Analysis of the Differences in Rhizosphere Microbial Communities and Pathogen Adaptability in Chili Root Rot Disease Between Continuous Cropping and Rotation Cropping Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection and Processing
2.2. Microbial Sequencing and Sequence Analysis
2.3. Pathogen Isolation and Identification
2.4. Determination of the Biological Characteristics of Pathogens
2.5. Statistical Analysis
3. Results
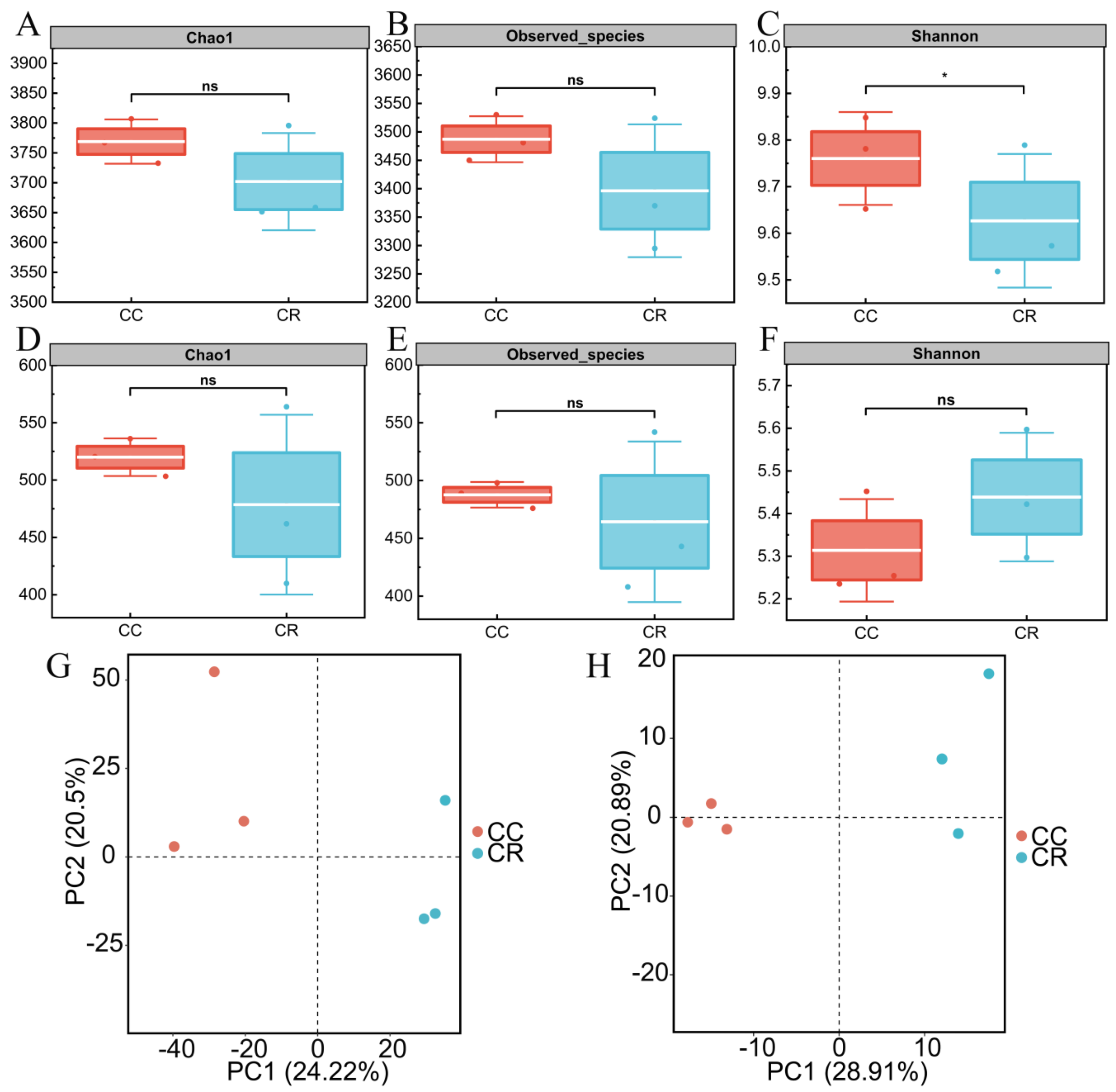
3.1. Diversity Analysis of Rhizosphere Soil Microbial Communities in the Continuous and Rotational Cropping of Chili
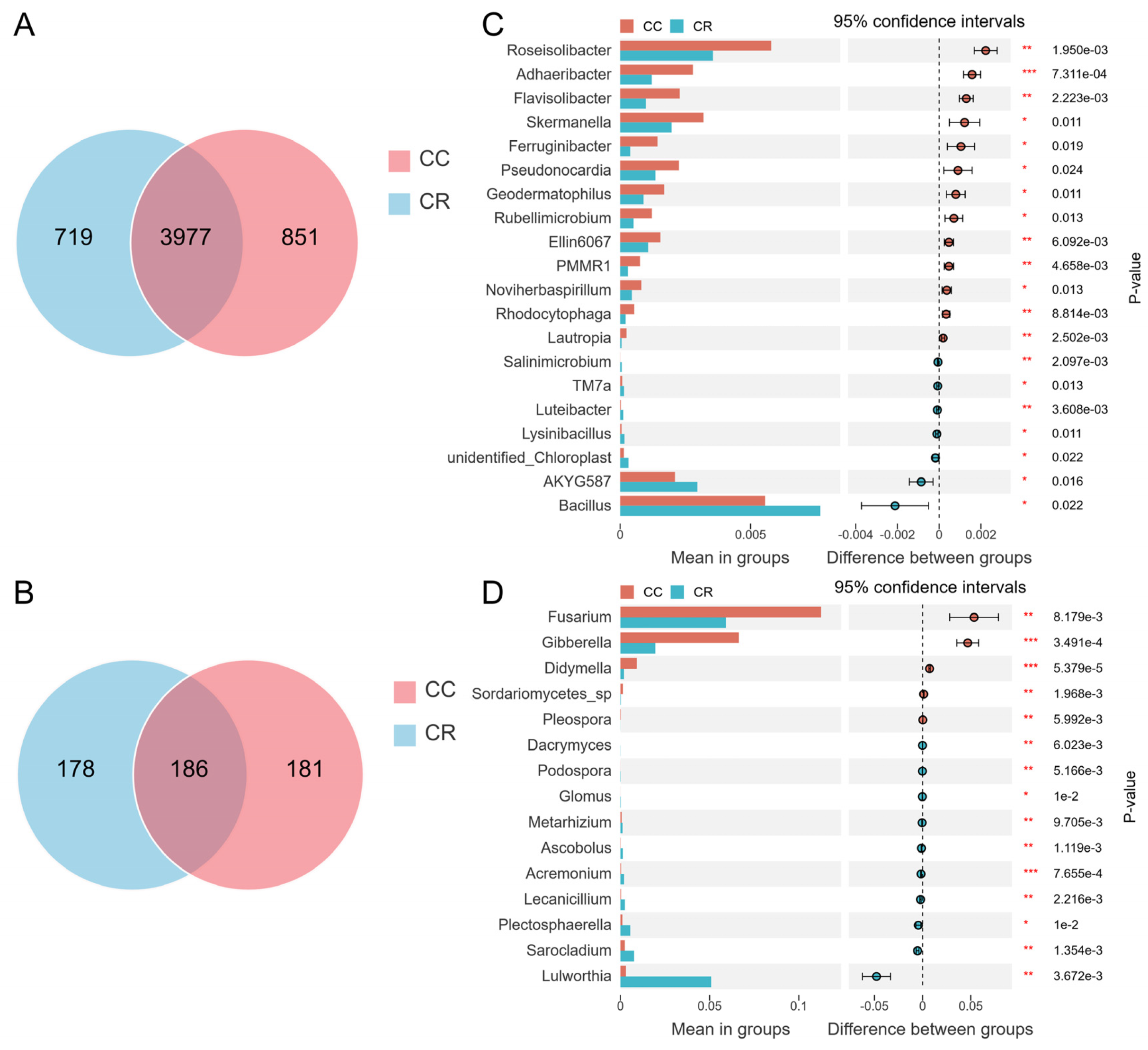
3.2. Relative Abundance Analysis of Dominant Groups in Rhizosphere Soil Microbial Communities Under Two Cropping Patterns
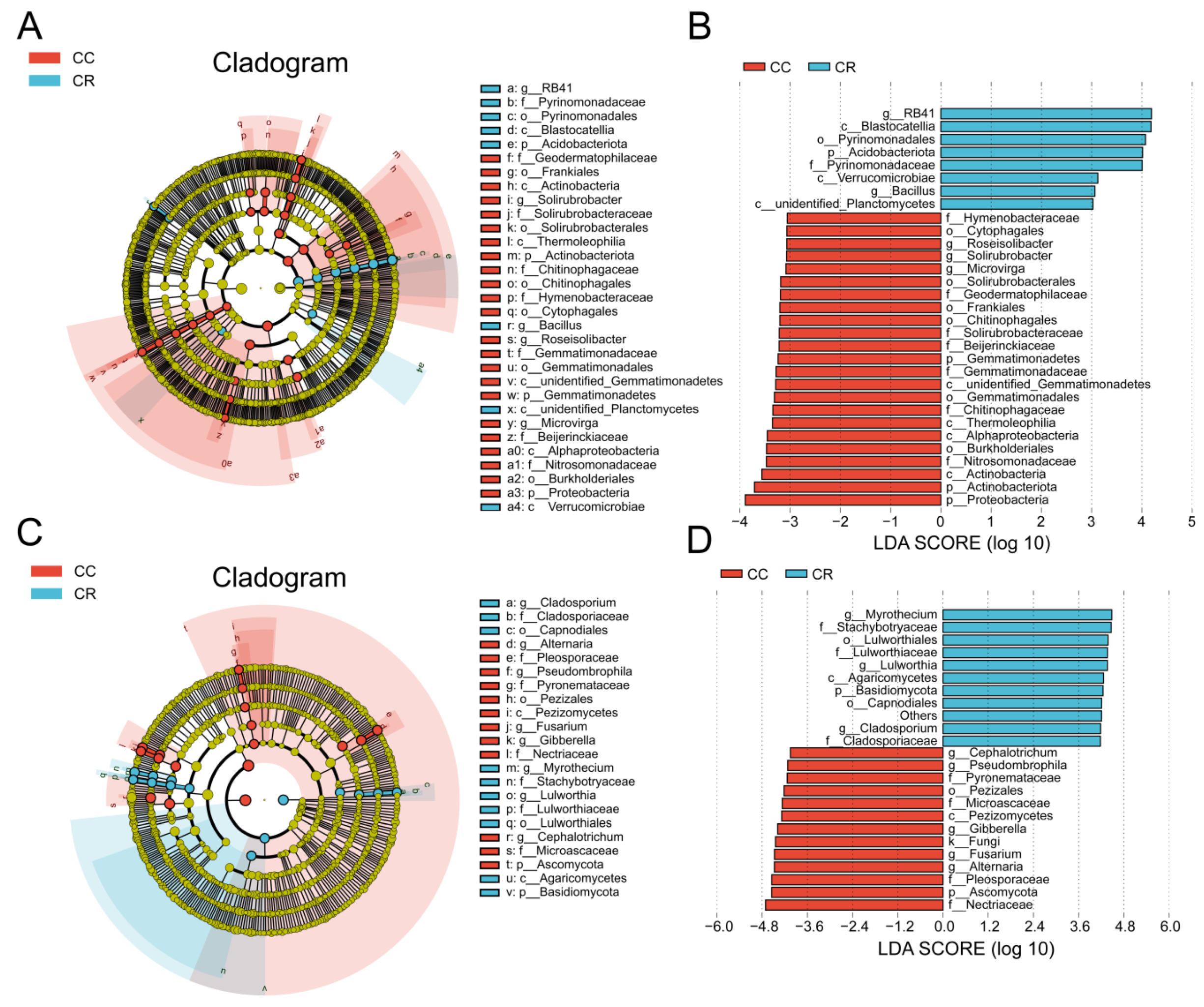
3.3. Analysis of Rhizosphere Microbial Community Differences in Chili Under Continuous and Rotation Cropping
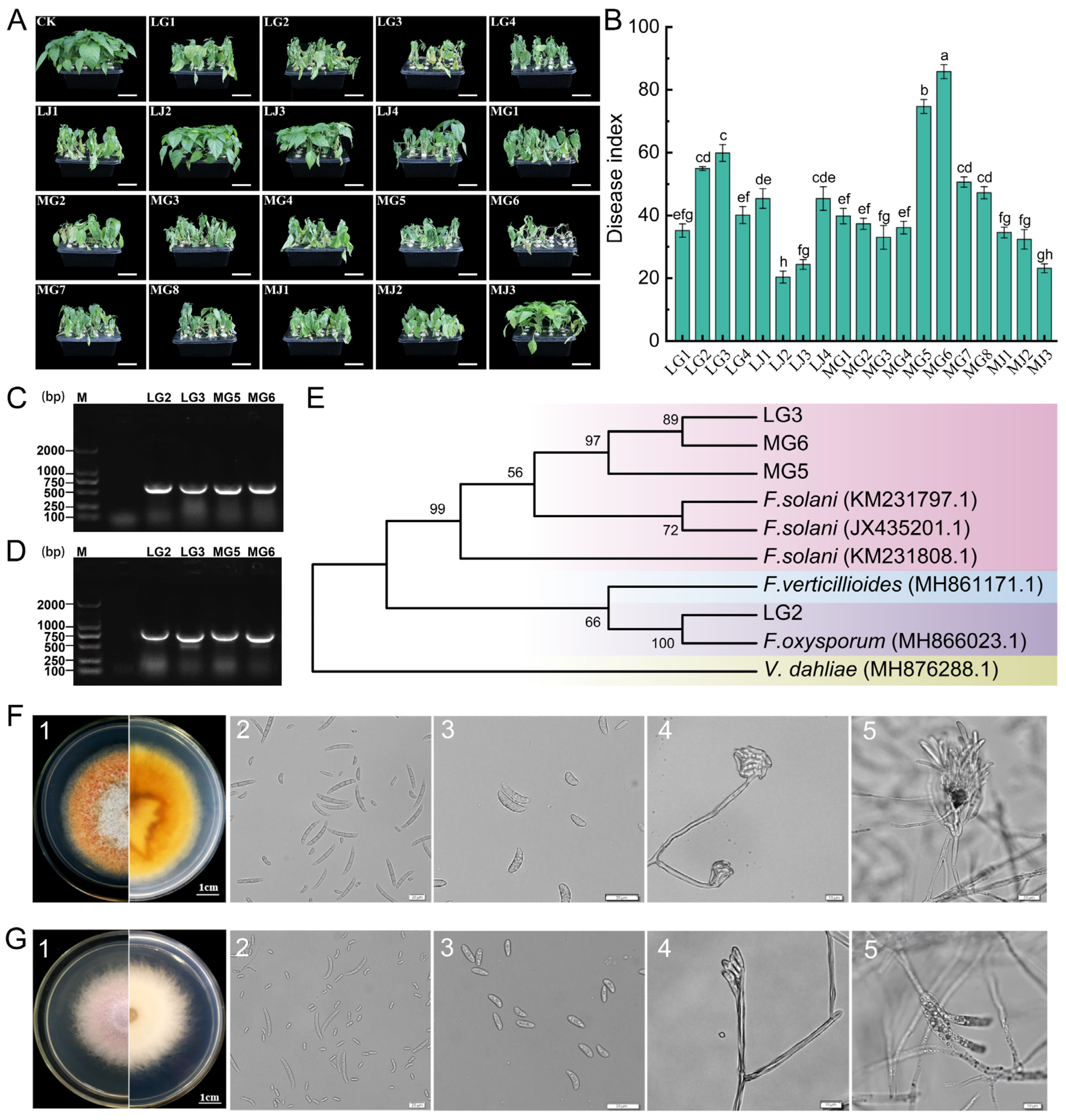
3.4. Isolation and Characterization of Chili Root Rot Pathogens
3.5. Biological Characterization of Pathogens
4. Discussion
4.1. Differences in Alpha/Beta Diversity of Soil Microorganisms in the Rhizosphere of Chili Under Continuous Cropping and Rotation Systems
4.2. Crop Rotation Suppresses Root Rot Pathogens by Regulating Microbial Community Composition
4.3. Stability of the Rhizosphere Core Microbiota
4.4. Effect of Crop Rotation on Chili Root Rot Pathogens
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Pérez, T.; Gómez-García, M.D.R.; Valverde, M.E.; Paredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, J.; Sun, H.; Xiong, C.; Sun, X.; Wang, X.; Wang, Z.; Jarret, R.; Wang, J.; Tang, B.; et al. Genomes of cultivated and wild Capsicum species provide insights into pepper domestication and population differentiation. Nat. Commun. 2023, 14, 5487–5501. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Rhizobiome engineering: Unveiling complex rhizosphere interactions to enhance plant growth and health. Microbiol. Res. 2022, 263, 127137–127152. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Ma, Y.; Dai, X.; Li, X.; Yang, S. Spread and industry development of pepper in china. Acta Hortic. Sinica 2020, 47, 1715–1726. [Google Scholar]
- Guo, H.; Yao, L. Development achievements and countermeasures of high quality development of pepper industry in Xinjiang. Southeast Hortic. 2024, 12, 525–535. [Google Scholar]
- Xu, W.; Li, H.; Ma, Q.; Mu, S.; Zhao, Z.; Gao, J.; Zhang, F.; Xie, H. Exploring the mitigation effect of microbial inoculants on the continuous cropping obstacle of capsicum. Sci. Hortic. 2024, 338, 113507–113522. [Google Scholar] [CrossRef]
