Abstract
Staphylococcus aureus is a Gram-positive bacterium that causes significant human morbidity and mortality. The capacity of S. aureus to cause disease is primarily attributed to an array of virulence factors produced by this pathogen that collectively overcome immune defenses and promote survival in a variety of host tissues. These include an arsenal of different cytotoxins that compromise plasma membrane integrity, with the specificity of each dependent upon the host organism and cell type. S. aureus encounters a variety of peripheral blood cell types during infection that play important roles in maintaining homeostasis and defending against microbial invasion, namely erythrocytes, thrombocytes, and leukocytes. S. aureus targets each of these cell types with specific cytotoxins to successfully establish disease. This review summarizes our current understanding of the susceptibility of different human peripheral blood cell types to each of these cytotoxins.
1. Introduction
Staphylococcus aureus (S. aureus) is a common Gram-positive bacterium that can colonize and infect a wide range of hosts. Though generally a benign commensal found in the anterior nares of approximately one third of the human population [1], this bacterium can also cause a spectrum of infections that range from superficial skin abscesses to life-threatening systemic disease [2]. Our capacity to treat these infections is complicated by the widespread acquisition of antibiotic resistance mechanisms. In addition, a vaccine to prevent S. aureus disease has been unsuccessful despite extensive efforts [3,4,5]. S. aureus remains a significant source of human morbidity and mortality worldwide [6], underscoring the need to further our understanding of S. aureus virulence to advance novel therapeutic strategies that limit pathogenesis.
The ability of S. aureus to infect numerous host tissues in a variety of organisms is largely attributed to an arsenal of diverse virulence factors that include multiple cytotoxins, host immunomodulatory proteins, and adhesins. Cytotoxins target host cells in a receptor-dependent or receptor-independent manner, compromising plasma membrane integrity to cause loss of function and lysis. Many excellent reviews detail the structure and function of each of these cytotoxins [7,8,9,10,11,12,13,14]. However, there have been no reviews that have focused on the susceptibility of human peripheral blood cells to these cytotoxins. Defining these susceptibility profiles will expand our understanding of how S. aureus specifically targets host cells to advance bacterial survival and pathogenesis.
Clinical S. aureus isolates express multiple cytotoxins known to be active against a variety of human cell types (Table 1). Perhaps the most characterized of these is α-hemolysin (Hla), which has been shown to recognize ADAM10 [15] and form a heptameric β-barrel pore composed of monomeric subunits on a wide range of target cells. The bicomponent leukocidins are a group of pore-forming cytotoxins that bind different host cell receptors and form an octameric β-barrel pore composed of dimeric subunits. These include γ-hemolysin A/B (HlgAB) that recognizes CCR2, CXCR1, CXCR2, and the Duffy antigen receptor for chemokines (DARC) [16,17]; γ-hemolysin C/B (HlgCB) that recognizes C5aR1, C5aR2 [16]; leukocidin E/D (LukED) that recognizes CCR5, CXCR1, CXCR2, and DARC [17,18,19]; leukocidin G/H (LukGH [20], also known as LukAB [21]) that recognizes CD11b and the voltage-gated hydrogen channel 1 (HVCN1) [22,23]; and the Panton-Valentine leukocidin (PVL) that recognizes C5aR1, C5aR2, and CD45 [24,25]. The phenol-soluble modulin peptides (PSMs) are small amphipathic peptides thought to act like detergents and interact with host cell membranes in a receptor-independent manner [14,26,27]. These include δ-toxin (Hld), the phenol-soluble modulin-α (PSMα) peptides, and the phenol-soluble modulin-β (PSMβ) peptides. Like the PSMs, β-toxin (Hlb) degrades plasma membranes but does not generate a true pore on susceptible host cells [7,28]. Membrane damage is generated by the sphingomyelinase activity of Hlb and may be mediated through a specific host cell receptor, though none have been identified to date [7]. Notably, the majority of S. aureus strains associated with humans have the Sa3Int prophage integrated into the gene encoding Hlb that deactivates expression of this cytotoxin [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. The number and apparent redundancy of these cytotoxins has made it difficult to parse out the relative importance of each during different facets of human disease. Further, host specificity [7,8,10,13,46,47,48,49,50,51,52,53,54] has limited the utility of non-human cell lines and animal models of infection to understand the contribution of each cytotoxin to S. aureus pathogenesis in humans.

Table 1.
The prevalence and specificity of S. aureus cytotoxins against human peripheral blood cell types.
S. aureus gene expression in vivo is largely dictated by the concerted influence of 16 two-component regulatory systems that recognize environmental stimuli and alter gene expression in response [104,105,106]. Two of these, the accessory gene regulator (Agr) two-component system and the S. aureus exoprotein (Sae) two-component system, are recognized as major regulators of cytotoxin production. Agr is a quorum-sensing system that increases expression of all cytotoxins at high bacterial concentrations [107,108]. This two-component system primarily regulates gene expression in a post-transcriptional manner via the action of RNAIII [109,110] but also through transcriptional regulation of PSMs [111]. In contrast, Sae is thought to recognize neutrophil-associated stimuli and respond by upregulating the transcription of almost all the cytotoxins but not PSMs [112,113,114,115,116,117,118]. As the activity of two-component regulatory systems is largely driven by environmental cues, exclusive examination of cytotoxicity caused by purified proteins or S. aureus during in vitro growth may not accurately reflect the relative contribution of each cytotoxin to S. aureus virulence in vivo.
S. aureus encounters a variety of circulating peripheral blood cell types during infection, namely erythrocytes (red blood cells) that primarily maintain host tissue perfusion, thrombocytes (platelets) that play a key role in tissue repair, and leukocytes (neutrophils, T cells, B cells, monocytes, eosinophils, and basophils) that are a vital defense against microbial infection. The ability of S. aureus cytotoxins to compromise these cell types is essential for bacterial survival and dissemination in the healthy human host. This review summarizes our current understanding of the importance of each of these cytotoxins in causing lysis of different peripheral blood cells in humans.
2. Erythrocytes
Erythrocytes are considerably more abundant than other peripheral blood cell types, with approximately 4 to 6 million cells per microliter of adult human blood [119]. These anucleated cells are specialized to transport oxygen from the lungs to host tissue using hemoglobin and each contains approximately 270 million copies of this protein. Reversible binding of oxygen by hemoglobin is enabled by four iron-containing heme molecules. Iron is an essential metabolite for nearly all organisms and failure of bacteria to obtain iron in the host limits pathogenesis [120,121]. S. aureus acquires this important metabolite during infection following hemolysis of host erythrocytes and sequestration of released heme using the iron-regulated surface determinant (Isd) system [122,123]. Thus, a clear understanding of the specific cytotoxins produced by S. aureus that cause significant hemolysis of human erythrocytes will underscore virulence factors that are important for iron acquisition and consequent pathogenesis in humans.
Traditionally, hemolysis produced by S. aureus has been measured using sheep blood agar and rabbit erythrocyte lysis assays. However, it has been shown that the susceptibility of animal erythrocytes to S. aureus cytotoxins differs significantly from human erythrocytes [46,48,49,50] and agar is known to limit the hemolytic activity of HlgAB [48]. Recent investigations have helped to clarify which cytotoxins target human erythrocytes using assays that examine these cell types in solution. These studies have measured the hemolytic activity of purified proteins, extracellular factors produced by S. aureus deletion mutants during in vitro growth, and the effects of co-culturing these mutants with human erythrocytes.
Using purified proteins, it has been shown that strong hemolysis of human erythrocytes is caused by HlgAB and LukED, while HlgCB, Hla, PVL and LukGH produce limited or no hemolysis [17,75,77,78,124]. Interestingly, noncanonical pairing of purified HlgA with LukD was shown to exhibit more potent hemolytic activity than other bicomponent leukocidin pairs [54]. It has also been demonstrated that hemolysis of both HlgAB and LukED require erythrocyte expression of the Duffy antigen receptor for chemokines (DARC) [17], a chemokine receptor also used by Plasmodium vivax and Plasmodium knowlesi to invade erythrocytes during malarial pathogenesis [125,126]. DARC is not expressed by many individuals of African descent, reducing susceptibility to malaria as well as hemolysis caused by HlgAB and LukED. Others have also shown that purified PSMα1, PSMα2, PSMα3, PSMβ1, and Hld exhibit pronounced hemolytic activity against human erythrocytes while PSMα4 and PSMβ2 are also hemolytic but to a lesser extent [74,127]. In addition, synergy between purified PSMs and Hlb has been shown to enhance hemolysis, whereas all but one study indicate that Hlb alone is not hemolytic against human erythrocytes [72,74,102,128].
Research examining hemolysis of human erythrocytes in suspension by cytotoxins produced by S. aureus during growth has been more limited. A study using extracellular factors produced by S. aureus laboratory strain Newman during in vitro growth has shown that HlgA plays a major role in hemolysis of human erythrocytes [76]. However, this same study indicated that hemolysis caused by extracellular factors produced by the clinically relevant S. aureus strain USA300 was largely independent of HlgAB. This discrepancy can be explained by a point mutation in the histidine kinase sensor SaeS in strain Newman that induces constitutive activation of the Sae two-component system and consequent overexpression of numerous secreted virulence factors [117,129]. Others have demonstrated that transcription of hlgAB is highly upregulated following exposure of USA300 to human blood, suggesting that specific in vivo environmental stimuli are needed to trigger HlgAB mediated cytotoxicity caused by this strain. Indeed, it was shown that USA300 grown in the presence of human erythrocytes expressing DARC caused hemolysis that was dependent upon HlgA [17]. In contrast, others have used extracellular factors produced by S. aureus lacking PSMα to show that these peptides are a dominant factor causing hemolysis of human erythrocytes [60,102]. Collectively, these studies indicate that HlgAB, LukED, and PSMα peptides play major roles in causing hemolysis during S. aureus infection in humans. However, a comprehensive analysis directly comparing the contribution of these cytotoxins in causing hemolysis of human erythrocytes has not been performed.
3. Thrombocytes
Thrombocytes, or platelets, are essential for maintaining vascular integrity following host tissue injury. Platelets are anucleated cell fragments shed from megakaryocyte extensions in the bone marrow and are abundant in circulation, with approximately 150,000 to 450,000 platelets per microliter of adult human blood [119]. Platelets play a major role in primary hemostasis by adhering to the site of vascular injury, forming aggregates that plug the damaged vessel, and releasing factors that trigger thrombosis. In addition to maintaining vascular integrity, platelets also have important roles in limiting infection that include alerting the immune system to invading microbes, performing antimicrobial functions, and enhancing the adaptive immune response [130,131,132,133,134].
Clinical evidence that thrombocytopenia in patients with S. aureus bacteremia corresponds to increased mortality [58,135,136] indicates that platelets are an important defense against systemic S. aureus infection in humans. Platelets have been shown to bind and trap different bacteria including S. aureus to promote neutrophil phagocytosis [137], and antimicrobial peptides produced by human platelets kill S. aureus and induce extracellular trap formation by neutrophils [58,137,138]. However, S. aureus produces numerous virulence factors that enhance platelet aggregation and activation, including ClfA, ClfB, SpA, FnBPA, FnBPB, SSL5, and Hla [56,139,140,141,142,143,144,145], suggesting this pathogen manipulates platelet function to enhance survival in vivo [146]. In addition, S. aureus produces at least one cytotoxin, Hla, that lyses human platelets.
Investigations have demonstrated that purified Hla compromises the viability of human platelets [56,57,58] and S. aureus expression of this cytotoxin enhances platelet destruction during ex vivo infection [58]. Evidence also indicates Hla triggers apoptosis of human platelets [57]. In contrast, purified LukGH, LukED, or PVL did not influence platelet activation or viability [57]. These results are supported by the observation that resting platelets express ADAM10, the host cell receptor recognized by Hla, but not other host cell receptors recognized by S. aureus cytotoxins [147,148]. However, activated platelets can express both CD11b [149] and C5aR [150,151,152], indicating that certain in vivo conditions can induce platelet susceptibility to LukGH, HlgCB, and PVL. In addition, the impact of PSMs on human platelet integrity has not been examined. Collectively, these findings indicate that Hla plays a prominent role in causing lysis of human platelets, but further investigations are needed to conclusively determine the influence of other S. aureus cytotoxins on platelet viability.
4. Neutrophils
Neutrophils are the most common circulating leukocytes, with approximately 2500 to 8000 cells per microliter of adult human blood [119]. These granulocytes are relatively short-lived, with a half-life of around 7 hours in circulation. Neutrophils are a major defense against microbial invasion and play a particularly important role in preventing S. aureus disease [41,153]. Neutrophils in circulation are primed following detection of host- and/or microbe-derived signaling molecules released during infection and migrate to the site of distressed host tissue through a process referred to as extravasation [154]. Phagocytosis of invading microbes is primarily mediated by various Fc receptors on the neutrophil cell membrane that bind to immunoglobulins and complement components that have opsonized the microbial cell surface. Following phagocytosis, bacteria are normally terminated by an array of antimicrobial peptides and reactive oxygen species released within the phagosome.
In contrast to most bacteria, S. aureus can not only survive phagocytosis by expressing factors such as superoxide dismutase [155], catalase [156], and SPIN [157,158], but can also subsequently compromise neutrophil integrity and function via the production of cytotoxins [159,160]. When a substantial number of S. aureus is present in host tissue, such as an established S. aureus abscess, high concentrations of secreted cytotoxins can also preemptively strike incoming neutrophils to impede direct engagement with this pathogen. Neutrophils express all the receptors recognized by the receptor-dependent cytotoxins produced by S. aureus [161,162,163] and numerous investigations have shown these cells are susceptible to each. These studies have tested the susceptibility of human neutrophils to purified proteins, intoxication by extracellular factors produced by S. aureus cytotoxin deletion mutants, and cell destruction following exposure to these deletion mutants. However, the relative importance of each cytotoxin in the lysis of these important immune cells following initial inoculation, dissemination through the lymphatic and circulatory systems, and colonization of distal host tissue is not entirely understood.
