Physiological Insights into Enhanced Epsilon-Poly-l-Lysine Production Induced by Extract Supplement from Heterogeneous Streptomyces Strain
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Media
2.2. ε-PL Production After the Addition of Other Microorganisms
2.3. Primary Extraction of Microbial Signal Mixture and Its Influence on ε-PL Production
2.4. ε-PL Production After the Addition of S. gilvosporeus Extracts in a 5 L Fermenter
2.5. Impacts of S. gilvosporeus Extracts on the Morphology of S. albulus IFO 14147
2.6. RNA Extraction and Transcriptomic Profiling
2.7. Transcriptional Performances of Key Genes in ε-PL Biosynthesis
2.8. Activity Assay of Key Enzymes and Electron Transport System in ε-PL Biosynthesis
2.9. Metabolomic Profiling via UPLC-ESI-MS/MS
2.10. Analytical Methods
2.11. Calculations
2.12. Statistical Processing
3. Results
3.1. ε-PL Production After the Addition of Heterogeneous Microorganisms’ Extracts
3.2. ε-PL Fermentation Profile After Addition of S. gilvosporeus Biomass Extract
3.3. Cell Morphology of S. albulus After S. gilvosporeus Extract Addition
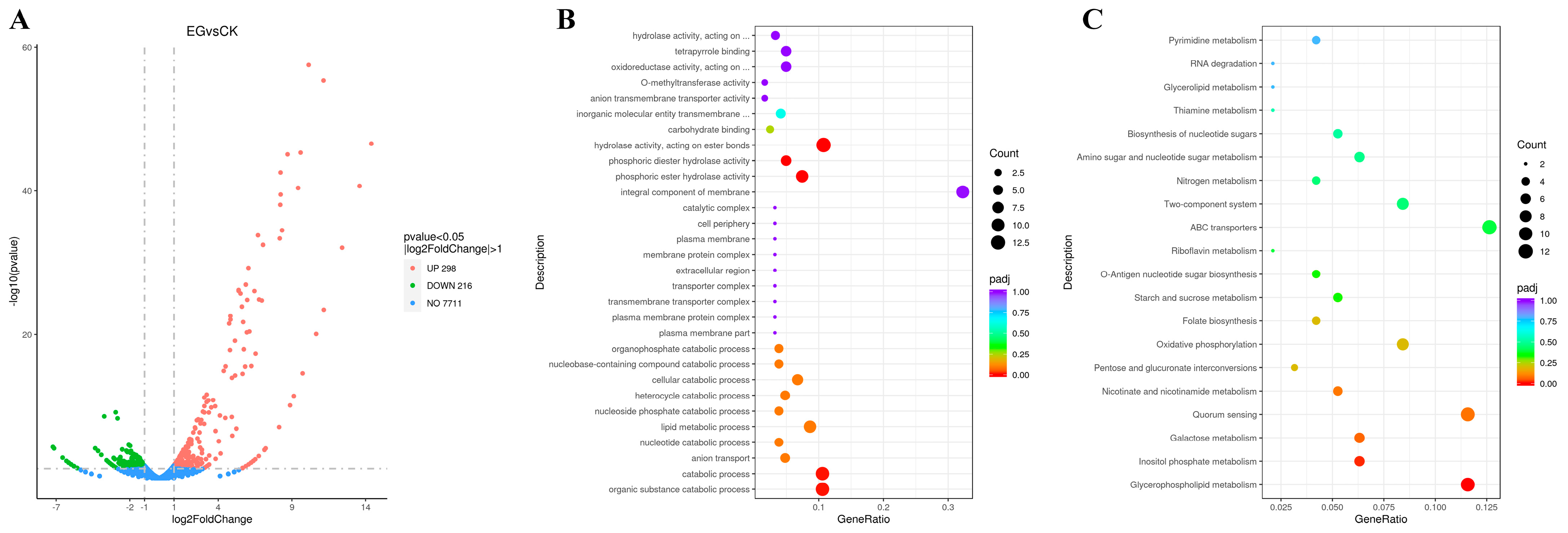
3.4. Transcriptome Performance After S. gilvosporeus Extract Addition
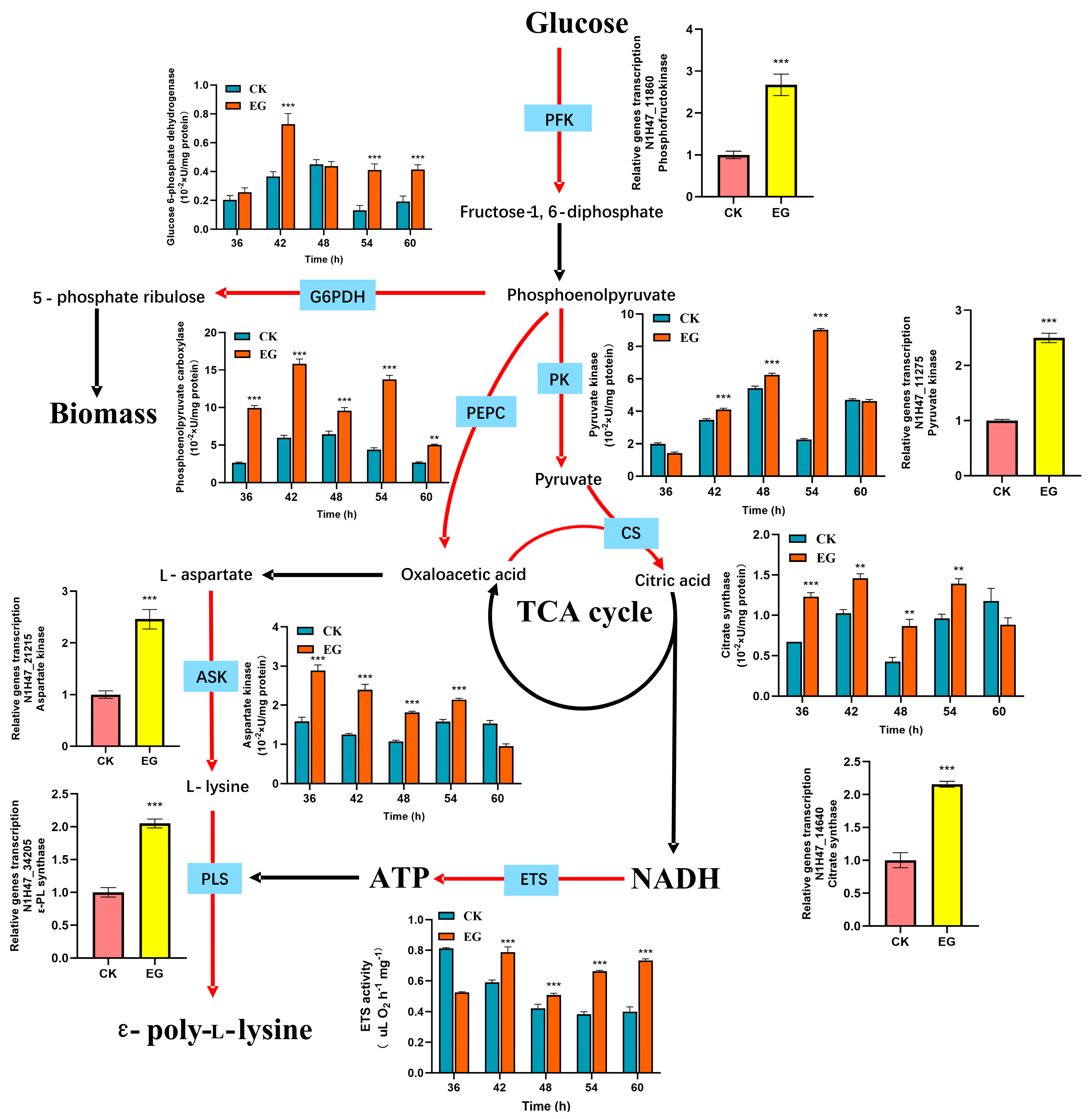
3.5. Transcriptional and Enzymatic Performance of Key Genes in ε-PL Biosynthesis After S. gilvosporeus Extract Addition
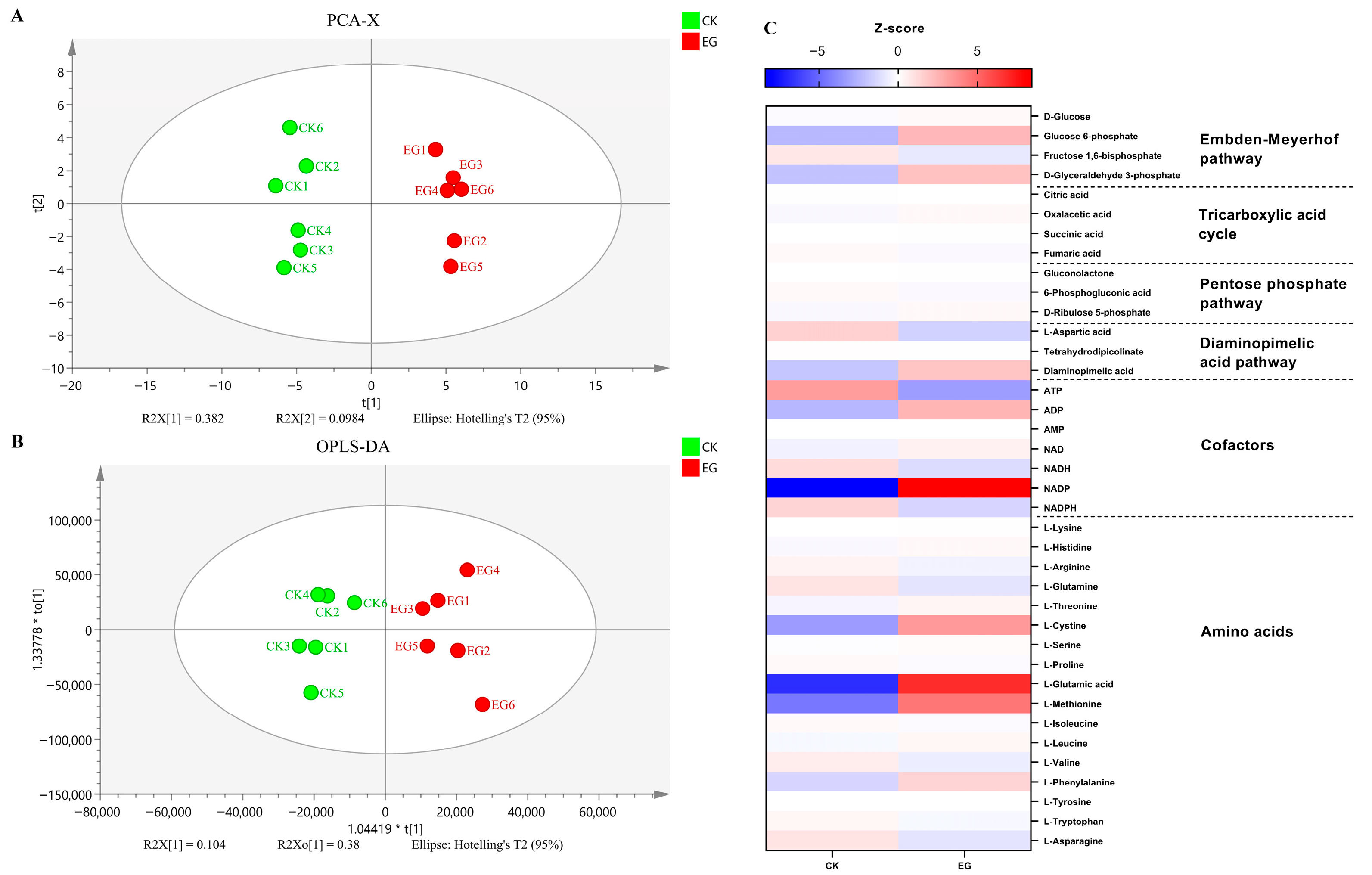
3.6. Effect of S. gilvosporeus Extracts on the Metabolic Pools of Intermediates in Pathways for ε-PL Biosynthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamanaka, K.; Maruyama, C.; Takagi, H.; Hamano, Y. ε-poly-L-lysine dispersity is controlled by a highly unusual nonribosomal peptide synthetase. Nat. Chem. Biol. 2008, 4, 766–772. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Dineshkumar, R.; Dhanarajan, G.; Sen, R.; Mishra, S. Improvement of ε-polylysine production by marine bacterium Bacillus licheniformis using artificial neural network modeling and particle swarm optimization technique. Biochem. Eng. J. 2017, 126, 8–15. [Google Scholar] [CrossRef]
- Dou, Y.; Routledge, M.N.; Gong, Y.; Godana, E.A.; Dhanasekaran, S.; Yang, Q.; Zhang, X.; Zhang, H. Efficacy of epsilon-poly-L-lysine inhibition of postharvest blue mold in apples and potential mechanisms. Postharvest Biol. Technol. 2021, 171, 111346. [Google Scholar] [CrossRef]