- Jing, J.; Cong, W.F.; Bezemer, T.M. Legacies at work: Plant-soil-microbiome interactions underpinning agricultural sustainability. Trends Plant Sci. 2022, 27, 781–792. [Google Scholar] [CrossRef]
- Tan, G.; Liu, Y.; Peng, S.; Yin, H.; Meng, D.; Tao, J.; Gu, Y.; Li, J.; Yang, S.; Xiao, N.; et al. Soil potentials to resist continuous cropping obstacle: Three field cases. Environ. Res. 2021, 200, 111319–111330. [Google Scholar] [CrossRef]
- Sun, J.; Zou, X.; Luo, Z.; Liu, M.; Dai, X. Research Progress in Continuous Cropping of Hot Pepper. J. China Capsicum 2011, 9, 1–7+27. [Google Scholar]
- Ge, S.; Gao, J.; Chang, D.; He, T.; Cai, H.; Wang, M.; Li, C.; Luo, Z.; Yang, E.; Meng, J.; et al. Biochar contributes to resistance against root rot disease by stimulating soil polyphenol oxidase. Biochar 2023, 5, 55–72. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Qian, X.; Gu, J.; Chen, W.; Shen, X.; Tao, S.; Jiao, S.; Wei, G. Metagenomics insights into responses of rhizobacteria and their alleviation role in licorice allelopathy. Microbiome 2023, 11, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ma, L.; Chen, G.; Wei, R.; Ma, Y.; Dang, J.; Cheng, Z.; Ma, S.; Li, S. Mechanisms of pathogenic microorganisms, root antioxidant systems, and secondary metabolites of Codonopsis pilosula (Franch.) Nannf. in response to continuous cropping obstacles. Ind. Crops Prod. 2025, 225, 120455–120471. [Google Scholar] [CrossRef]
- Ahsan, T.; Tian, P.C.; Gao, J.; Wang, C.; Liu, C.; Huang, Y.Q. Effects of microbial agent and microbial fertilizer input on soil microbial community structure and diversity in a peanut continuous cropping system. J. Adv. Res. 2024, 64, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Zhang, J.; Duan, Y.; Zhou, W.; He, P. Optimized fertilization for effective suppression of soil-borne disease: Differential effects on bulk and rhizosphere soils. Field Crop. Res. 2025, 322, 109725–109735. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Barbetti, M.J.; Chilvers, M.I.; Pandey, A.K.; Steinberg, C. Exploiting root exudates to manage soil-borne disease complexes in a changing climate. Trends Microbiol. 2024, 32, 27–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Chen, F.; Yan, J.; Wu, J.; Wang, J.; Ge, S. Transcriptome Analyses Reveal Distinct Defense Strategies in Chili Plants under Soilborne Disease Intervention. Horticulturae 2023, 9, 1267. [Google Scholar] [CrossRef]