Purified HlgAB, HlgCB, LukED, LukGH, PVL, and PSMα peptides have been shown to cause plasma membrane permeability of primary human neutrophils [16,18,21,22,23,24,25,27,51,52,79,80,81,83,84,86,92,93,94,95,96,97,98,99,100]. Of these, purified HlgCB, LukGH, and PVL appear to have the most potent activity against these cell types [79,81]. Interestingly, non-canonical pairing of HlgCB and PVL appears to exhibit enhanced cytotoxicity [81]. Intoxication by extracellular proteins produced by S. aureus deletion mutants has indicated that secreted LukGH, HlgAB and/or HlgCB, PVL, and the PSMα peptides all play a role in compromising neutrophil plasma membrane permeability, with in vitro culture conditions and type of S. aureus strain largely dictating the relative importance of each [20,21,27,52,82,87,94]. Alternatively, studies measuring neutrophil integrity following exposure to live S. aureus have also shown that Hla, LukGH, PVL, and PSMα peptides all contribute to destruction of these immune cells to varying degrees that appear to be dependent upon the S. aureus strain examined [20,21,22,52,59,60,61,80,82,86,87,88,103]. Taken together, these studies indicate that most cytotoxins produced by S. aureus have the capacity to destroy human neutrophils and the context of intoxication influences their potency (Figure 1).
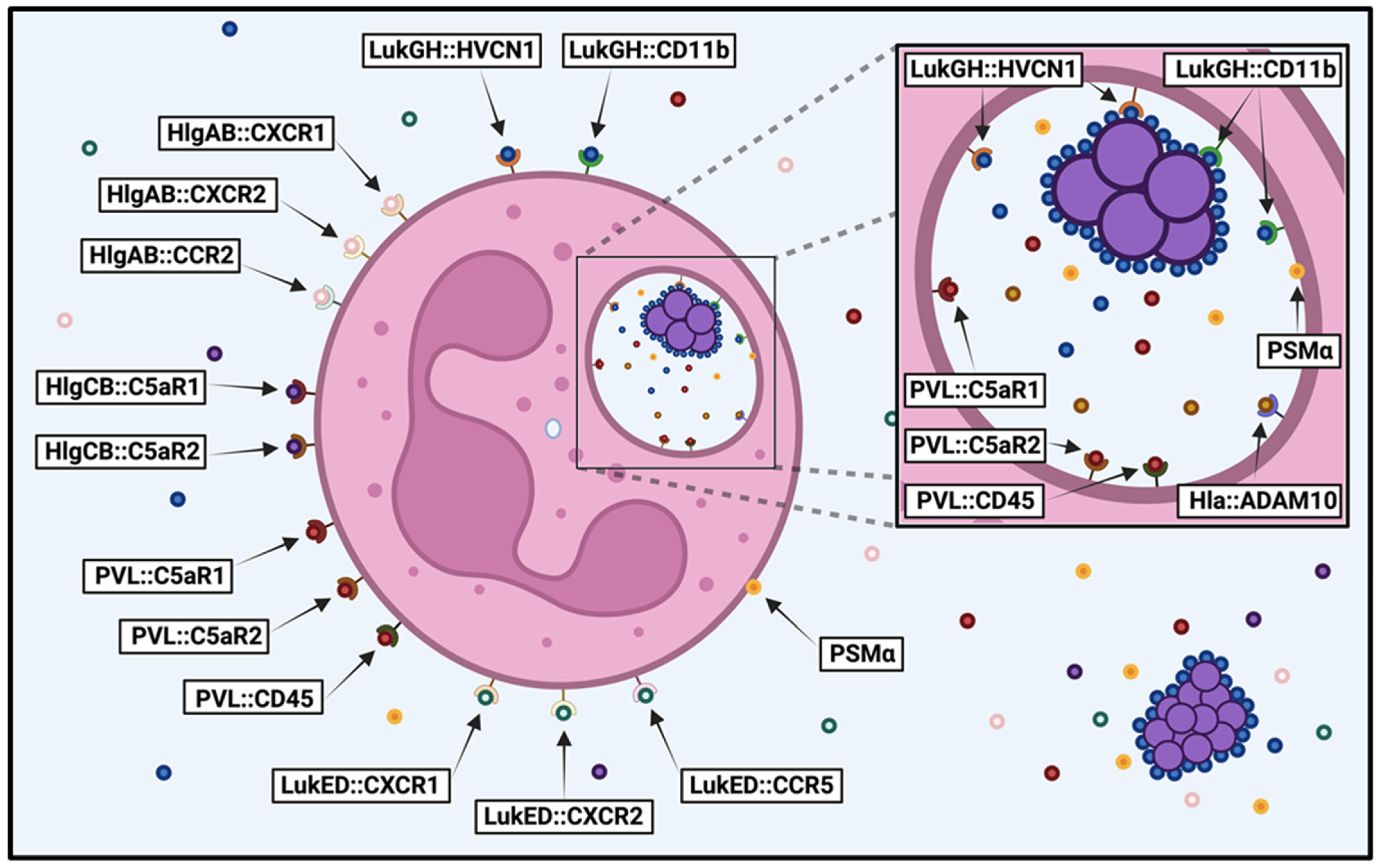
Figure 1.
Susceptibility of human neutrophils to S. aureus cytotoxins. Diagram illustrating the S. aureus cytotoxins that target human neutrophils before and after phagocytosis, detailing the host cell receptors recognized by each cytotoxin (cytotoxin::host cell receptor). Image created in BioRender.
Though numerous cytotoxins have been shown to target human neutrophils, which of these are most important for causing lysis of these important innate immune cells is not clear. To clarify the relative contribution of each cytotoxin to human neutrophil destruction, we recently used a library of deletion mutants in S. aureus strain USA300 to examine neutrophil lysis following intoxication by extracellular proteins and after phagocytosis [89]. We found that PVL played a dominant role in initially compromising neutrophil integrity caused by extracellular proteins produced by USA300 during growth in a variety of media. In contrast, LukGH was the primary cytotoxin causing neutrophil destruction immediately following phagocytosis. The different context-dependent cytotoxicity exhibited by these two apparently redundant bicomponent leukocidins can be explained by the unique location and structure of LukGH. There is only 26–40% sequence homology between LukGH and the other bicomponent leukocidins [20,21,164]. Unlike other cytotoxins, LukGH is expressed at high levels on the surface of S. aureus [20,165] and is preassembled in its activated form prior to engagement with neutrophils [166,167]. In conjunction with our results, and as previously speculated by others [7], this suggests that LukGH is poised on the surface of S. aureus to immediately engage with phagocytes upon direct contact. Thus, PVL appears to play a major role in lysing incoming neutrophils when S. aureus is established in host tissue and producing high concentrations of cytotoxins, while LukGH is the primary cause of lysis following direct contact with neutrophils and may be important for survival following initial inoculation or dissemination of this pathogen when the concentration of other cytotoxins is relatively low.
However, there are several caveats to our study. Cytotoxicity was only examined using S. aureus strain USA300 and substantial genetic diversity is found between different S. aureus isolates [168]. Notably, a single point mutation in the 5′ untranslated region of the hlgCB operon of USA300 minimizes translation of HlgC relative to HlgB [169]. Although neutrophil phagocytosis triggers significant alterations in S. aureus gene expression [159], other stimuli associated with infection of host tissue were largely lacking from this study [170]. For example, it has been shown that transcription of the hlgABC operon is immediately upregulated following exposure to human blood [82,171] and transcription of the hlgABC operon, hla, lukED, and pvl are upregulated during cutaneous infection in humans [172,173]. In addition, the activation state of neutrophils influences the expression levels of host cell receptors that are recognized by cytotoxins [162], suggesting the potency of these cytotoxins can vary in vivo under different conditions. Future studies that incorporate these factors may elucidate more profound roles for other cytotoxins in causing human neutrophil lysis.
5. T Cells
T cells are the most common circulating lymphocytes, with approximately 800 to 2500 cells per microliter of adult human blood [119], and are the primary constituents of the adaptive cellular immune response. T cells have been broadly classified as CD4+ helper T cells (Th cells) that direct the immune response through cytokine expression or CD8+ cytotoxic T cells that eliminate diseased host cells. Each T cell expresses a unique T cell receptor that induces cell activation and proliferation upon binding a specific antigen in conjunction with additional signals provided during antigen presentation. The distinct antigen-binding capacity of each T cell receptor on Th cells elicits a discrete cytokine expression profile directed towards specifically eliminating the source of that antigen [174]. Given the consistent failure to produce a S. aureus vaccine, the importance of T cells in protecting against S. aureus infection has gained appreciation in the last several decades [175,176,177,178].
S. aureus produces numerous virulence factors that specifically target T cell integrity and function, namely superantigenic proteins and cytotoxins. Superantigenic proteins bind directly to the T cell receptor on T cells and MHC Class II on antigen presenting cells to generate antigen-independent activation of approximately 20% of T cells, roughly 25,000-fold greater than a normal T cell response [179,180,181,182,183]. This produces robust inflammation and is thought to drown out an effective Th cell response that would otherwise limit pathogenesis. In addition to superantigens that manipulate T cell function, there are several S. aureus cytotoxins known to compromise T cell plasma membrane integrity.
Primary human T cells appear to be more resistant to S. aureus cytotoxicity than human neutrophils or monocytes [63,68]. T cells can express ADAM10 [184], CCR2 [185,186,187], CCR5 [185,187], CD11b [188,189], CD45 [190], CXCR1 [191,192,193], and C5aR [194,195] to varying degrees in vivo, indicating these lymphocytes are susceptible to intoxication by Hla, HlgAB, HlgCB, LukED, LukGH, and PVL. However, studies using purified proteins have shown that primary human T cells are susceptible to Hla [62,63,64,65,66,67] and LukED [19], but resistant to PVL [24,93] and other bicomponent leukocidins [79]. Others have shown that purified Hlb is cytotoxic against proliferating [28] but not resting human T cells [73]. Intoxication of human T cells with extracellular factors produced by an isogenic deletion mutant of Hla in S. aureus strain USA300 and incubation of this isolate with human T cells further demonstrated the importance of this pore-forming toxin for lysis of these lymphocytes [63]. Evidence indicates that T cells exposed to low concentrations of Hla form small pores that only allow passage of monovalent ions and trigger programmed cell death while high concentrations of Hla result in plasma membrane permeability of larger molecules and immediate cell lysis [63,66]. Other investigations using an antibody that neutralizes LukGH-mediated cytotoxicity indicated this bicomponent leukocidin does not target human T cells [80]. T cell expression of CCR2 [186,187], CCR5 [187], CD11b [188,189], CXCR1 [191,193], and C5aR [194] is influenced by activation state, suggesting that the potency of HlgAB, HlgCB, LukED, LukGH, and PVL against these cells may vary under different in vivo conditions. Though evidence indicates that Hla and LukED are the primary factors produced by S. aureus that cause human T cell lysis, a comprehensive analysis examining the relative contribution of these and other cytotoxins towards the destruction of these lymphocytes has not been performed.
6. B Cells
B cells produce immunoglobulins, or antibodies, that are the foundation of the adaptive humoral immune response. These lymphocytes are less abundant than T cells, with approximately 100 to 450 B cells per microliter of adult human blood [119]. Akin to T cells, each B cell possesses a unique B cell receptor that is composed of an immunoglobulin anchored to the cell membrane and associated with embedded accessory proteins. B cell activation, proliferation, and differentiation into antibody-producing plasma cells requires antigen binding to the B cell receptor and secondary signals generally provided by Th cells. Circulating antibodies not only bind to microbes to promote phagocytosis and inhibit adhesion but also bind to secreted microbial virulence factors to neutralize their activity. As such, B cells play an essential role in adaptive immunity against microbes.
The importance of B cells in preventing infections caused by S. aureus is not clear. Humans with deficiencies in B cell numbers or function are not more susceptible to S. aureus disease [5,196,197,198], suggesting that B cells are dispensable for preventing S. aureus pathogenesis. This might explain why the same strain of S. aureus can cause repeated infections in humans and vaccine efforts against this bacterium have universally failed. However, it is also possible that S. aureus is extremely adept at limiting an efficient adaptive humoral immune response. Indeed, S. aureus produces virulence factors that not only manipulate Th cell function to indirectly impede appropriate B cell activation, as described above, but also directly minimize the function of antibodies and B cells. These include the Staphylococcal protein A (SpA) and staphylococcal binder of immunoglobulin (Sbi) that both bind to human antibodies to inhibit their activity [199,200,201,202]. In addition, SpA is superantigenic against human B cells that express VH3-encoded immunoglobulin, causing widespread antigen-independent activation and subsequent apoptosis of these cells [203,204,205,206]. S. aureus also expresses cytotoxins that cause human B cell destruction, though these lymphocytes appear to be more resistant to S. aureus cytotoxicity than other peripheral blood cell types [63,68].
Given the repeated failures to produce an S. aureus vaccine and that B cell integrity is critical for an efficient adaptive humoral immune response, it is surprising that there have been few investigations that have examined the potency of S. aureus cytotoxins against human B cells. These lymphocytes express ADAM10 [207], CCR2 [186], and CD45 [190], indicating that they are theoretically susceptible to Hla, HlgAB, and PVL. However, currently published studies have only demonstrated that primary human B cells are susceptible to Hla. These have shown that purified Hla targets primary human B cells [62,63,65]. Further analysis of extracellular factors produced by an isogenic deletion mutant of Hla in USA300 as well as following infection of B cells with this mutant confirmed the importance of this cytotoxin for causing B cell lysis [63]. This study also demonstrated that Hla causes programmed cell death of B cells. However, it was shown that USA300 lacking Hla still causes B cell lysis [63], suggesting that other cytotoxins also contribute to destruction of these cell types. Other studies have indicated that human B cells are resistant to cytotoxicity caused by LukGH [80] or PVL [83,93] while the impact of PSMs on the viability of these lymphocytes has not been addressed. Collectively, these findings demonstrate that B cells are susceptible to Hla but also indicate that other cytotoxins produced by S. aureus target these important adaptive immune cells.
