Periodontal Microbial Profiles Across Periodontal Conditions in Pediatric Subjects: A Narrative Review
Abstract
1. Introduction
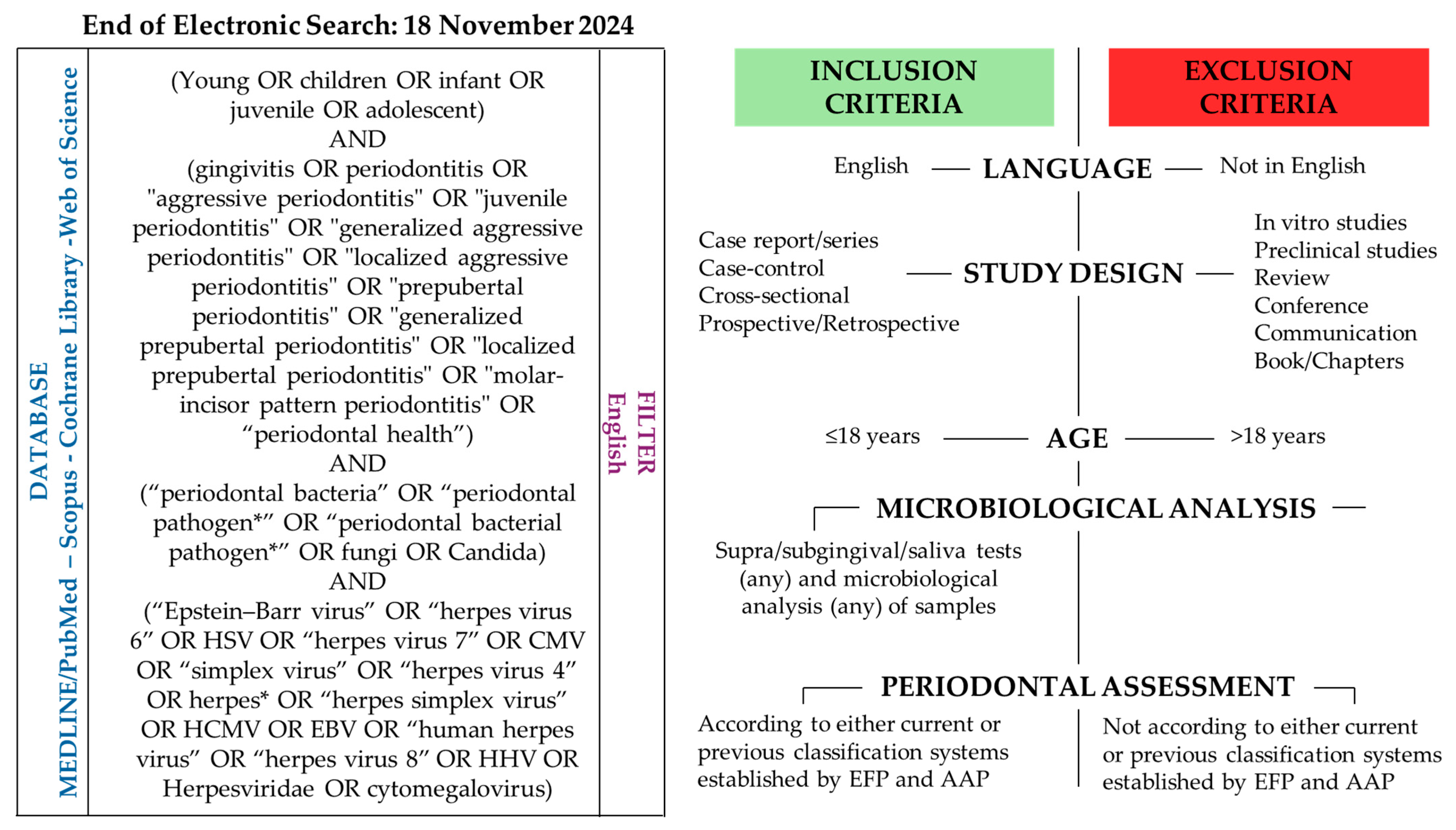
2. Materials and Methods
3. Microbiological Sampling and Analysis of Periodontal Microbiota in Pediatric Subjects
3.1. Periodontal Microbiological Sampling
3.2. Periodontal Microbiological Analysis
4. Periodontal Microbiota in Pediatric Subjects
4.1. Periodontal Bacteria in Pediatric Subjects
4.2. Periodontal Viruses in Pediatric Subjects
4.3. Periodontal Fungi in Pediatric Subjects
4.4. Periodontal Microbial Interactions in Pediatric Subjects
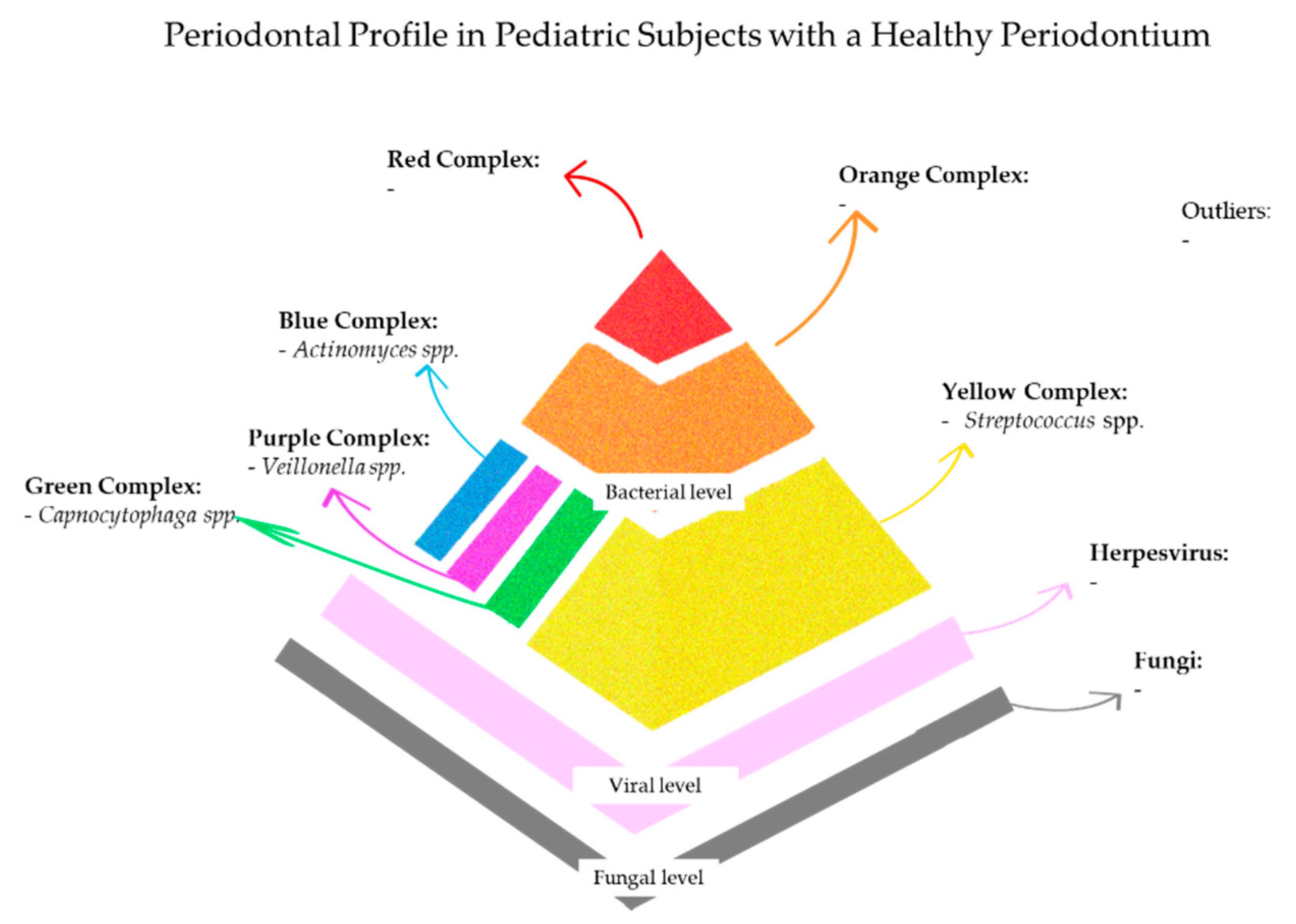
5. Periodontal Profile in Pediatric Subjects with a Healthy Periodontium
5.1. Periodontal Profile in Systemically Healthy Pediatric Subjects with a Healthy Periodontium
5.2. Periodontal Profile in Systemically Compromised Pediatric Subjects with a Healthy Periodontium
6. Periodontal Profile in Pediatric Subjects with Gingivitis
6.1. Periodontal Profile in Systemically Healthy Pediatric Subjects with Gingivitis
6.2. Periodontal Profile in Systemically Compromised Pediatric Subjects with Gingivitis
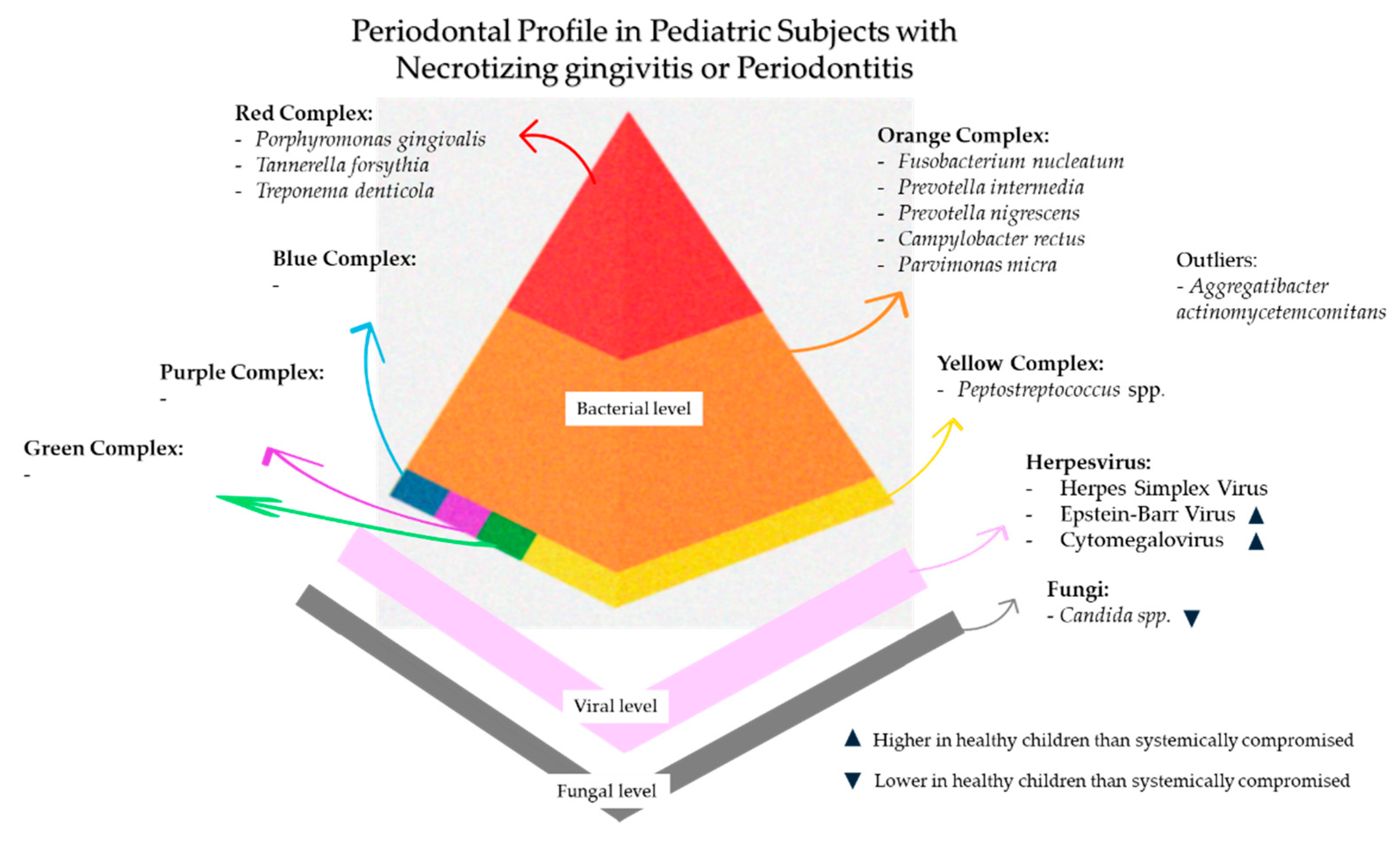
7. Periodontal Profile in Pediatric Subjects with Necrotizing Gingivitis/Periodontitis
7.1. Periodontal Profile in Systemically Healthy Pediatric Subjects with Necrotizing Gingivitis/Periodontitis
7.2. Periodontal Profile in Systemically Compromised Pediatric Subjects with Necrotizing Gingivitis/Periodontitis
8. Periodontal Profile in Pediatric Subjects with Periodontitis
8.1. Periodontal Profile in Systemically Healthy Pediatric Subjects with Periodontitis
8.2. Periodontal Profile in Systemically Compromised Pediatric Subjects with Periodontitis
9. General Health, Periodontal Status, and Related Microbial Profile in Pediatric Subjects
9.1. Rare Primary Immunodeficiencies and Genetic Immune Dysregulation Syndromes
9.2. Secondary Immunosuppression Resulting from Infectious or Nutritional Etiologies
9.3. Multisystem Genetic Syndromes
9.4. Metabolic and Hematologic Disorders Affecting the Immune System
10. Discussion
10.1. Which Microbial Shifts Herald Progression from Health to Gingivitis?
10.2. How Does the Pediatric Bacterial Periodontal Profile Compare to the Adult One?
10.3. What Roles Do Viruses Play on Pediatric Periodontal Status?
10.4. What Roles Do Viruses and Fungi Play on Pediatric Periodontal Status?
10.5. Study Limits and Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiebe, C.B.; Putnins, E.E. The Periodontal Disease Classification System of the American Academy of Periodontology—An Update. J. Can. Dent. Assoc. 2000, 66, 594–597. [Google Scholar]
- Ranney, R.R. Diagnosis of Periodontal Diseases. Adv. Dent. Res. 1991, 5, 21–36. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-implant Diseases and Conditions—Introduction and Key Changes from the 1999 Classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172, Erratum in: J. Periodontol. 2018, 89, 1475. https://doi.org/10.1002/jper.10239. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, N.; Thurnheer, T.; Kreutzer, S.; Gmür, R.D.; Attin, T.; Russo, G.; Karygianni, L. Necrotizing Gingivitis: Microbial Diversity and Quantification of Protein Secretion in Necrotizing Gingivitis. Antibiotics 2021, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Alonso, B.; De Arriba, L.; Santa Cruz, I.; Serrano, C.; Sanz, M. Acute Periodontal Lesions. Periodontology 2000 2014, 65, 149–177. [Google Scholar] [CrossRef]
- Albandar, J.M.; Susin, C.; Hughes, F.J. Manifestations of Systemic Diseases and Conditions That Affect the Periodontal Attachment Apparatus: Case Definitions and Diagnostic Considerations. J. Periodontol. 2018, 89, S183–S203. [Google Scholar] [CrossRef]
- Botero, J.E.; Rodríguez-Medina, C.; Amaya-Sanchez, S.; Salazar, C.L.; Contreras, A. A Comprehensive Review of the Relationship Between Oral Health and Down Syndrome. Curr. Oral Health Rep. 2024, 11, 15–22. [Google Scholar] [CrossRef]
- Duque, C.; João, M.F.D.; Camargo, G.A.d.C.G.; Teixeira, G.S.; Machado, T.S.; Azevedo, R.d.S.; Mariano, F.S.; Colombo, N.H.; Vizoto, N.L.; Mattos-Graner, R.d.O. Microbiological, Lipid and Immunological Profiles in Children with Gingivitis and Type 1 Diabetes Mellitus. J. Appl. Oral Sci. 2017, 25, 217–226. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a Classification System for Periodontal Diseases and Conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Ting, M.; Contreras, A.; Slots, J. Herpesviruses in Localized Juvenile Periodontitis. J. Periodontal Res. 2000, 35, 17–25. [Google Scholar] [CrossRef]
- Slots, J. Herpesviral-Bacterial Interactions in Periodontal Diseases. Periodontology 2000 2010, 52, 117–140. [Google Scholar] [CrossRef]
- Grinde, B. Herpesviruses: Latency and Reactivation—Viral Strategies and Host Response. J. Oral Microbiol. 2013, 5, 22766. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The Keystone-Pathogen Hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Grevich, S.; Lee, P.; Leroux, B.; Ringold, S.; Darveau, R.; Henstorf, G.; Berg, J.; Kim, A.; Velan, E.; Kelly, J.; et al. Oral Health and Plaque Microbial Profile in Juvenile Idiopathic Arthritis. Pediatr. Rheumatol. 2019, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Lanau, N.; Mareque-Bueno, J.; Zabalza, M. Prevalence of high blood pressure in periodontal patients: A pilot study. Dent. Med. Probl. 2023, 60, 635–640. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pisano, M.; Di Palo, M.P.; De Benedetto, G.; Rizki, I.; Franci, G.; Amato, M. Periodontal Status and Herpesiviridae, Bacteria, and Fungi in Gingivitis and Periodontitis of Systemically Compromised Pediatric Subjects: A Systematic Review. Children 2025, 12, 375. [Google Scholar] [CrossRef]
- Sánchez-Romero, M.I.; García-Lechuz Moya, J.M.; González López, J.J.; Orta Mira, N. Recogida, Transporte y Procesamiento General de Las Muestras En El Laboratorio de Microbiología. Enferm. Infecc. Microbiol. Clin. 2019, 37, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Ramette, A. Multivariate Analyses in Microbial Ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pisano, M.; Caggiano, M.; De Benedetto, G.; Di Palo, M.P.; Franci, G.; Amato, M. Human Herpesviruses, Bacteria, and Fungi in Gingivitis and Periodontitis Pediatric Subjects: A Systematic Review. Children 2024, 12, 39. [Google Scholar] [CrossRef]
- Bang, E.; Oh, S.; Ju, U.; Chang, H.E.; Hong, J.-S.; Baek, H.-J.; Kim, K.-S.; Lee, H.-J.; Park, K.U. Factors Influencing Oral Microbiome Analysis: From Saliva Sampling Methods to next-Generation Sequencing Platforms. Sci. Rep. 2023, 13, 10086. [Google Scholar] [CrossRef]
- Rams, T.E.; Contreras, A.; Slots, J. Aggressive Periodontitis in Southwestern American Indian Adolescents. J. Periodontol. 2024, 95, 594–602. [Google Scholar] [CrossRef]