- Kaur, N.; Lozada, D.N.; Bhatta, M.; Barchenger, D.W.; Khokhar, E.S.; Nourbakhsh, S.S.; Sanogo, S. Insights into the genetic architecture of Phytophthora capsici root rot resistance in chile pepper (Capsicum spp.) from multi-locus genome-wide association study. BMC Plant Biol. 2024, 24, 416–434. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Belal, E.B.; El-Khateeb, N.M.M.; Shreef, B.A. Utilizing bio-synthesis of nanomaterials as biological agents for controlling soil-borne diseases in pepper plants: Root-knot nematodes and root rot fungus. BMC Plant Biol. 2024, 24, 110–131. [Google Scholar] [CrossRef]
- Arora, H.; Sharma, A.; Sharma, S.; Haron, F.F.; Gafur, A.; Sayyed, R.Z.; Datta, R. Pythium damping-off and root rot of Capsicum annuum L.: Impacts, diagnosis, and management. Microorganisms 2021, 9, 823. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, R.; Liu, Y.; Liu, Y. Evaluation of the biocontrol efficiency of Bacillus subtilis wettable powder on pepper root rot caused by Fusarium solani. Pathogens 2023, 12, 225. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Rhizosphere engineering for soil carbon sequestration. Trends Plant Sci. 2024, 29, 447–468. [Google Scholar] [CrossRef]
- Ren, T.; Feng, H.; Xu, C.; Xu, Q.; Fu, B.; Azwar, E.; Wei, Y.; Lam, S.S.; Liu, G. Exogenous application and interaction of biochar with environmental factors for improving functional diversity of rhizosphere’s microbial community and health. Chemosphere 2022, 294, 133710–133719. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Khashi, U.; Rahman, M.; Gao, D.; Wei, Z.; Wu, F.; Dini-Andreote, F. Interspecific plant interaction via root exudates structures the disease suppressiveness of rhizosphere microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103–125. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.I.A.; Moreira, H.; Argyras, K.; Castro, P.M.L.; Marques, A.P.G.C. Promotion of sunflower growth under saline water irrigation by the inoculation of beneficial microorganisms. Appl. Soil Ecol. 2016, 105, 36–47. [Google Scholar] [CrossRef]
- Avis, T.J.; Gravel, V.; Antoun, H.; Tweddell, R.J. Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol. Biochem. 2008, 40, 1733–1740. [Google Scholar] [CrossRef]
- Gu, Y.; Banerjee, S.; Dini-Andreote, F.; Xu, Y.; Shen, Q.; Jousset, A.; Wei, Z. Small changes in rhizosphere microbiome composition predict disease outcomes earlier than pathogen density variations. ISME J. 2022, 16, 2448–2456. [Google Scholar] [CrossRef]