7. Monocytes
Monocytes are professional phagocytes that play important roles in both the innate and adaptive immune responses. These cells are less abundant in peripheral circulation relative to other leukocytes, with approximately 100 to 700 monocytes per microliter in adult human blood [119]. Though monocytes were traditionally thought to primarily differentiate into macrophages and dendritic cells following recruitment to host tissue, current evidence suggests that these antigen-presenting cells can also remain undifferentiated and exhibit both pro-inflammatory and anti-inflammatory characteristics [208]. Monocytes play an important role in limiting infection by recognizing microbial invaders and coordinating an appropriate immune response through cytokine signaling and antigen presentation.
Though monocytes likely play an important role in the immune response against S. aureus and numbers of these cells significantly increase during systemic S. aureus disease [209], evidence suggests that this pathogen can undermine the function of these leukocytes to further bacterial survival and dissemination [210,211,212]. Studies have shown that S. aureus can survive phagocytosis by primary human monocytes and monocyte-derived macrophages and it has been proposed that this furthers dissemination in the host [211,212,213]. Others have indicated that the cytokine response to live S. aureus during infection of human blood is suppressed relative to heat-killed S. aureus [210], S. aureus lacking the Sae two-component system [210], or S. aureus lacking Hla [68]. The mechanisms by which S. aureus manipulates monocyte function are not entirely clear, but cytotoxins have been implicated in this process [68,210,211].
Relative to other human leukocytes, evidence suggests monocytes are highly susceptible to cytotoxicity caused by S. aureus [63,68]. As with neutrophils, these cell types express all the receptors recognized by the receptor-dependent cytotoxins [214,215,216,217,218]. Indeed, studies have shown that purified Hla, Hlb, HlgAB, HlgCB, LukED, LukGH, and PVL can all cause lysis of primary human monocytes [16,19,23,24,62,63,73,75,83,90,93,101]. Infection assays using isogenic deletion mutants of LukGH and Hla confirmed the importance of these cytotoxins for causing monocyte lysis [63,68,90]. Though PSMs have not been demonstrated to lyse primary human monocytes, it has been shown that PSMα peptides and Hld can cause lysis of human monocyte-derived dendritic cells [219]. In addition, assays examining the cytotoxicity of USA300 deletion mutants of Sae that are defective in producing all cytotoxins except PSMs demonstrated these mutants could still cause lysis of primary human monocytes, though significantly less so than wild-type USA300 [63,65]. Collectively, these findings indicate that the majority of cytotoxins produced by S. aureus are capable of lysing human monocytes. However, which of these cytotoxins is most important for causing lysis of human monocytes during different contexts of intoxication is not clear.
8. Eosinophils and Basophils
Eosinophils and basophils are the least abundant circulating leukocytes, with approximately 50 to 500 eosinophils and 25 to 100 basophils per microliter of adult human blood [119]. These granulocytes are known to play important roles in protection against parasitic infections and in driving allergic responses. In addition, a correlation has been observed between S. aureus colonization and atopic diseases that include atopic dermatitis, allergic asthma, and allergic rhinitis [220,221,222,223,224,225,226]. Consequently, studies have examined the influence of S. aureus on eosinophil and basophil function with a primary focus on superantigenic proteins produced by this pathogen [227,228,229,230,231,232,233,234,235,236,237,238]. However, the S. aureus cytotoxins that target these cell types in humans are almost entirely unknown; a single study has shown that Hla lyses primary human eosinophils [69], while there are no published reports on the susceptibility of human basophils to cytotoxins produced by S. aureus. Eosinophils have been shown to express CCR2 [239], CD11b [228,240], CD45 [241], CXCR2 [242], and C5aR [243,244] indicating that these cells are susceptible to HlgAB, HlgCB, LukED, LukGH, and PVL. Basophils are also theoretically susceptible to these bicomponent leukocidins, as they have been shown to express CCR2 [245,246,247], CCR5 [245,246], CD11b [245,248,249,250], CD45 [251], CXCR1 [245,246], CXCR2 [246], and C5aR [243,245,250]. Given that eosinophils and basophils play important roles driving atopic diseases that are associated with S. aureus colonization, future studies that define the susceptibility or resistance of these cell types to S. aureus cytotoxins may provide insight into the complex manifestation of these diseases.
9. Conclusions and Future Studies
Our knowledge of S. aureus cytotoxins has made significant advances in the last two decades. These include the characterization of the previously undescribed LukGH, PSMα peptides, and PSMβ peptides, as well as the identification of the host cell receptors recognized by S. aureus cytotoxins. However, our understanding of the susceptibility of human peripheral blood cells to each cytotoxin produced by S. aureus remains incomplete. Though the cytotoxins that target human neutrophils have been largely defined, the relative susceptibility profiles of other human peripheral blood cell types to these cytotoxins are incomplete or lacking entirely. In addition, how the activation state of host cells influences susceptibility to receptor-dependent cytotoxins is not clear and may be important, as activated host cells alter the expression of cell surface receptors. Though in vitro and ex vivo studies have measured host cell lysis using live S. aureus, in vivo stimuli that promote virulence factor expression are absent under these conditions. Moreover, the high genetic variability of S. aureus dictates the need for studies comparing cytotoxin production and subsequent host cell lysis caused by different clinical S. aureus lineages. Thus, future investigations are needed to fully understand the contribution of each cytotoxin to the lysis of different human peripheral blood cells in vivo by S. aureus. Clearly defining the susceptibility profiles of human peripheral blood cells to S. aureus cytotoxins will not only advance our understanding of how this bacterium causes a diverse range of human ailments but also lay the foundation for novel therapeutic strategies that limit disease.
Author Contributions
Conceptualization, T.K.N.; resources, T.K.N. and J.M.V.; writing—original draft preparation, T.K.N.; writing—review and editing, T.K.N. and J.M.V.; funding acquisition, T.K.N. and J.M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Montana INBRE, grant number P20GM103474, and the National Institute of Health, grant number R01AI149491.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank Mark T. Quinn and Timothy R. Borgogna for their help in proofreading the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kuehnert, M.J.; Kruszon-Moran, D.; Hill, H.A.; McQuillan, G.; McAllister, S.K.; Fosheim, G.; McDougal, L.K.; Chaitram, J.; Jensen, B.; Fridkin, S.K.; et al. Prevalence of Staphylococcus aureus Nasal Colonization in the United States, 2001–2002. J. Infect. Dis. 2006, 193, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus aureus Vaccine Research and Development: The Past, Present and Future, Including Novel Therapeutic Strategies. Front. Immunol. 2021, 12, 705360. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Fowler, V.G., Jr.; Shukla, S.K.; Rose, W.E.; Proctor, R.A. Development of a Vaccine against Staphylococcus aureus Invasive Infections: Evidence Based on Human Immunity, Genetics and Bacterial Evasion Mechanisms. FEMS Microbiol. Rev. 2020, 44, 123–153. [Google Scholar] [CrossRef]
- Proctor, R.A. Immunity to Staphylococcus aureus: Implications for Vaccine Development. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Aguilar, G.R.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Weaver, N.D.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Vandenesch, F.; Lina, G.; Henry, T. Staphylococcus aureus Hemolysins, Bi-Component Leukocidins, and Cytolytic Peptides: A Redundant Arsenal of Membrane-Damaging Virulence Factors? Front. Cell. Infect. Microbiol. 2012, 2, 12. [Google Scholar] [CrossRef]
- Spaan, A.N.; Van Strijp, J.A.G.; Torres, V.J. Leukocidins: Staphylococcal Bi-Component Pore-Forming Toxins Find Their Receptors. Nat. Rev. Microbiol. 2017, 15, 435–447. [Google Scholar] [CrossRef]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.P.; Molle, V. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Tam, K.; Torres, V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus aureus. Virulence 2021, 31, 547–569. [Google Scholar] [CrossRef]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef]
- Alonzo, F.; Torres, V.J. The Bicomponent Pore-Forming Leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 2014, 78, 199–230. [Google Scholar] [CrossRef] [PubMed]
- Peschel, A.; Otto, M. Phenol-Soluble Modulins and Staphylococcal Infection. Nat. Rev. Microbiol. 2013, 11, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Wilke, G.A.; Wardenburg, J.B. Role of a Disintegrin and Metalloprotease 10 in Staphylococcus aureus α-Hemolysin–Mediated Cellular Injury. Proc. Natl. Acad. Sci. USA 2010, 107, 13473–13478. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Vrieling, M.; Wallet, P.; Badiou, C.; Reyes-Robles, T.; Ohneck, E.A.; Benito, Y.; De Haas, C.J.C.; Day, C.J.; Jennings, M.P.; et al. The Staphylococcal Toxins γ-Haemolysin AB and CB Differentially Target Phagocytes by Employing Specific Chemokine Receptors. Nat. Commun. 2014, 5, 5438. [Google Scholar] [CrossRef]
- Spaan, A.N.; Reyes-Robles, T.; Badiou, C.; Cochet, S.; Boguslawski, K.M.; Yoong, P.; Day, C.J.; de Haas, C.J.C.; van Kessel, K.P.M.; Vandenesch, F.; et al. Staphylococcus aureus Targets the Duffy Antigen Receptor for Chemokines (DARC) to Lyse Erythrocytes. Cell Host Microbe 2015, 18, 363–370. [Google Scholar] [CrossRef]
- Reyes-Robles, T.; Alonzo, F.; Kozhaya, L.; Lacy, D.B.; Unutmaz, D.; Torres, V.J. Staphylococcus aureus Leukotoxin ED Targets the Chemokine Receptors CXCR1 and CXCR2 to Kill Leukocytes and Promote Infection. Cell Host Microbe 2013, 14, 453–459. [Google Scholar] [CrossRef]
- Alonzo, F.; Kozhaya, L.; Rawlings, S.A.; Reyes-Robles, T.; DuMont, A.L.; Myszka, D.G.; Landau, N.R.; Unutmaz, D.; Torres, V.J. CCR5 Is a Receptor for Staphylococcus aureus Leukotoxin ED. Nature 2013, 493, 51–55. [Google Scholar] [CrossRef]
- Ventura, C.L.; Malachowa, N.; Hammer, C.H.; Nardone, G.A.; Robinson, M.A.; Kobayashi, S.D.; DeLeo, F.R. Identification of a Novel Staphylococcus aureus Two-Component Leukotoxin Using Cell Surface Proteomics. PLoS ONE 2010, 5, e11634. [Google Scholar] [CrossRef]
- Dumont, A.L.; Nygaard, T.K.; Watkins, R.L.; Smith, A.; Kozhaya, L.; Kreiswirth, B.N.; Shopsin, B.; Unutmaz, D.; Voyich, J.M.; Torres, V.J. Characterization of a New Cytotoxin That Contributes to Staphylococcus aureus Pathogenesis. Mol. Microbiol. 2011, 79, 814–825. [Google Scholar] [CrossRef] [PubMed]
- DuMont, A.L.; Yoong, P.; Day, C.J.; Alonzo, F.; McDonald, W.H.; Jennings, M.P.; Torres, V.J. Staphylococcus aureus LukAB Cytotoxin Kills Human Neutrophils by Targeting the CD11b Subunit of the Integrin Mac-1. Proc. Natl. Acad. Sci. USA 2013, 110, 10794–10799. [Google Scholar] [CrossRef] [PubMed]