- Jiao, W.; Liu, X.; Chen, Q.; Du, Y.; Li, Y.; Yue, F.; Dong, X.; Fu, M. Epsilon-poly-L-lysine (ε-PL) exhibits antifungal activity in vivo and in vitro against Botrytis cinerea and the mechanism involved. Postharvest Biol. Technol. 2020, 168, 111270. [Google Scholar] [CrossRef]
- Li, F.; Wu, S.; Xu, B. Preservation of stewed beef chunks by using ε-polylysine and tea polyphenols. LWT–Food Sci. Technol. 2021, 147, 111595. [Google Scholar] [CrossRef]
- Li, W.; Lv, J.; Dong, T.; Li, X.; Li, X.; Tan, Z.; Jia, S. Effects of amino acids and overexpression of dapA gene on the production of ε-poly-L-lysine by Streptomyces diastatochromogenes strains. Curr. Microbiol. 2021, 78, 2640–2647. [Google Scholar] [CrossRef] [PubMed]
- Tsukatani, T.; Kuroda, R.; Kawaguchi, T. Screening biofilm eradication activity of ethanol extracts from foodstuffs: Potent biofilm eradication activity of glabridin, a major flavonoid from licorice (Glycyrrhiza glabra), alone and in combination with ɛ-poly-L-lysine. World J. Microbiol. Biotechnol. 2022, 38, 24. [Google Scholar] [CrossRef]
- Chen, S.; Huang, S.; Li, Y.; Zhou, C. Recent advances in epsilon-poly-L-lysine and L-lysine-based dendrimer synthesis, modification, and biomedical applications. Front. Chem. 2021, 9, 659304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Shen, B.X.; Li, X.Z.; Tong, M.Q.; Xue, P.P.; Chen, R.; Yao, Q.; Chen, B.; Xiao, J.; Xu, H.L. Tumor cellular membrane camouflaged liposomes as a non-invasive vehicle for genes: Specific targeting toward homologous gliomas and traversing the blood–brain barrier. Nanoscale 2020, 12, 15473–15494. [Google Scholar] [CrossRef]
- Li, S.; Chen, N.; Li, Y.; Li, X.; Zhan, Q.; Ban, J.; Zhao, J.; Hou, X.; Yuan, X. Metal-crosslinked ε-poly-L-lysine tissue adhesives with high adhesive performance: Inspiration from mussel adhesive environment. Int. J. Biol. Macromol. 2020, 153, 1251–1261. [Google Scholar] [CrossRef]
- Hyon, W.; Shibata, S.; Ozaki, E.; Fujimura, M.; Hyon, S.H.; Matsumura, K. Elucidating the degradation mechanism of a self-degradable dextran-based medical adhesive. Carbohydr. Polym. 2022, 278, 118949. [Google Scholar] [CrossRef]
- Shen, S.; Liu, X.; Huang, J.; Sun, Y.; Liu, B.; Song, W.; Meng, L.; Du, M.; Feng, Q. Efficacy of a mouthwash containing ε-poly-L-lysine, funme peptides, and domiphen in reducing halitosis and supragingival plaque: A randomized clinical trial. BMC Oral Health 2024, 24, 525. [Google Scholar] [CrossRef]
