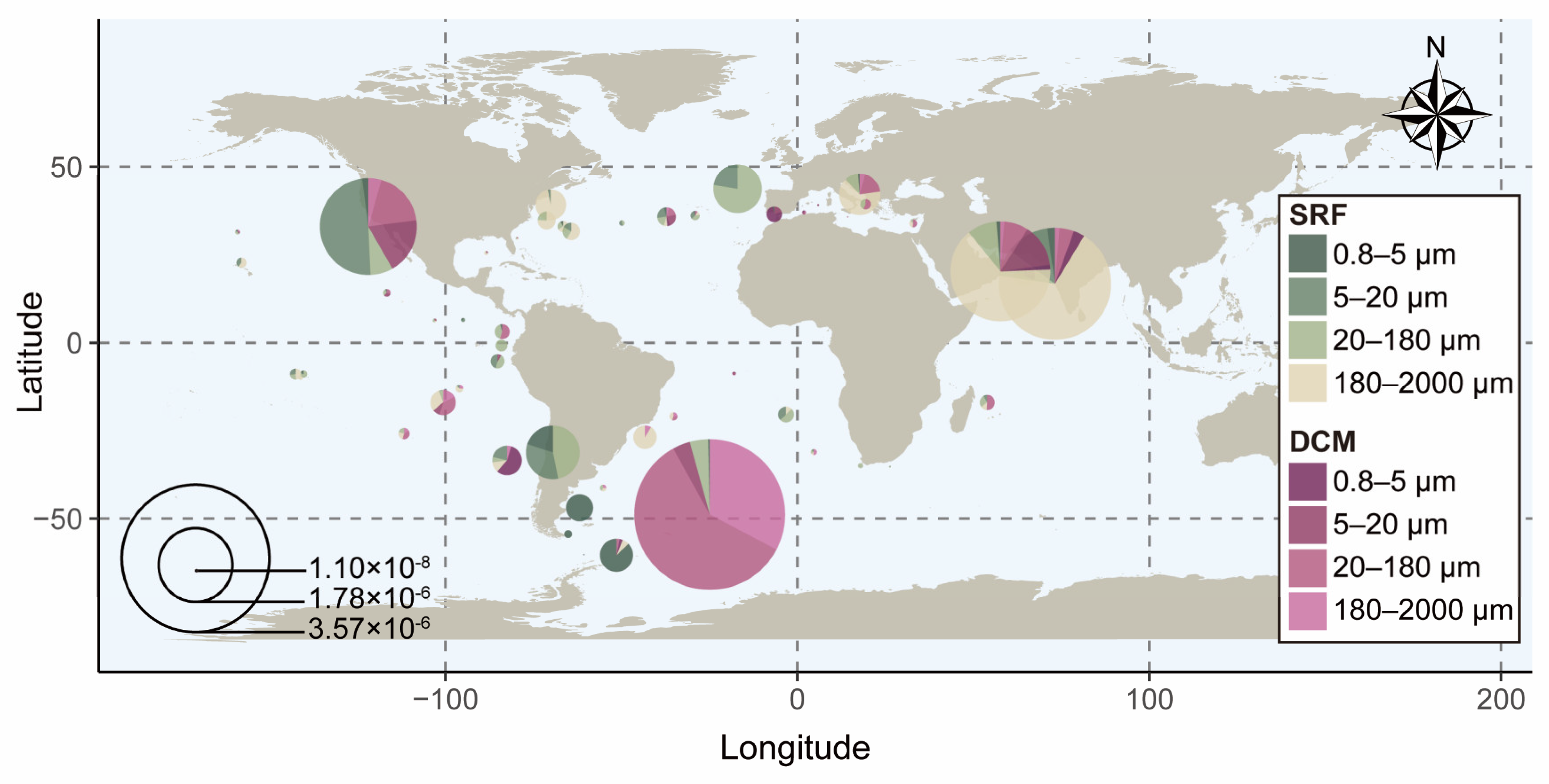
3.2. Global Distribution of dino-SPXc
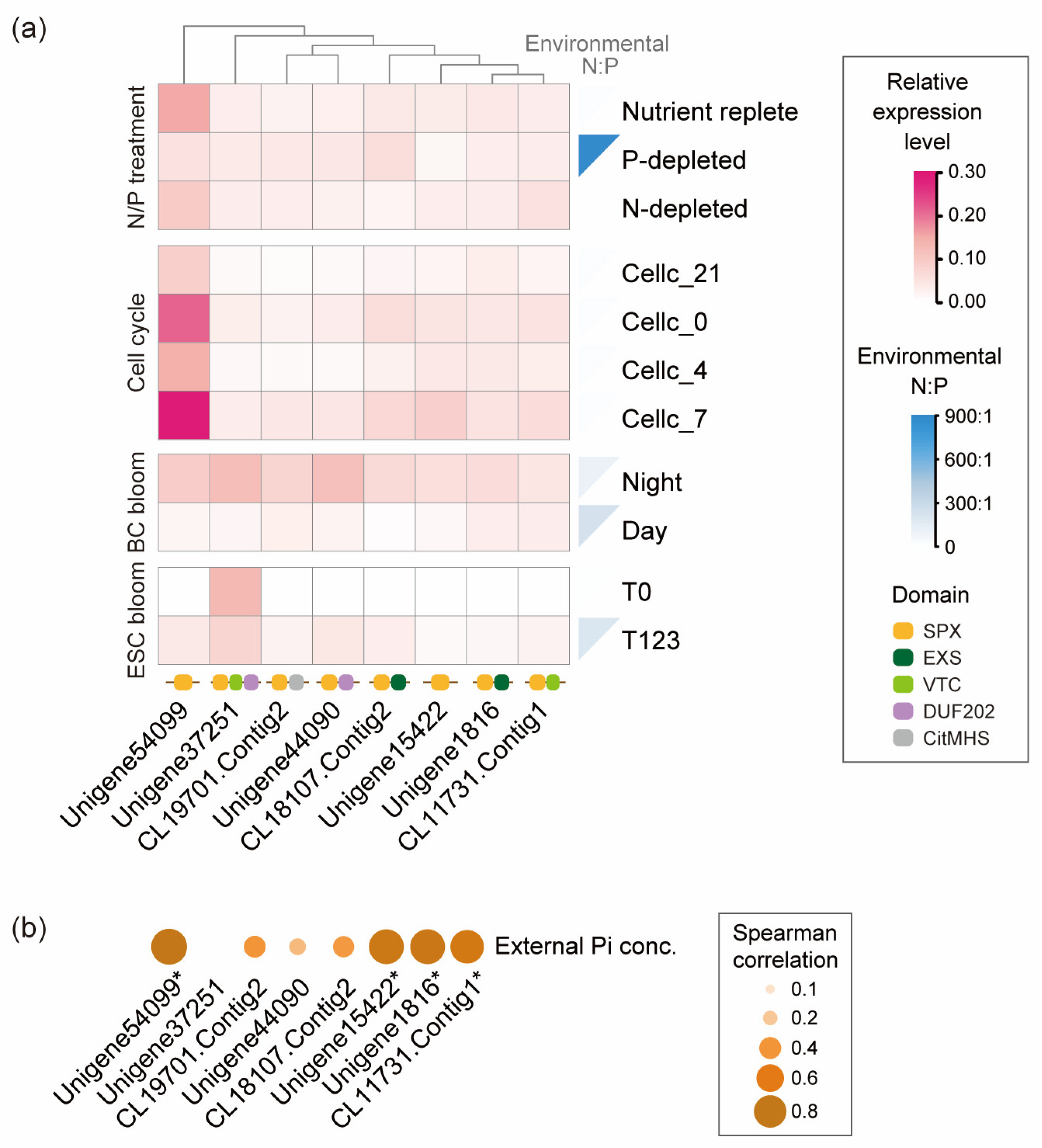
3.3. Expression Pattern of SPXc Genes in P. shikokuense and Symbiodiniaceae
4. Discussion
4.1. Wide Taxonomic and Geographic Distribution of dino-SPXc
4.2. Potential P Homeostasis Regulatory Mechanism in Dinoflagellate by dino-SPXc Proteins
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| P | phosphorus |
| N | nitrogen |
| SPX | named after SYG1/PHO81/XPR1 |
| PHR | phosphate starvation response protein |
| dino-SPXc | genes encoding SPX domain-containing proteins in dinoflagellate |
| Pshi-SPXc | genes encoding SPX domain-containing proteins in Prorocentrum shikokuense |
| SPX-EXS | proteins containing SPX and EXS domains |
| SPX-VTC | proteins containing SPX and VTC domains |
| SPX-other | proteins containing SPX domain plus DUF202 or CitMHS domain |
| PSI | Pi starvation-induced genes |
| HAB | harmful algal bloom |
| CDD | conserved domain database |
| SMART | simple modular architecture research tool |
| OGA | Ocean Gene Atlas |
| DCM | deep chlorophyll maximum |
| SRF | surface water |
| TPM | transcript per million |
| SAGER | Symbiodiniceae and algal genomic resource |
References
- Lin, S.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef]
- David, L.; Nelson; Cox, M.M. Principles of Biochemistry; Worth Publishers: New York, NY, USA, 1993. [Google Scholar]
- Dyhrman, S.T. Nutrients and their acquisition: Phosphorus physiology in microalgae. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer: Cham, Switzerland, 2016; Volume 6, pp. 155–183. [Google Scholar]
- Paytan, A.; McLaughlin, K. The oceanic phosphorus cycle. Chem. Rev. 2007, 107, 563–576. [Google Scholar] [CrossRef]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of oceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef]
- Duhamel, S.; Diaz, J.M.; Adams, J.C.; Djaoudi, K.; Steck, V.; Waggoner, E.M. Phosphorus as an integral component of global marine biogeochemistry. Nat. Geosci. 2021, 14, 359–368. [Google Scholar] [CrossRef]
