Abstract
Background: Chlamydia trachomatis is the most prevalent bacterial sexually transmitted infection (STI) globally, linked to severe complications such as pelvic inflammatory disease and infertility. In the Brazilian Amazon, socioeconomic vulnerability and the absence of screening policies exacerbate risks, particularly among female sex workers (FSWs). Objective: This study aimed to determine the seroprevalence of anti-C. trachomatis IgG antibodies among FSWs in five municipalities of Pará State, Brazilian Amazon, and identify epidemiological factors associated with infection. Methods: A retrospective cross-sectional study (2005–2007) included 348 FSWs recruited via convenience sampling. Sociodemographic and behavioral data were collected through questionnaires, and blood samples were analyzed by ELISA for anti-C. trachomatis IgG. Statistical analyses included Fisher’s exact tests, odds ratios (ORs), and 95% confidence intervals (CIs), using SPSS 21.0. Results: Overall seroprevalence was 93.9% (327/348; 95% CI: 83.1–90%). Significant associations included a household income of 1–3 minimum wages (98.4%; p = 0.0002), sexual partners from the same region (98.8%; p = 0.0421), and age >42 years (96.3%). Most reported inconsistent condom use (43.7%), multiple monthly partners (54.6%), and illicit drug use (53.4%). Discussion: The extremely high seroprevalence reflects chronic C. trachomatis exposure, driven by socioeconomic deprivation and limited healthcare access. Comparisons with global data underscore the urgent need for screening policies, absent in Brazil for FSWs, and highlight the vulnerability of this population. Conclusions: The findings reveal an alarming burden of C. trachomatis exposure among Amazonian FSWs. Integrated strategies, including routine screening, sexual health education, and inclusion of FSWs in priority health programs, are critical to reduce transmission and associated complications.
1. Introduction
Chlamydia trachomatis causes the most prevalent bacterial sexually transmitted infection (STI) in the world, with approximately 128.5 million new annual cases, predominantly affecting women aged 15–49 years [1]. It is classified into 19 genotypes that are involved in three different clinical conditions, such as trachoma, caused by genotypes A to C [2], lymphogranuloma venereum, caused by genotypes L1 to L3, and urogenital infection, caused by genotypes D to K [3], which can be asymptomatic in up to 80% of women and lead to pelvic inflammatory disease (PID), which is an ascending and systemic inflammatory/infectious process that manifests with salpingitis, endometritis, ovaritis, cervicitis, and urethritis [4,5]. Fibrosis caused by PID is responsible for tubal occlusion and other sequelae such as preventable infertility due to tubal factor and ectopic pregnancy [6]. In addition, it favors infection by the Human Immunodeficiency Virus (HIV) [7,8]. The highest rates of STIs caused by C. trachomatis are found in the United States (69.9–73.7%) [9,10], the Emirate of Abu Dhabi, and United Arab Emirates (18.9%) [11], followed by China (18.8%), Mexico (14.8%), Peru (13.9%), Germany (10.9%), Poland (7.4%) [12], and the Netherlands (10.5%) [13].
The Brazilian health system is universal and decentralized, and although there is an updated treatment protocol for STIs caused by C. trachomatis [14], it does not include asymptomatic young women of reproductive age, and the epidemiological scenario of C. trachomatis remains unnoticed, with hospitalization rates for PID exceeding 45,000 annually [15,16]. The Brazilian Amazon has low demographics and socioeconomic conditions, which impact the high socioeconomic vulnerability of hard-to-reach communities, and logistical and political–ideological problems hinder the adherence, territorialization, and expansion of primary health services in these locations [17,18]. The prevalence of anti-C. trachomatis antibodies in the Amazon has been reported as 33.3% (in STI clinics), 97.1% (in indigenous people of Parakanã village) [19], 30.9% in residents of Marajó Island [20], 64.8% in the population of people living with HIV [21], and 22.2% in individuals from a riverside community [22].
Female sex workers (FSWs) are a population with high vulnerability to STIs because they are often subjected to a lifestyle that exposes them to risk factors, such as multiple partners and group sex [23], discontinued condom use during sexual intercourse, and early sexual initiation [24]. In addition, asymptomatic FSWs seek sexual health services only when they have moderate or severe clinical symptoms [25]. There are few studies on STIs in FSWs in the Brazilian Amazon, but they report high rates of these infections in people in prostitution [26,27]. The investigation of anti-C. trachomatis serological markers in remote regions of the Amazon offers an important tool for assessing the exposure profile of women to sexual infection by C. trachomatis, as they are easy to perform and low-cost [28]. Therefore, the aim of this study was to identify IgG antibodies against C. trachomatis infection in FSWs from five municipalities in the state of Pará, the Amazon region of Brazil.