- Garrido-Sanz, D.; Čaušević, S.; Vacheron, J.; Heiman, C.M.; Sentchilo, V.; van der Meer, J.R.; Keel, C. Changes in structure and assembly of a species-rich soil natural community with contrasting nutrient availability upon establishment of a plant-beneficial Pseudomonas in the wheat rhizosphere. Microbiome 2023, 11, 214–231. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Liu, J.; Li, X.; Wang, X.; Dai, C.; Zhang, T.; Carrión, V.J.; Wei, Z.; Cao, F.; et al. Crop rotation and native microbiome inoculation restore soil capacity to suppress a root disease. Nat. Commun. 2023, 14, 8126–8140. [Google Scholar] [CrossRef]
- Li, Z.; Bai, X.; Jiao, S.; Li, Y.; Li, P.; Yang, Y.; Zhang, H.; Wei, G. A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant-induced systemic resistance. Microbiome 2021, 9, 217–237. [Google Scholar] [CrossRef]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.M.; Zhou, X.; Zhang, A.M.; Cai, L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 2021, 9, 187–205. [Google Scholar] [CrossRef]
- Nawaz, K.; Anwar, W.; Subhani, M.N.; Akhter, A.; Iftikhar, S.; Khan, H.A.A.; Shahid, A.A. Glucanase gene of Trichoderma; new strategy for the management of root rot disease in chili. J. Soil Sci. Plant Nutr. 2023, 24, 354–370. [Google Scholar] [CrossRef]
- Qin, L.; Gao, Y.; Wang, L.; Ran, J.; Ou, X.; Wang, Y.; Jiang, W.; Zhou, T.; Yuan, Q.-S. Trichoderma consortium compost corncob promotes the growth of Pseudostellaria heterophylla and alleviates its diseases incidence via reshaping the soil microbiome and improving the soil nutrients under continuous monocropping conditions. Ind. Crops Prod. 2024, 216, 118800–118812. [Google Scholar] [CrossRef]
- Fang, H.; Guo, C.; Mei, X.; Hao, M.; Zhang, J.; Luo, L.; Liu, H.; Liu, Y.; Huang, H.; He, X.; et al. Light stress elicits soilborne disease suppression mediated by root-secreted flavonoids in Panax notoginseng. Hortic. Res. 2024, 11, uhae213. [Google Scholar] [CrossRef] [PubMed]
- Town, J.R.; Dumonceaux, T.; Tidemann, B.; Helgason, B.L. Crop rotation significantly influences the composition of soil, rhizosphere, and root microbiota in canola (Brassica napus L.). Environ. Microbiome 2023, 18, 40. [Google Scholar] [CrossRef]
- Wang, B.; Li, R.; Ruan, Y.; Ou, Y.; Zhao, Y.; Shen, Q. Pineapple–banana rotation reduced the amount of Fusarium oxysporum more than maize–banana rotation mainly through modulating fungal communities. Soil Boil. Biochem. 2015, 86, 77–86. [Google Scholar] [CrossRef]
- Benitez, M.-S.; Ewing, P.M.; Osborne, S.L.; Lehman, R.M. Rhizosphere microbial communities explain positive effects of diverse crop rotations on maize and soybean performance. Soil Biol. Biochem. 2021, 159, 108309. [Google Scholar] [CrossRef]