- Morey, J.S.; Monroe, E.A.; Kinney, A.L.; Beal, M.; Johnson, J.G.; Hitchcock, G.L.; Van Dolah, F.M. Transcriptomic response of the red tide dinoflagellate, Karenia brevis, to nitrogen and phosphorus depletion and addition. BMC Genom. 2011, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Guisande, C.; Frangópulos, M.; Maneiro, I.; Vergara, A.R.; Riveiro, I. Ecological advantages of toxin production by the dinoflagellate Alexandrium minutum under phosphorus limitation. Mar. Ecol. Prog. Ser. 2002, 225, 169–176. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Ren, H.; Shen, C.; Li, Y.; Ling, H.Q.; Wu, C.; Lian, X.; Wu, P. OsSPX1 suppresses the function of OsPHR2 in the regulation of expression of OsPT2 and phosphate homeostasis in shoots of rice. Plant J. 2010, 62, 508–517. [Google Scholar] [CrossRef]
- Secco, D.; Wang, C.; Arpat, B.A.; Wang, Z.; Poirier, Y.; Tyerman, S.D.; Wu, P.; Shou, H.; Whelan, J. The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol. 2012, 193, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhong, Y.; Wang, Y.; Wang, Z.; Zhang, L.; Shi, J.; Wu, Z.; Liu, Y.; Mao, C.; Yi, K.; et al. SPX4 negatively regulates phosphate signaling and homeostasis through its interaction with PHR2 in rice. Plant Cell 2014, 26, 1586–1597. [Google Scholar] [CrossRef]
- Wang, Z.; Ruan, W.; Shi, J.; Zhang, L.; Xiang, D.; Yang, C.; Li, C.; Wu, Z.; Liu, Y.; Yu, Y.; et al. Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc. Natl. Acad. Sci. USA 2014, 111, 14953–14958. [Google Scholar] [CrossRef]
- Du, H.; Yang, C.; Ding, G.; Shi, L.; Xu, F. Genome-wide identification and characterization of SPX domain-containing members and their responses to phosphate deficiency in Brassica napus. Front. Plant Sci. 2017, 8, 35. [Google Scholar] [CrossRef]
- Jung, J.Y.; Ried, M.K.; Hothorn, M.; Poirier, Y. Control of plant phosphate homeostasis by inositol pyrophosphates and the SPX domain. Curr. Opin. Biotechnol. 2018, 49, 156–162. [Google Scholar] [CrossRef]
- Puga, M.I.; Mateos, I.; Charukesi, R.; Wang, Z.; Franco-Zorrilla, J.M.; de Lorenzo, L.; Irigoyen, M.L.; Masiero, S.; Bustos, R.; Rodriguez, J.; et al. SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14947–14952. [Google Scholar] [CrossRef]
- Wege, S.; Khan, G.A.; Jung, J.Y.; Vogiatzaki, E.; Pradervand, S.; Aller, I.; Meyer, A.J.; Poirier, Y. The EXS domain of PHO1 participates in the response of shoots to phosphate deficiency via a root-to-shoot signal. Plant Physiol. 2016, 170, 385–400. [Google Scholar] [CrossRef]