2. Material and Methods
2.1. Study Area, Design, and Ethics Aspects
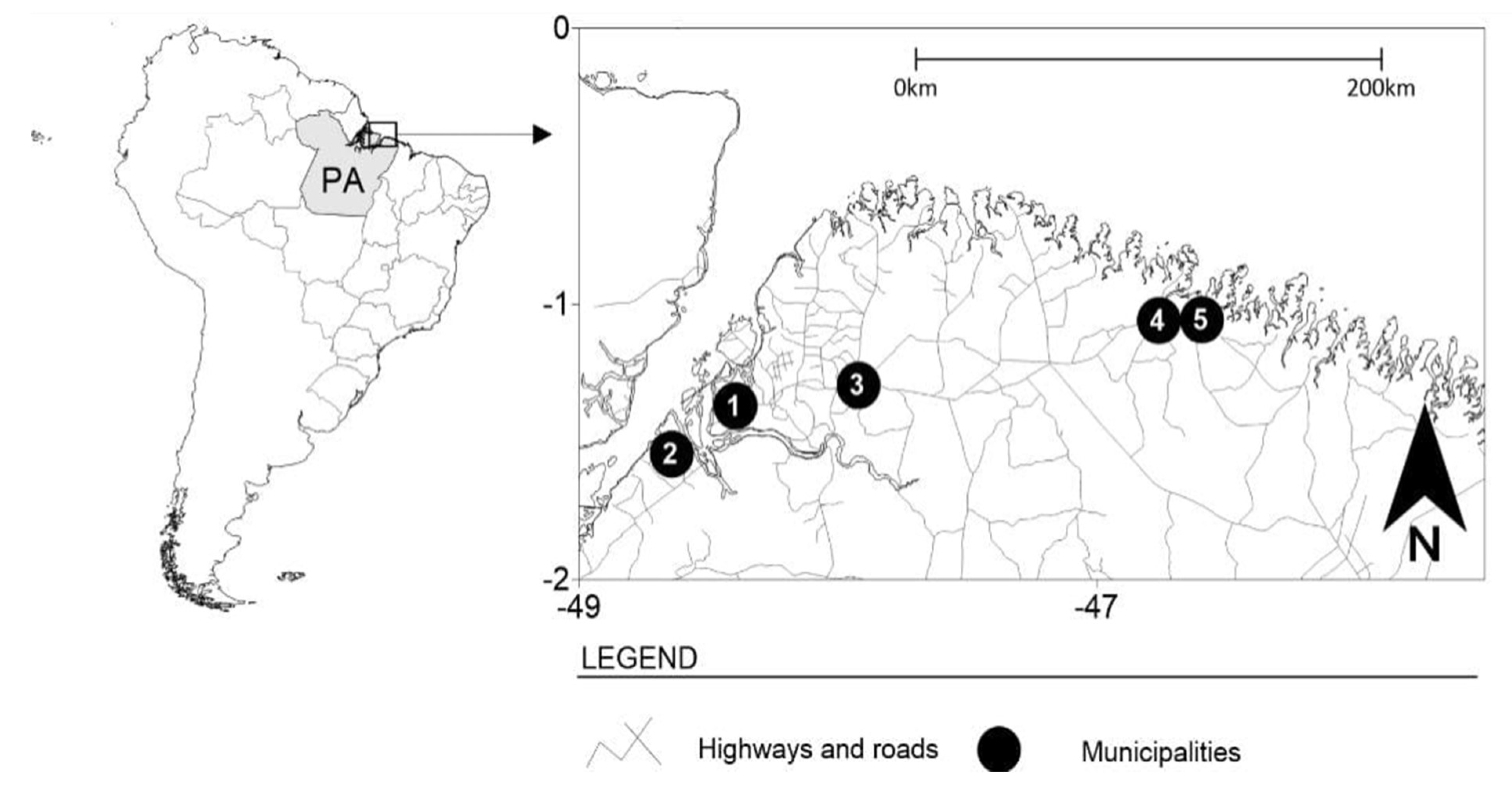
This was a cross-sectional, retrospective, and analytical study, whose study population was FSWs from five locations in the state of Pará, northern Brazil: Belém (capital), Bragança, Augusto Corrêa, Barcarena, and Castanhal (Figure 1). These municipalities are areas of intense flow of people and circulation of products, with many historical, cultural, and tourist attractions that stand out in the context of the Amazon region; however, they are marked by areas of prostitution.
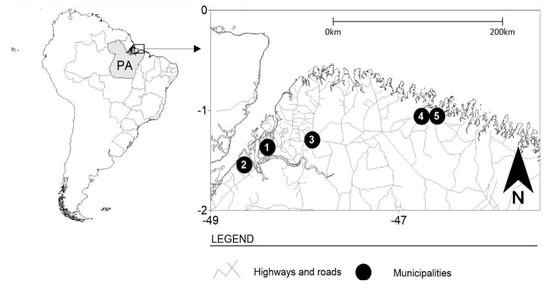
Figure 1.
The municipalities where data collection was performed for this study. Belém—1, Barcarena—2, Castanhal—3, Bragança—4, and Augusto Corrêa—5.
This study was approved by the Human Research Ethics Committee of the HEMOPA Foundation, under number 12/2005.