- De Corato, U.; Patruno, L.; Avella, N.; Salimbeni, R.; Lacolla, G.; Cucci, G.; Crecchio, C. Soil management under tomato-wheat rotation increases the suppressive response against Fusarium wilt and tomato shoot growth by changing the microbial composition and chemical parameters. Appl. Soil Ecol. 2020, 154, 103601–103619. [Google Scholar] [CrossRef]
- Edwards, J.; Santos-Medellín, C.; Sundaresan, V. Extraction and 16S rRNA sequence analysis of microbiomes associated with rice roots. Bio Protoc. 2018, 8, e2884–e2900. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Stach, J.E.; Maldonado, L.A.; Masson, D.G.; Ward, A.C.; Goodfellow, M.; Bull, A.T. Statistical approaches for estimating actinobacterial diversity in marine sediments. Appl. Environ. Microbiol. 2003, 69, 6189–6200. [Google Scholar] [CrossRef]
- Jin, L.; Yang, L.; Li, W.; Xu, D.; Yang, N.; Li, G.; Wan, P. Diversity and Biocontrol Potential of Culturable Endophytic Fungi in Cotton. Front. Microbiol. 2021, 12, 698930. [Google Scholar] [CrossRef]
- Zhu, T.; Luo, X.; Hao, C.; Zhu, Z.; Liu, L.; Deng, Z.; Cao, Y.; Ma, X. Isolation, identification and pathogenicity of two root rot pathogens Fusarium solani in Citrus. Hortic Plant J. 2025. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 2019, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Whitaker, B.K.; Laraba, I.; Proctor, R.H.; Brown, D.W.; Broders, K.; Kim, H.-S.; McCormick, S.P.; Busman, M.; Aoki, T.; et al. DNA sequence-based identification of Fusarium: A work in progress. Plant Dis. 2022, 106, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Qu, H.; Zhao, S.; Guo, H.; Ma, M. Pathogen identification of processing chili pepper root rot disease in Xinjiang. North. Hortic. 2016, 108–112. [Google Scholar] [CrossRef]
- Lian, Y.; Li, H.; Li, H.; Jiang, W.; Li, J.; Shi, M.; Han, B. Pathogen identification and biological characteristics of Fusarium root rot of pepper in Qingyang of Gansu Province. J. Gansu Agric. Univ. 2021, 56, 55–61+68. [Google Scholar]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef]
- Zeeshan Ul Haq, M.; Yu, J.; Yao, G.; Yang, H.; Iqbal, H.A.; Tahir, H.; Cui, H.; Liu, Y.; Wu, Y. A systematic review on the continuous cropping obstacles and control strategies in medicinal plants. Int. J. Mol. Sci. 2023, 24, 12470. [Google Scholar] [CrossRef]