- Zhou, Y.; Ni, M. SHORT HYPOCOTYL UNDER BLUE1 truncations and mutations alter its association with a signaling protein complex in Arabidopsis. Plant Cell 2010, 22, 703–715. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Luan, M.; Wang, Y.; Zhang, C.; Zhang, B.; Shi, J.; Zhao, F.G.; Lan, W.; Luan, S. A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, E6571–E6578. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Saroussi, S.; Huang, W.; Akkawi, N.; Grossman, A.R. Metabolic control of acclimation to nutrient deprivation dependent on polyphosphate synthesis. Sci. Adv. 2020, 6, eabb5351. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Zhang, Y.; Xu, L.; Menand, B.; Zhao, H.; Zeng, H.; Dolan, L.; Zhu, Y.; Yi, K. Loss of two families of SPX domain-containing proteins required for vacuolar polyphosphate accumulation coincides with the transition to phosphate storage in green plants. Mol. Plant 2021, 14, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Huang, T.K.; Chiou, T.J. Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 2013, 25, 4061–4074. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Peng, M.; Rothstein, S.J. Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLoS Genet. 2011, 7, e1002021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, Z.; Li, J.; Wang, J.; Yu, L.; Lin, S. SPX-related genes regulate phosphorus homeostasis in the marine phytoplankton, Phaeodactylum tricornutum. Commun. Biol. 2021, 4, 797. [Google Scholar] [CrossRef]
- Yu, L.; Li, T.; Li, L.; Lin, X.; Li, H.; Liu, C.; Guo, C.; Lin, S. SAGER: A database of Symbiodiniaceae and Algal Genomic Resource. Database 2020, 2020, baaa051. [Google Scholar] [CrossRef] [PubMed]
- Tara Oceans Consortium, Coordinators; Tara Oceans Expedition, Participants. Registry of all samples from the Tara Oceans Expedition (2009–2013). Pangaea 2017. [CrossRef]
- Vernette, C.; Lecubin, J.; Sanchez, P.; Tara Oceans, C.; Sunagawa, S.; Delmont, T.O.; Acinas, S.G.; Pelletier, E.; Hingamp, P.; Lescot, M. The Ocean Gene Atlas v2.0: Online exploration of the biogeography and phylogeny of plankton genes. Nucleic Acids Res. 2022, 50, W516–W526. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Li, L.; Wang, Y.; Lin, S. Comparative genomics illuminates adaptive evolution of DVNP with lifestyle and with loss of histone H1 in dinoflagellates. bioRxiv 2024, in press. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Yu, L.; Yang, X.; Shi, X.; Wang, J.; Li, J.; Lin, S. Transcriptome profiling reveals versatile dissolved organic nitrogen utilization, mixotrophy, and N conservation in the dinoflagellate Prorocentrum shikokuense under N deficiency. Sci. Total Environ. 2021, 763, 143013. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lin, X.; Li, L.; Li, M.; Palenik, B.; Lin, S. Transcriptomic and microRNAomic profiling reveals multi-faceted mechanisms to cope with phosphate stress in a dinoflagellate. ISME J. 2017, 11, 2209–2218. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Li, M.; Wang, C.; Lin, X.; Li, L.; Shi, X.; Guo, C.; Lin, S. Comparative metatranscriptomic profiling and microRNA sequencing to reveal active metabolic pathways associated with a dinoflagellate bloom. Sci. Total Environ. 2019, 699, 134323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Shi, X.; Lin, L.; Luo, H.; Li, L.; Lin, S. Metatranscriptomic Signatures Associated With Phytoplankton Regime Shift From Diatom Dominance to a Dinoflagellate Bloom. Front. Microbiol. 2019, 10, 590. [Google Scholar] [CrossRef]
- Baumgarten, S.; Bayer, T.; Aranda, M.; Liew, Y.J.; Carr, A.; Micklem, G.; Voolstra, C.R. Integrating microRNA and mRNA expression profiling in Symbiodinium microadriaticum, a dinoflagellate symbiont of reef-building corals. BMC Genom. 2013, 14, 704. [Google Scholar] [CrossRef]
- Shoguchi, E.; Beedessee, G.; Tada, I.; Hisata, K.; Kawashima, T.; Takeuchi, T.; Arakaki, N.; Fujie, M.; Koyanagi, R.; Roy, M.C.; et al. Two divergent Symbiodinium genomes reveal conservation of a gene cluster for sunscreen biosynthesis and recently lost genes. BMC Genom. 2018, 19, 458. [Google Scholar] [CrossRef]
- Parkinson, J.E.; Baumgarten, S.; Michell, C.T.; Baums, I.B.; LaJeunesse, T.C.; Voolstra, C.R. Gene expression variation resolves species and individual strains among coral-associated dinoflagellates within the genus Symbiodinium. Genome Biol. Evol. 2016, 8, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Li, L.; Wang, Y.; Lin, S. Phosphorus nutrition strategies in a Symbiodiniacean species: Implications in coral-alga symbiosis facing increasing phosphorus deficiency in future warmer oceans. Glob. Change Biol. 2023, 29, 6558–6571. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, L.; Zhang, H. Transcriptomic responses to thermal stress and varied phosphorus conditions in Fugacium kawagutii. Microorganisms 2019, 7, 96. [Google Scholar] [CrossRef]
