A Severe Form of Mpox Infection and the Current Epidemiological Status in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Samples
- Electronic Patients Records: Data were extracted from the digital system implemented at INBIMB for epidemiological and clinical analysis, for both suspected and confirmed cases. An Mpox case surveillance form was completed according to INSP guidelines and subsequently reported per Government Decision No. 657/2022 and Ministry of Health Order No. 1738/2022 [14]. Epidemiological, demographic, and clinical data were systematically recorded and processed using Excel software 2024, ensuring adherence to standardized Mpox case classification protocols.
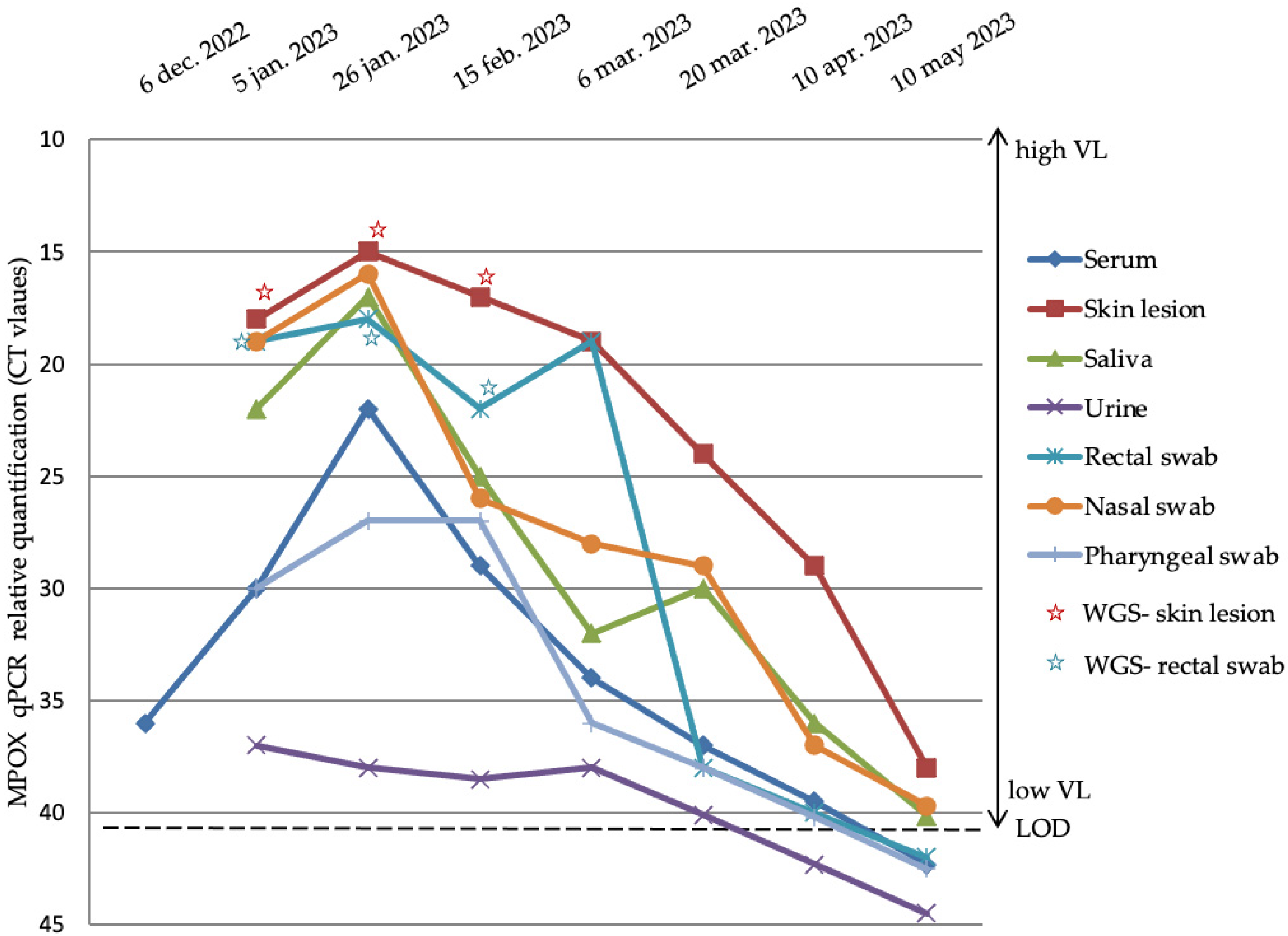
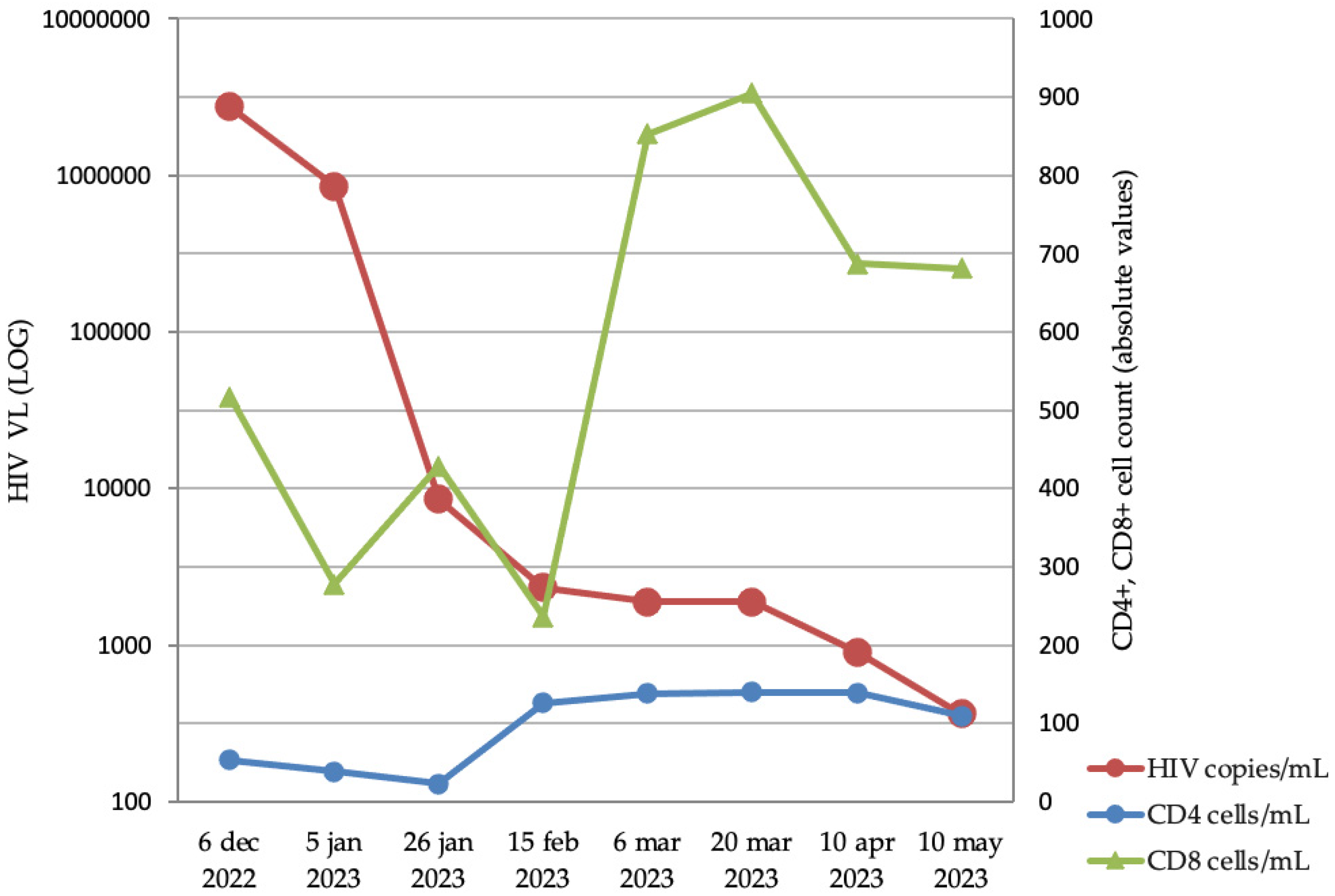
- Clinical and laboratory analysis: Clinical data regarding symptoms and other potentially complicating infections (especially HIV, hepatitis B and C virus, and sexually transmitted infections—STIs) were collected at the patients’ first hospital presentation in the emergency unit. Furthermore, in patients with suspected or living with HIV-1 infection, HIV-1 viral load (VL) and CD4+ and CD8+ T cells in peripheral blood were quantified. Multiple samples were dynamically (approximately every 3 weeks for 5 months) collected from different sanctuaries (sera, skin lesion, saliva, urine, rectal, nasal, and pharyngeal swabs) from one Mpox-positive patient with HIV-1 in order to evaluate MPXV VL evolution. All clinical samples were collected in VTM (viral transport medium) tubes. Approximately 300 μL of VTM media was used for nucleic acid isolation using the QIAmp DSP Virus Kit (Qiagen, Germany) according to the manufacturer’s instructions. MPXV genome detection was performed with the monkeypox virus genesig® Advanced Kit (Primer design, UK) according to the manufacturer’s instructions. The qPCR reactions were performed on the CFX96 thermal cycler (Bio-Rad, USA).