- Hanookai, D.; Nowzari, H.; Contreras, A.; Morrison, J.L.; Slots, J. Herpesviruses and Periodontopathic Bacteria in Trisomy 21 Periodontitis. J. Periodontol. 2000, 71, 376–384. [Google Scholar] [CrossRef]
- Otero, R.A.; Nascimento, F.N.N.; Souza, I.P.R.; Silva, R.C.; Lima, R.S.; Robaina, T.F.; Câmara, F.P.; Santos, N.; Castro, G.F. Lack of Association Between Herpesvirus Detection in Saliva and Gingivitis in Hiv-Infected Children. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 221–225. [Google Scholar] [CrossRef]
- Contreras, A.; Slots, J. Active Cytomegalovirus Infection in Human Periodontitis. Oral Microbiol. Immunol. 1998, 13, 225–230. [Google Scholar] [CrossRef]
- Yildirim, S.; Yapar, M.; Kubar, A. Detection and Quantification of Herpesviruses in Kostmann Syndrome Periodontitis Using Real-time Polymerase Chain Reaction: A Case Report. Oral Microbiol. Immunol. 2006, 21, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Schmidlin, P.R.; Sahrmann, P. Molecular Microbiological Evaluation of Subgingival Biofilm Sampling by Paper Point and Curette. APMIS 2014, 122, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Giordano, F.; Di Palo, M.P.; D’Ambrosio, F.; Scognamiglio, B.; Sangiovanni, G.; Caggiano, M.; Gasparro, R. Microbiota of Peri-Implant Healthy Tissues, Peri-Implant Mucositis, and Peri-Implantitis: A Comprehensive Review. Microorganisms 2024, 12, 1137. [Google Scholar] [CrossRef] [PubMed]
- Council for International Organizations of Medical Sciences (CIOMS). International Ethical Guidelines for Health-Related Research Involving Humans, 4th ed.; Council for International Organizations of Medical Sciences (CIOMS): Geneva, Switzerland, 2016. [Google Scholar]
- Lagier, J.-C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.-M.; Fournier, P.-E.; Raoult, D. Culturing the Human Microbiota and Culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal Microbial Ecology. Periodontology 2000 2005, 38, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.; Teles, F.; Frias-Lopez, J.; Paster, B.; Haffajee, A. Lessons Learned and Unlearned in Periodontal Microbiology. Periodontology 2000 2013, 62, 95–162. [Google Scholar] [CrossRef]
- Reyes, A.; Semenkovich, N.P.; Whiteson, K.; Rohwer, F.; Gordon, J.I. Going Viral: Next-Generation Sequencing Applied to Phage Populations in the Human Gut. Nat. Rev. Microbiol. 2012, 10, 607–617. [Google Scholar] [CrossRef]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun Metagenomics, from Sampling to Analysis. Nat. Biotechnol. 2017, 35, 833–844. [Google Scholar] [CrossRef]
- Radaic, A.; Kapila, Y.L. The Oralome and Its Dysbiosis: New Insights into Oral Microbiome-Host Interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Panduwawala, C.P.; Samaranayake, L.P. Biodiversity of the Human Oral Mycobiome in Health and Disease. Oral Dis. 2019, 25, 363–371. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Slots, J. Herpesvirus Periodontitis: Infection Beyond Biofilm. J. Calif. Dent. Assoc. 2011, 39, 393–399. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial Interactions in Dental Biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef]
- Iniesta, M.; Chamorro, C.; Ambrosio, N.; Marín, M.J.; Sanz, M.; Herrera, D. Subgingival Microbiome in Periodontal Health, Gingivitis and Different Stages of Periodontitis. J. Clin. Periodontol. 2023, 50, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Mashima, I.; Nakazawa, F. The Influence of Oral Veillonella Species on Biofilms Formed by Streptococcus Species. Anaerobe 2014, 28, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ciantar, M.; Gilthorpe, M.S.; Hurel, S.J.; Newman, H.N.; Wilson, M.; Spratt, D.A. Capnocytophaga Spp. in Periodontitis Patients Manifesting Diabetes Mellitus. J. Periodontol. 2005, 76, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Beyond the Red Complex and into More Complexity: The Polymicrobial Synergy and Dysbiosis (PSD) Model of Periodontal Disease Etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef]
- Berezow, A.B.; Darveau, R.P. Microbial Shift and Periodontitis. Periodontology 2000 2011, 55, 36–47. [Google Scholar] [CrossRef]
- González, R.; Elena, S.F. The Interplay between the Host Microbiome and Pathogenic Viral Infections. Mbio 2021, 12, e02496-21. [Google Scholar] [CrossRef]
- Messina, B.M.; Grippaudo, C.; Polizzi, A.; Blasi, A.; Isola, G. The Key Role of Porphyromonas Gingivalis in the Pathogenesis of Periodontitis Linked with Systemic Diseases. Appl. Sci. 2025, 15, 6847. [Google Scholar] [CrossRef]
- Ishihara, K. Virulence Factors of Treponema Denticola. Periodontology 2000 2010, 54, 117–135. [Google Scholar] [CrossRef]
- Yousefi, L.; Leylabadlo, H.E.; Pourlak, T.; Eslami, H.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Kafil, H.S. Oral Spirochetes: Pathogenic Mechanisms in Periodontal Disease. Microb. Pathog. 2020, 144, 104193. [Google Scholar] [CrossRef]
- Larsson, S.C.; Burgess, S. Appraising the Causal Role of Smoking in Multiple Diseases: A Systematic Review and Meta-Analysis of Mendelian Randomization Studies. EBioMedicine 2022, 82, 104154. [Google Scholar] [CrossRef]
- Nishihara, T.; Ishihara, Y.; Koseki, T.; Boutsi, E.A.; Senpuku, H.; Hanada, N. Membrane-Associated Interleukin-1 on Macrophages Stimulated with Actinobacillus Actinomycetemcomitans Lipopolysaccharide Induces Osteoclastic Bone Resorption in Vivo. Cytobios 1995, 81, 229–237. [Google Scholar]
- Silveira, V.R.S.; Nogueira, M.V.B.; Nogueira, N.A.P.; Lima, V.; Furlaneto, F.A.C.; Rego, R.O. Leukotoxicity of Aggregatibacter Actinomycetemcomitans in Generalized Aggressive Periodontitis in Brazilians and Their Family Members. J. Appl. Oral Sci. 2013, 21, 430–436. [Google Scholar] [CrossRef]
- Naqvi, A.R.; Slots, J. Human and Herpesvirus MicroRNAs in Periodontal Disease. Periodontology 2000 2021, 87, 325–339. [Google Scholar] [CrossRef]
- Wade, W.G. The Oral Microbiome in Health and Disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Slots, J. Herpesviruses in Periodontal Diseases. Periodontology 2000 2005, 38, 33–62. [Google Scholar] [CrossRef]
- Amit, R.; Morag, A.; Ravid, Z.; Hochman, N.; Ehrlich, J.; Zakay-Rones, Z. Detection of Herpes Simplex Virus in Gingival Tissue. J. Periodontol. 1992, 63, 502–506. [Google Scholar] [CrossRef]
- Chen, C.; Feng, P.; Slots, J. Herpesvirus-bacteria Synergistic Interaction in Periodontitis. Periodontology 2000 2020, 82, 42–64. [Google Scholar] [CrossRef]
- Botero, J.E.; Parra, B.; Jaramillo, A.; Contreras, A. Subgingival Human Cytomegalovirus Correlates with Increased Clinical Periodontal Parameters and Bacterial Coinfection in Periodontitis. J. Periodontol. 2007, 78, 2303–2310. [Google Scholar] [CrossRef]
- Saygun, I.; Kubar, A.; Özdemir, A.; Yapar, M.; Slots, J. Herpesviral–Bacterial Interrelationships in Aggressive Periodontitis. J. Periodontal Res. 2004, 39, 207–212. [Google Scholar] [CrossRef]
- SUBRAMANIAM, A.; BRITT, W.J. Herpesviridae Infection: Prevention, Screening, and Management. Clin. Obstet. Gynecol. 2018, 61, 157–176. [Google Scholar] [CrossRef]