This study included FSWs and lasted from January 2005 to August 2007. Furthermore, a non-probabilistic convenience sample sampling method was used to recruit FSWs at their workplaces (streets, nightclubs, strip clubs, etc.) twice a week. To achieve an adequate sample size, we referred to previous prevalence in Belém do Pará (33%) [19]. The assumed sampling error (ε) was 5%, and the power of 80% was chosen, generating a minimum size of 345 participants. This study included cisgender women who engaged in sex work for money and had worked in the selected locations for at least six months. Transgender women, those under the influence of illicit drugs or alcohol at the time of data collection, and those unable to adequately respond to the epidemiological questionnaire were excluded. In each municipality included in this study, a data survey was carried out to identify and recognize the places that FSWs used as prostitution points. Subsequently, an invitation was made to the leaders of the FSW groups so that they could inform the other FSWs and extend the invitation to participate in this study. The variables investigated were as follows: age group (year), marital status, education level (years of study), family income (minimum wage), condom use, STI history, use of illicit drugs, number of partners (monthly), partners from another region, and partners from other countries.
The FSWs were informed of the objectives of this study prior to being invited to participate. The Informed Consent Form was signed by the participant after verbal agreement and acceptance. They provided information on social status and sexual behavior through the data collection questionnaire.
2.2. Laboratory Tests
A peripheral blood sample (5 mL) was collected from each FSW using a vacuum collection system with EDTA as an anticoagulant. The plasma was separated by centrifugation (9500 rpm for 15 min) and stored at −20 °C until use, in the Virology Laboratory of the Institute of Biological Sciences at the Federal University of Pará. Each sample was subjected to an enzyme-linked immunosorbent assay (ELISA) to detect specific IgG antibodies against C. trachomatis (Diagnostic Automation Inc.—Microwell Elisa, Calabasas, CA, USA; Specificity, 98.5%; Sensitivity, 91.1%) according to the manufacturer’s instructions. It is worth noting that all serological tests were performed at the time the samples were collected.
2.3. Statistical Analysis
Statistical analysis involved the correlation of serological results with the epidemiological data of the study population. The Statistical Package for Social Sciences (SPSS) version 21.0 (SPSS, Chicago, IL, USA) was used for analysis. The Proportion Parameter Estimation was used to verify the value of the proportion of positives over the total sample. For categorical variables with only two options, Fisher’s exact test and the odds ratio test were used. For variables with more than two options, the G test of independence was used. The 95% confidence interval (CI) was calculated for these comparisons, no adjustments for multiple comparisons (e.g., Bonferroni correction) were applied, as this study prioritized exploratory sensitivity over Type I error reduction in a high-prevalence context (93.9% seropositivity), where avoiding Type II errors (false negatives) was critical to identifying potential public health risks. A significance level of p ≤ 0.05 was considered for all analyses.
2.4. Ethics Statement
The investigations were conducted in accordance with the principles outlined in the Declaration of Helsinki (1975, revised in 2013), according to point 23 of this declaration and in accordance with Resolution 466/2012 of the Brazilian National Health Council [29]. This study is part of the project “Epidemiology of viral (HIV, HTLV, HBV and HCV) and bacterial (T. pallidum and C. trachomatis) infections in female sex workers in the states of Pará, Amapá and Acre, Northern region of Brazil”. The methodological steps of this study followed the pertinent ethical guidelines, as it was authorized by the Research Ethics Committee of the João de Barros Barreto University Hospital, Federal University of Pará (process number: 2089/05). In all cases, capital and interior, only participants who had read and signed the Free and Informed Consent Form (FICF) and were aged 18 years and older were included in this study. After this, data and biological sample collection continued while maintaining complete anonymity. Participants who tested positive for antibodies against C. trachomatis received appropriate guidance and referral for medical evaluation and treatment.
3. Results
A total of 348 FSWs participated in this study, with a mean age of 43.1 years (15 to 71 years), and most of the participants (61.2%; 213/348) were between 23 and 42 years of age. The total seroprevalence of antibodies against C. trachomatis infection was 93.9% (327/348; 95% CI: 83.1–90%), and in relative frequency, it was found significantly in women who had a family income between one and three Brazilian minimum wages (98.4%, p = 0.0002) and had fewer than 20 sexual partners per month (94.3%), with partners from the same region (98.8%, p = 0.0421) and from this country (95.5%). The epidemiological information of the FSWs participating in this study is contained in Table 1.

Table 1.
Seroprevalence, epidemiological characteristics, and sexual behavior of female sex workers included in this study.
4. Discussion
In this study, we found high seroprevalence of antibodies against infection by C. trachomatis in FSWs from the capital and four other municipalities of the state of Pará, in the Amazon region of Brazil. The seroprevalence observed here far exceeds rates reported in asymptomatic populations from other countries [11,12,13,28,30], most of which are from developed countries that have an official screening policy for C. trachomatis in young adult women [31,32,33]. Interestingly, high seroprevalences were found in American women with active clinical disease with evident symptoms of PID (73.7%) [9] and tubal factor infertility (69.9%) [10]. We did not assess the clinical conditions or gynecological complaints of the participants to verify any correlation. High seroprevalence rates were also found in female populations with socioeconomic vulnerability, such as HIV-positive married women in Nigeria (60.9%) [34], and asymptomatic women without access to health services in Puerto Rico (47%) [35] and China (47.46%) [36].