- Whole-genome sequencing (WGS): In order to understand the evolution of MPXV intra-host diversity from patients whose samples were dynamically collected, we performed WGS analyzing mutational sites. The best cycle threshold (Ct) samples (Ct < 25) were selected from two different sanctuaries (zygomatic skin lesion and rectal swabs) to identify potential viral evolution. Approximately 250 ng of extracted nucleic acid was used for next-generation sequencing (NGS) by Illumina DNA Prep (Illumina, USA) following the manufacturer’s recommendations. The reads were polished, trimmed, and de novo-assembled using the shovill pipeline with SPADES 3.12.067 already implemented [15,16]. The resulting contigs were further used for a BLAST reference sequence search [17]. The contigs as well as the raw reads were mapped on the best hit reference sequence. The MPXV strains underwent reference mapping, with trimming in a 5-repeat iteration fashion and the addition of a Q30 quality factor threshold, all performed with Geneious Prime 2023.0.1. The resulting consensus sequences for each of the sequenced strains were further subject to mutational diversity analysis. The sequences were aligned using Mafft [18].
2.2. Ethical Considerations
3. Results
3.1. Epidemiological Situation in Romania
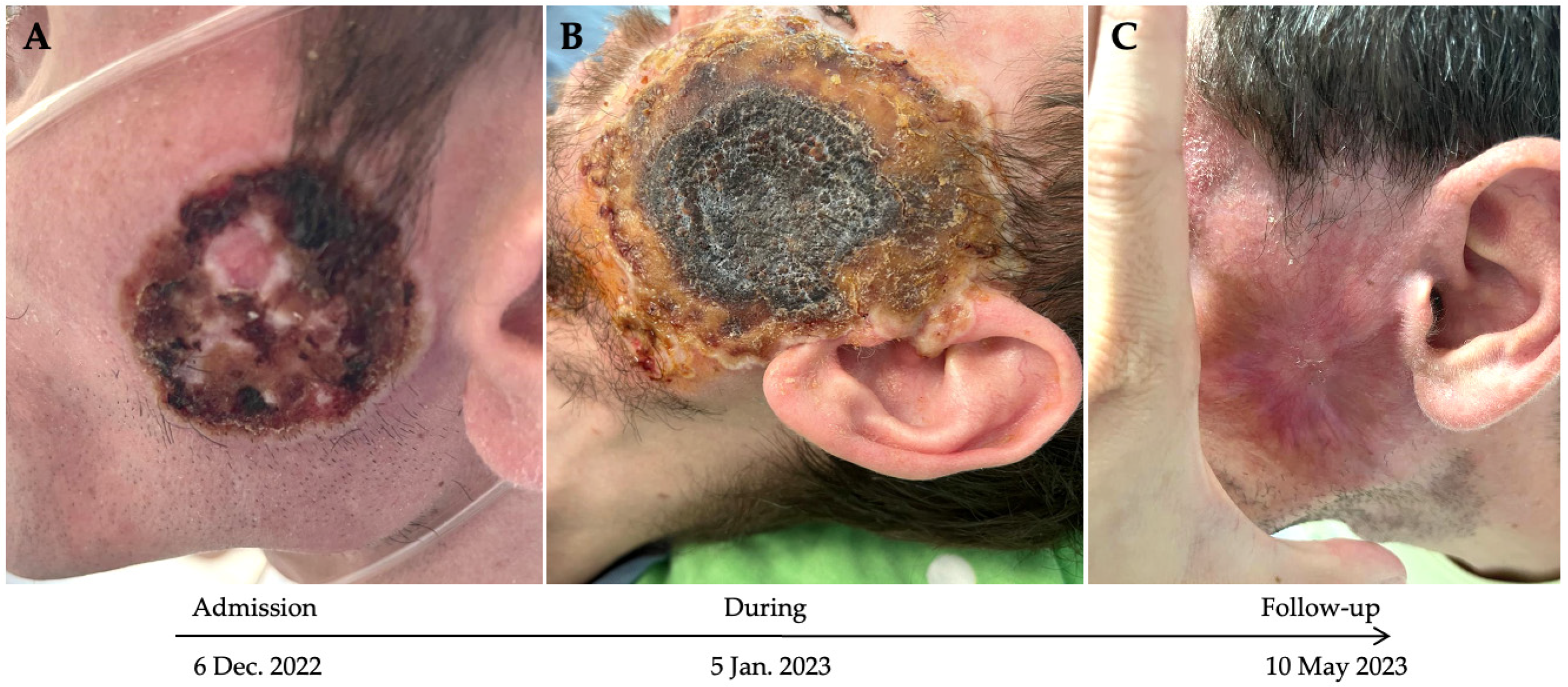
3.2. Case Description
4. Discussion
4.1. Epidemiological Situation of Mpox Infection Worldwide and in Romania
4.2. Mpox Infection in Immunocompromised Patients
4.3. Virological Evolution of Mpox Infection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization—Mpox. Available online: https://www.who.int/news-room/fact-sheets/detail/mpox (accessed on 27 January 2025).