- Wu, Y.M.; Yan, J.; Ojcius, D.M.; Chen, L.L.; Gu, Z.Y.; Pan, J.P. Correlation between infections with different genotypes of human cytomegalovirus and Epstein-Barr virus in subgingival samples and periodontal status of patients. J. Clin. Microbiol. 2007, 45, 3665–3670, Erratum in: J. Clin. Microbiol. 2008, 46, 836. [Google Scholar] [CrossRef] [PubMed]
- Kolokotronis, A.; Louloudiadis, K.; Fotiou, G.; Matiais, A. Oral Manifestations of Infections Due to Varicella Zoster Virus in Otherwise Healthy Children. J. Clin. Pediatr. Dent. 2001, 25, 107–112. [Google Scholar] [CrossRef]
- Contaldo, M.; Di Stasio, D.; Romano, A.; Fiori, F.; Della Vella, F.; Rupe, C.; Lajolo, C.; Petruzzi, M.; Serpico, R.; Lucchese, A. Oral Candidiasis and Novel Therapeutic Strategies: Antifungals, Phytotherapy, Probiotics, and Photodynamic Therapy. Curr. Drug Deliv. 2023, 20, 441–456. [Google Scholar] [CrossRef]
- Contaldo, M.; Santoro, R.; Romano, A.; Loffredo, F.; Di Stasio, D.; Della Vella, F.; Scivetti, M.; Petruzzi, M.; Serpico, R.; Lucchese, A. Oral Manifestations in Children and Young Adults with Down Syndrome: A Systematic Review of the Literature. Appl. Sci. 2021, 11, 5408. [Google Scholar] [CrossRef]
- Slazhneva, E.; Tikhomirova, E.; Tsarev, V.; Orekhova, L.; Loboda, E.; Atrushkevich, V. Candida Species Detection in Patients with Chronic Periodontitis: A Systematic Review and Meta-analysis. Clin. Exp. Dent. Res. 2022, 8, 1354–1375. [Google Scholar] [CrossRef]
- Patini, R.; Gioco, G.; Rupe, C.; Contaldo, M.; Serpico, R.; Giuliani, M.; Lajolo, C. Oral Candida and Psoriasis: Is There Association? A Systematic Review and Trial Sequential Analysis. Oral Dis. 2023, 29, 3121–3135. [Google Scholar] [CrossRef]
- Hu, Y.; Ren, B.; Cheng, L.; Deng, S.; Chen, Q. Candida Species in Periodontitis: A New Villain or a New Target? J. Dent. 2024, 148, 105138. [Google Scholar] [CrossRef]
- Kröger, A.; Hülsmann, C.; Fickl, S.; Spinell, T.; Hüttig, F.; Kaufmann, F.; Heimbach, A.; Hoffmann, P.; Enkling, N.; Renvert, S.; et al. The Severity of Human Peri-implantitis Lesions Correlates with the Level of Submucosal Microbial Dysbiosis. J. Clin. Periodontol. 2018, 45, 1498–1509. [Google Scholar] [CrossRef]
- Bachtiar, E.W.; Bachtiar, B.M.; Jarosz, L.M.; Amir, L.R.; Sunarto, H.; Ganin, H.; Meijler, M.M.; Krom, B.P. AI-2 of Aggregatibacter Actinomycetemcomitans Inhibits Candida Albicans Biofilm Formation. Front. Cell. Infect. Microbiol. 2014, 4, 94. [Google Scholar] [CrossRef]
- Jabri, B.; Iken, M.; Ait- Ou-amar, S.; Rida, S.; Bouziane, A.; Ennibi, O.K. Candida Albicans and Candida Dubliniensis in Periodontitis in Adolescents and Young Adults. Int. J. Microbiol. 2022, 2022, 4625368. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Di Spirito, F.; D’Ambrosio, F.; Boccia, G.; Moccia, G.; De Caro, F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms 2022, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S74–S84. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef]
- Ma, Z.; Jiang, Z.; Dong, H.; Xu, W.; Yan, S.; Chen, J.; Li, A.; Wang, X. Microbial Communities and Functional Genes in Periodontitis and Healthy Controls. Int. Dent. J. 2024, 74, 638–646. [Google Scholar] [CrossRef]
- Uriarte, S.M.; Edmisson, J.S.; Jimenez-Flores, E. Human Neutrophils and Oral Microbiota: A Constant Tug-of-war between a Harmonious and a Discordant Coexistence. Immunol. Rev. 2016, 273, 282–298. [Google Scholar] [CrossRef]
- Zannella, C.; Shinde, S.; Vitiello, M.; Falanga, A.; Galdiero, E.; Fahmi, A.; Santella, B.; Nucci, L.; Gasparro, R.; Galdiero, M.; et al. Antibacterial Activity of Indolicidin-Coated Silver Nanoparticles in Oral Disease. Appl. Sci. 2020, 10, 1837. [Google Scholar] [CrossRef]
- Stadler, A.F.; Angst, P.D.M.; Arce, R.M.; Gomes, S.C.; Oppermann, R.V.; Susin, C. Gingival Crevicular Fluid Levels of Cytokines/Chemokines in Chronic Periodontitis: A Meta-analysis. J. Clin. Periodontol. 2016, 43, 727–745. [Google Scholar] [CrossRef]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and Chemokines in Periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- de Lima, C.L.; Acevedo, A.C.; Grisi, D.C.; Taba, M.; Guerra, E.; De Luca Canto, G. Host-derived Salivary Biomarkers in Diagnosing Periodontal Disease: Systematic Review and Meta-analysis. J. Clin. Periodontol. 2016, 43, 492–502. [Google Scholar] [CrossRef]
- Faria Carrada, C.; Almeida Ribeiro Scalioni, F.; Evangelista Cesar, D.; Lopes Devito, K.; Ribeiro, L.C.; Almeida Ribeiro, R. Salivary Periodontopathic Bacteria in Children and Adolescents with Down Syndrome. PLoS ONE 2016, 11, e0162988. [Google Scholar] [CrossRef] [PubMed]
- Martinon, P.; Fraticelli, L.; Giboreau, A.; Dussart, C.; Bourgeois, D.; Carrouel, F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Aren, G.; Sepet, E.; Özdemır, D.; Dınççağ, N.; Güvener, B.; Firatli, E. Periodontal Health, Salivary Status, and Metabolic Control in Children with Type 1 Diabetes Mellitus. J. Periodontol. 2003, 74, 1789–1795. [Google Scholar] [CrossRef]
- Van Der Meulen, T.; Harmsen, H.; Bootsma, H.; Spijkervet, F.; Kroese, F.; Vissink, A. The Microbiome–Systemic Diseases Connection. Oral Dis. 2016, 22, 719–734. [Google Scholar] [CrossRef]
- Gomez, A.; Nelson, K.E. The Oral Microbiome of Children: Development, Disease, and Implications Beyond Oral Health. Microb. Ecol. 2017, 73, 492–503. [Google Scholar] [CrossRef]
- Nemec, M.; Mittinger, N.; Bertl, M.; Liu, E.; Jonke, E.; Andrukhov, O.; Rausch-Fan, X. Salivary MRP-8/14 and the Presence of Periodontitis-Associated Bacteria in Children with Bonded Maxillary Expansion Treatment. Clin. Oral Investig. 2021, 25, 3767–3774. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Davey, M.E.; O’toole, G.A. Microbial Biofilms: From Ecology to Molecular Genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef]
- Höglund Åberg, C.; Kwamin, F.; Claesson, R.; Dahlén, G.; Johansson, A.; Haubek, D. Progression of Attachment Loss Is Strongly Associated with Presence of the JP2 Genotype of Aggregatibacter Actinomycetemcomitans: A Prospective Cohort Study of a Young Adolescent Population. J. Clin. Periodontol. 2014, 41, 232–241. [Google Scholar] [CrossRef]
- Ludovichetti, F.S. Periodontitis in the Developmental Age: Pathogenesis, Epidemiology, Differential Diagnosis and Treatment. A Narrative Review. Interv. Pediatr. Dent. Open Access J. 2020, 3, 256–264. [Google Scholar] [CrossRef]
- Crielaard, W.; Zaura, E.; Schuller, A.A.; Huse, S.M.; Montijn, R.C.; Keijser, B.J.F. Exploring the Oral Microbiota of Children at Various Developmental Stages of Their Dentition in the Relation to Their Oral Health. BMC Med. Genom. 2011, 4, 22. [Google Scholar] [CrossRef]
- Dzidic, M.; Collado, M.C.; Abrahamsson, T.; Artacho, A.; Stensson, M.; Jenmalm, M.C.; Mira, A. Oral Microbiome Development during Childhood: An Ecological Succession Influenced by Postnatal Factors and Associated with Tooth Decay. ISME J. 2018, 12, 2292–2306. [Google Scholar] [CrossRef]