Most FSWs are single mothers and therefore choose a larger number of clients to ensure higher income, guarantee the subsistence of their families and fit within the average Brazilian family income, which is approximately BRL 2927.70, equivalent to USD 526.70 [37]. This may be reflected in our study in the high levels of antibodies against C. trachomatis found in women who had a family income between one and three Brazilian minimum wages (equivalent to USD 250–USD 750) (p = 0.0002), possibly due to frequent exposure to this infection. In Brazil, socioeconomic instability associated with high STI rates is strongly present in peripheral societies and is commonly reported as a factor that favors risky sexual behavior [38,39] and encourages the activity of exchanging sex for money or goods [40,41,42].
The populations of the Amazon suffer from basic deficiencies in prevention, promotion, diagnosis, and health education and are constantly reported with high prevalence of this and other STIs [16,17]. Furthermore, we consider that the understanding of the epidemiology of C. trachomatis in Brazil is the “tip of the iceberg”, as it is based only on studies in isolated regions. Since its first notification in 1988 [43], STI caused by C. trachomatis in the Amazon has been characterized by high seroprevalence among symptomatic patients, in patients recently diagnosed with HIV (64.8%) [21], and in indigenous villages (23.9–90.7%) [44]. High frequencies continue to be confirmed today with nucleic acid amplification tests in the Marajó region (4%) [45], in gynecological clinic attendees (4.6%) [46], public maternity hospitals (11–18%) [47,48], and in asymptomatic university students (11.2%) [49].
Since 1984, Brazil has been concerned with including the promotion of women’s reproductive health in primary care [50], but there are no public policies aimed at FSW reproductive health in the National Program for Comprehensive Women’s Health Care (PNAISM) even after the universalization of the health system [51,52], and their participation in debates for the construction and/or reformulation of health policies is still rare. In addition, the logistical difficulties and political–administrative problems suffered by populations in remote and hard-to-reach regions of the Amazon are an aggravating factor to be considered [17,18]. In Brazil, FSWs work as sex workers in their own businesses and in small groups [53], but a systematic commercial network with mediators, promoters, or assistants of sex work, such as the creation of “brothels”, procuring and pimping websites, is a crime [54]. The vast majority of them use the streets, highways, and rivers of the Amazon, which have a large flow of people, as temporary professional addresses, since travelers or men from other locations in the same region are routinely associated with the use of FSWs’ services [26,27]. Perhaps for this reason, our study showed that FSWs who had partners from this region had a higher seroprevalence of C. trachomatis (p = 0.0421), and many of them have little access to and reception in health services or do not declare themselves to be FSWs [54,55], due to stigmatization and social discrimination [56], which leads to hesitation in seeking these services for this population group [57,58].
The main limitations of this study were the limited sample size with the use of secondary data and the occurrence of incomplete or lost medical records that prevented us from performing multivariate analyses. Another limitation in our study is the lack of clinical correlation with C. trachomatis seropositivity. Future clinical studies are needed to verify the natural history of this infection to adverse clinical outcomes such as PID, infertility, and HIV co-infection. In addition, there is a possibility of cross-reaction in women who had previous trachoma events and who had a false-positive result, as well as in participants who were affected by active asymptomatic sexual infection at the time of this study, which may have implications for the false-negative result.
5. Conclusions
We concluded that FSWs who had a family income of one to three Brazilian minimum wages and those who receive clients from other states showed higher seroprevalence for anti-C. trachomatis antibodies. Brazilian public health policies should implement routine C. trachomatis screening programs for women and include FSWs as a priority group in official women’s health programs, which should be complemented by innovative actions for sexual and reproductive health education. These actions should be intensified in the FSW population, as this is a population group that is strongly present in Brazilian society and deserves to be integrated into STI prevention measures. Future studies will be necessary to verify a possible association between associated tubal immunopathologies and high seropositivity for antibodies against C. trachomatis infection in FSWs.
Author Contributions
Conceptualization: R.V.L., R.R.d.S.F. and L.F.A.M.; Data curation: D.A.P. and R.N.M.F.; Investigation and methodology: L.G.C.P.d.F., L.M.d.S., F.B.F., R.V.L. and M.M.d.C.; Formal analysis: A.B.O.-F., P.d.S.d.O.d.C.L. and L.F.A.M.; Writing—original draft: A.B.O.-F., R.V.L., L.M.d.S. and L.F.A.M.; Writing—review and editing: J.C.M., L.M.d.S., D.O.d.A., R.V.L., A.B.O.-F. and L.F.A.M.; Project administration: L.F.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from the National Council of Science and Technology (CNPq/MCTI/FNDCT # 407219/2021-8 and CNPq # 445041/2023-4). L.F.A.M. is a CNPq Grantee (#314209/2021-2). Publication of the article was supported by Public Notice PAPQ, PROPESP/FADESP of the Federal University of Pará.