- Hiraki, J.; Hatakeyama, M.; Morita, H.; Izumi, Y. Improved ε-poly-L-lysine production of an S-(2-aminoethyl)-L-cysteine resistant mutant of Streptomyces albulus. Seibutsu Kogaku Kaishi 1998, 76, 487–493. [Google Scholar]
- Liu, Y.J.; Wang, K.F.; Pan, L.; Chen, X.S. Improved production of ε-poly-L-lysine in Streptomyces albulus using genome shuffling and its high-yield mechanism analysis. Front. Microbiol. 2022, 13, 923526. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Zhao, J.; Liu, Y.; Chen, X.; Tang, L.; Mao, Z. Efficiently activated ε-poly-L-lysine production by multiple antibiotic-resistance mutations and acidic pH shock optimization in Streptomyces albulus. MicrobiologyOpen 2019, 8, e00728. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tian, W.; Cheng, L.; Xu, Y.; Wang, X.; Qin, J.; Yu, B. Enhanced ε-poly-L-lysine production by the synergistic effect of ε-poly-L-lysine synthetase overexpression and citrate in Streptomyces albulus. Front. Bioeng. Biotechnol. 2020, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, H.; Wu, M.; Zhang, H.; Zhang, J.; Chen, X. Enhanced ε-poly-L-lysine production in Streptomyces albulus through multi-omics-guided metabolic engineering. Biomolecules 2024, 14, 752. [Google Scholar] [CrossRef]
- Kahar, P.; Iwata, T.; Hiraki, J.; Park, E.Y.; Okabe, M. Enhancement of ε-poly-L-lysine production by Streptomyces albulus strain 410 using pH control. J. Biosci. Bioeng. 2001, 91, 190–194. [Google Scholar] [CrossRef]
- Jia, S.R.; Wang, G.L.; Sun, Y.F.; Tan, Z.L. Improvement of ε-poly-L-lysine production by Streptomyces albulus TUST2 employing a feeding strategy. In Proceedings of the International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Beijing, China, 11–13 June 2009; pp. 1–4. [Google Scholar]
- Xu, Z.; Bo, F.; Xia, J.; Sun, Z.; Li, S.; Feng, X.; Xu, H. Effects of oxygen-vectors on the synthesis of epsilon-poly-lysine and the metabolic characterization of Streptomyces albulus PD-1. Biochem. Eng. J. 2015, 94, 58–64. [Google Scholar] [CrossRef]
- Xia, J.; Xu, Z.; Xu, H.; Feng, X.; Bo, F. The regulatory effect of citric acid on the co-production of poly(ε-lysine) and poly(L-diaminopropionic acid) in Streptomyces albulus PD-1. Bioproc. Biosyst. Eng. 2014, 37, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, Q.; Zhang, J.; Mo, S. Production of ε-poly-L-lysine by Streptomyces sp. using resin-based, in situ product removal. Biotechnol. Lett. 2011, 33, 1581–1585. [Google Scholar] [CrossRef]