- World Health Organization—Mpox (Monkeypox)—Democratic Republic of the Congo. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON493 (accessed on 28 January 2025).
- Center For Disease Control—Mpox in the United States and Around the World: Current Situation. Available online: https://www.cdc.gov/mpox/situation-summary/index.html (accessed on 25 January 2025).
- Isaacs, S.N.; Mitjà, O. Epidemiology, Clinical Manifestations, and Diagnosis of Mpox (Formerly Monkeypox)—Up to Date. Available online: https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-mpox-formerly-monkeypox/print (accessed on 28 January 2025).
- Naga, N.G.; Nawar, E.A.; Mobarak, A.A.; Faramawy, A.G.; Al-Kordy, H.M.H. Monkeypox: A re-emergent virus with global health implications—A comprehensive review. Trop. Dis. Travel Med. Vaccines 2025, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: Epidemiology, pathogenesis, treatment and prevention. Sig. Transduct. Target. Ther. 2022, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Reda, A.; Hemmeda, L.; Brakat, A.M.; Sah, R.; El-Qushayri, A.E. The clinical manifestations and severity of the 2022 monkeypox outbreak among 4080 patients. Travel Med. Infect. Dis. 2022, 50, 102456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Leng, P.; Zhou, H. Global transmission of monkeypox virus-a potential threat under the COVID-19 pandemic. Front. Immunol. 2023, 14, 1174223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Dabbagh, J.; Mohammad Deeb, E.; Younis, R.; Eissa, R. The dermatological manifestations and differential diagnosis of monkeypox: A narrative review. Medicine 2024, 103, e40359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, H.; Yamamoto, K. Mpox in people with HIV: A narrative review. HIV Med. 2024, 25, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Mpox Outbreak: Global Trends. Geneva: World Health Organization, 2025. Available online: https://worldhealthorg.shinyapps.io/mpx_global/ (accessed on 1 June 2025).
- National Institute for Public Health. Analysis of the Evolution of Communicable Diseases Under Surveillance: Report for the Years 2022 and 2023. Available online: https://insp.gov.ro/centrul-national-de-supraveghere-si-control-al-bolilor-transmisibile-cnscbt/rapoarte-anuale/ (accessed on 24 January 2025).
- CNSCBT.Metodologie-de-Supraveghere-Infectia-cu-Virusul-Variolei-Maimutei-Monkeypox_12.07.2022. Available online: https://insp.gov.ro/centrul-national-de-supraveghere-si-control-al-bolilor-transmisibile-cnscbt/metodologii/ (accessed on 24 January 2025).
- Assemble Bacterial Isolate Genomes from Illumina Paired-End Reads. Available online: https://github.com/tseemann/shovill (accessed on 5 July 2025).