- Velsko, I.M.; Fellows Yates, J.A.; Aron, F.; Hagan, R.W.; Frantz, L.A.F.; Loe, L.; Martinez, J.B.R.; Chaves, E.; Gosden, C.; Larson, G.; et al. Microbial Differences between Dental Plaque and Historic Dental Calculus Are Related to Oral Biofilm Maturation Stage. Microbiome 2019, 7, 102. [Google Scholar] [CrossRef]
- Gu, Y.; Han, X. Toll-Like Receptor Signaling and Immune Regulatory Lymphocytes in Periodontal Disease. Int. J. Mol. Sci. 2020, 21, 3329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Chen, X.; Jiang, W.; Wang, S.; Xu, L.; Tu, Y.; Zheng, P.; Wang, Y.; Lin, X.; et al. Profiling of Oral Microbiota in Early Childhood Caries Using Single-Molecule Real-Time Sequencing. Front. Microbiol. 2017, 8, 2244. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Kuraji, R.; Kapila, Y.L. The Human Oral Virome: Shedding Light on the Dark Matter. Periodontology 2000 2021, 87, 282–298. [Google Scholar] [CrossRef]
- Pride, D.T.; Salzman, J.; Haynes, M.; Rohwer, F.; Davis-Long, C.; White, R.A.; Loomer, P.; Armitage, G.C.; Relman, D.A. Evidence of a Robust Resident Bacteriophage Population Revealed through Analysis of the Human Salivary Virome. ISME J. 2012, 6, 915–926. [Google Scholar] [CrossRef]
- Contreras, A.; Botero, J.E.; Slots, J. Biology and Pathogenesis of Cytomegalovirus in Periodontal Disease. Periodontology 2000 2014, 64, 40–56. [Google Scholar] [CrossRef]
- Contaldo, M.; Lucchese, A.; Lajolo, C.; Rupe, C.; Di Stasio, D.; Romano, A.; Petruzzi, M.; Serpico, R. The Oral Microbiota Changes in Orthodontic Patients and Effects on Oral Health: An Overview. J. Clin. Med. 2021, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, Y.; Xie, X.; Wang, H.; Li, X.; Fang, D.; Bai, Y. Candida albicans Enriched in Orthodontic Derived White Spot Lesions and Shaped Focal Supragingival Bacteriome. Front. Microbiol. 2023, 14, 1084850. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.; Flores-Fraile, J.; Lo Giudice, R.; Marchetti, E.; Nart, J.; Greethurst, A.R.; Voltas, F.R.; Tarallo, F.; Galletti, C. Association Between Alzheimer’s Disease and Periodontal Inflammatory Parameters: A Systematic Review. J. Clin. Exp. Dent. 2025, 17, e310–e323. [Google Scholar] [CrossRef] [PubMed]
- Suresh Unniachan, A.; Krishnavilasom Jayakumari, N.; Sethuraman, S. Association between Candida Species and Periodontal Disease: A Systematic Review. Curr. Med. Mycol. 2020, 6, 63–68. [Google Scholar] [CrossRef]
- Contreras, A.; Falkler, W.A.; Enwonwu, C.O.; Idigbe, E.O.; Savage, K.O.; Afolabi, M.B.; Onwujekwe, D.; Rams, T.E.; Slots, J. Human Herpesviridae in Acute Necrotizing Ulcerative Gingivitis in Children in Nigeria. Oral Microbiol. Immunol. 1997, 12, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Vocale, C.; Montevecchi, M.; D’Alessandro, G.; Gatto, M.; Piana, G.; Nibali, L.; Re, M.C.; Sambri, V. Subgingival Periodontal Pathogens in Down Syndrome Children without Periodontal Breakdown. A Case-Control Study on Deciduous Teeth. Eur. J. Paediatr. Dent. 2021, 22, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Lyko, K.; Bonfim, C.; Benelli, E.M.; Torres-Pereira, C.C.; Amenábar, J.M. Salivary Detection of Periodontopathic Bacteria in Fanconi’s Anemia Patients. Anaerobe 2013, 24, 32–35. [Google Scholar] [CrossRef]
- Pachoński, M.; Koczor-Rozmus, A.; Mocny-Pachońska, K.; Łanowy, P.; Mertas, A.; Jarosz-Chobot, P. Oral Microbiota in Children with Type 1 Diabetes Mellitus. Pediatr. Endocrinol. Diabetes Metab. 2021, 27, 100–108. [Google Scholar] [CrossRef]
- Ho, D.Y.; Enriquez, K.; Multani, A. Herpesvirus Infections Potentiated by Biologics. Infect. Dis. Clin. N. Am. 2020, 34, 311–339. [Google Scholar] [CrossRef]
- Pólvora, T.L.S.; Nobre, Á.V.V.; Tirapelli, C.; Taba, M.; Macedo, L.D.d.; Santana, R.C.; Pozzetto, B.; Lourenço, A.G.; Motta, A.C.F. Relationship between Human Immunodeficiency Virus (HIV-1) Infection and Chronic Periodontitis. Expert Rev. Clin. Immunol. 2018, 14, 315–327. [Google Scholar] [CrossRef]
- Ford, E.S.; Magaret, A.S.; Spak, C.W.; Selke, S.; Kuntz, S.; Corey, L.; Wald, A. Increase in HSV Shedding at Initiation of Antiretroviral Therapy and Decrease in Shedding over Time on Antiretroviral Therapy in HIV and HSV-2 Infected Persons. AIDS 2018, 32, 2525–2531. [Google Scholar] [CrossRef]
- Nastri, B.M.; Pagliano, P.; Zannella, C.; Folliero, V.; Masullo, A.; Rinaldi, L.; Galdiero, M.; Franci, G. HIV and Drug-Resistant Subtypes. Microorganisms 2023, 11, 221. [Google Scholar] [CrossRef]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Fairlie, K.; Ferrandiz, J.; Nasri, C.; McKiernan, M.; Gunsolley, J. Aggregatibacter Actinomycetemcomitans and Its Relationship to Initiation of Localized Aggressive Periodontitis: Longitudinal Cohort Study of Initially Healthy Adolescents. J. Clin. Microbiol. 2007, 45, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Barton, E.S.; White, D.W.; Cathelyn, J.S.; Brett-McClellan, K.A.; Engle, M.; Diamond, M.S.; Miller, V.L.; Virgin, H.W. Herpesvirus Latency Confers SyMbiotic Protection from Bacterial Infection. Nature 2007, 447, 326–329. [Google Scholar] [CrossRef]
- Teles, F.; Wang, Y.; Hajishengallis, G.; Hasturk, H.; Marchesan, J.T. Impact of Systemic Factors in Shaping the Periodontal Microbiome. Periodontology 2000 2021, 85, 126–160. [Google Scholar] [CrossRef]
- Nel Van Zyl, K.; Matukane, S.R.; Hamman, B.L.; Whitelaw, A.C.; Newton-Foot, M. Effect of Antibiotics on the Human Microbiome: A Systematic Review. Int. J. Antimicrob. Agents 2022, 59, 106502. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Li, S.; Wang, X.; Liu, J.; Li, X. The Prevalence of Gingivitis and Related Risk Factors in Schoolchildren Aged 6–12 Years Old. BMC Oral Health 2022, 22, 623. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Silva, C.O.; Tatakis, D.N. Plaque-induced Gingivitis: Case Definition and Diagnostic Considerations. J. Clin. Periodontol. 2018, 45, S44–S67. [Google Scholar] [CrossRef]
- Wirth, R.; Maróti, G.; Lipták, L.; Mester, M.; Al Ayoubi, A.; Pap, B.; Madléna, M.; Minárovits, J.; Kovács, K.L. Microbiomes in Supragingival Biofilms and Saliva of Adolescents with Gingivitis and Gingival Health. Oral Dis. 2022, 28, 2000–2014. [Google Scholar] [CrossRef]
- Di Spirito, F.; D’Ambrosio, F.; Cannatà, D.; D’Antò, V.; Giordano, F.; Martina, S. Impact of Clear Aligners versus Fixed Appliances on Periodontal Status of Patients Undergoing Orthodontic Treatment: A Systematic Review of Systematic Reviews. Healthcare 2023, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental Plaque–Induced Gingival Conditions. J. Periodontol. 2018, 89, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.; Eber, R.; Wang, H. Periodontal Diseases in the Child and Adolescent. J. Clin. Periodontol. 2002, 29, 400–410. [Google Scholar] [CrossRef]
- Al-Ghutaimel, H.; Riba, H.; Al-Kahtani, S.; Al-Duhaimi, S. Common Periodontal Diseases of Children and Adolescents. Int. J. Dent. 2014, 2014, 850674. [Google Scholar] [CrossRef]