- Perelman, S.S.; James, D.B.A.; Boguslawski, K.M.; Nelson, C.W.; Ilmain, J.K.; Zwack, E.E.; Prescott, R.A.; Mohamed, A.; Tam, K.; Chan, R.; et al. Genetic Variation of Staphylococcal LukAB Toxin Determines Receptor Tropism. Nat. Microbiol. 2021, 6, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.M.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.C.; van Kessel, K.P.M.; Vandenesch, F.; et al. The Staphylococcal Toxin Panton-Valentine Leukocidin Targets Human C5a Receptors. Cell Host Microbe 2013, 13, 584–594. [Google Scholar] [CrossRef]
- Tromp, A.T.; Van Gent, M.; Abrial, P.; Martin, A.; Jansen, J.P.; De Haas, C.J.C.; Van Kessel, K.P.M.; Bardoel, B.W.; Kruse, E.; Bourdonnay, E.; et al. Human CD45 Is an F-Component-Specific Receptor for the Staphylococcal Toxin Panton-Valentine Leukocidin. Nat. Microbiol. 2018, 3, 708–717. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Kretschmer, D.; Queck, S.Y.; Joo, H.-S.; Wang, R.; Duong, A.C.; Nguyen, T.H.; Bach, T.-H.L.; Porter, A.R.; DeLeo, F.R.; et al. Insight into Structure-Function Relationship in Phenol-Soluble Modulins Using an Alanine Screen of the Phenol-Soluble Modulin (PSM) A3 Peptide. FASEB J. 2014, 28, 153–161. [Google Scholar] [CrossRef]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.-H.L.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of Novel Cytolytic Peptides as Key Virulence Determinants for Community-Associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef]
- Huseby, M.; Shi, K.; Brown, C.K.; Digre, J.; Mengistu, F.; Seo, K.S.; Bohach, G.A.; Schlievert, P.M.; Ohlendorf, D.H.; Earhart, C.A. Structure and Biological Activities of Beta Toxin from Staphylococcus aureus. J. Bacteriol. 2007, 189, 8719–8726. [Google Scholar] [CrossRef]
- Rohmer, C.; Wolz, C. The Role of Hlb-Converting Bacteriophages in Staphylococcus aureus Host Adaption. Microb. Physiol. 2021, 31, 109–122. [Google Scholar] [CrossRef]
- Spoor, L.E.; McAdam, P.R.; Weinert, L.A.; Rambaut, A.; Hasman, H.; Aarestrup, F.M.; Kearns, A.M.; Larsen, A.R.; Skov, R.L.; Ross Fitzgerald, J. Livestock Origin for a Human Pandemic Clone of Community-Associated Methicillin-Resistant Staphylococcus aureus. mBio 2013, 4, e00356-13. [Google Scholar] [CrossRef]
- Resch, G.; François, P.; Morisset, D.; Stojanov, M.; Bonetti, E.J.; Schrenzel, J.; Sakwinska, O.; Moreillon, P. Human-to-Bovine Jump of Staphylococcus aureus CC8 Is Associated with the Loss of a β-Hemolysin Converting Prophage and the Acquisition of a New Staphylococcal Cassette Chromosome. PLoS ONE 2013, 8, e58187. [Google Scholar] [CrossRef]
- Chaguza, C.; Smith, J.T.; Bruce, S.A.; Gibson, R.; Martin, I.W.; Andam, C.P. Prophage-Encoded Immune Evasion Factors Are Critical for Staphylococcus aureus Host Infection, Switching, and Adaptation. Cell Genomics 2022, 2, 100194. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, C.; Dobritz, R.; Tuncbilek-Dere, D.; Lehmann, E.; Gerlach, D.; George, S.E.; Bae, T.; Nieselt, K.; Wolz, C. Influence of Staphylococcus aureus Strain Background on Sa3int Phage Life Cycle Switches. Viruses 2022, 14, 2471. [Google Scholar] [CrossRef] [PubMed]
- Verkaik, N.J.; Benard, M.; Boelens, H.A.; De Vogel, C.P.; Nouwen, J.L.; Verbrugh, H.A.; Melles, D.C.; Van Belkum, A.; Van Wamel, W.J.B. Immune Evasion Cluster-Positive Bacteriophages Are Highly Prevalent among Human Staphylococcus aureus Strains, but They Are Not Essential in the First Stages of Nasal Colonization. Clin. Microbiol. Infect. 2011, 17, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, M.; Murray, G.G.R.; Harrison, E.M.; Holmes, M.A.; Weinert, L.A. The Evolutionary Genomics of Host Specificity in Staphylococcus aureus. Trends Microbiol. 2020, 28, 465–477. [Google Scholar] [CrossRef]
- Bouiller, K.; Bertrand, X.; Hocquet, D.; Chirouze, C. Human Infection of Methicillin-susceptible Staphylococcus aureus Cc398: A Review. Microorganisms 2020, 8, 1737. [Google Scholar] [CrossRef]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host Adaptation and Emergence of Methicillin Resistance in Livestock. mBio 2012, 3, 10-1128. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Witney, A.A.; Gould, K.A.; Moodley, A.; Guardabassi, L.; Voss, A.; Denis, O.; Broens, E.M.; Hinds, J.; Lindsay, J.A. The Distribution of Mobile Genetic Elements (MGEs) in MRSA CC398 Is Associated with Both Host and Country. Genome Biol. Evol. 2011, 3, 1164–1174. [Google Scholar] [CrossRef]
- Cuny, C.; Abdelbary, M.; Layer, F.; Werner, G.; Witte, W. Prevalence of the Immune Evasion Gene Cluster in Staphylococcus aureus CC398. Vet. Microbiol. 2015, 177, 219–223. [Google Scholar] [CrossRef]
- Xia, G.; Wolz, C. Phages of Staphylococcus aureus and Their Impact on Host Evolution. Infect. Genet. Evol. 2014, 21, 593–601. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus Host Interactions and Adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Pantůček, R.; Doškař, J.; Růžičková, V.; Kašpárek, P.; Oráčová, E.; Kvardová, V.; Rosypal, S. Identification of Bacteriophage Types and Their Carriage in Staphylococcus aureus. Arch. Virol. 2004, 149, 1689–1703. [Google Scholar] [CrossRef] [PubMed]
- Goerke, C.; Pantucek, R.; Holtfreter, S.; Schulte, B.; Zink, M.; Grumann, D.; Bröker, B.M.; Doskar, J.; Wolz, C. Diversity of Prophages in Dominant Staphylococcus aureus Clonal Lineages. J. Bacteriol. 2009, 191, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Goerke, C.; Wirtz, C.; Flückiger, U.; Wolz, C. Extensive Phage Dynamics in Staphylococcus aureus Contributes to Adaptation to the Human Host during Infection. Mol. Microbiol. 2006, 61, 1673–1685. [Google Scholar] [CrossRef]
- Van Wamel, W.J.B.; Rooijakkers, S.H.M.; Ruyken, M.; Van Kessel, K.P.M.; Van Strijp, J.A.G. The Innate Immune Modulators Staphylococcal Complement Inhibitor and Chemotaxis Inhibitory Protein of Staphylococcus aureus Are Located on β-Hemolysin-Converting Bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef]
- Freer, J.H.; Arbuthnottt, J.P. Toxins of Staphylococcus aureus. Pharmacol. Ther. 1982, 19, 55–106. [Google Scholar] [CrossRef]
- Mrochen, D.M.; Fernandes de Oliveira, L.M.; Raafat, D.; Holtfreter, S. Staphylococcus aureus Host Tropism and Its Implications for Murine Infection Models. Int. J. Mol. Sci. 2020, 21, 7061. [Google Scholar] [CrossRef]
- Wiseman, G.M. The Hemolysins of Staphylococcus aureus. Bacteriol. Rev. 1975, 39, 317–344. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Avigad, L.S.; Kim, K.S. Staphylococcal Sphingomyelinase (β-Hemolysin). Ann. N. Y. Acad. Sci. 1974, 236, 292–306. [Google Scholar] [CrossRef]
- Elek, S.D.; Levy, E. Distribution of Hæmolysins in Pathogenic and Non-pathogenic Staphylococci. J. Pathol. 1950, 62, 541–554. [Google Scholar] [CrossRef]
- Malachowa, N.; Kobayashi, S.D.; Braughton, K.R.; Whitney, A.R.; Parnell, M.J.; Gardner, D.J.; Deleo, F.R. Staphylococcus aureus Leukotoxin GH Promotes Inflammation. J. Infect. Dis. 2012, 206, 1185–1193. [Google Scholar] [CrossRef]
- Löffler, B.; Hussain, M.; Grundmeier, M.; Brück, M.; Holzinger, D.; Varga, G.; Roth, J.; Kahl, B.C.; Proctor, R.A.; Peters, G. Staphylococcus aureus Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils. PLoS Pathog. 2010, 6, e1000715. [Google Scholar] [CrossRef] [PubMed]
- Trstenjak, N.; Milić, D.; Graewert, M.A.; Rouha, H.; Svergun, D.; Djinović-Carugo, K.; Nagy, E.; Badarau, A. Molecular Mechanism of Leukocidin GH-Integrin CD11b/CD18 Recognition and Species Specificity. Proc. Natl. Acad. Sci. USA 2020, 117, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.P.; Kort, T.; Shulenin, S.; Kanipakala, T.; Ganjbaksh, N.; Roghmann, M.-C.; Holtsberg, F.W.; Aman, M.J. Antibodies to S. Aureus LukS-PV Attenuated Subunit Vaccine Neutralize a Broad Spectrum of Canonical and Non-Canonical Bicomponent Leukotoxin Pairs. PLoS ONE 2015, 10, e0137874. [Google Scholar] [CrossRef]
- Bennett, M.R.; Thomsen, I.P. Epidemiological and Clinical Evidence for the Role of Toxins in S. aureus Human Disease. Toxins 2020, 12, 408. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Schwertz, H.; Weyrich, A.S.; Franks, Z.G.; Lindemann, S.; Otto, M.; Behr, H.; Loppnow, H.; Schlitt, A.; Russ, M.; et al. Staphylococcus aureus α-Toxin Triggers the Synthesis of B-Cell Lymphoma 3 by Human Platelets. Toxins 2011, 3, 120–133. [Google Scholar] [CrossRef]
- Jahn, K.; Handtke, S.; Palankar, R.; Kohler, T.P.; Wesche, J.; Wolff, M.; Bayer, J.; Wolz, C.; Greinacher, A.; Hammerschmidt, S. α-Hemolysin of Staphylococcus aureus Impairs Thrombus Formation. J. Thromb. Haemost. 2022, 20, 1464–1475. [Google Scholar] [CrossRef]
- Sun, J.; Uchiyama, S.; Olson, J.; Morodomi, Y.; Cornax, I.; Ando, N.; Kohno, Y.; Kyaw, M.M.T.; Aguilar, B.; Haste, N.M.; et al. Repurposed Drugs Block Toxin-Driven Platelet Clearance by the Hepatic Ashwell-Morell Receptor to Clear Staphylococcus aureus Bacteremia. Sci. Transl. Med. 2021, 13, eabd6737. [Google Scholar] [CrossRef]
- Rungelrath, V.; Porter, A.R.; Malachowa, N.; Freedman, B.A.; Leung, J.M.; Voyich, J.M.; Otto, M.; Kobayashi, S.D.; DeLeo, F.R. Further Insight into the Mechanism of Human PMN Lysis Following Phagocytosis of Staphylococcus aureus. Microbiol. Spectr. 2021, 9, e0088821. [Google Scholar] [CrossRef]
- Li, M.; Dai, Y.; Zhu, Y.; Fu, C.-L.; Tan, V.Y.; Wang, Y.; Wang, X.; Hong, X.; Liu, Q.; Li, T.; et al. Virulence Determinants Associated with the Asian Community-Associated Methicillin-Resistant Staphylococcus aureus Lineage ST59. Sci. Rep. 2016, 6, 27899. [Google Scholar] [CrossRef]
- Pang, Y.Y.; Schwartz, J.; Thoendel, M.; Ackermann, L.W.; Horswill, A.R.; Nauseef, W.M. Agr-Dependent Interactions of Staphylococcus aureus USA300 with Human Polymorphonuclear Neutrophils. J. Innate Immun. 2010, 2, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Tsuiji, M.; Shiohara, K.; Takei, Y.; Shinohara, Y.; Nemoto, S.; Yamaguchi, S.; Kanto, M.; Itoh, S.; Oku, T.; Miyashita, M.; et al. Selective Cytotoxicity of Staphylococcal α-Hemolysin (α-Toxin) against Human Leukocyte Populations. Biol. Pharm. Bull. 2019, 42, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, T.K.; Pallister, K.B.; DuMont, A.L.; DeWald, M.; Watkins, R.L.; Pallister, E.Q.; Malone, C.; Griffith, S.; Horswill, A.R.; Torres, V.J.; et al. Alpha-Toxin Induces Programmed Cell Death of Human T Cells, B Cells, and Monocytes during USA300 Infection. PLoS ONE 2012, 7, e36532. [Google Scholar] [CrossRef] [PubMed]
- Valeva, A.; Walev, I.; Pinkernell, M.; Walker, B.; Bayley, H.; Palmer, M.; Bhakdi, S. Transmembrane β-Barrel of Staphylococcal α-Toxin Forms in Sensitive but Not in Resistant Cells. Proc. Natl. Acad. Sci. USA 1997, 94, 11607–11611. [Google Scholar] [CrossRef]
- Kleinhenz, M.; Li, Z.; Chidella, U.; Picard, W.; Wolfe, A.; Popelka, J.; Alexander, R.; Montgomery, C.P. Toxin-Neutralizing Abs Are Associated with Improved T Cell Function Following Recovery from Staphylococcus aureus Infection. JCI Insight 2024, 9, e173526. [Google Scholar] [CrossRef]
- Jonas, D.; Walev, I.; Berger, T.; Liebetrau, M.; Palmer, M.; Bhakdi, S. Novel Path to Apoptosis: Small Transmembrane Pores Created by Staphylococcal Alpha-Toxin in T Lymphocytes Evoke Internucleosomal DNA Degradation. Infect. Immun. 1994, 62, 1304–1312. [Google Scholar] [CrossRef]
- Blümel, E.; Munir Ahmad, S.; Nastasi, C.; Willerslev-Olsen, A.; Gluud, M.; Fredholm, S.; Hu, T.; Surewaard, B.G.J.; Lindahl, L.M.; Fogh, H.; et al. Staphylococcus aureus Alpha-Toxin Inhibits CD8+ T Cell-Mediated Killing of Cancer Cells in Cutaneous T-Cell Lymphoma. Oncoimmunology 2020, 9, 1751561. [Google Scholar] [CrossRef]