Institutional Review Board Statement
The investigations were conducted in accordance with the principles outlined in the Declaration of Helsinki (1975, revised in 2013), according to point 23 of this declaration and in accordance with Resolution 466/2012 (12 December 2012) of the Brazilian National Health Council.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge all subjects enrolled in this study and UREDIPE and Executive Secretariat of Public Health of the State of Pará. The author S.P.A. received fellowships of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES), Finance Code 001, to develop the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, C.; Ong, J.; Tang, W.; Wang, C. Chlamydia trachomatis infection: Epidemiology, prevention, clinical, and basic science research. Front. Public Health 2023, 11, 1167690. [Google Scholar] [CrossRef]
- Tedijanto, C.; Solomon, A.W.; Martin, D.L.; Nash, S.D.; Keenan, J.D.; Lietman, T.M.; Lammie, P.J.; Aiemjoy, K.; Amza, A.; Aragie, S.; et al. Monitoring transmission intensity of trachoma with serology. Nat. Commun. 2023, 14, 3269. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B.; Folga, B.A. Chlamydia trachomatis—An Emerging Old Entity? Microorganisms 2023, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Heijer, C.D.J.; Hoebe, C.J.P.A.; Driessen, J.H.M.; Wolffs, P.; Broek, I.V.F.; Hoenderboom, B.M.; Williams, R.; Vries, R.; Dukers-Muijrers, N.H.T.M. Chlamydia trachomatis and the Risk of Pelvic Inflammatory Disease, Ectopic Pregnancy, and Female Infertility: A Retrospective Cohort Study Among Primary Care Patients. Clin. Infect. Dis. 2019, 69, 1517–1525. [Google Scholar] [CrossRef]
- Hunt, S.; Vollenhoven, B. Pelvic inflammatory disease and infertility. Aust. J. Gen. Pract. 2023, 52, 215–218. [Google Scholar] [CrossRef]
- Tang, W.; Mao, J.; Li, K.T.; Walker, J.S.; Chou, R.; Fu, R.; Chen, W.; Darville, T.; Klausner, J.; Tucker, J.D. Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: A global systematic review and meta-analysis. Sex. Transm. Infect. 2020, 96, 322–329. [Google Scholar] [PubMed]
- Veretennikova, A.; Chang, T.L. Chlamydia trachomatis Enhances HIV Infection of Non-Activated PBMCs. EC Microbiol. 2022, 18, 13–17. [Google Scholar] [PubMed]
- Dzakah, E.E.; Zhao, J.; Wang, L.; Rashid, F.; Xu, R.; Yang, L.; Wan, Z.; Huang, L.; Wang, H.; Chen, S.; et al. Chlamydia trachomatis Stimulation Enhances HIV-1 Susceptibility through the Modulation of a Member of the Macrophage Inflammatory Proteins. J. Investig. Dermatol. 2022, 142, 1338–1348.e6. [Google Scholar] [CrossRef]
- Anyalechi, G.E.; Hong, J.; Danavall, D.C.; Martin, D.L.; Gwyn, S.E.; Horner, P.J.; Raphael, B.H.; Kirkcaldy, R.D.; Kersh, E.N.; Bernstein, K.T. High Plasmid Gene Protein 3 (Pgp3) Chlamydia trachomatis Seropositivity, Pelvic Inflammatory Disease, and Infertility Among Women, National Health and Nutrition Examination Survey, United States, 2013–2016. Clin. Infect. Dis. 2021, 73, 1507–1516. [Google Scholar] [CrossRef]
- Anyalechi, G.E.; Hong, J.; Kirkcaldy, R.D.; Wiesenfeld, H.C.; Horner, P.; Wills, G.S.; McClure, M.O.; Hammond, K.R.; Haggerty, C.L.; Kissin, D.M.; et al. Chlamydial Pgp3 Seropositivity and Population-Attributable Fraction Among Women with Tubal Factor Infertility. Sex. Transm. Dis. 2022, 49, 527–533. [Google Scholar] [CrossRef]
- Abdo, N.M.; Aslam, I.; Irfan, S.; George, J.A.; Alsuwaidi, A.R.; Ahmed, L.A.; Al-Rifai, R.H. Chlamydia trachomatis Seroepidemiology and Associated Factors in Fertility Treatment-Seeking Patients in the Abu Dhabi Emirate, United Arab Emirates. Sex. Transm. Dis. 2023, 50, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, J.M.; Pollmann, M.; Borchardt-Lohölter, V.; Moreira-Soto, A.; Kaya, S.; Sener, A.G.; Gómez-Guzmán, E.; Figueroa-Hernández, L.; Li, W.; Li, F.; et al. Seroprevalences of antibodies against ToRCH infectious pathogens in women of childbearing age residing in Brazil, Mexico, Germany, Poland, Turkey and China. Epidemiol. Infect. 2020, 148, e271. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, Z.W.; van Aar, F.; Hoenderboom, B.M.; Morre, S.A.; Heijne, J.C.M. Trends in Chlamydia trachomatis IgG seroprevalence in the general population of the Netherlands over 20 years. Sex. Transm. Infect. 2024, 100, 31–38. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Protocolo Clínico e Diretrizes Terapêuticas para Atenção Integral às Pessoas com Infecções Sexualmente Transmissíveis—IST [Recurso Eletrônico]; Ministério da Saúde: Brasília, Brazil, 2022; Volume 211, p. Il. Available online: https://www.gov.br/aids/pt-br/central-de-conteudo/pcdts/2022/ist/pcdt-ist-2022_isbn-1.pdf/view (accessed on 27 June 2024).