- Fan, W.; Liu, C.; Zhang, Y.; Yang, Z.; Li, J.; Huang, S. Epidemiology and Associated Factors of Gingivitis in Adolescents in Guangdong Province, Southern China: A Cross-Sectional Study. BMC Oral Health 2021, 21, 311. [Google Scholar] [CrossRef]
- Funieru, C.; Klinger, A.; Băicuș, C.; Funieru, E.; Dumitriu, H.T.; Dumitriu, A. Epidemiology of Gingivitis in Schoolchildren in Bucharest, Romania: A Cross-sectional Study. J. Periodontal Res. 2017, 52, 225–232. [Google Scholar] [CrossRef]
- Feldens, E.G.; Kramer, P.F.; Feldens, C.A.; Ferreira, S.H. Distribution of Plaque and Gingivitis and Associated Factors in 3- to 5-Year-Old Brazilian Children. J. Dent. Child. 2006, 73, 4–10. [Google Scholar]
- Mombelli, A.; Gusberti, F.A.; van Oosten, M.A.C.; Lang, N.P. Gingival Health and Gingivitis Development during Puberty. J. Clin. Periodontol. 1989, 16, 451–456. [Google Scholar] [CrossRef]
- Tankova, H.; Mitova, N.; Lazarova, Z. Clinical and microbiological diagnosis of plaque-induced gingivitis in children and adolescents. J. IMAB—Annu. Proceeding (Sci. Pap.) 2022, 28, 4501–4505. [Google Scholar] [CrossRef]
- Mitova, N.; Rashkova, M.; Popova, C. Quantity, Diversity and Complexity of Subgingival Microorganisms in Children with Plaque-Induced Gingivitis. Biotechnol. Biotechnol. Equip. 2019, 33, 620–626. [Google Scholar] [CrossRef]
- Papaioannou, W.; Gizani, S.; Haffajee, A.D.; Quirynen, M.; Mamai-Homata, E.; Papagiannoulis, L. The Microbiota on Different Oral Surfaces in Healthy Children. Oral Microbiol. Immunol. 2009, 24, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Blufstein, A.; Pejcic, N.; Spettel, K.; Hausmann, B.; Seki, D.; Ertekin, T.; Hinrichs-Priller, J.; Altner, S.; Nehr, M.; Bekes, K.; et al. Salivary Microbiome and MRP-8/14 Levels in Children with Gingivitis, Healthy Children, and Their Mothers. J. Periodontol. 2024, 95, 1035–1047. [Google Scholar] [CrossRef]
- Yang, H.; Ma, Y.; Gao, H.; Xie, X.; Wang, H.; Li, X.; Bai, Y. Supragingival Microbiome Variations and the Influence of Candida Albicans in Adolescent Orthodontic Patients with Gingivitis. J. Oral Microbiol. 2024, 16, 2366056. [Google Scholar] [CrossRef]
- Marty, M.; Palmieri, J.; Noirrit-Esclassan, E.; Vaysse, F.; Bailleul-Forestier, I. Necrotizing Periodontal Diseases in Children: A Literature Review and Adjustment of Treatment. J. Trop. Pediatr. 2016, 62, 331–337. [Google Scholar] [CrossRef]
- Özdemir Kabalak, M.; Aytac, E.N.; Tarhan, N.; Karabulut, E.; Keceli, H.G. Potential barriers to the rational antibiotic use in dental and periodontal practice: A questionnaire-based online survey. Dent. Med. Probl. 2024, 61, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Solari, J.; Barrionuevo, P.; Mastronardi, C.A. Periodontal Disease and Its Systemic Associated Diseases. Mediat. Inflamm. 2015, 2015, 153074. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute Periodontal Lesions (Periodontal Abscesses and Necrotizing Periodontal Diseases) and Endo-periodontal Lesions. J. Clin. Periodontol. 2018, 45, S78–S94. [Google Scholar] [CrossRef]
- Yokoe, S.; Hasuike, A.; Watanabe, N.; Tanaka, H.; Karahashi, H.; Wakuda, S.; Takeichi, O.; Kawato, T.; Takai, H.; Ogata, Y.; et al. Epstein-Barr Virus Promotes the Production of Inflammatory Cytokines in Gingival Fibroblasts and RANKL-Induced Osteoclast Differentiation in RAW264.7 Cells. Int. J. Mol. Sci. 2022, 23, 809. [Google Scholar] [CrossRef]
- Mouchrek, M.M.M.; Franco, M.M.; da Silva, L.A.; Martins, K.A.C.; da Conceição, S.I.O.; de Azevedo dos Santos, A.P.S.; Rodrigues, V.P.; Ribeiro, C.C.C.; Benatti, B.B. Cytokine Levels in the Gingival Crevicular Fluid and Their Association with Periodontal Status of down Syndrome Patients: A Cross-Sectional Study. Clin. Oral Investig. 2024, 28, 391. [Google Scholar] [CrossRef]
- Ghaffarpour, M.; Karami-Zarandi, M.; Rahdar, H.A.; Feyisa, S.G.; Taki, E. Periodontal Disease in down Syndrome: Predisposing Factors and Potential non-surgical Therapeutic Approaches. J. Clin. Lab. Anal. 2024, 38, e25002. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Miyake, T.; Hirai, Y.; Yamamoto, T. Hydroa Vacciniforme: A Distinctive Form of Epstein-Barr Virus-Associated T-Cell Lymphoproliferative Disorders. Eur. J. Dermatol. 2019, 29, 21–28. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pantaleo, G.; Di Palo, M.P.; Amato, A.; Raimondo, A.; Amato, M. Oral Human Papillomavirus Benign Lesions and HPV-Related Cancer in Healthy Children: A Systematic Review. Cancers 2023, 15, 1096. [Google Scholar] [CrossRef]
- Slots, J. Human Viruses in Periodontitis. Periodontology 2000 2010, 53, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Cassai, E.; Galvan, M.; Trombelli, L.; Rotola, A. HHV-6, HHV-7, HHV-8 in Gingival Biopsies from Chronic Adult Periodontitis Patients. J. Clin. Periodontol. 2003, 30, 184–191. [Google Scholar] [CrossRef]
- Marchetti, E.; Pizzolante, T.; Americo, L.M.; Bizzarro, S.; Quinzi, V.; Mummolo, S. Periodontology Part 4: Periodontal Disease in Children and Adolescents. Eur. J. Paediatr. Dent. 2022, 23, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Scelza, G.; Di Spirito, F.; Amato, A.; Rosa, D.; Gallotti, A.; Martina, S. Prevalence of Dental Anomalies in a Sample of Growing Subjects: A Retrospective Study. Epidemiol. Prev. 2022, 46, 376–381. [Google Scholar] [CrossRef]
- Wirth, R.; Pap, B.; Maróti, G.; Vályi, P.; Komlósi, L.; Barta, N.; Strang, O.; Minárovits, J.; Kovács, K.L. Toward Personalized Oral Diagnosis: Distinct Microbiome Clusters in Periodontitis Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 747814. [Google Scholar] [CrossRef]
- Miguel, M.M.V.; Shaddox, L.M. Grade C Molar-Incisor Pattern Periodontitis in Young Adults: What Have We Learned So Far? Pathogens 2024, 13, 580. [Google Scholar] [CrossRef]
- Botero, J.E.; Contreras, A.; Parra, B. Effects of Cytomegalovirus Infection on the MRNA Expression of Collagens and Matrix Metalloproteinases in Gingival Fibroblasts. J. Periodontal Res. 2008, 43, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Maulani, C.; Auerkari, E.I.; Masulili, S.L.C.; Soeroso, Y.; Djoko Santoso, W.; Kusdhany, L.S. Association between Epstein-Barr Virus and Periodontitis: A Meta-Analysis. PLoS ONE 2021, 16, e0258109. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Lv, J.; Wang, M. Epstein–Barr Virus Is Associated with Periodontal Diseases. Medicine 2017, 96, e5980. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, F.R.; Littringer, K.; Altmeier, S.; Tran, V.D.T.; Schönherr, F.; Lemberg, C.; Pagni, M.; Sanglard, D.; Joller, N.; LeibundGut-Landmann, S. Persistence of Candida Albicans in the Oral Mucosa Induces a Curbed Inflammatory Host Response That Is Independent of Immunosuppression. Front. Immunol. 2019, 10, 330. [Google Scholar] [CrossRef]
- Sudhakara, P.; Gupta, A.; Bhardwaj, A.; Wilson, A. Oral Dysbiotic Communities and Their Implications in Systemic Diseases. Dent. J. 2018, 6, 10. [Google Scholar] [CrossRef]