- Nygaard, T.K.; Pallister, K.B.; Zurek, O.W.; Voyich, J.M. The Impact of α-Toxin on Host Cell Plasma Membrane Permeability and Cytokine Expression during Human Blood Infection by CA-MRSA USA300. J. Leukoc. Biol. 2013, 94, 971–979. [Google Scholar] [CrossRef]
- Prince, L.R.; Graham, K.J.; Connolly, J.; Anwar, S.; Ridley, R.; Sabroe, I.; Foster, S.J.; Whyte, M.K.B. Staphylococcus aureus Induces Eosinophil Cell Death Mediated by α-Hemolysin. PLoS ONE 2012, 7, e31506. [Google Scholar] [CrossRef]
- Monecke, S.; Kuhnert, P.; Hotzel, H.; Slickers, P.; Ehricht, R. Microarray Based Study on Virulence-Associated Genes and Resistance Determinants of Staphylococcus aureus Isolates from Cattle. Vet. Microbiol. 2007, 125, 128–140. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; Larsen, H.D.; Eriksen, N.H.R.; Elsberg, C.S.; Jensen, N.E. Frequency of α- and β-Haemolysin in Staphylococcus aureus of Bovine and Human Origin. Apmis 1999, 107, 425–430. [Google Scholar] [CrossRef]
- Marshall, M.; Bohach, G.; Boehm, D. Characterization of Staphylococcus aureus Beta-Toxin Induced Leukotoxicity. J. Nat. Toxins 2000, 9, 125–138. [Google Scholar] [PubMed]
- Walev, I.; Weller, U.; Strauch, S.; Foster, T.; Bhakdi, S. Selective Killing of Human Monocytes and Cytokine Release Provoked by Sphingomyelinase (Beta-Toxin) of Staphylococcus aureus. Infect. Immun. 1996, 64, 2974–2979. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Duong, A.C.; Otto, M. Direct and Synergistic Hemolysis Caused by Staphylococcus Phenol-Soluble Modulins: Implications for Diagnosis and Pathogenesis. Microbes Infect. 2012, 14, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Ferreras, M.; Höper, F.; Dalla Serra, M.; Colin, D.A.; Prévost, G.; Menestrina, G. The Interaction of Staphylococcus aureus Bi-Component Gamma-Hemolysins and Leucocidins with Cells and Lipid Membranes. Biochim. Biophys. Acta 1998, 1414, 108–126. [Google Scholar] [CrossRef]
- Venkatasubramaniam, A.; Kanipakala, T.; Ganjbaksh, N.; Mehr, R.; Mukherjee, I.; Krishnan, S.; Bae, T.; Aman, M.J.; Adhikari, R.P. A Critical Role for HlgA in Staphylococcus aureus Pathogenesis Revealed by A Switch in the SaeRS Two-Component Regulatory System. Toxins 2018, 10, 377. [Google Scholar] [CrossRef]
- Kaneko, J.; Ozawa, T.; Tomita, T.; Kamio, Y. Sequential Binding of Staphylococcal Gamma-Hemolysin to Human Erythrocytes and Complex Formation of the Hemolysin on the Cell Surface. Biosci. Biotechnol. Biochem. 1997, 61, 846–851. [Google Scholar] [CrossRef]
- Peng, Z.; Takeshita, M.; Shibata, N.; Tada, H.; Tanaka, Y.; Kaneko, J. Rim Domain Loops of Staphylococcal β-Pore Forming Bi-Component Toxin S-Components Recognize Target Human Erythrocytes in a Coordinated Manner. J. Biochem. 2018, 164, 93–102. [Google Scholar] [CrossRef]
- Berends, E.T.M.; Zheng, X.; Zwack, E.E.; Ménager, M.M.; Cammer, M.; Shopsin, B.; Torres, V.J. Staphylococcus aureus Impairs the Function of and Kills Human Dendritic Cells via the LukAB Toxin. mBio 2019, 10, e01918-18. [Google Scholar] [CrossRef]
- Rouha, H.; Weber, S.; Janesch, P.; Maierhofer, B.; Gross, K.; Dolezilkova, I.; Mirkina, I.; Visram, Z.C.; Malafa, S.; Stulik, L.; et al. Disarming Staphylococcus aureus from Destroying Human Cells by Simultaneously Neutralizing Six Cytotoxins with Two Human Monoclonal Antibodies. Virulence 2018, 9, 231–247. [Google Scholar] [CrossRef]
- Yanai, M.; Rocha, M.A.; Matolek, A.Z.; Chintalacharuvu, A.; Taira, Y.; Chintalacharuvu, K.; Beenhouwer, D.O. Separately or Combined, LukG/LukH Is Functionally Unique Compared to Other Staphylococcal Bicomponent Leukotoxins. PLoS ONE 2014, 9, e89308. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; Whitney, A.R.; Kobayashi, S.D.; Sturdevant, D.E.; Kennedy, A.D.; Braughton, K.R.; Shabb, D.W.; Diep, B.A.; Chambers, H.F.; Otto, M.; et al. Global Changes in Staphylococcus aureus Gene Expression in Human Blood. PLoS ONE 2011, 6, e18617. [Google Scholar] [CrossRef] [PubMed]
- Hodille, E.; Plesa, A.; Bourrelly, E.; Belmont, L.; Badiou, C.; Lina, G.; Dumitrescu, O. Staphylococcal Panton-Valentine Leucocidin and Gamma Haemolysin Target and Lyse Mature Bone Marrow Leucocytes. Toxins 2020, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Spaan, A.N.; Schiepers, A.; de Haas, C.J.C.; van Hooijdonk, D.D.J.J.; Badiou, C.; Contamin, H.; Vandenesch, F.; Lina, G.; Gerard, N.P.; Gerard, C.; et al. Differential Interaction of the Staphylococcal Toxins Panton-Valentine Leukocidin and γ-Hemolysin CB with Human C5a Receptors. J. Immunol. 2015, 195, 1034–1043. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Lindsay, J.A. Staphylococcus aureus Innate Immune Evasion Is Lineage-Specific: A Bioinfomatics Study. Infect. Genet. Evol. 2013, 19, 7–14. [Google Scholar] [CrossRef]
- Janesch, P.; Rouha, H.; Weber, S.; Malafa, S.; Gross, K.; Maierhofer, B.; Badarau, A.; Visram, Z.C.; Stulik, L.; Nagy, E. Selective Sensitization of Human Neutrophils to LukGH Mediated Cytotoxicity by Staphylococcus aureus and IL-8. J. Infect. 2017, 74, 473–483. [Google Scholar] [CrossRef]
- DuMont, A.L.; Yoong, P.; Surewaard, B.G.J.; Benson, M.A.; Nijland, R.; van Strijp, J.A.G.; Torres, V.J. Staphylococcus aureus Elaborates Leukocidin AB to Mediate Escape from within Human Neutrophils. Infect. Immun. 2013, 81, 1830–1841. [Google Scholar] [CrossRef]
- Yang, D.; Ho, Y.X.; Cowell, L.M.; Jilani, I.; Foster, S.J.; Prince, L.R. A Genome-Wide Screen Identifies Factors Involved in S. aureus-Induced Human Neutrophil Cell Death and Pathogenesis. Front. Immunol. 2019, 10, 45. [Google Scholar] [CrossRef]
- Nygaard, T.K.; Borgogna, T.R.; Pallister, K.B.; Predtechenskaya, M.; Burroughs, O.S.; Gao, A.; Lubick, E.G.; Voyich, J.M. The Relative Importance of Cytotoxins Produced by Methicillin-Resistant Staphylococcus aureus Strain USA300 for Causing Human PMN Destruction. Microorganisms 2024, 12, 1782. [Google Scholar] [CrossRef]
- Melehani, J.H.; James, D.B.A.; DuMont, A.L.; Torres, V.J.; Duncan, J.A. Staphylococcus aureus Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC When Extracellular, but Not Intracellular. PLoS Pathog. 2015, 11, e1004970. [Google Scholar] [CrossRef]
- Brown, M.L.; O’Hara, F.P.; Close, N.M.; Mera, R.M.; Miller, L.A.; Suaya, J.A.; Amrine-Madsen, H. Prevalence and Sequence Variation of Panton-Valentine Leukocidin in Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus Strains in the United States. J. Clin. Microbiol. 2012, 50, 86–90. [Google Scholar] [CrossRef]
- Grebe, T.; Sarkari, M.T.; Cherkaoui, A.; Schaumburg, F. Exploration of Compounds to Inhibit the Panton-Valentine Leukocidin of Staphylococcus aureus. Med. Microbiol. Immunol. 2024, 213, 19. [Google Scholar] [CrossRef]
- Holzinger, D.; Gieldon, L.; Mysore, V.; Nippe, N.; Taxman, D.J.; Duncan, J.A.; Broglie, P.M.; Marketon, K.; Austermann, J.; Vogl, T.; et al. Staphylococcus aureus Panton-Valentine Leukocidin Induces an Inflammatory Response in Human Phagocytes via the NLRP3 Inflammasome. J. Leukoc. Biol. 2012, 92, 1069–1081. [Google Scholar] [CrossRef]
- Graves, S.F.; Kobayashi, S.D.; Braughton, K.R.; Diep, B.A.; Chambers, H.F.; Otto, M.; Deleo, F.R. Relative Contribution of Panton-Valentine Leukocidin to PMN Plasma Membrane Permeability and Lysis Caused by USA300 and USA400 Culture Supernatants. Microbes Infect. 2010, 12, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Hongo, I.; Baba, T.; Oishi, K.; Morimoto, Y.; Ito, T.; Hiramatsu, K. Phenol-Soluble Modulin Alpha 3 Enhances the Human Neutrophil Lysis Mediated by Panton-Valentine Leukocidin. J. Infect. Dis. 2009, 200, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Genestier, A.-L.; Michallet, M.-C.; Prévost, G.; Bellot, G.; Chalabreysse, L.; Peyrol, S.; Thivolet, F.; Etienne, J.; Lina, G.; Vallette, F.M.; et al. Staphylococcus aureus Panton-Valentine Leukocidin Directly Targets Mitochondria and Induces Bax-Independent Apoptosis of Human Neutrophils. J. Clin. Investig. 2005, 115, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Mairpady Shambat, S.; Chen, P.; Nguyen Hoang, A.T.; Bergsten, H.; Vandenesch, F.; Siemens, N.; Lina, G.; Monk, I.R.; Foster, T.J.; Arakere, G.; et al. Modelling Staphylococcal Pneumonia in a Human 3D Lung Tissue Model System Delineates Toxin-Mediated Pathology. Dis. Models Mech. 2015, 8, 1413–1425. [Google Scholar] [CrossRef]
- Gauduchon, V.; Cozon, G.; Vandenesch, F.; Genestier, A.; Eyssade, N.; Peyrol, S.; Etienne, J.; Lina, G. Neutralization of Staphylococcus aureus Panton Valentine Leukocidin by Intravenous Immunoglobulin In Vitro. J. Infect. Dis. 2004, 189, 346–353. [Google Scholar] [CrossRef]
- Hermos, C.R.; Yoong, P.; Pier, G.B. High Levels of Antibody to Panton-Valentine Leukocidin Are Not Associated with Resistance to Staphylococcus aureus—Associated Skin and Soft-Tissue Infection. Clin. Infect. Dis. 2010, 51, 1138–1146. [Google Scholar] [CrossRef]
- Meyer, F.; Girardot, R.; Piémont, Y.; Prévost, G.; Colin, D.A. Analysis of the Specificity of Panton-Valentine Leucocidin and Gamma-Hemolysin F Component Binding. Infect. Immun. 2009, 77, 266–273. [Google Scholar] [CrossRef]
- Jeannoel, M.; Casalegno, J.-S.; Ottmann, M.; Badiou, C.; Dumitrescu, O.; Lina, B.; Lina, G. Synergistic Effects of Influenza and Staphylococcus aureus Toxins on Inflammation Activation and Cytotoxicity in Human Monocytic Cell Lines. Toxins 2018, 10, 286. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, T.; Wang, Y.; Huang, M.; Wang, Y.; Luo, Z. New Insight into the Virulence and Inflammatory Response of Staphylococcus aureus Strains Isolated from Diabetic Foot Ulcers. Front. Cell. Infect. Microbiol. 2023, 13, 1234994. [Google Scholar] [CrossRef]
- Surewaard, B.G.J.; de Haas, C.J.C.; Vervoort, F.; Rigby, K.M.; DeLeo, F.R.; Otto, M.; van Strijp, J.A.G.; Nijland, R. Staphylococcal Alpha-Phenol Soluble Modulins Contribute to Neutrophil Lysis after Phagocytosis. Cell. Microbiol. 2013, 15, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Haag, A.F.; Bagnoli, F. The Role of Two-Component Signal Transduction Systems in Staphylococcus aureus Virulence Regulation. Curr. Top. Microbiol. Immunol. 2017, 409, 145–198. [Google Scholar] [CrossRef] [PubMed]
- Bleul, L.; Francois, P.; Wolz, C. Two-Component Systems of S. Aureus: Signaling and Sensing Mechanisms. Genes 2021, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.; Nagai, Y.; et al. Whole Genome Sequencing of Meticillin-Resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Le, K.Y.; Otto, M. Quorum-Sensing Regulation in Staphylococci—An Overview. Front. Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef]
- Yarwood, J.M.; Schlievert, P.M. Quorum Sensing in Staphylococcus Infections. J. Clin. Investig. 2003, 112, 1620–1625. [Google Scholar] [CrossRef]
- Novick, R.P.; Ross, H.F.; Projan, S.J.; Kornblum, J.; Kreiswirth, B.; Moghazeh, S. Synthesis of Staphylococcal Virulence Factors Is Controlled by a Regulatory RNA Molecule. EMBO J. 1993, 12, 3967–3975. [Google Scholar] [CrossRef]
- Boisset, S.; Geissmann, T.; Huntzinger, E.; Fechter, P.; Bendridi, N.; Possedko, M.; Chevalier, C.; Helfer, A.C.; Benito, Y.; Jacquier, A.; et al. Staphylococcus aureus RNAIII Coordinately Represses the Synthesis of Virulence Factors and the Transcription Regulator Rot by an Antisense Mechanism. Genes Dev. 2007, 21, 1353–1366. [Google Scholar] [CrossRef]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.-H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-Independent Target Gene Control by the Agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef]