- Azevedo, C.; Saiardi, A. Eukaryotic phosphate homeostasis: The inositol pyrophosphate perspective. Trends Biochem. Sci. 2017, 42, 219–231. [Google Scholar] [CrossRef]
- Zhang, S.-F.; Yuan, C.-J.; Chen, Y.; Lin, L.; Wang, D.-Z. Transcriptomic response to changing ambient phosphorus in the marine dinoflagellate Prorocentrum donghaiense. Sci. Total Environ. 2019, 692, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, V.; Berdieva, M.; Aksenov, N.; Skarlato, S. Phosphorus deficiency induces sexual reproduction in the dinoflagellate Prorocentrum cordatum. Sci. Rep. 2023, 13, 14191. [Google Scholar] [CrossRef]
- Helliwell, K.E. Emerging trends in nitrogen and phosphorus signalling in photosynthetic eukaryotes. Trends Plant Sci. 2022, 28, 344–358. [Google Scholar] [CrossRef]
- Janouskovec, J.; Gavelis, G.S.; Burki, F.; Dinh, D.; Bachvaroff, T.R.; Gornik, S.G.; Bright, K.J.; Imanian, B.; Strom, S.L.; Delwiche, C.F.; et al. Major transitions in dinoflagellate evolution unveiled by phylotranscriptomics. Proc. Natl. Acad. Sci. USA 2017, 114, E171–E180. [Google Scholar] [CrossRef]
- Ustick, L.J.; Larkin, A.A.; Garcia, C.A.; Garcia, N.S.; Brock, M.L.; Lee, J.A.; Wiseman, N.A.; Moore, J.K.; Martiny, A.C. Metagenomic analysis reveals global-scale patterns of ocean nutrient limitation. Science 2021, 372, 287–291. [Google Scholar] [CrossRef]
- You, Y.; Sun, X.; Ma, M.; He, J.; Li, L.; Porto, F.W.; Lin, S. Trypsin is a coordinate regulator of N and P nutrients in marine phytoplankton. Nat. Commun. 2022, 13, 4022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.Z.; Ouyang, L.L.; Shen, A.L.; Wang, Y.L. The cell cycle of phytoplankton: A review. J. World Aquac. Soc. 2022, 53, 799–815. [Google Scholar] [CrossRef]
- Li, M.Z.; Li, L.; Shi, X.G.; Lin, L.X.; Lin, S.J. Effects of phosphorus deficiency and adenosine 5′-triphosphate (ATP) on growth and cell cycle of the dinoflagellate Prorocentrum donghaiense. Harmful Algae 2015, 47, 35–41. [Google Scholar] [CrossRef]