- Ren, X.D.; Chen, X.S.; Zeng, X.; Wang, L.; Tang, L.; Mao, Z.G. Acidic pH shock induced overproduction of ε-poly-L-lysine in fed-batch fermentation by Streptomyces sp. M-Z18 from agro-industrial by-products. Bioproc. Biosyst. Eng. 2015, 38, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chen, X.S.; Liu, M.M.; Liu, Y.J.; Mao, Z.G. Efficient production of ε-poly-L-lysine from glucose by two-stage fermentation using pH shock strategy. Process Biochem. 2017, 63, 8–15. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.S.; Wang, K.F.; Mao, Z.G. Understanding of high ε-poly-L-lysine production by Streptomyces albulus using pH shock strategy in the level of transcriptomics. J. Ind. Microbiol. Biot. 2019, 46, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chen, X.S.; Wang, K.F.; Mao, Z.G. A temporal transcriptomic dynamics study reveals the reason of enhanced ε-poly-L-lysine production in Streptomyces albulus M-Z18 by pH shock. Process Biochem. 2019, 85, 1–11. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.S.; Wang, K.F.; Mao, Z.G. Mechanisms of response to pH shock in microbial fermentation. Bioproc. Biosyst. Eng. 2020, 43, 361–372. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, C.; Yue, C.; Su, Z.; Tai, B.; Tang, H.; Zeng, H.; Xin, B.; Zhu, M. Transcriptome and metabolome analysis revealing the improved ε-poly-L-lysine production induced by a microbial call from Botrytis cinerea. Appl. Environ. Microbiol. 2022, 88, e00952-22. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, X.S.; Gao, Y.; Ren, X.D.; Wang, L.; Mao, Z.G. Continuously high reactive oxygen species generation decreased the specific ε-poly-L-lysine formation rate in fed-batch fermentation using glucose and glycerol as a mixed carbon source. Process Biochem. 2015, 50, 1993–2003. [Google Scholar] [CrossRef]
- Zeng, X.; Miao, W.Y.; Wen, B.B.; Mao, Z.G.; Zhu, M.Z.; Chen, X.S. Transcriptional study of the enhanced ε-poly-L-lysine productivity in culture using glucose and glycerol as a mixed carbon source. Bioproc. Biosyst. Eng. 2019, 42, 555–566. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Cheng, Y.; Ni, N.; Tong, S.; Da, W.; Liu, C.; Diao, Q.; Chen, Z.; Xin, B.; et al. Transcriptional analysis revealing the improvement of ε-poly-L-lysine production from intracellular ROS elevation after Botrytis cinerea induction. J. Fungi 2024, 10, 324. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, X.S.; Ren, X.D.; Liu, Q.R.; Wang, L.; Sun, Q.X.; Tang, L.; Mao, Z.G. Insights into the role of glucose and glycerol as a mixed carbon source in the improvement of ε-poly-L-lysine productivity. Appl. Biochem. Biotech. 2014, 173, 2211–2224. [Google Scholar] [CrossRef]