- Wang, G.; Ren, Y.; Bai, X.; Su, Y.; Han, J. Contributions of beneficial microorganisms in soil remediation and quality improvement of medicinal plants. Plants 2022, 11, 3200. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Shen, Z.; Yang, Z.; Fu, X.; Wang, X.; Li, S.; Chai, H.; Wang, R.; Liu, X.; et al. Alfalfa with forage crop rotation alleviates continuous alfalfa obstacles through regulating soil enzymes and bacterial community structures. Agronomy 2024, 14, 1349. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, Y.; Qin, S.; Zhang, W.; Shi, M.; Fan, Y.; Yang, X. Ridge–mulch tillage and rotation with broad bean affect soil microbial community, diversity and crop yield in a long-term potato continuous cropping field. Soil Use Manag. 2020, 37, 677–688. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, X.; Zhang, Z.; He, H.; Yu, J.; Zhou, Y. Effects of different cultivation patterns on soil microbial community and enzyme activity in continuous cropped pepper field. Acta Agric. Zhejiangensis 2019, 31, 1485–1492. [Google Scholar]
- Zhao, X.; Dong, Q.; Han, Y.; Zhang, K.; Shi, X.; Yang, X.; Yuan, Y.; Zhou, D.; Wang, K.; Wang, X.; et al. Maize/peanut intercropping improves nutrient uptake of side-row maize and system microbial community diversity. BMC Microbiol. 2022, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, V.; Costa, J.L. Soil quality indicators under continuous cropping systems in the Argentinean Pampas. Soil Tillage Res. 2007, 96, 155–165. [Google Scholar] [CrossRef]
- Qin, J.; Bian, C.; Duan, S.; Wang, W.; Li, G.; Jin, L. Effects of different rotation cropping systems on potato yield, rhizosphere microbial community and soil biochemical properties. Front. Plant Sci. 2022, 13, 999730–999744. [Google Scholar] [CrossRef]
- Zong, D.; Zhou, Y.; Zhou, J.; Zhao, Y.; Hu, X.; Wang, T. Soil microbial community composition by crop type under rotation diversification. BMC Microbiol. 2024, 24, 435–447. [Google Scholar] [CrossRef]
- Cho, G.; Kim, D.R.; Kwak, Y.S. Ecological shifts in soil microbiota and root rot disease progress during ginseng monoculture. Front. Microbiol. 2024, 15, 1442208–1442219. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2008, 321, 341–361. [Google Scholar] [CrossRef]
- Wang, C.; Ma, H.; Feng, Z.; Yan, Z.; Song, B.; Wang, J.; Zheng, Y.; Hao, W.; Zhang, W.; Yao, M.; et al. Integrated organic and inorganic fertilization and reduced irrigation altered prokaryotic microbial community and diversity in different compartments of wheat root zone contributing to improved nitrogen uptake and wheat yield. Sci. Total Environ. 2022, 842, 156952–156966. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Zhang, S.; Li, N.; Wei, X.; Wei, Y.; Wei, L.; Li, J.; Huang, S.; Chen, Q.; et al. Soil organic carbon stability mediate soil phosphorus in greenhouse vegetable soil by shifting phoD-harboring bacterial communities and keystone taxa. Sci. Total Environ. 2023, 873, 162400. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Sun, W.; Guo, J.; Liu, W.; Zhao, M. Microbial communities in the rhizosphere soil of Ambrosia artemisiifolia facilitate its growth. Plant Soil 2023, 492, 353–365. [Google Scholar] [CrossRef]