- Ezzat, L.; Maguer, J.F.; Grover, R.; Ferrier-Pages, C. Limited phosphorus availability is the Achilles heel of tropical reef corals in a warming ocean. Sci. Rep. 2016, 6, 31768. [Google Scholar] [CrossRef] [PubMed]

| Clade A a | Clade B b | Clade C * c | Clade F d | |||||
|---|---|---|---|---|---|---|---|---|
| SmicGene7450 | SymA3.s891_g10 | SymA3.s6604_g1 | SymbB.v1.2.009528 | SymbC1.Scaffold4357.3 | Symbiodinium-sp-C1- 20140214|13393_1 | Fkaw26060 | Fkaw03036 | |
| P-replete | - | - | - | - | 32.233 | 25.43 | 14.69 | 15.27 |
| P-depleted | - | - | - | - | 34.13 | 22.6 | 2.52 | 0.93 |
| DOP (G3P) | - | - | - | - | 25.52 | 27.01 | 0 | 0.97 |
| DOP (PA) | - | - | - | - | 36.1 | 23.65 | - | - |
| Normal temperature | 22.01 | 18.52 | 9.9 | 12.75 | 15.65 | - | - | - |
| Heat stress | 14.26 | 21.7 | 9.97 | - | 11.57 | - | - | - |
| Heat shock | 21.25 | - | - | - | - | - | - | - |
| Cold stress | 24.38 | - | - | - | - | - | - | - |
| Cold shock | 16.22 | - | - | - | - | - | - | - |
| Light period | 22.01 | 18.52 | 9.9 | - | - | - | - | - |
| Dark period | 23.29 | - | - | - | - | - | - | - |
| Dark stress | 18.78 | 23.34 | 8.16 | - | - | - | - | - |
| Dark + heat stress | - | 22.45 | 10.27 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, J.; Wang, X.; Zhang, K.; Lin, S. Widespread Presence of SPX and Its Potential Role as a Phosphorus Nutrient Regulator in Dinoflagellates. Microorganisms 2025, 13, 1867. https://doi.org/10.3390/microorganisms13081867
Li J, Wang J, Wang X, Zhang K, Lin S. Widespread Presence of SPX and Its Potential Role as a Phosphorus Nutrient Regulator in Dinoflagellates. Microorganisms. 2025; 13(8):1867. https://doi.org/10.3390/microorganisms13081867
Chicago/Turabian StyleLi, Jiashun, Jingtian Wang, Xiaoyu Wang, Kaidian Zhang, and Senjie Lin. 2025. "Widespread Presence of SPX and Its Potential Role as a Phosphorus Nutrient Regulator in Dinoflagellates" Microorganisms 13, no. 8: 1867. https://doi.org/10.3390/microorganisms13081867
APA StyleLi, J., Wang, J., Wang, X., Zhang, K., & Lin, S. (2025). Widespread Presence of SPX and Its Potential Role as a Phosphorus Nutrient Regulator in Dinoflagellates. Microorganisms, 13(8), 1867. https://doi.org/10.3390/microorganisms13081867