- Herrera, A.; Gómez, M.; Packard, T.T.; Reglero, P.; Blanco, E.; Barberá-Cebrián, C. Potential respiration estimated by electron transport system activity in deep-sea suprabenthic crustaceans off Balearic Islands (Western Mediterranean). J. Marine Syst. 2014, 138, 104–111. [Google Scholar] [CrossRef]
- Schalk, P.H. Respiratory electron transport system (ETS) activities in zooplankton and micronekton of the Indo-Pacific region. Mar. Ecol. Prog. Ser. 1988, 44, 25–35. [Google Scholar] [CrossRef]
- Cammen, L.M.; Corwin, S.; Christensen, J. Electron transport system (ETS) activity as a measure of benthic macrofaunal metabolism. Mar. Ecol. Prog. Ser. 1990, 65, 171–182. [Google Scholar] [CrossRef]
- Yue, C.; Su, Z.; Tai, B.; Tang, H.; Da, W.; Xu, H.; Zeng, H.; Xin, B.; Zeng, X. Physiological analysis of the improved ε-poly-L-lysine production induced by reactive oxygen species. Appl. Microbiol. Biotechnol. 2023, 107, 881–896. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, Y. Metal ions driven production, characterization and bioactivity of extracellular melanin from Streptomyces sp. ZL-24. Int. J. Biol. Macromol. 2019, 123, 521–530. [Google Scholar] [CrossRef]
- Kawai, K.; Wang, G.; Okamoto, S.; Ochi, K. The rare earth, scandium, causes antibiotic overproduction in Streptomyces spp. FEMS Microbiol. Lett. 2007, 274, 311–315. [Google Scholar]
- Wang, C.; Huang, D.; Liang, S. Identification and metabolomic analysis of chemical elicitors for tacrolimus accumulation in Streptomyces tsukubaensis. Appl. Microbiol. Biot. 2018, 102, 7541–7553. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Yuan, J.; Yuan, J.; Jiang, L.; Jiang, X.; Yang, B.; Zhao, G.; Liu, B.; Huang, D. Generation of Streptomyces hygroscopicus cell factories with enhanced ascomycin production by combined elicitation and pathway-engineering strategies. Biotechnol. Bioeng. 2019, 116, 3382–3395. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Lee, B.R.; Sathiyanarayanan, G.; Song, H.S.; Kim, J.; Jeon, J.M.; Kim, J.H.; Park, S.H.; Yu, J.H.; Park, K.; et al. Medium engineering for enhanced production of undecylprodigiosin antibiotic in Streptomyces coelicolor using oil palm biomass hydrolysate as a carbon source. Bioresour. Technol. 2016, 217, 141–149. [Google Scholar] [CrossRef]