- Adamczyk, B.; Kitunen, V.; Smolander, A. Polyphenol oxidase, tannase and proteolytic activity in relation to tannin concentration in the soil organic horizon under silver birch and Norway spruce. Soil Biol. Biochem. 2009, 41, 2085–2093. [Google Scholar] [CrossRef]
- Hirozawa, M.T.; Ono, M.A.; Suguiura, I.M.d.S.; Bordini, J.G.; Ono, E.Y.S. Lactic acid bacteria and Bacillus spp. as fungal biological control agents. J. Appl. Microbiol. 2023, 134, lxac083. [Google Scholar] [CrossRef]
- Jia, F.; Chang, F.; Guan, M.; Jia, Q.; Sun, Y.; Li, Z. Effects of rotation and Bacillus on the changes of continuous cropping soil fungal communities in American ginseng. World J. Microbiol. Biotechnol. 2023, 39, 354–365. [Google Scholar] [CrossRef]
- Su, L.; Zhang, J.; Fan, J.; Li, D.; Zhao, M.; Wang, Y.; Pan, H.; Zhao, L.; Zhang, X. Antagonistic mechanism analysis of Bacillus velezensis JLU-1, a biocontrol agent of rice pathogen Magnaporthe oryzae. J. Agric. Food Chem. 2024, 72, 19657–19666. [Google Scholar] [CrossRef]
- Liu, J.; Qin, D.; Huang, W.; Wang, X.; Li, Y.; Zhang, R. Biocontrol ability and action mechanism of Bacillus amyloliquefaciens Baf1 against Fusarium incarnatum causing fruit rot in postharvest muskmelon (cv. Yugu) fruit. Lwt 2023, 181, 114714–114726. [Google Scholar] [CrossRef]
- Hao, Y.; Rasheed, A.; Zhu, Z.; Wulff, B.B.H.; He, Z. Harnessing wheat Fhb1 for Fusarium resistance. Trends Plant Sci. 2020, 25, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Wang, Z.; Liu, S.; Lv, N.; Deng, X.; Xiong, W.; Shen, Z.; Zhang, N.; Geisen, S.; Li, R.; et al. Additive fungal interactions drive biocontrol of Fusarium wilt disease. New Phytol. 2023, 238, 1198–1214. [Google Scholar] [CrossRef]
- Ren, J.; Cao, T.; Zang, X.; Liu, J.; Yang, D. Antifungal mechanisms and characteristics of Pseudomonas fluorescens: Promoting peanut growth and combating Fusarium oxysporum-induced root rot. Plant Physiol. Biochem. 2024, 216, 109092–109102. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Yu, S.; Liu, S.; Cui, T.; Huang, R.; Wang, Y.; Wang, J.; Tan, K.; Li, X. Crop rotations reduce pathogenic fungi compared to continuous cropping. Rhizosphere 2025, 34, 101074–101083. [Google Scholar] [CrossRef]