- Fitzpatrick, R.E.; Wijeyewickrema, L.C.; Pike, R.N. The Gingipains: Scissors and Glue of the Periodontal Pathogen, Porphyromonas Gingivalis. Future Microbiol. 2009, 4, 471–487. [Google Scholar] [CrossRef]
- Ciaston, I.; Budziaszek, J.; Satala, D.; Potempa, B.; Fuchs, A.; Rapala-Kozik, M.; Mizgalska, D.; Dobosz, E.; Lamont, R.J.; Potempa, J.; et al. Proteolytic Activity-Independent Activation of the Immune Response by Gingipains from Porphyromonas Gingivalis. Mbio 2022, 13, e03787-21. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, T.; Li, Y.; Huang, L.; Yin, D. Fusobacterium Nucleatum: The Opportunistic Pathogen of Periodontal and Peri-Implant Diseases. Front. Microbiol. 2022, 13, 860149. [Google Scholar] [CrossRef]
- Biggs, C.M.; Keles, S.; Chatila, T.A. DOCK8 Deficiency: Insights into Pathophysiology, Clinical Features and Management. Clin. Immunol. 2017, 181, 75–82. [Google Scholar] [CrossRef]
- Carlsson, G.; Wahlin, Y.; Johansson, A.; Olsson, A.; Eriksson, T.; Claesson, R.; Hänström, L.; Henter, J. Periodontal Disease in Patients From the Original Kostmann Family With Severe Congenital Neutropenia. J. Periodontol. 2006, 77, 744–751. [Google Scholar] [CrossRef]
- Doke, M.; Fukamachi, H.; Morisaki, H.; Arimoto, T.; Kataoka, H.; Kuwata, H. Nucleases from Prevotella Intermedia Can Degrade Neutrophil Extracellular Traps. Mol. Oral Microbiol. 2017, 32, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C.; Cullinan, M.P. Comparison of the Clinical Features of Chronic and Aggressive Periodontitis. Periodontology 2000 2010, 53, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Nuernberg, M.A.A.; Ivanaga, C.A.; Haas, A.N.; Aranega, A.M.; Casarin, R.C.V.; Caminaga, R.M.S.; Garcia, V.G.; Theodoro, L.H. Periodontal Status of Individuals with Down Syndrome: Sociodemographic, Behavioural and Family Perception Influence. J. Intellect. Disabil. Res. 2019, 63, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Roshna, T.; Nandakumar, K. Generalized Aggressive Periodontitis and Its Treatment Options: Case Reports and Review of the Literature. Case Rep. Med. 2012, 2012, 535321. [Google Scholar] [CrossRef]
- Debureaux, P.E.; Sicre de Fontbrune, F.; Bonfim, C.; Dalle, J.H.; Buchbinder, N.; Bertrand, Y.; Renard, C.; Forcade, E.; Fernandes, J.; Talbot, A.; et al. FLAG-Sequential Regimen Followed by Bone Marrow Transplantation for Myelodysplastic Syndrome or Acute Leukemia in Patients with Fanconi Anemia: A Franco-Brazilian Study. Bone Marrow Transplant. 2021, 56, 285–288. [Google Scholar] [CrossRef]
- Dufour, C.; Pierri, F. Modern Management of Fanconi Anemia. Hematology 2022, 2022, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.M.; Darwazeh, A.M.; Idrees, M.M. The Effect of Glycemic Control on Candida Colonization of the Tongue and the Subgingival Plaque in Patients with Type II Diabetes and Periodontitis. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2013, 116, 321–326. [Google Scholar] [CrossRef]
- Shinjo, T.; Nishimura, F. The Bidirectional Association between Diabetes and Periodontitis, from Basic to Clinical. Jpn. Dent. Sci. Rev. 2024, 60, 15–21. [Google Scholar] [CrossRef]
- Sanz, M.; Teughels, W. Innovations in Non-surgical Periodontal Therapy: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 3–7. [Google Scholar] [CrossRef]
- Zhang, Q.; Davis, J.C.; Lamborn, I.T.; Freeman, A.F.; Jing, H.; Favreau, A.J.; Matthews, H.F.; Davis, J.; Turner, M.L.; Uzel, G.; et al. Combined Immunodeficiency Associated with DOCK8 Mutations. N. Engl. J. Med. 2009, 361, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Betts, K.; Abusleme, L.; Freeman, A.F.; Sarmadi, M.; Fahle, G.; Pittaluga, S.; Cuellar-Rodriguez, J.; Hickstein, D.; Holland, S.M.; Su, H.; et al. A 17-Year Old Patient with DOCK8 Deficiency, Severe Oral HSV-1 and Aggressive Periodontitis—A Case of Virally Induced Periodontitis? J. Clin. Virol. 2015, 63, 46–50. [Google Scholar] [CrossRef]
- Satoh, T.; Nishizawa, A.; Takayama, K.; Yokozeki, H. Hydroa Vacciniforme with Mucosal Involvement and Recalcitrant Periodontitis and Multiple Virus Re-Activators after Sun-Exposure. Acta Derm. Venereol. 2010, 90, 498–501. [Google Scholar] [CrossRef]
- Velazco, C.H.; Coelho, C.; Salazar, F.; Contreras, A.; Slots, J.; Pacheco, J.J. Microbiological Features of Papillon-Lefèvre Syndrome Periodontitis. J. Clin. Periodontol. 1999, 26, 622–627. [Google Scholar] [CrossRef]
- Nowzari, H.; Jorgensen, M.G.; Ta, T.T.; Contreras, A.; Slots, J. Aggressive Periodontitis Associated With Fanconi’s Anemia. A Case Report. J. Periodontol. 2001, 72, 1601–1606. [Google Scholar] [CrossRef]
- Graves, D.T.; Corrêa, J.D.; Silva, T.A. The Oral Microbiota Is Modified by Systemic Diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Georges, F.M.; Do, N.T.; Seleem, D. Oral Dysbiosis and Systemic Diseases. Front. Dent. Med. 2022, 3, 995423. [Google Scholar] [CrossRef]
- Pisano, M.; Giordano, F.; Sangiovanni, G.; Capuano, N.; Acerra, A.; D’Ambrosio, F. The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiol. Res. 2023, 14, 1862–1878. [Google Scholar] [CrossRef]
- Taylor, J.J.; Preshaw, P.M.; Lalla, E. A Review of the Evidence for Pathogenic Mechanisms That May Link Periodontitis and Diabetes. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S113–S134. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, F.; Kirschner, F.; Staderini, E.; Iavarone, F.; Fiorino, A.; Gallenzi, P. Proteomic analysis of salivary inflammatory biomarkers of developmental gingival enlargements in patients with West and Noonan syndromes: A preliminary pilot single-center retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 11093–11102. [Google Scholar] [CrossRef]
- Kitamoto, S.; Kamada, N. Periodontal Connection with Intestinal Inflammation: Microbiological and Immunological Mechanisms. Periodontology 2000 2022, 89, 142–153. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Mitchell, H.M. Campylobacter Concisus—A New Player in Intestinal Disease. Front. Cell. Infect. Microbiol. 2012, 2, 4. [Google Scholar] [CrossRef]
- Gowri, V.; Chougule, A.; Gupta, M.; Taur, P.; Iyengar, V.V.; Sivasankaran, M.; Munirathnam, D.; Krishna, S.; Bargir, U.A.; Dalvi, A.; et al. Clinical, Immunological and Molecular Findings of Patients with DOCK-8 Deficiency from India. Scand. J. Immunol. 2023, 98, e13276. [Google Scholar] [CrossRef] [PubMed]
- Su, H.C.; Jing, H.; Angelus, P.; Freeman, A.F. Insights into Immunity from Clinical and Basic Science Studies of DOCK8 Immunodeficiency Syndrome. Immunol. Rev. 2019, 287, 9–19. [Google Scholar] [CrossRef]
- Tamiya, H.; Abe, M.; Nagase, T.; Mitani, A. The Link. between Periodontal Disease and Asthma: How Do These Two Diseases Affect. Each Other? J. Clin. Med. 2023, 12, 6747. [Google Scholar] [CrossRef]
- Hyyppä, T. Gingival IgE and Histamine Concentrations in Patients with Asthma and in Patients with Periodontitis. J. Clin. Periodontol. 1984, 11, 132–137. [Google Scholar] [CrossRef]