- Flack, C.E.; Zurek, O.W.; Meishery, D.D.; Pallister, K.B.; Malone, C.L.; Horswill, A.R.; Voyich, J.M. Differential Regulation of Staphylococcal Virulence by the Sensor Kinase SaeS in Response to Neutrophil-Derived Stimuli. Proc. Natl. Acad. Sci. USA 2014, 111, E2037–E2045. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, T.K.; Borgogna, T.R.; Sward, E.W.; Guerra, F.E.; Dankoff, J.G.; Collins, M.M.; Pallister, K.B.; Chen, L.; Kreiswirth, B.N.; Voyich, J.M. Aspartic Acid Residue 51 of SaeR Is Essential for Staphylococcus aureus Virulence. Front. Microbiol. 2018, 9, 3085. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, T.K.; Pallister, K.B.; Ruzevich, P.; Griffith, S.; Vuong, C.; Voyich, J.M. SaeR Binds a Consensus Sequence within Virulence Gene Promoters to Advance USA300 Pathogenesis. J. Infect. Dis. 2010, 201, 241–254. [Google Scholar] [CrossRef]
- Voyich, J.M.; Vuong, C.; DeWald, M.; Nygaard, T.K.; Kocianova, S.; Griffith, S.; Jones, J.; Iverson, C.; Sturdevant, D.E.; Braughton, K.R.; et al. The SaeR/S Gene Regulatory System Is Essential for Innate Immune Evasion by Staphylococcus aureus. J. Infect. Dis. 2009, 199, 1698–1706. [Google Scholar] [CrossRef]
- Liu, Q.; Yeo, W.-S.; Bae, T. The SaeRS Two-Component System of Staphylococcus aureus. Genes 2016, 7, 81. [Google Scholar] [CrossRef]
- Geiger, T.; Goerke, C.; Mainiero, M.; Kraus, D.; Wolz, C. The Virulence Regulator Sae of Staphylococcus aureus: Promoter Activities and Response to Phagocytosis-Related Signals. J. Bacteriol. 2008, 190, 3419–3428. [Google Scholar] [CrossRef]
- Boguslawski, K.M.; McKeown, A.N.; Day, C.J.; Lacey, K.A.; Tam, K.; Vozhilla, N.; Kim, S.Y.; Jennings, M.P.; Koralov, S.B.; Elde, N.C.; et al. Exploiting Species Specificity to Understand the Tropism of a Human-Specific Toxin. Sci. Adv. 2020, 6, eaax7515. [Google Scholar] [CrossRef]
- Pagana, K.D.; Pagana, T.J.; Pagana, T.N. Mosby’s® Diagnostic and Laboratory Test Reference; Mosby: St. Louis, MO, USA, 2024; ISBN 978-0-323-82866-6. [Google Scholar]
- Skaar, E.P.; Schneewind, O. Iron-Regulated Surface Determinants (Isd) of Staphylococcus aureus: Stealing Iron from Heme. Microbes Infect. 2004, 6, 390–397. [Google Scholar] [CrossRef]
- Van Dijk, M.C.; De Kruijff, R.M.; Hagedoorn, P.-L. The Role of Iron in Staphylococcus aureus Infection and Human Disease: A Metal Tug of War at the Host—Microbe Interface. Front. Cell Dev. Biol. 2022, 10, 857237. [Google Scholar] [CrossRef]
- Torres, V.J.; Pishchany, G.; Humayun, M.; Schneewind, O.; Skaar, E.P. Staphylococcus aureus IsdB Is a Hemoglobin Receptor Required for Heme Iron Utilization. J. Bacteriol. 2006, 188, 8421–8429. [Google Scholar] [CrossRef] [PubMed]
- Skaar, E.P.; Humayun, M.; Bae, T.; DeBord, K.L.; Schneewind, O. Iron-Source Preference of Staphylococcus aureus Infections. Science 2004, 305, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, A.; Pohl, M.; Bhakdi, S. Staphylococcus aureus Alpha-Toxin. Dual Mechanism of Binding to Target Cells. J. Biol. Chem. 1991, 266, 17195–17200. [Google Scholar] [CrossRef] [PubMed]
- Sanger, R.; Race, R.R.; Jack, J. The Duffy Blood Groups of New York Negroes: The Phenotype Fy (A−b−). Br. J. Haematol. 1955, 1, 370–374. [Google Scholar] [CrossRef]
- Miller, L.H.; Mason, S.J.; Clyde, D.F.; McGinniss, M.H. The Resistance Factor to Plasmodium Vivax in Blacks. N. Engl. J. Med. 1976, 295, 302–304. [Google Scholar] [CrossRef]
- Wolfmeier, H.; Mansour, S.C.; Liu, L.T.; Pletzer, D.; Draeger, A.; Babiychuk, E.B.; Hancock, R.E.W. Liposomal Therapy Attenuates Dermonecrosis Induced by Community-Associated Methicillin-Resistant Staphylococcus aureus by Targeting α-Type Phenol-Soluble Modulins and α-Hemolysin. EBioMedicine 2018, 33, 211–217. [Google Scholar] [CrossRef]
- Hébert, G.A.; Hancock, G.A. Synergistic Hemolysis Exhibited by Species of Staphylococci. J. Clin. Microbiol. 1985, 22, 409–415. [Google Scholar] [CrossRef]
- Mainiero, M.; Goerke, C.; Geiger, T.; Gonser, C.; Herbert, S.; Wolz, C. Differential Target Gene Activation by the Staphylococcus aureus Two-Component System saeRS. J. Bacteriol. 2010, 192, 613–623. [Google Scholar] [CrossRef]
- Guo, L.; Rondina, M.T. The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases. Front. Immunol. 2019, 10, 2204. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Ni, H. Crosstalk Between Platelets and Microbial Pathogens. Front. Immunol. 2020, 11, 1962. [Google Scholar] [CrossRef]
- Yeaman, M.R. Platelets: At the Nexus of Antimicrobial Defence. Nat. Rev. Microbiol. 2014, 12, 426–437. [Google Scholar] [CrossRef]
- Hamzeh-Cognasse, H.; Damien, P.; Chabert, A.; Pozzetto, B.; Cognasse, F.; Garraud, O. Platelets and Infections—Complex Interactions with Bacteria. Front. Immunol. 2015, 6, 82. [Google Scholar] [CrossRef]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet Biology and Functions: New Concepts and Clinical Perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Douglas-Louis, R.; Lou, M.; Lee, B.; Minejima, E.; Bubeck-Wardenburg, J.; Wong-Beringer, A. Prognostic Significance of Early Platelet Dynamics in Staphylococcus aureus Bacteremia. BMC Infect. Dis. 2023, 23, 82. [Google Scholar] [CrossRef]
- Gafter-Gvili, A.; Mansur, N.; Bivas, A.; Zemer-Wassercug, N.; Bishara, J.; Leibovici, L.; Paul, M. Thrombocytopenia in Staphylococcus aureus Bacteremia: Risk Factors and Prognostic Importance. Mayo Clin. Proc. 2011, 86, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Ahmad, Z.; Rosenberger, G.; Fan, S.; Nicolai, L.; Busch, B.; Yavuz, G.; Luckner, M.; Ishikawa-Ankerhold, H.; Hennel, R.; et al. Migrating Platelets Are Mechano-Scavengers That Collect and Bundle Bacteria. Cell 2017, 171, 1368–1382.e23. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, B.F.; Campbell, R.A.; Schwertz, H.; Cody, M.J.; Franks, Z.; Tolley, N.D.; Kahr, W.H.A.; Lindemann, S.; Seizer, P.; Yost, C.C.; et al. Novel Anti-Bacterial Activities of β-Defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation. PLoS Pathog. 2011, 7, e1002355. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.R.; Loughman, A.; Keane, F.; Brennan, M.; Knobel, M.; Higgins, J.; Visai, L.; Speziale, P.; Cox, D.; Foster, T.J. Fibronectin-Binding Proteins of Staphylococcus aureus Mediate Activation of Human Platelets via Fibrinogen and Fibronectin Bridges to Integrin GPIIb/IIIa and IgG Binding to the FcγRIIa Receptor. Mol. Microbiol. 2006, 59, 212–230. [Google Scholar] [CrossRef]
- Vanassche, T.; Kauskot, A.; Verhaegen, J.; Peetermans, W.E.; van Ryn, J.; Schneewind, O.; Hoylaerts, M.F.; Verhamme, P. Fibrin Formation by Staphylothrombin Facilitates Staphylococcus aureus-Induced Platelet Aggregation. Thromb. Haemost. 2012, 107, 1107–1121. [Google Scholar] [CrossRef]
- Miajlovic, H.; Loughman, A.; Brennan, M.; Cox, D.; Foster, T.J. Both Complement- and Fibrinogen-Dependent Mechanisms Contribute to Platelet Aggregation Mediated by Staphylococcus aureus Clumping Factor B. Infect. Immun. 2007, 75, 3335–3343. [Google Scholar] [CrossRef]
- O’Brien, L.; Kerrigan, S.W.; Kaw, G.; Hogan, M.; Penadés, J.; Litt, D.; Fitzgerald, D.J.; Foster, T.J.; Cox, D. Multiple Mechanisms for the Activation of Human Platelet Aggregation by Staphylococcus aureus: Roles for the Clumping Factors ClfA and ClfB, the Serine-Aspartate Repeat Protein SdrE and Protein A. Mol. Microbiol. 2002, 44, 1033–1044. [Google Scholar] [CrossRef]
- Hawiger, J.; Steckley, S.; Hammond, D.; Cheng, C.; Timmons, S.; Glick, A.D.; Des Prez, R.M. Staphylococci-Induced Human Platelet Injury Mediated by Protein A and Immunoglobulin G Fc Fragment Receptor. J. Clin. Investig. 1979, 64, 931–937. [Google Scholar] [CrossRef] [PubMed]
- De Haas, C.J.C.; Weeterings, C.; Vughs, M.M.; De Groot, P.G.; Van Strijp, J.A.; Lisman, T. Staphylococcal Superantigen-like 5 Activates Platelets and Supports Platelet Adhesion under Flow Conditions, Which Involves Glycoprotein Ibα and αIIbβ3. J. Thromb. Haemost. 2009, 7, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Surewaard, B.G.J.; Thanabalasuriar, A.; Zeng, Z.; Tkaczyk, C.; Cohen, T.S.; Bardoel, B.W.; Jorch, S.K.; Deppermann, C.; Bubeck Wardenburg, J.; Davis, R.P.; et al. α-Toxin Induces Platelet Aggregation and Liver Injury during Staphylococcus aureus Sepsis. Cell Host Microbe 2018, 24, 271–284.e3. [Google Scholar] [CrossRef] [PubMed]
- Liesenborghs, L.; Verhamme, P.; Vanassche, T. Staphylococcus aureus, Master Manipulator of the Human Hemostatic System. J. Thromb. Haemost. 2018, 16, 441–454. [Google Scholar] [CrossRef]
- Colciaghi, F.; Borroni, B.; Pastorino, L.; Marcello, E.; Zimmermann, M.; Cattabeni, F.; Padovani, A.; Di Luca, M. α-Secretase ADAM10 as Well as αAPPs Is Reduced in Platelets and CSF of Alzheimer Disease Patients. Mol. Med. 2002, 8, 67–74. [Google Scholar] [CrossRef]
- Raab, S.; Kropp, K.N.; Steinle, A.; Klein, G.; Kanz, L.; Kopp, H.-G.; Salih, H.R. Platelet-Derived Proteases ADAM10 and ADAM17 Impair NK Cell Immunosurveillance of Metastasizing Tumor Cells by Diminishing NKG2D Ligand Surface Expression. Blood 2014, 124, 4164. [Google Scholar] [CrossRef]
- Philippeaux, M.M.; Vesin, C.; Tacchini-Cottier, F.; Piguet, P.F. Activated Human Platelets Express Beta2 Integrin. Eur. J. Haematol. 1996, 56, 130–137. [Google Scholar] [CrossRef]
- Nording, H.; Baron, L.; Haberthür, D.; Emschermann, F.; Mezger, M.; Sauter, M.; Sauter, R.; Patzelt, J.; Knoepp, K.; Nording, A.; et al. The C5a/C5a Receptor 1 Axis Controls Tissue Neovascularization through CXCL4 Release from Platelets. Nat. Commun. 2021, 12, 3352. [Google Scholar] [CrossRef]
- Patzelt, J.; Mueller, K.A.L.; Breuning, S.; Karathanos, A.; Schleicher, R.; Seizer, P.; Gawaz, M.; Langer, H.F.; Geisler, T. Expression of Anaphylatoxin Receptors on Platelets in Patients with Coronary Heart Disease. Atherosclerosis 2015, 238, 289–295. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Sarkar, A.; Giannini, H.M.; Goel, R.R.; Mathew, D.; Suzuki, A.; Baxter, A.E.; Greenplate, A.R.; Alanio, C.; Abdel-Hakeem, M.; et al. Signaling through FcγRIIA and the C5a-C5aR Pathway Mediates Platelet Hyperactivation in COVID-19. bioRxiv 2021. [Google Scholar] [CrossRef]
- Guerra, F.E.; Borgogna, T.R.; Patel, D.M.; Sward, E.W.; Voyich, J.M. Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2017, 7, 286. [Google Scholar] [CrossRef]
- Nygaard, T.; Malachowa, N.; Kobayashi, S.D.; DeLeo, F.R. Phagocytes. In Management of Infections in the Immunocompromised Host; Segal, B.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–25. ISBN 978-3-319-77672-9. [Google Scholar]
- Karavolos, M.H.; Horsburgh, M.J.; Ingham, E.; Foster, S.J. Role and Regulation of the Superoxide Dismutases of Staphylococcus aureus. Microbiology 2003, 149, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, M.J.; Clements, M.O.; Crossley, H.; Ingham, E.; Foster, S.J. PerR Controls Oxidative Stress Resistance and Iron Storage Proteins and Is Required for Virulence in Staphylococcus aureus. Infect. Immun. 2001, 69, 3744–3754. [Google Scholar] [CrossRef] [PubMed]
- de Jong, N.W.M.; Ramyar, K.X.; Guerra, F.E.; Nijland, R.; Fevre, C.; Voyich, J.M.; McCarthy, A.J.; Garcia, B.L.; van Kessel, K.P.M.; van Strijp, J.A.G.; et al. Immune Evasion by a Staphylococcal Inhibitor of Myeloperoxidase. Proc. Natl. Acad. Sci. USA 2017, 114, 9439–9444. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.E.; Addison, C.B.; de Jong, N.W.M.; Azzolino, J.; Pallister, K.B.; van Strijp, J.A.G.; Voyich, J.M. Staphylococcus aureus SaeR/S-Regulated Factors Reduce Human Neutrophil Reactive Oxygen Species Production. J. Leukoc. Biol. 2016, 100, 1005–1010. [Google Scholar] [CrossRef]