- van Bruggen, A.H.C.; Semenov, A.M. In search of biological indicators for soil health and disease suppression. Appl. Soil Ecol. 2000, 15, 13–24. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, X.; Solanki, M.K.; Pang, F. A synthetic microbial community of plant core microbiome can be a potential biocontrol tool. J. Agric. Food Chem. 2023, 71, 5030–5041. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, T.; Huang, Q.; Guo, H.; Zhang, H.; Xu, Q.; Shen, Q.; Ling, N. Core species impact plant health by enhancing soil microbial cooperation and network complexity during community coalescence. Soil Boil. Biochem. 2024, 188, 109231–109243. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, D.; Wang, H. Pepper root rot resistance and pepper yield are enhanced through biological agent G15 soil amelioration. PeerJ 2021, 9, e11768–e11785. [Google Scholar] [CrossRef] [PubMed]
- Cerkauskas, R.F. Etiology and management of Fusarium crown and root rot (Fusarium oxysporum) on greenhouse pepper in Ontario, Canada. Can. J. Plant Pathol. 2017, 39, 121–132. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Cao, X.; Zhang, L.; Hu, X.; Zeng, X.; Wei, Y.; Zhang, D.; Xiao, X.; Xi, H.; Zhao, S. Analysis of the Differences in Rhizosphere Microbial Communities and Pathogen Adaptability in Chili Root Rot Disease Between Continuous Cropping and Rotation Cropping Systems. Microorganisms 2025, 13, 1806. https://doi.org/10.3390/microorganisms13081806
Zhao Q, Cao X, Zhang L, Hu X, Zeng X, Wei Y, Zhang D, Xiao X, Xi H, Zhao S. Analysis of the Differences in Rhizosphere Microbial Communities and Pathogen Adaptability in Chili Root Rot Disease Between Continuous Cropping and Rotation Cropping Systems. Microorganisms. 2025; 13(8):1806. https://doi.org/10.3390/microorganisms13081806
Chicago/Turabian StyleZhao, Qiuyue, Xiaolei Cao, Lu Zhang, Xin Hu, Xiaojian Zeng, Yingming Wei, Dongbin Zhang, Xin Xiao, Hui Xi, and Sifeng Zhao. 2025. "Analysis of the Differences in Rhizosphere Microbial Communities and Pathogen Adaptability in Chili Root Rot Disease Between Continuous Cropping and Rotation Cropping Systems" Microorganisms 13, no. 8: 1806. https://doi.org/10.3390/microorganisms13081806
APA StyleZhao, Q., Cao, X., Zhang, L., Hu, X., Zeng, X., Wei, Y., Zhang, D., Xiao, X., Xi, H., & Zhao, S. (2025). Analysis of the Differences in Rhizosphere Microbial Communities and Pathogen Adaptability in Chili Root Rot Disease Between Continuous Cropping and Rotation Cropping Systems. Microorganisms, 13(8), 1806. https://doi.org/10.3390/microorganisms13081806