- Slots, J.; Slots, H. Periodontal Herpesvirus Morbidity and Treatment. Periodontology 2000 2019, 79, 210–220. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614, Erratum in: Microorganisms 2024, 12, 1961. https://doi.org/10.3390/microorganisms12101961. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.M.; Santiago, L.M.; Lettieri, G.C.; Borges, L.G.d.A.; Marconatto, L.; de Oliveira, L.A.; Damé-Teixeira, N.; Salles, L.P. Oral Phenotype and Salivary Microbiome of Individuals With Papillon–Lefèvre Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 720790. [Google Scholar] [CrossRef]
- Pereira, L.L.; Veiga Siqueira Amorim, D.; Brito Sampaio, W.; Almeida Cruz Azevêdo, T.; Bispo Pereira Cardoso, V.; Barreto Lemos, F.; Silva Chang, A.; Machado, F.; Pereira Lima, F.; Sampaio Neves, F.; et al. Factors Associated with Periodontitis in Patients with and without HIV. Int. J. Dent. 2023, 2023, 9929835. [Google Scholar] [CrossRef] [PubMed]
- Suryana, K.; Suharsono, H.; Antara, I.G.P.J. Factors Associated with Oral Candidiasis in People Living with HIV/AIDS: A Case Control Study. HIV AIDS 2020, 12, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lomelí-Martínez, S.M.; González-Hernández, L.A.; Ruiz-Anaya, A.d.J.; Lomelí-Martínez, M.A.; Martínez-Salazar, S.Y.; Mercado González, A.E.; Andrade-Villanueva, J.F.; Varela-Hernández, J.J. Oral Manifestations Associated with HIV/AIDS Patients. Medicina 2022, 58, 1214. [Google Scholar] [CrossRef]
- Kato, H.; Imamura, A. Unexpected Acute Necrotizing Ulcerative Gingivitis in a Well-Controlled HIV-Infected Case. Intern. Med. 2017, 56, 2223–2227. [Google Scholar] [CrossRef][Green Version]
- Menezes, S.A.F.d.; Araújo, V.C.d.; Napimoga, M.H.; Menezes, T.O.d.A.; Nogueira, B.M.L.; Fonseca, R.R.d.S.; Martinez, E.F. Interleukin-6 and Interferon-α Levels in Gingival Crevicular Fluid in HIV-1 Patients with Chronic Periodontitis. Int. J. Odontostomatol. 2018, 12, 219–224. [Google Scholar] [CrossRef]
- Moscicki, A.; Yao, T.; Russell, J.S.; Farhat, S.; Scott, M.; Magpantay, L.; Halec, G.; Shiboski, C.H.; Ryder, M.I. Biomarkers of Oral Inflammation in Perinatally HIV-infected and Perinatally HIV-exposed, Uninfected Youth. J. Clin. Periodontol. 2019, 46, 1072–1082. [Google Scholar] [CrossRef]
- Sheng, S.; Kim, H.H.; Meng, H.-W.; Tribble, G.D.; Chang, J. Necrotizing Periodontal Disease in a Nutritionally Deficient Patient: A Case Report. Front. Dent. Med. 2022, 3, 994442. [Google Scholar] [CrossRef]
- Mwene-Batu, P.; Bisimwa, G.; Donnen, P.; Bisimwa, J.; Tshongo, C.; Dramaix, M.; Hermans, M.P.; Briend, A. Risk of Chronic Disease after an Episode of Marasmus, Kwashiorkor or Mixed–Type Severe Acute Malnutrition in the Democratic Republic of Congo: The Lwiro Follow-Up Study. Nutrients 2022, 14, 2465. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pisano, M.; Di Palo, M.P.; Franci, G.; Rupe, A.; Fiorino, A.; Rengo, C. Peri-Implantitis-Associated Microbiota before and after Peri-Implantitis Treatment, the Biofilm “Competitive Balancing” Effect: A Systematic Review of Randomized Controlled Trials. Microorganisms 2024, 12, 1965. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.F.R.; März, D.; Krüger, M. The Effects of Stress Hormones on Growth of Selected Periodontitis Related Bacteria. Anaerobe 2013, 24, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.M.; Guzmán, I.C. Association of P Orphyromonas gingivalis with High Levels of Stress-Induced Hormone Cortisol in Chronic Periodontitis Patients. J. Investig. Clin. Dent. 2016, 7, 361–367. [Google Scholar] [CrossRef]
- Casarin, M.; da Silveira, T.M.; Bezerra, B.; Pirih, F.Q.; Pola, N.M. Association between Different Dietary Patterns and Eating Disorders and Periodontal Diseases. Front. Oral Health 2023, 4, 1152031. [Google Scholar] [CrossRef] [PubMed]
- Morishima, S.; Takeda, K.; Greenan, S.; Maki, Y. Salivary Microbiome in Children with Down Syndrome: A Case-Control Study. BMC Oral Health 2022, 22, 438. [Google Scholar] [CrossRef]
- Contaldo, M.; Lucchese, A.; Romano, A.; Della Vella, F.; Di Stasio, D.; Serpico, R.; Petruzzi, M. Oral Microbiota Features in Subjects with Down Syndrome and Periodontal Diseases: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9251. [Google Scholar] [CrossRef]
- Khocht, A.; Yaskell, T.; Janal, M.; Turner, B.F.; Rams, T.E.; Haffajee, A.D.; Socransky, S.S. Subgingival Microbiota in Adult Down Syndrome Periodontitis. J. Periodontal Res. 2012, 47, 500–507. [Google Scholar] [CrossRef]
- Willis, J.R.; Iraola-Guzmán, S.; Saus, E.; Ksiezopolska, E.; Cozzuto, L.; Bejarano, L.A.; Andreu-Somavilla, N.; Alloza-Trabado, M.; Puig-Sola, A.; Blanco, A.; et al. Oral Microbiome in down Syndrome and Its Implications on Oral Health. J. Oral Microbiol. 2021, 13, 1865690. [Google Scholar] [CrossRef]
- Nóvoa, L.; Sánchez, M.d.C.; Blanco, J.; Limeres, J.; Cuenca, M.; Marín, M.J.; Sanz, M.; Herrera, D.; Diz, P. The Subgingival Microbiome in Patients with Down Syndrome and Periodontitis. J. Clin. Med. 2020, 9, 2482. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Liu, X.; Zhang, W.; Li, Y. Interrelationship between Diabetes and Periodontitis: Role of Hyperlipidemia. Arch. Oral Biol. 2015, 60, 667–674. [Google Scholar] [CrossRef]
- Di Spirito, F.; Di Palo, M.P.; Folliero, V.; Cannatà, D.; Franci, G.; Martina, S.; Amato, M. Oral Bacteria, Virus and Fungi in Saliva and Tissue Samples from Adult Subjects with Oral Squamous Cell Carcinoma: An Umbrella Review. Cancers 2023, 15, 5540. [Google Scholar] [CrossRef]
- Lalla, E.; Papapanou, P.N. Diabetes Mellitus and Periodontitis: A Tale of Two Common Interrelated Diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef]
- Polak, D.; Shapira, L. An Update on the Evidence for Pathogenic Mechanisms That May Link Periodontitis and Diabetes. J. Clin. Periodontol. 2018, 45, 150–166. [Google Scholar] [CrossRef]
- Jia, L.; Han, N.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Pathogenesis of Important Virulence Factors of Porphyromonas Gingivalis via Toll-Like Receptors. Front. Cell. Infect. Microbiol. 2019, 9, 262. [Google Scholar] [CrossRef]
- Tanner, A.; Maiden, M.F.J.; Macuch, P.J.; Murray, L.L.; Kent, R.L. Microbiota of Health, Gingivitis, and Initial Periodontitis. J. Clin. Periodontol. 1998, 25, 85–98. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’Addona, A. Oral Microbiota in Human Health and Disease: A Perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.A.; Soliman, Z.S.; Edrees, M.F. Oral Microbiota Associated with Gingiva of Healthy, Gingivitis and Periodontitis Cases. Microb. Pathog. 2022, 171, 105724. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; D’Arena, G.; Crispo, A.; Tecce, M.F.; Nocerino, F.; Grimaldi, M.; Rotondo, E.; D’Ursi, A.M.; Scrima, M.; Galdiero, M.; et al. Role of Viral MiRNAs and Epigenetic Modifications in Epstein-Barr Virus-Associated Gastric Carcinogenesis. Oxid. Med. Cell. Longev. 2016, 2016, 6021934. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter Actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef]