- Voyich, J.M.; Braughton, K.R.; Sturdevant, D.E.; Whitney, A.R.; Saïd-Salim, B.; Porcella, S.F.; Long, R.D.; Dorward, D.W.; Gardner, D.J.; Kreiswirth, B.N.; et al. Insights into Mechanisms Used by Staphylococcus aureus to Avoid Destruction by Human Neutrophils. J. Immunol. 2005, 175, 3907–3919. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Braughton, K.R.; Palazzolo-Ballance, A.M.; Kennedy, A.D.; Sampaio, E.; Kristosturyan, E.; Whitney, A.R.; Sturdevant, D.E.; Dorward, D.W.; Holland, S.M.; et al. Rapid Neutrophil Destruction Following Phagocytosis of Staphylococcus aureus. J. Innate Immun. 2010, 2, 560–575. [Google Scholar] [CrossRef]
- Seifert, A.; Düsterhöft, S.; Wozniak, J.; Koo, C.Z.; Tomlinson, M.G.; Nuti, E.; Rossello, A.; Cuffaro, D.; Yildiz, D.; Ludwig, A. The Metalloproteinase ADAM10 Requires Its Activity to Sustain Surface Expression. Cell. Mol. Life Sci. 2020, 78, 715–732. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil Chemoattractant Receptors in Health and Disease: Double-Edged Swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil Cell Surface Receptors and Their Intracellular Signal Transduction Pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef]
- Nygaard, T.K.; DeLeo, F.R.; Voyich, J.M. Community-Associated Methicillin-Resistant Staphylococcus aureus Skin Infections: Advances toward Identifying the Key Virulence Factors. Curr. Opin. Infect. Dis. 2008, 21, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Marsman, G.; Lacey, K.A.; Chapman, J.R.; Goosmann, C.; Ueberheide, B.M.; Torres, V.J. The Cell Envelope of Staphylococcus aureus Selectively Controls the Sorting of Virulence Factors. Nat. Commun. 2021, 12, 6193. [Google Scholar] [CrossRef] [PubMed]
- DuMont, A.L.; Yoong, P.; Liu, X.; Day, C.J.; Chumbler, N.M.; James, D.B.A.; Alonzo, F.; Bode, N.J.; Lacy, D.B.; Jennings, M.P.; et al. Identification of a Crucial Residue Required for Staphylococcus aureus LukAB Cytotoxicity and Receptor Recognition. Infect. Immun. 2014, 82, 1268–1276. [Google Scholar] [CrossRef]
- Badarau, A.; Rouha, H.; Malafa, S.; Logan, D.T.; Håkansson, M.; Stulik, L.; Dolezilkova, I.; Teubenbacher, A.; Gross, K.; Maierhofer, B.; et al. Structure-Function Analysis of Heterodimer Formation, Oligomerization, and Receptor Binding of the Staphylococcus aureus Bi-Component Toxin LukGH. J. Biol. Chem. 2015, 290, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.R.; Sturdevant, D.E.; Mackie, S.M.; Gill, S.R.; Musser, J.M. Evolutionary Genomics of Staphylococcus aureus: Insights into the Origin of Methicillin-Resistant Strains and the Toxic Shock Syndrome Epidemic. Proc. Natl. Acad. Sci. USA 2001, 98, 8821–8826. [Google Scholar] [CrossRef]
- Pivard, M.; Caldelari, I.; Brun, V.; Croisier, D.; Jaquinod, M.; Anzala, N.; Gilquin, B.; Teixeira, C.; Benito, Y.; Couzon, F.; et al. Complex Regulation of Gamma-Hemolysin Expression Impacts Staphylococcus aureus Virulence. Microbiol. Spectr. 2023, 11, e01073-23. [Google Scholar] [CrossRef]
- Dastgheyb, S.S.; Otto, M. Staphylococcal Adaptation to Diverse Physiologic Niches: An Overview of Transcriptomic and Phenotypic Changes in Different Biological Environments. Future Microbiol. 2015, 10, 1981–1995. [Google Scholar] [CrossRef]
- Den Reijer, P.M.; Lemmens-den Toom, N.; Kant, S.; Snijders, S.V.; Boelens, H.; Tavakol, M.; Verkaik, N.J.; Van Belkum, A.; Verbrugh, H.A.; Van Wamel, W.J.B. Characterization of the Humoral Immune Response during Staphylococcus aureus Bacteremia and Global Gene Expression by Staphylococcus aureus in Human Blood. PLoS ONE 2013, 8, e53391. [Google Scholar] [CrossRef]
- Loughman, J.A.; Fritz, S.A.; Storch, G.A.; Hunstad, D.A. Virulence Gene Expression in Human Community-Acquired Staphylococcus aureus Infection. J. Infect. Dis. 2009, 199, 294–301. [Google Scholar] [CrossRef]
- Date, S.V.; Modrusan, Z.; Lawrence, M.; Morisaki, J.H.; Toy, K.; Shah, I.M.; Kim, J.; Park, S.; Xu, M.; Basuino, L.; et al. Global Gene Expression of Methicillin-Resistant Staphylococcus aureus USA300 during Human and Mouse Infection. J. Infect. Dis. 2014, 209, 1542–1550. [Google Scholar] [CrossRef]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef]
- Armentrout, E.I.; Liu, G.Y.; Martins, G.A. T Cell Immunity and the Quest for Protective Vaccines against Staphylococcus aureus Infection. Microorganisms 2020, 8, 1936. [Google Scholar] [CrossRef]
- Kolata, J.B.; Kühbandner, I.; Link, C.; Normann, N.; Vu, C.H.; Steil, L.; Weidenmaier, C.; Bröker, B.M. The Fall of a Dogma? Unexpected High T-Cell Memory Response to Staphylococcus aureus in Humans. J. Infect. Dis. 2015, 212, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.S.; Cho, J.S. Immunity against Staphylococcus aureus Cutaneous Infections. Nat. Rev. Immunol. 2011, 11, 505–518. [Google Scholar] [CrossRef]
- Hendriks, A.; Mnich, M.E.; Clemente, B.; Cruz, A.R.; Tavarini, S.; Bagnoli, F.; Soldaini, E. Staphylococcus aureus-Specific Tissue-Resident Memory CD4+ T Cells Are Abundant in Healthy Human Skin. Front. Immunol. 2021, 12, 642711. [Google Scholar] [CrossRef] [PubMed]
- Marrack, P.; Kappler, J. The Staphylococcal Enterotoxins and Their Relatives. Science 1990, 248, 1066. [Google Scholar] [CrossRef] [PubMed]
- Proft, T.; Fraser, J.D. Bacterial Superantigens. Clin. Exp. Immunol. 2003, 133, 299–306. [Google Scholar] [CrossRef]
- Deacy, A.M.; Gan, S.K.-E.; Derrick, J.P. Superantigen Recognition and Interactions: Functions, Mechanisms and Applications. Front. Immunol. 2021, 12, 731845. [Google Scholar] [CrossRef]
- Choi, Y.; Lafferty, J.A.; Clements, J.R.; Todd, J.K.; Gelfand, E.W.; Kappler, J.; Marrack, P.; Kotzin, B.L. Selective Expansion of T Cells Expressing V Beta 2 in Toxic Shock Syndrome. J. Exp. Med. 1990, 172, 981–984. [Google Scholar] [CrossRef]
- Kotzin, B.L.; Leung, D.Y.; Kappler, J.; Marrack, P. Superantigens and Their Potential Role in Human Disease. Adv. Immunol. 1993, 54, 99–166. [Google Scholar] [CrossRef]
- Sezin, T.; Selvakumar, B.; Scheffold, A. The Role of A Disintegrin and Metalloproteinase (ADAM)-10 in T Helper Cell Biology. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2022, 1869, 119192. [Google Scholar] [CrossRef]
- Bonecchi, R.; Bianchi, G.; Bordignon, P.P.; D’Ambrosio, D.; Lang, R.; Borsatti, A.; Sozzani, S.; Allavena, P.; Gray, P.A.; Mantovani, A.; et al. Differential Expression of Chemokine Receptors and Chemotactic Responsiveness of Type 1 T Helper Cells (Th1s) and Th2s. J. Exp. Med. 1998, 187, 129–134. [Google Scholar] [CrossRef]
- Frade, J.M.; Mellado, M.; del Real, G.; Gutierrez-Ramos, J.C.; Lind, P.; Martinez-A, C. Characterization of the CCR2 Chemokine Receptor: Functional CCR2 Receptor Expression in B Cells. J. Immunol. 1997, 159, 5576–5584. [Google Scholar] [CrossRef] [PubMed]
- Nansen, A.; Marker, O.; Bartholdy, C.; Thomsen, A.R. CCR2+ and CCR5+ CD8+ T Cells Increase during Viral Infection and Migrate to Sites of Infection. Eur. J. Immunol. 2000, 30, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, S.; Licenziati, S.; Alessandri, G.; Castelli, F.; Caligaris, S.; Bonafede, M.; Grassi, M.; Garrafa, E.; Balsari, A.; Turano, A.; et al. CD11b Expression Identifies CD8+CD28+ T Lymphocytes with Phenotype and Function of Both Naive/Memory and Effector Cells. J. Immunol. 2001, 166, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Roberts, T.J.; Sriram, V.; Cho, S.; Brutkiewicz, R.R. Myeloid Marker Expression on Antiviral CD8+ T Cells Following an Acute Virus Infection. Eur. J. Immunol. 2003, 33, 2736–2743. [Google Scholar] [CrossRef]
- Hermiston, M.L.; Xu, Z.; Weiss, A. CD45: A Critical Regulator of Signaling Thresholds in Immune Cells. Annu. Rev. Immunol. 2003, 21, 107–137. [Google Scholar] [CrossRef]
- Francis, J.N.; Jacobson, M.R.; Lloyd, C.M.; Sabroe, I.; Durham, S.R.; Till, S.J. CXCR1+CD4+ T Cells in Human Allergic Disease. J. Immunol. 2004, 172, 268–273. [Google Scholar] [CrossRef]
- Takata, H.; Tomiyama, H.; Fujiwara, M.; Kobayashi, N.; Takiguchi, M. Cutting Edge: Expression of Chemokine Receptor CXCR1 on Human Effector CD8+ T Cells1. J. Immunol. 2004, 173, 2231–2235. [Google Scholar] [CrossRef]
- Gasser, O.; Missiou, A.; Eken, C.; Hess, C. Human CD8+ T Cells Store CXCR1 in a Distinct Intracellular Compartment and Up-Regulate It Rapidly to the Cell Surface upon Activation. Blood 2005, 106, 3718–3724. [Google Scholar] [CrossRef]
- Nataf, S.; Davoust, N.; Ames, R.S.; Barnum, S.R. Human T Cells Express the C5a Receptor and Are Chemoattracted to C5a1. J. Immunol. 1999, 162, 4018–4023. [Google Scholar] [CrossRef]
- Verghese, D.A.; Chun, N.; Paz, K.; Fribourg, M.; Woodruff, T.M.; Flynn, R.; Hu, Y.; Xiong, H.; Zhang, W.; Yi, Z.; et al. C5aR1 Regulates T Follicular Helper Differentiation and Chronic Graft-versus-Host Disease Bronchiolitis Obliterans. JCI Insight 2018, 3, e124646. [Google Scholar] [CrossRef]
- Pozzi, C.; Lofano, G.; Mancini, F.; Soldaini, E.; Speziale, P.; De Gregorio, E.; Rappuoli, R.; Bertholet, S.; Grandi, G.; Bagnoli, F. Phagocyte Subsets and Lymphocyte Clonal Deletion behind Ineffective Immune Response to Staphylococcus aureus. FEMS Microbiol. Rev. 2015, 39, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Karauzum, H.; Datta, S.K. Adaptive Immunity against Staphylococcus aureus. Curr. Top. Microbiol. Immunol. 2017, 409, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Daum, R. Development of a Vaccine against Staphylococcus aureus. Semin. Immunopathol. 2012, 34, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Deisenhofer, J. Crystallographic Refinement and Atomic Models of a Human Fc Fragment and Its Complex with Fragment B of Protein A from Staphylococcus aureus at 2.9- and 2.8-.ANG. Resolution. Biochemistry 1981, 20, 2361–2370. [Google Scholar] [CrossRef]
- Moks, T.; Abrahmsén, L.; Nilsson, B.; Hellman, U.; Sjöquist, J.; Uhlén, M. Staphylococcal Protein A Consists of Five IgG-Binding Domains. Eur. J. Biochem. 1986, 156, 637–643. [Google Scholar] [CrossRef]
- Zhang, L.; Jacobsson, K.; Vasi, J.; Lindberg, M.; Frykberg, L. A Second IgG-Binding Protein in Staphylococcus aureus. Microbiology 1998, 144 Pt 4, 985–991. [Google Scholar] [CrossRef]
- Atkins, K.L.; Burman, J.D.; Chamberlain, E.S.; Cooper, J.E.; Poutrel, B.; Bagby, S.; Jenkins, A.T.A.; Feil, E.J.; van den Elsen, J.M.H.S. Aureus IgG-Binding Proteins SpA and Sbi: Host Specificity and Mechanisms of Immune Complex Formation. Mol. Immunol. 2008, 45, 1600–1611. [Google Scholar] [CrossRef]
- Romagnani, S.; Giudizi, M.G.; del Prete, G.; Maggi, E.; Biagiotti, R.; Almerigogna, F.; Ricci, M. Demonstration on Protein A of Two Distinct Immunoglobulin-Binding Sites and Their Role in the Mitogenic Activity of Staphylococcus aureus Cowan I on Human B Cells. J. Immunol. 1982, 129, 596–602. [Google Scholar] [CrossRef]
- Silverman, G.J.; Goodyear, C.S. A Model B-Cell Superantigen and the Immunobiology of B Lymphocytes. Clin. Immunol. 2002, 102, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, C.S.; Silverman, G.J. Death by a B Cell Superantigen. J. Exp. Med. 2003, 197, 1125–1139. [Google Scholar] [CrossRef]
- Goodyear, C.S.; Silverman, G.J. Staphylococcal Toxin Induced Preferential and Prolonged In Vivo Deletion of Innate-like B Lymphocytes. Proc. Natl. Acad. Sci. USA 2004, 101, 11392–11397. [Google Scholar] [CrossRef]
- Lownik, J.C.; Luker, A.J.; Damle, S.R.; Cooley, L.F.; El Sayed, R.; Hutloff, A.; Pitzalis, C.; Martin, R.K.; El Shikh, M.E.M.; Conrad, D.H. ADAM10-Mediated ICOSL Shedding on B Cells Is Necessary for Proper T Cell ICOS Regulation and TFH Responses. J. Immunol. 2017, 199, 2305–2315. [Google Scholar] [CrossRef]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte Differentiation and Antigen-Presenting Functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Ardura, M.I.; Banchereau, R.; Mejias, A.; Di Pucchio, T.; Glaser, C.; Allantaz, F.; Pascual, V.; Banchereau, J.; Chaussabel, D.; Ramilo, O. Enhanced Monocyte Response and Decreased Central Memory T Cells in Children with Invasive Staphylococcus aureus Infections. PLoS ONE 2009, 4, e5446. [Google Scholar] [CrossRef]