- Menezes, M.L.B.; Giraldo, P.C.; Linhares, I.M.; Boldrini, N.A.T.; Aragón, M.G. Brazilian Protocol for Sexually Transmitted Infections 2020: Pelvic inflammatory disease. Epidemiol. Serv. Saude 2021, 30, e2020602. [Google Scholar]
- Perciney, P.; Costa, A.L.S.; Leite, I.C.G.; Nogueira, M.C. Pelvic inflammatory disease hospitalizations in Brazil: Time trend from 2000 to 2019. Rev. Bras. Saude Mater. Infant. 2022, 22, 767–773. [Google Scholar] [CrossRef]
- Machado, L.F.A.; Fonseca, R.R.S.; Oliveira-Filho, M.A.F.; Cayres-Vallinoto, I.M.V.; Vallinoto, A.C.R.; de Oliveira Guimarães Ishak, M.; Ishak, R. The Epidemiological Impact of STIs among General and Vulnerable Populations of the Amazon Region of Brazil: 30 Years of Surveillance. Viruses 2021, 13, 855. [Google Scholar] [CrossRef]
- Faria, R.M. The territorialization of Primary Health Care of the Brazilian Unified Health System. Cien. Saude Colet. 2020, 25, 4521–4530. [Google Scholar] [CrossRef] [PubMed]
- Ishak, M.O.; Ishak, R.; Cruz, A.C.; Santos, D.E.; Salgado, U. Chlamydial infection in the Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 60–62. [Google Scholar]
- Ferreira, G.R.O.N.; Freitas, F.B.; Queiroz, M.A.F.; Lima, S.S.; Vallinoto, A.C.R.; de O Guimarães Ishak, M.; Ishak, R. Epidemiology and risk factors for Chlamydia trachomatis, treponema pallidum, Hepatitis B Virus and Hepatitis C Virus in the Marajó Archipelago, Brazilian Amazon. J. Community Med. Health Educ. 2019, 9, 643. [Google Scholar]
- Góes, S.D.S.; Fonseca, R.R.S.; Avelino, M.E.S.; Lima, S.S.; Lima, M.S.G.A.; Laurentino, R.V.; Queiroz, M.A.F.; Freitas, F.B.; Vallinoto, A.C.R.; Ishak, R.; et al. Exposure to Chlamydia trachomatis Infection in Individuals Who Are Newly Diagnosed with HIV and Antiretroviral-Naive from Belem, Northern Brazil. Vaccines 2022, 10, 1719. [Google Scholar] [CrossRef]
- Galvão, J.J.D.S.; Cunha, C.L.F.; Pinho, E.C.C.; Paiva, D.J.D.S.; de Castro, N.J.C.; Nascimento, V.G.C.; de Azevedo Junior, W.S.; da Silva, R.A.R.; Feitosa, R.N.M.; Vallinoto, A.C.R.; et al. Seroprevalence of Chlamydia trachomatis and Associated Factors among Vulnerable Riverine in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2022, 19, 15969. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Luo, J.; Chen, Y.; Chen, L.; Hu, H.; Qiu, T.; Liu, X.; Xu, X.; Chen, Y.; Zhang, Z.; et al. Prevalence of syphilis and Chlamydia trachomatis infection among female sex workers in Jiangsu, China: Results from a multicenter cross-sectional and venue-based study. Front. Public Health 2022, 10, 1018724. [Google Scholar] [CrossRef]
- Tremblay, F.; Courtemanche, Y.; Bélanger, R.E.; Turcotte-Tremblay, A.M. A systematic review of the association between history of sexually transmitted infections and subsequent condom use in adolescents. BMC Public Health 2024, 24, 1000. [Google Scholar] [CrossRef]
- Birger, L.; Peled, E.; Benyamini, Y. Stigmatizing and inaccessible: The perspectives of female sex workers on barriers to reproductive healthcare utilization—A scoping review. J. Adv. Nurs. 2024, 80, 2273–2289. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.B.; Aires, D.W.F.; Cavalcante, N.S.; Raiol, N.C.; Lisboa, B.L.A.; Frade, P.C.R.; da Costa, L.M.; Pinheiro, L.M.L.; Machado, L.F.A.; Martins, L.C.; et al. Hepatitis C Virus among Female Sex Workers: A Cross-Sectional Study Conducted along Rivers and Highways in the Amazon Region. Pathogens 2019, 8, 236. [Google Scholar] [CrossRef]
- Coelho, E.C.; Souza, S.B.; Costa, C.C.S.; Costa, L.M.; Pinheiro, L.M.L.; Machado, L.F.A.; Silva-Oliveira, G.C.; Martins, L.C.; Frade, P.C.R.; Oliveira-Filho, A.B. Treponema pallidum in female sex workers from the Brazilian Marajo Archipelago: Prevalence, risk factors, drug-resistant mutations and coinfections. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.R.; Patel, E.U.; Grabowski, M.K.; Gaydos, C.A.; Quinn, T.C.; Tobian, A.A.R. Seroprevalence of Chlamydia trachomatis Among Female Adults in the United States: The National Health and Nutrition Examination Surveys. Clin. Infect. Dis. 2021, 73, e629–e637. [Google Scholar] [CrossRef] [PubMed]
- Conselho Nacional de Saúde (Brasil). Resolução N° 466, de 12 de Dezembro de 2012. Brasília, 2012. [citado 11 March 2014]. Available online: https://www.gov.br/conselho-nacional-de-saude/pt-br (accessed on 4 January 2025).