- Sekurova, O.N.; Zhang, J.; Kristiansen, K.A.; Zotchev, S.B. Activation of chloramphenicol biosynthesis in Streptomyces venezuelae ATCC 10712 by ethanol shock: Insights from the promoter fusion studies. Microb. Cell Fact. 2016, 15, 85. [Google Scholar] [CrossRef]
- Liu, X.; Tang, J.; Wang, L.; Liu, R. Mechanism of CuO nano-particles on stimulating production of actinorhodin in Streptomyces coelicolor by transcriptional analysis. Sci. Rep. 2019, 9, 11253. [Google Scholar] [CrossRef]
- Liu, X.; Tang, J.; Wang, L.; Tang, J.; Wang, L.; Giesy, J.P. Al2O3 nanoparticles promote secretion of antibiotics in Streptomyces coelicolor by regulating gene expression through the nano effect. Chemosphere 2019, 226, 687–695. [Google Scholar] [CrossRef]
- Bursy, J.; Kuhlmann, A.U.; Pittelkow, M.; Hartmann, H.; Jebbar, M.; Pierik, A.J.; Bremer, E. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl. Environ. Microbiol. 2008, 74, 7286–7295. [Google Scholar] [CrossRef]
- Bucca, G.; Pothi, R.; Hesketh, A.; Möller-Levet, C.; Hodgson, D.A.; Laing, E.E.; Stewart, G.R.; Smith, C.P. Translational control plays an important role in the adaptive heatshock response of Streptomyces coelicolor. Nucleic Acids Res. 2018, 46, 5692–5703. [Google Scholar] [CrossRef]
- Mo, S.; Kim, J.H.; Oh, C.H. Different effects of acidic pH shock on the prodiginine production in Streptomyces coelicolor M511 and SJM1 mutants. J. Microbiol. Biotechn. 2013, 23, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Sun, Y.F.; Tang, X.; He, C.N.; Shao, Y.L.; Tang, Y.; Zhou, W.W. Alkaline pH shock enhanced production of validamycin A in fermentation of Streptomyces hygroscopicus. Bioresource Technol. 2018, 249, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Onken, U.; Sattler, I.; Zeeck, A. Influence of increased dissolved oxygen concentration on the formation of secondary metabolites by manumycin-producing Streptomyces parvulus. Appl. Microbiol. Biot. 1994, 41, 309–312. [Google Scholar] [CrossRef]
- Song, Z.; Ma, Z.; Bechthold, A.; Yu, X. Effects of addition of elicitors on rimocidin biosynthesis in Streptomyces rimosus M527. Appl. Microbiol. Biot. 2020, 104, 4445–4455. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, J.; Gu, S.; Shi, Q. Influence of fungal elicitors on biosynthesis of natamycin by Streptomyces natalensis HW-2. Appl. Microbiol. Biot. 2013, 97, 5527–5534. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, L.; Zhang, Y.; Zhang, M.; Gu, S. Physicochemical and microbial responses of Streptomyces natalensis HW-2 to fungal elicitor. Appl. Microbiol. Biotechnol. 2017, 101, 6705–6712. [Google Scholar] [CrossRef]