- Subbarao, K.; Nattuthurai, G.; Sundararajan, S.; Sujith, I.; Joseph, J.; Syedshah, Y. Gingival Crevicular Fluid: An Overview. J. Pharm. Bioallied Sci. 2019, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Preianò, M.; Savino, R.; Villella, C.; Pelaia, C.; Terracciano, R. Gingival Crevicular Fluid Peptidome Profiling in Healthy and in Periodontal Diseases. Int. J. Mol. Sci. 2020, 21, 5270. [Google Scholar] [CrossRef] [PubMed]
- Serretiello, E.; Astorri, R.; Chianese, A.; Stelitano, D.; Zannella, C.; Folliero, V.; Santella, B.; Galdiero, M.; Franci, G.; Galdiero, M. The Emerging Tick-Borne Crimean-Congo Haemorrhagic Fever Virus: A Narrative Review. Travel Med. Infect. Dis. 2020, 37, 101871. [Google Scholar] [CrossRef] [PubMed]

| Bacterium | Socransky Complex | Microbiological Features | Main Associated Periodontal Conditions | Key Periodontal Role |
|---|---|---|---|---|
| Streptococcus spp. (S. oralis, S. mitis, S. sanguis) [42,43] | Yellow | Gram-positive cocci Facultative anaerobes | Periodontal health | Early colonizers Maintaining oral eubiosis |
| Actinomyces spp. (A. naeslundii, A. oris) [42,43] | Blue | Gram-positive rods Facultative anaerobes/anaerobic | Periodontal health | Early colonizers Maintaining oral eubiosis |
| Veillonella parvula [44] | Purple | Gram-negative coccus Anaerobic | Periodontal health | Early colonizers |
| Capnocytophaga spp. [45] | Green | Gram-negative rods Facultative anaerobes/microaerophiles | Gingivitis Periodontal health | Early colonizers |
| Fusobacterium nucleatum [46,47,48] | Orange | Gram-negative rod Anaerobic | Gingivitis Periodontitis Necrotizing forms | Bridge bacterium, which facilitates co-aggregation |
| Prevotella intermedia [46,47,48] | Orange | Gram-negative rod Microaerophiles | Gingivitis Periodontitis Necrotizing forms | Involved in inflammatory response and biofilm accumulation |
| Campylobacter rectus [29] | Orange | Gram-negative rod, motile Anaerobic | Gingivits Periodontitis | Involved in inflammatory response and tissue invasion |
| Parvimonas micra [29] | Orange | Gram-positive coccus Anaerobic | Gingivits Periodontitis | Enhances inflammation |
| Porphyromonas gingivalis [49] | Red | Gram-negative rod Anaerobic | Gingivitis Periodontitis | Evades immune response and disrupts host–microbiome balance |
| Tannerella forsythia [21] | Red | Gram-negative rod Anaerobic | Gingivitis Periodontitis | Produces proteolytic enzymes and surface-layer proteins that promote epithelial invasion and immune evasion |
| Treponema denticola [50,51] | Red | Gram-negative spirochete, motile Anaerobic | Gingivitis Periodontitis Necrotizing forms | Highly proteolytic spirochete; invades tissues and promotes necrotizing forms |
| Aggregatibacter actinomycetemcomitans [52,53,54] | None | Gram-negative coccobacillus Facultative anaerobic | Aggressive periodontitis | Major pathogen in aggressive periodontitis; JP2 clone produces leukotoxin and impairs immune defense in adolescents (12–19 years old) |
| Microorganisms Species | Periodontal Diseases | Interactions |
|---|---|---|
| Porphyromonas gingivalis, Fusobacterium nucleatum, and EBV-I, EBV-II, CMV | Generalized periodontitis MIPP | Porphyromonas gingivalis and Fusobacterium nucleatum trigger viral reactivation, exacerbating the inflammatory status by the relapse of metabolites like butyric acid [70]. |
| Porphyromonas gingivalis and Candida albicans | Periodontitis | Candida albicans promoted Porphyromonas gingivalis growth and virulence, creating an anaerobic environment and promoting bacterial invasion and toxin release. Porphyromonas gingivalis favors Candida albicans adhesion [67,69]. |
| A.actinomycetemcomitans, Fusobacterium nucleatum, and Candida albicans/dubliniensis | Periodontitis | Fusobacterium nucleatum and A. actinomycetemcomitans promoted co-aggregation and inhibited Candida filamentation and growth, releasing quorum-sensing molecules as autoinducer-2 [67,69,71,72]. |
| A.actinomycetemcomitans and CMV | Periodontitis Necrotizing gingivitis | Active CMV infection enhanced the initial bacterial colonization, destroying the epithelial cells of the periodontal pocket and exposing new attachment receptors for bacteria, thus enhancing adherence and growth in a dose-dependent manner [13]. |
| Porphyromonas gingivalis and HHVs | Periodontitis | HHV infections impair neutrophil, macrophage, and complement functions, reducing antibody-mediated bacterial clearance and favoring the overgrowth of Porphyromonas gingivalis; the bacteria, conversely, release proteases as gingipains, degrade antiviral cytokines (IL-6 and IL-8), and contribute to the reactivation of latent herpesviruses, further amplifying inflammation and tissue destruction [13]. |
| EBV and/or CMV and/or HSV | Active periodontitis Acute necrotizing gingivitis | The co-infection of EBV and CMV enhanced the cytotoxic T-cell responses and the release of pro-inflammatory cytokines. Furthermore, the co-infection by two HHVs leads to a reciprocal transactivation of the latent viral genomes and the viral reactivation [13]. |
| HPV or human parvovirus B-19 and HHVs | Necrotizing gingivitis Periodontitis | A putative negative competitive interaction suggested that HHVs inhibit replication of non-herpesvirus such as HPV and human parvovirus B-19, modulating immune system responses. In fact, in both necrotizing gingivitis and periodontitis, HPV and human parvovirus B-19 were not detected in positive HHV samples in pediatric subjects [18]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Spirito, F.; Di Palo, M.P.; De Benedetto, G.; Piedepalumbo, F.; Galdi, M.; Cannatà, D.; Cafà, N.; Contaldo, M. Periodontal Microbial Profiles Across Periodontal Conditions in Pediatric Subjects: A Narrative Review. Microorganisms 2025, 13, 1813. https://doi.org/10.3390/microorganisms13081813
Di Spirito F, Di Palo MP, De Benedetto G, Piedepalumbo F, Galdi M, Cannatà D, Cafà N, Contaldo M. Periodontal Microbial Profiles Across Periodontal Conditions in Pediatric Subjects: A Narrative Review. Microorganisms. 2025; 13(8):1813. https://doi.org/10.3390/microorganisms13081813
Chicago/Turabian StyleDi Spirito, Federica, Maria Pia Di Palo, Giuseppina De Benedetto, Federica Piedepalumbo, Marzio Galdi, Davide Cannatà, Noemi Cafà, and Maria Contaldo. 2025. "Periodontal Microbial Profiles Across Periodontal Conditions in Pediatric Subjects: A Narrative Review" Microorganisms 13, no. 8: 1813. https://doi.org/10.3390/microorganisms13081813
APA StyleDi Spirito, F., Di Palo, M. P., De Benedetto, G., Piedepalumbo, F., Galdi, M., Cannatà, D., Cafà, N., & Contaldo, M. (2025). Periodontal Microbial Profiles Across Periodontal Conditions in Pediatric Subjects: A Narrative Review. Microorganisms, 13(8), 1813. https://doi.org/10.3390/microorganisms13081813