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.A.; Korobeynikov, A.; Lapidus, A.; Prjibelski, A.D.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J. Comput. Biol. 2013, 20, 714–737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hohan, R.; Vlaicu, O.; Bănică, L.; Tudor, A.I.; Negru, A.; Paraschiv, S.; Oțelea, D. Clinical, epidemiological and molecular aspects of patients with mpox in Romania. Germs 2024, 14, 126–135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oprea, C.; Ianache, I.; Piscu, S.; Tardei, G.; Nica, M.; Ceausu, E.; Popescu, C.P.; Florescu, S.A. First report of monkeypox in a patient living with HIV from Romania. Travel Med. Infect. Dis. 2022, 49, 102395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Colina-García, J.F.; Caso, J.M.; González-García, C.; Folgueira, M.D.; Hernández, A.M.; Auñón, P.; Andrés, A.; Cavero, T.; López-Medrano, F.; Morales, E. Severe Presentation of Mpox With Skin, Lung and Pleural Involvement in a Non-HIV-Infected Kidney Transplant Recipient. Am. J. Kidney Dis. 2024, 84, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Mohamed, M.G.; Dabou, E.A.; Abuijlan, I.; Chandran, D.; El-Shall, N.A.; Chopra, H.; Dhama, K. Monkeypox (mpox) in immunosuppressed patients. F1000Research 2023, 12, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitjà, O.; Alemany, A.; Marks, M.; Mora, J.I.L.; Rodríguez-Aldama, J.C.; Silva, M.S.T.; Herrera, E.A.C.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. SHARE-NET writing group. Mpox in people with advanced HIV infection: A global case series. Lancet 2023, 401, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Higgins, E.; Ranganath, N.; Mehkri, O.; Majeed, A.; Walker, J.; Spivack, S.; Bhaimia, E.; Benamu, E.; Hand, J.; Keswani, S.; et al. Clinical features, treatment, and outcomes of mpox in solid organ transplant recipients: A multicenter case series and literature review. Am. J. Transplant. 2023, 23, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Aldama, J.C.; Pérez-Barragán, E.; Hernández-Silva, G.; Lezama-Mora, J.I.; Olin-López, A.K.; González-Flores, B.; Cruz-Flores, R.A.; Crabtree-Ramírez, B. Immune Reconstitution Inflammatory Syndrome Related to Antiretroviral Therapy Initiation in People With HIV and Mpox: An Observational Retrospective Study. Open Forum Infect. Dis. 2024, 11, S133–S136. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Factsheet for Health Professionals on Mpox. Available online: https://www.ecdc.europa.eu/en/all-topics-z/monkeypox/factsheet-health-professionals (accessed on 7 July 2025).
- Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents With HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/mpox (accessed on 7 July 2025).
- Smith, T.G.; Gigante, C.M.; Wynn, N.T.; Matheny, A.; Davidson, W.; Yang, Y.; Condori, R.E.; O’cOnnell, K.; Kovar, L.; Williams, T.L.; et al. Tecovirimat Resistance in Mpox Patients, United States, 2022–2023. Emerg. Infect. Dis. 2023, 29, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Bapolisi, W.A.; Krasemann, S.; Wayengera, M.; Kirenga, B.; Bahizire, E.; Malembaka, E.B.; Fodjo, J.N.S.; Colebunders, R.; Katoto, P.D. Mpox outbreak-tecovirimat resistance, management approaches, and challenges in HIV-endemic regions. Lancet Infect. Dis. 2024, 24, e672–e673. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number of Cases |
|---|---|
| Male | 47/47 |
| Age group | |
| 20–29 years | 12/47 |
| 30–39 years | 25/47 |
| 40–49 years | 8/47 |
| >50 years | 2/47 |
| Residence | |
| Bucharest (capital) | 38/47 |
| Other districts | 9/47 |
| Country | Number of Sexual Partners in the Last 3 Months | n |
|---|---|---|
| Romania and other countries | 1 2 3 10 | 16/47 13/47 5/47 1/47 |
| Not available data | n = 12/47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negru, A.R.; Mangaloiu, D.V.; Vlaicu, O.; Cornovac, A.; Molagic, V.; Duport-Dodot, I.; Tilișcan, C.; Stratan, L.; Marinescu, A.; Cavaropol, L.; et al. A Severe Form of Mpox Infection and the Current Epidemiological Status in Romania. Microorganisms 2025, 13, 1814. https://doi.org/10.3390/microorganisms13081814
Negru AR, Mangaloiu DV, Vlaicu O, Cornovac A, Molagic V, Duport-Dodot I, Tilișcan C, Stratan L, Marinescu A, Cavaropol L, et al. A Severe Form of Mpox Infection and the Current Epidemiological Status in Romania. Microorganisms. 2025; 13(8):1814. https://doi.org/10.3390/microorganisms13081814
Chicago/Turabian StyleNegru, Anca Ruxandra, David Valentin Mangaloiu, Ovidiu Vlaicu, Alexandra Cornovac, Violeta Molagic, Irina Duport-Dodot, Cătălin Tilișcan, Laurențiu Stratan, Adrian Marinescu, Lia Cavaropol, and et al. 2025. "A Severe Form of Mpox Infection and the Current Epidemiological Status in Romania" Microorganisms 13, no. 8: 1814. https://doi.org/10.3390/microorganisms13081814
APA StyleNegru, A. R., Mangaloiu, D. V., Vlaicu, O., Cornovac, A., Molagic, V., Duport-Dodot, I., Tilișcan, C., Stratan, L., Marinescu, A., Cavaropol, L., Bercea, M. N., Păuna, A. M., Pițigoi, D., Aramă, V., & Aramă, S.-S. (2025). A Severe Form of Mpox Infection and the Current Epidemiological Status in Romania. Microorganisms, 13(8), 1814. https://doi.org/10.3390/microorganisms13081814