- Öhman, H.; Rantsi, T.; Joki-Korpela, P.; Tiitinen, A.; Surcel, H.M. Prevalence and persistence of Chlamydia trachomatis-specific antibodies after occasional and recurrent infections. Sex. Transm. Infect. 2020, 96, 277–282. [Google Scholar] [CrossRef]
- U.S. Preventive Services Task Force. Screening for Chlamydial Infection: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 2007, 147, 128–134. [Google Scholar] [PubMed]
- European Centre for Disease Prevention and Control. Chlamydia Control in Europe: Literature Review; ECDC: Stockholm, Sweden, 2014; Available online: www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/chlamydia-control-europe.pdf (accessed on 2 July 2024).
- Low, N.; Hocking, J.S.; van Bergen, J. The changing landscape of chlamydia control strategies. Lancet 2021, 398, 1386–1388. [Google Scholar] [CrossRef]
- Omosigho, P.O.; Ajide, T.E.; Izevbuwa, O.E.; Okesanya, O.J.; Oladejo, J.M.; Uyigue, P.O. Seroprevalence of Chlamydia trachomatis and associated risk factors among HIV positive women in North Central Nigeria. Infez. Med. 2024, 32, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Avila, M.A.; Suárez-Pérez, E.; Bernabe-Dones, R.; Unger, E.R.; Panicker, G.; Ortiz, A.P. Chlamydia trachomatis and Human Papillomavirus Serostatus in Puerto Rican Women. P. R. Health Sci. J. 2020, 39, 28–33. [Google Scholar] [PubMed]
- Zhou, Q.; Li, J.; Luo, L.; Min, S.; Wang, L.; Peng, L.; Hou, Y.; He, P.; He, S.; Tang, S.; et al. Characterization of genital Chlamydia trachomatis infection among women attending infertility and gynecology clinics in Hunan, China. BMC Infect. Dis. 2024, 24, 405. [Google Scholar] [CrossRef]
- Brasil Instituto de Pesquisa Econômica Aplicada. Renda Média do Trabalhador Brasileiro Cresce 4.0% no Primeiro Trimestre de 2024 na Comparação com o Primeiro Trimestre de 2023. Available online: https://www.ipea.gov.br/portal/categorias/45-todas-as-noticias/noticias/15092-renda-media-do-trabalhador-brasileiro-cresce-4-0-no-primeiro-trimestre-de-2024-na-comparacao-com-o-primeiro-trimestre-de-2023 (accessed on 28 February 2025).
- Tabler, J.; Mykyta, L.; Schmitz, R.M.; Kamimura, A.; Martinez, D.A.; Martinez, R.D.; Flores, P.; Gonzalez, K.; Marquez, A.; Marroquin, G.; et al. Social Determinants of Sexual Behavior and Awareness of Sexually Transmitted Infections (STI) Among Low-Income HIV+ or STI At-Risk Hispanic Residents Receiving Care at the U.S.-Mexico Border. J. Community Health 2019, 44, 127–136. [Google Scholar] [CrossRef]
- Jiménez-Morón, A.; Hueso-Montoro, C.; Caparros-González, R.; Pérez-Morente, M.Á. Risk factors for the acquisition of Sexually Transmitted Infections in sex workers: A systematic review. Rev. Esp. Salud Publica 2024, 98, e2024023019. [Google Scholar]
- Karandikar, S.; Gezinski, L.B.; Meshelemiah, J.C. A qualitative examination of women involved in prostitution in Mumbai, India: The role of family and acquaintances. Int. Soc. Work. 2013, 56, 496–515. [Google Scholar]
- Footer, K.H.A.; White, R.H.; Park, J.N.; Decker, M.R.; Lutnick, A.; Sherman, S.G. Entry to Sex Trade and Long-Term Vulnerabilities of Female Sex Workers Who Enter the Sex Trade Before the Age of Eighteen. J. Urban Health 2020, 97, 406–417. [Google Scholar] [CrossRef]
- Yoosefi Lebni, J.; Irandoost, S.F.; Dehghan, A.A.; Ziapour, A.; Khosravi, B.; Mehedi, N. Exploring the reasons for women to engage in sex work in Tehran, Iran: A qualitative study. Heliyon 2021, 7, e08512. [Google Scholar] [CrossRef]
- Ishak MO, G.; Mumtaz, G.; Ishak, R.; Ridgway, G.L. Prevalence of antibodies to Chlamydia trachomatis in population groups of Brazil, England and Portugal. Rev. Inst. Med. Trop. Săo Paulo 1988, 30, 40–44. [Google Scholar]
- Ishak Mde, O.; Costa, M.M.; Almeida, N.C.; Santiago, A.M.; Brito, W.B.; Vallinoto, A.C.; Azevedo, V.N.; Ishak, R. Chlamydia trachomatis serotype A infections in the Amazon region of Brazil: Prevalence, entry and dissemination. Rev. Soc. Bras. Med. Trop. 2015, 48, 170–174. [Google Scholar] [CrossRef][Green Version]