- Shen, W.; Wang, D.; Wei, L.; Zhang, Y. Fungal elicitor-induced transcriptional changes of genes related to branched-chain amino acid metabolism in Streptomyces natalensis HW-2. Appl. Microbiol. Biotechnol. 2020, 104, 4471–4482. [Google Scholar] [CrossRef]
- Zong, G.; Fu, J.; Zhang, P.; Zhang, W.; Xu, Y.; Cao, G.; Zhang, R. Use of elicitors to enhance or activate the antibiotic production in Streptomyces. Crit. Rev. Biotechnol. 2022, 42, 1260–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.Y.; Zhang, J.H.; Rao, Z.M.; Xu, X.M.; Mao, Z.G.; Chen, X.S. Epsilon-poly-L-lysine: Recent advances in biomanufacturing and applications. Front. Bioeng. Biotechnol. 2021, 9, 748976. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.M.; Nilsson, A.Y.; Roy, I.; Harrop, A.; Dixon, K.; Keshavarz, T. Enhanced intracellular Ca2+ concentrations in Escherichia coli and Bacillus subtilis after addition of oligosaccharide elicitors. Biotechnol. Lett. 2011, 33, 985–991. [Google Scholar] [CrossRef] [PubMed]



| Strains Role | Strains | Origin |
|---|---|---|
| ε-PL-producing strain | Streptomyces albulus IFO14147 | CICC 11022 |
| Inducing strain (bacteria) | Escherichia coli | CICC 10389 |
| Bacillus subtilis | CICC 10002 | |
| Micrococcus luteus | CICC 10269 | |
| Bacillus thuringiensis | CICC 10061 | |
| Corynebacterium glutamicum | CICC 20182 | |
| Pseudomonas aeruginosa | CICC 10419 | |
| Inducing strain (fungi) | Botrytis cinerea | CGMCC 3.3790 |
| Aspergillus niger | CICC 40102 | |
| Monascus purpureus | CICC 40942 | |
| Aspergillus oryzae | CGMCC 3.7084 | |
| Penicillium chrysogenum | CGMCC 3.15725 | |
| Saccharomyces cerevisiae | CICC 1302 | |
| Inducing strain (actinomycetes) | Streptomyces gilvosporeus | ATCC 13326 |
| Streptomyces coelicolor | CGMCC 4.3587 | |
| Streptomyces lividans | CGMCC 4.7169 | |
| Streptomyces diastatochromogenes | CICC 11011 | |
| Streptomyces atratus | CICC 11048 | |
| Streptomyces aquilus | CICC 11055 |
| Gene ID | Log2(EG/CK) | p-Value | Description |
|---|---|---|---|
| N1H47_00700 | 1.48759231 | 4.55 × 10−4 | Phospholipase D |
| N1H47_29515 | 3.218336643 | 2.46 × 10−12 | Phospholipase C |
| N1H47_18160 | 4.370954313 | 1.21 × 10−15 | Phospholipase C |
| N1H47_18130 | −0.115759649 | 0.819 | Phospholipase D |
| N1H47_33410 | −0.105715175 | 0.827 | Phospholipase C |