- Zwack, E.E.; Chen, Z.; Devlin, J.C.; Li, Z.; Zheng, X.; Weinstock, A.; Lacey, K.A.; Fisher, E.A.; Fenyö, D.; Ruggles, K.V.; et al. Staphylococcus aureus Induces a Muted Host Response in Human Blood That Blunts the Recruitment of Neutrophils. Proc. Natl. Acad. Sci. USA 2022, 119, e2123017119. [Google Scholar] [CrossRef] [PubMed]
- Kubica, M.; Guzik, K.; Koziel, J.; Zarebski, M.; Richter, W.; Gajkowska, B.; Golda, A.; Maciag-Gudowska, A.; Brix, K.; Shaw, L.; et al. A Potential New Pathway for Staphylococcus aureus Dissemination: The Silent Survival of S. aureus Phagocytosed by Human Monocyte-Derived Macrophages. PLoS ONE 2008, 3, e1409. [Google Scholar] [CrossRef] [PubMed]
- Musilova, J.; Mulcahy, M.E.; Kuijk, M.M.; McLoughlin, R.M.; Bowie, A.G. Toll-like Receptor 2–Dependent Endosomal Signaling by Staphylococcus aureus in Monocytes Induces Type I Interferon and Promotes Intracellular Survival. J. Biol. Chem. 2019, 294, 17031–17042. [Google Scholar] [CrossRef]
- Thwaites, G.E.; Gant, V. Are Bloodstream Leukocytes Trojan Horses for the Metastasis of Staphylococcus aureus? Nat. Rev. Microbiol. 2011, 9, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Tai, J.J.-Y.; Wong, W.-C.; Han, H.; Sem, X.; Yeap, W.-H.; Kourilsky, P.; Wong, S.-C. Gene Expression Profiling Reveals the Defining Features of the Classical, Intermediate, and Nonclassical Human Monocyte Subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, V.; Cuschieri, J.; Garcia, I.; Knoll, M.; Billgren, J.; Jelacic, S.; Bulger, E.; Maier, R. The priming effect of C5a on monocytes is predominantly mediated by the p38 MAPK pathway. Shock 2007, 27, 623. [Google Scholar] [CrossRef] [PubMed]
- Möller-Hackbarth, K.; Dewitz, C.; Schweigert, O.; Trad, A.; Garbers, C.; Rose-John, S.; Scheller, J. A Disintegrin and Metalloprotease (ADAM) 10 and ADAM17 Are Major Sheddases of T Cell Immunoglobulin and Mucin Domain 3 (Tim-3). J. Biol. Chem. 2013, 288, 34529–34544. [Google Scholar] [CrossRef]
- Trebst, C.; Sørensen, T.L.; Kivisäkk, P.; Cathcart, M.K.; Hesselgesser, J.; Horuk, R.; Sellebjerg, F.; Lassmann, H.; Ransohoff, R.M. CCR1+/CCR5+ Mononuclear Phagocytes Accumulate in the Central Nervous System of Patients with Multiple Sclerosis. Am. J. Pathol. 2001, 159, 1701–1710. [Google Scholar] [CrossRef]
- Ales, E.; Sackstein, R. Analysis of CD45 Isoforms Displayed By Human Peripheral Blood Mononuclear Cells. Blood 2022, 140, 11202. [Google Scholar] [CrossRef]
- Richardson, J.R.; Armbruster, N.S.; Günter, M.; Henes, J.; Autenrieth, S.E. Staphylococcus aureus PSM Peptides Modulate Human Monocyte-Derived Dendritic Cells to Prime Regulatory T Cells. Front. Immunol. 2018, 9, 2603. [Google Scholar] [CrossRef]
- Vickery, T.W.; Ramakrishnan, V.R.; Suh, J.D. The Role of Staphylococcus aureus in Patients with Chronic Sinusitis and Nasal Polyposis. Curr. Allergy Asthma Rep. 2019, 19, 21. [Google Scholar] [CrossRef]
- Teufelberger, A.R.; Bröker, B.M.; Krysko, D.V.; Bachert, C.; Krysko, O. Staphylococcus aureus Orchestrates Type 2 Airway Diseases. Trends Mol. Med. 2019, 25, 696–707. [Google Scholar] [CrossRef]
- Jorde, I.; Schreiber, J.; Stegemann-Koniszewski, S. The Role of Staphylococcus aureus and Its Toxins in the Pathogenesis of Allergic Asthma. Int. J. Mol. Sci. 2022, 24, 654. [Google Scholar] [CrossRef]
- Bachert, C.; Humbert, M.; Hanania, N.A.; Zhang, N.; Holgate, S.; Buhl, R.; Bröker, B.M. Staphylococcus aureus and Its IgE-Inducing Enterotoxins in Asthma: Current Knowledge. Eur. Respir. J. 2020, 55, 1901592. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Perricone, C.; Olivieri, G.; Cipriano, E.; Spinelli, F.R.; Valesini, G.; Conti, F. Staphylococcus aureus Nasal Carriage and Autoimmune Diseases: From Pathogenic Mechanisms to Disease Susceptibility and Phenotype. Int. J. Mol. Sci. 2019, 20, 5624. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; He, Y.; Lv, Z.; Liang, Z.; Chen, J.; Li, P.; Liu, J.; Yang, H.; Tao, A.; et al. Exploring the Role of Staphylococcus aureus in Inflammatory Diseases. Toxins 2022, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- DeVore, S.B.; Gonzalez, T.; Sherenian, M.G.; Herr, A.B.; Hershey, G.K.K. On the Surface: Skin Microbial Exposure Contributes to Allergic Disease. Ann. Allergy Asthma Immunol. 2020, 125, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, E.; Zhang, N.; Krysko, O.; Lan, F.; Holtappels, G.; De Ruyck, N.; Nauwynck, H.; Yousefi, S.; Simon, H.-U.; Bachert, C. Extracellular Eosinophilic Traps in Association with Staphylococcus aureus at the Site of Epithelial Barrier Defects in Patients with Severe Airway Inflammation. J. Allergy Clin. Immunol. 2017, 139, 1849–1860.e6. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, R.S.; Levi-Schaffer, F. sCD48 Is Anti-Inflammatory in Staphylococcus aureus Enterotoxin B-Induced Eosinophilic Inflammation. Allergy 2016, 71, 829–839. [Google Scholar] [CrossRef]
- Minai-Fleminger, Y.; Gangwar, R.S.; Migalovich-Sheikhet, H.; Seaf, M.; Leibovici, V.; Hollander, N.; Feld, M.; Moses, A.E.; Homey, B.; Levi-Schaffer, F. The CD48 Receptor Mediates Staphylococcus aureus Human and Murine Eosinophil Activation. Clin. Exp. Allergy 2014, 44, 1335–1346. [Google Scholar] [CrossRef]
- Leyva-Castillo, J.-M.; Das, M.; Kane, J.; Strakosha, M.; Singh, S.; Wong, D.S.H.; Horswill, A.R.; Karasuyama, H.; Brombacher, F.; Miller, L.S.; et al. Basophil-Derived IL-4 Promotes Cutaneous Staphylococcus aureus Infection. JCI Insight 2021, 6, e149953. [Google Scholar] [CrossRef]
- Bachert, C.; van Steen, K.; Zhang, N.; Holtappels, G.; Cattaert, T.; Maus, B.; Buhl, R.; Taube, C.; Korn, S.; Kowalski, M.; et al. Specific IgE against Staphylococcus aureus Enterotoxins: An Independent Risk Factor for Asthma. J. Allergy Clin. Immunol. 2012, 130, 376–381.e8. [Google Scholar] [CrossRef]
- Kowalski, M.L.; Cieślak, M.; Pérez-Novo, C.A.; Makowska, J.S.; Bachert, C. Clinical and Immunological Determinants of Severe/Refractory Asthma (SRA): Association with Staphylococcal Superantigen-Specific IgE Antibodies. Allergy 2011, 66, 32–38. [Google Scholar] [CrossRef]
- Haruna, T.; Kariya, S.; Fujiwara, T.; Higaki, T.; Makihara, S.; Kanai, K.; Fujiwara, R.; Iwasaki, S.; Noguchi, Y.; Nishizaki, K.; et al. Association between Impaired IL-10 Production Following Exposure to Staphylococcus aureus Enterotoxin B and Disease Severity in Eosinophilic Chronic Rhinosinusitis. Allergol. Int. 2018, 67, 392–398. [Google Scholar] [CrossRef]
- Hellings, P.W.; Hens, G.; Meyts, I.; Bullens, D.; Vanoirbeek, J.; Gevaert, P.; Jorissen, M.; Ceuppens, J.L.; Bachert, C. Aggravation of Bronchial Eosinophilia in Mice by Nasal and Bronchial Exposure to Staphylococcus aureus Enterotoxin B. Clin. Exp. Allergy 2006, 36, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Pastacaldi, C.; Lewis, P.; Howarth, P. Staphylococci and Staphylococcal Superantigens in Asthma and Rhinitis: A Systematic Review and Meta-Analysis. Allergy 2011, 66, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Colantuono, S.; Ciasca, G.; Basile, U.; Di Santo, R.; Bagnasco, D.; Passalacqua, G.; Caminati, M.; Michele, S.; Senna, G.; et al. Different Aspects of Severe Asthma in Real Life: Role of Staphylococcus aureus Enterotoxins and Correlation to Comorbidities and Disease Severity. Allergy 2023, 78, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Tamburini, M.; Giudizi, M.G.; Biagiotti, R.; Almerigogna, F.; Romagnani, S. Mechanism of Activation of Human Basophils by Staphylococcus aureus Cowan 1. Infect. Immun. 1987, 55, 803–809. [Google Scholar] [CrossRef]
- Gaur, P.; Seaf, M.; Trabelsi, N.; Marcu, O.; Gafarov, D.; Schueler-Furman, O.; Mandelboim, O.; Ben-Zimra, M.; Levi-Schaffer, F. 2B4: A Potential Target in Staphylococcus aureus Associated Allergic Inflammation. Clin. Exp. Immunol. 2024, 215, 37–46. [Google Scholar] [CrossRef]
- Dunzendorfer, S.; Kaneider, N.C.; Kaser, A.; Woell, E.; Frade, J.M.R.; Mellado, M.; Martínez-Alonso, C.; Wiedermann, C.J. Functional Expression of Chemokine Receptor 2 by Normal Human Eosinophils. J. Allergy Clin. Immunol. 2001, 108, 581–587. [Google Scholar] [CrossRef]
- Suzukawa, M.; Koketsu, R.; Iikura, M.; Nakae, S.; Matsumoto, K.; Nagase, H.; Saito, H.; Matsushima, K.; Ohta, K.; Yamamoto, K.; et al. Interleukin-33 Enhances Adhesion, CD11b Expression and Survival in Human Eosinophils. Lab. Investig. 2008, 88, 1245–1253. [Google Scholar] [CrossRef]
- Blaylock, M.G.; Lipworth, B.J.; Dempsey, O.J.; Duncan, C.J.A.; Lee, D.K.C.; Lawrie, A.; Douglas, J.G.; Walsh, G.M. Eosinophils from Patients with Asthma Express Higher Levels of the Pan-Leucocyte Receptor CD45 and the Isoform CD45RO. Clin. Exp. Allergy 2003, 33, 936–941. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Munitz, A.; Ackerman, S.J.; Drake, M.G.; Jackson, D.J.; Wardlaw, A.J.; Dougan, S.K.; Berdnikovs, S.; Schleich, F.; Matucci, A.; et al. Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clin. Proc. 2021, 96, 2694–2707. [Google Scholar] [CrossRef]
- Zwirner, J.; Götze, O.; Begemann, G.; Kapp, A.; Kirchhoff, K.; Werfel, T. Evaluation of C3a Receptor Expression on Human Leucocytes by the Use of Novel Monoclonal Antibodies. Immunology 1999, 97, 166–172. [Google Scholar] [CrossRef]
- Emtenani, S.; Holtsche, M.M.; Stahlkopf, R.; Seiler, D.L.; Burn, T.; Liu, H.; Parker, M.; Yilmaz, K.; Dikmen, H.O.; Lang, M.H.; et al. Differential Expression of C5aR1 and C5aR2 in Innate and Adaptive Immune Cells Located in Early Skin Lesions of Bullous Pemphigoid Patients. Front. Immunol. 2022, 13, 942493. [Google Scholar] [CrossRef]
- Steiner, M.; Huber, S.; Harrer, A.; Himly, M. The Evolution of Human Basophil Biology from Neglect towards Understanding of Their Immune Functions. Biomed. Res. Int. 2016, 2016, 8232830. [Google Scholar] [CrossRef] [PubMed]
- Iikura, M.; Miyamasu, M.; Yamaguchi, M.; Kawasaki, H.; Matsushima, K.; Kitaura, M.; Morita, Y.; Yoshie, O.; Yamamoto, K.; Hirai, K. Chemokine Receptors in Human Basophils: Inducible Expression of Functional CXCR4. J. Leukoc. Biol. 2001, 70, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Feng, Y.; Peng, Y.; Zhou, H.; Deng, Z.; Li, L.; Han, H.; Lin, J.; Shi, L.; Wang, S.; et al. Basophil Recruitment to Skin Lesions of Patients with Systemic Lupus Erythematosus Mediated by CCR1 and CCR2. Cell. Physiol. Biochem. 2017, 43, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.S.; McKelvey, A.A.; Sterbinsky, S.A.; Hildreth, J.E.; Derse, C.P.; Klunk, D.A.; Lichtenstein, L.M.; Schleimer, R.P. IL-3 Augments Adhesiveness for Endothelium and CD11b Expression in Human Basophils but Not Neutrophils. J. Immunol. 1990, 145, 1832–1837. [Google Scholar] [CrossRef]
- Han, X.; Jorgensen, J.L.; Brahmandam, A.; Schlette, E.; Huh, Y.O.; Shi, Y.; Awagu, S.; Chen, W. Immunophenotypic Study of Basophils by Multiparameter Flow Cytometry. Arch. Pathol. Lab. Med. 2008, 132, 813–819. [Google Scholar] [CrossRef]
- Füreder, W.; Agis, H.; Willheim, M.; Bankl, H.C.; Maier, U.; Kishi, K.; Müller, M.R.; Czerwenka, K.; Radaszkiewicz, T.; Butterfield, J.H.; et al. Differential Expression of Complement Receptors on Human Basophils and Mast Cells. Evidence for Mast Cell Heterogeneity and CD88/C5aR Expression on Skin Mast Cells. J. Immunol. 1995, 155, 3152–3160. [Google Scholar] [CrossRef]
- Gane, P.; Pecquet, C.; Lambin, P.; Abuaf, N.; Leynadier, F.; Rouger, P. Flow Cytometric Evaluation of Human Basophils. Cytometry 1993, 14, 344–348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).