- Santos, L.M.; Vieira, M.R.M.D.S.; Oliveira, J.F.G.; Trindade, J.Q.; Brasiliense, D.M.; Ferrari, S.F.; Tsutsumi, M.Y.; Fuzii, H.T.; Junior, E.C.S.; Ishikawa, E.A.Y.; et al. High prevalence of sexual Chlamydia trachomatis infection in young women from Marajó Island, in the Brazilian Amazon. PLoS ONE 2018, 13, e0207853. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.; Souza, J.D.; Mbakwa, H.A.; Nobre, A.F.S.; Vieira, R.C.; Ferrari, S.F.; Rodrigues, A.R.; Ishikawa, E.A.Y.; Guerreiro, J.F.; Sousa, M.S. High prevalence of sexual infection by human papillomavirus and Chlamydia trachomatis in sexually-active women from a large city in the Amazon region of Brazil. PLoS ONE 2022, 17, e0270874. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.; Souza, I.R.A.; Holanda, L.H.C.; Vaz, J.O.; Tsutsumi, M.Y.; Ishikawa, E.A.Y.; de Sousa, M.S. Alta incidência da infecção urogenital por Chlamydia trachomatis em mulheres parturientes de Belém, Estado do Pará, Brasil. Rev. Pan-Amaz. Saúde 2016, 7, 101–106. [Google Scholar] [CrossRef]
- Brasiliense, D.M.; Borges, B.N.; Ferreira, W.A. Genotyping and prevalence of Chlamydia trachomatis infection among women in Belém, Pará, northern Brazil. J. Infect. Dev. Ctries 2016, 10, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.M.S.; Vieira, M.R.M.S.; Vieira, R.C.; Silva, L.B.L.; Macêdo, G.M.M.; Miranda, A.E.; Brasiliense, D.M.; Guimarães, R.J.P.S.; Sousa Junior, E.C.; Ferrari, S.F.; et al. Prevalence and circulant genotypes of Chlamydia trachomatis in university women from cities in the Brazilian Amazon. PLoS ONE 2024, 19, e0287119. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. Assistência Integral à Saúde da Mulher: Bases da Ação Programática; Ministério da Saúde: Brasília, Brazil, 1984. [Google Scholar]
- Brasil. Lei N° 8.080 de, de 19 de Setembro de 1990. Dispõe Sobre as Condições para a Promoção, Proteção e Recuperação da Saúde, a Organização e o Funcionamento dos Serviços Correspondentes e dá Outras Providências. Available online: http://www.planalto.gov.br/ccivil_03/leis/L8080.htm (accessed on 4 January 2025).
- Brasil Ministério da Saúde. Secretaria de Atenção à Saúde. Departamento de Ações Programáticas Estratégicas. Política Nacional de Atenção Integral à Saúde da Mulher: Princípios e Diretrizes; Ministério da Saúde: Brasília, Brazil, 2004. [Google Scholar]
- Brasil Ministério do Trabalho—MST. Código Brasileiro de Ocupações N° 5198-05 (Profissionais do Sexo) de 02 de Fevereiro de 2015. Available online: https://cbo.mte.gov.br/cbosite/pages/home.jsf (accessed on 28 February 2025).
- BRASIL. Decreto-Lei 2.848, de 07 de Dezembro de 1940. Código Penal. Available online: https://www2.camara.leg.br/legin/fed/declei/1940-1949/decreto-lei-2848-7-dezembro-1940-412868-publicacaooriginal-1-pe.html (accessed on 28 February 2025).
- Dourado, I.; Guimarães, M.D.C.; Damacena, G.N.; Magno, L.; Souza Júnior, P.R.B.; Szwarcwald, C.L. Sex work stigma and non-disclosure to health care providers: Data from a large RDS study among FSW in Brazil. BMC Int. Health Hum. Rights 2019, 19, 8. [Google Scholar] [CrossRef]
- Pastori, B.G.; Colmanetti, A.B.; Aguiar, C.A. Perceptions of sex workers about the care received in the health care context. J. Hum. Growth Dev. 2022, 32, 275–282. [Google Scholar] [CrossRef]
- Lima, F.S.; Merchán-Hamann, E.; Urdaneta, M.; Damacena, G.N.; Szwarcwald, C.L. Factors associated with violence against female sex workers in ten Brazilian cities. Cad. Saude Publica 2017, 33, e00157815. [Google Scholar] [CrossRef]
- Muhindo, R.; Mujugira, A.; Castelnuovo, B.; Sewankambo, N.K.; Parkes-Ratanshi, R.; Tumwesigye, N.M.; Nakku-Joloba, E.; Kiguli, J. I felt very small and embarrassed by the health care provider when I requested to be tested for syphilis”: Barriers and facilitators of regular syphilis and HIV testing among female sex workers in Uganda. BMC Public Health 2021, 21, 1982. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).