| N1H47_33420 | −0.28392415 | 0.584 | Phospholipase C |
| N1H47_39580 | −0.037235596 | 0.954 | Phospholipase D |
| Gene ID | Log2(EG/CK) | p-Value | Description |
|---|---|---|---|
| N1H47_17980 | 1.40 | 0.001 | NADH-quinone oxidoreductase subunit D |
| N1H47_17995 | 1.36 | 0.001 | NADH-quinone oxidoreductase subunit A |
| N1H47_17970 | 1.25 | 0.003 | NADH-quinone oxidoreductase subunit F |
| N1H47_17985 | 1.20 | 0.004 | NADH-quinone oxidoreductase subunit C |
| N1H47_17990 | 1.20 | 0.004 | NADH-quinone oxidoreductase subunit B |
| N1H47_17975 | 1.15 | 0.006 | NADH-quinone oxidoreductase subunit E |
| N1H47_17960 | 1.01 | 0.015 | NADH-quinone oxidoreductase subunit H |
| N1H47_26260 | 0.24 | 0.561 | F0F1 ATP synthase subunit beta |
| N1H47_26255 | 0.28 | 0.503 | F0F1 ATP synthase subunit gamma |
| N1H47_26240 | 0.07 | 0.860 | F0F1 ATP synthase subunit B |
| N1H47_26245 | 0.01 | 0.985 | F0F1 ATP synthase subunit delta |
| N1H47_26235 | 0.11 | 0.789 | F0F1 ATP synthase subunit C |
| N1H47_26265 | 0.38 | 0.363 | F0F1 ATP synthase subunit epsilon |
| N1H47_26230 | 0.03 | 0.936 | F0F1 ATP synthase subunit A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, S.; Zhang, C.; Zhang, Z.; Zeng, H.; Xin, B.; Zhao, M.; Zhao, D.; Zeng, X.; Zhang, F. Physiological Insights into Enhanced Epsilon-Poly-l-Lysine Production Induced by Extract Supplement from Heterogeneous Streptomyces Strain. Microorganisms 2025, 13, 1868. https://doi.org/10.3390/microorganisms13081868
Tong S, Zhang C, Zhang Z, Zeng H, Xin B, Zhao M, Zhao D, Zeng X, Zhang F. Physiological Insights into Enhanced Epsilon-Poly-l-Lysine Production Induced by Extract Supplement from Heterogeneous Streptomyces Strain. Microorganisms. 2025; 13(8):1868. https://doi.org/10.3390/microorganisms13081868
Chicago/Turabian StyleTong, Siyu, Chen Zhang, Zhanyang Zhang, Huawei Zeng, Bingyue Xin, Mingtao Zhao, Deyin Zhao, Xin Zeng, and Fei Zhang. 2025. "Physiological Insights into Enhanced Epsilon-Poly-l-Lysine Production Induced by Extract Supplement from Heterogeneous Streptomyces Strain" Microorganisms 13, no. 8: 1868. https://doi.org/10.3390/microorganisms13081868
APA StyleTong, S., Zhang, C., Zhang, Z., Zeng, H., Xin, B., Zhao, M., Zhao, D., Zeng, X., & Zhang, F. (2025). Physiological Insights into Enhanced Epsilon-Poly-l-Lysine Production Induced by Extract Supplement from Heterogeneous Streptomyces Strain. Microorganisms, 13(8), 1868. https://doi.org/10.3390/microorganisms13081868






