Fecal Virome Transplantation Confirms Non-Bacterial Components (Virome and Metabolites) Participate in Fecal Microbiota Transplantation-Mediated Growth Performance Enhancement and Intestinal Development in Broilers with Spatial Heterogeneity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Broilers Management.
2.2. Preparation of FVT Suspension
2.3. Diets and Compositional Analysis
2.4. Growth Performance
2.5. Immunological and Antioxidant
2.6. Measurement of Intestinal Histomorphological Parameters
2.7. RNA Extraction and qRT-PCR Analysis
2.8. Cecal Microbial Diversity
2.9. Statistical Analysis
3. Results
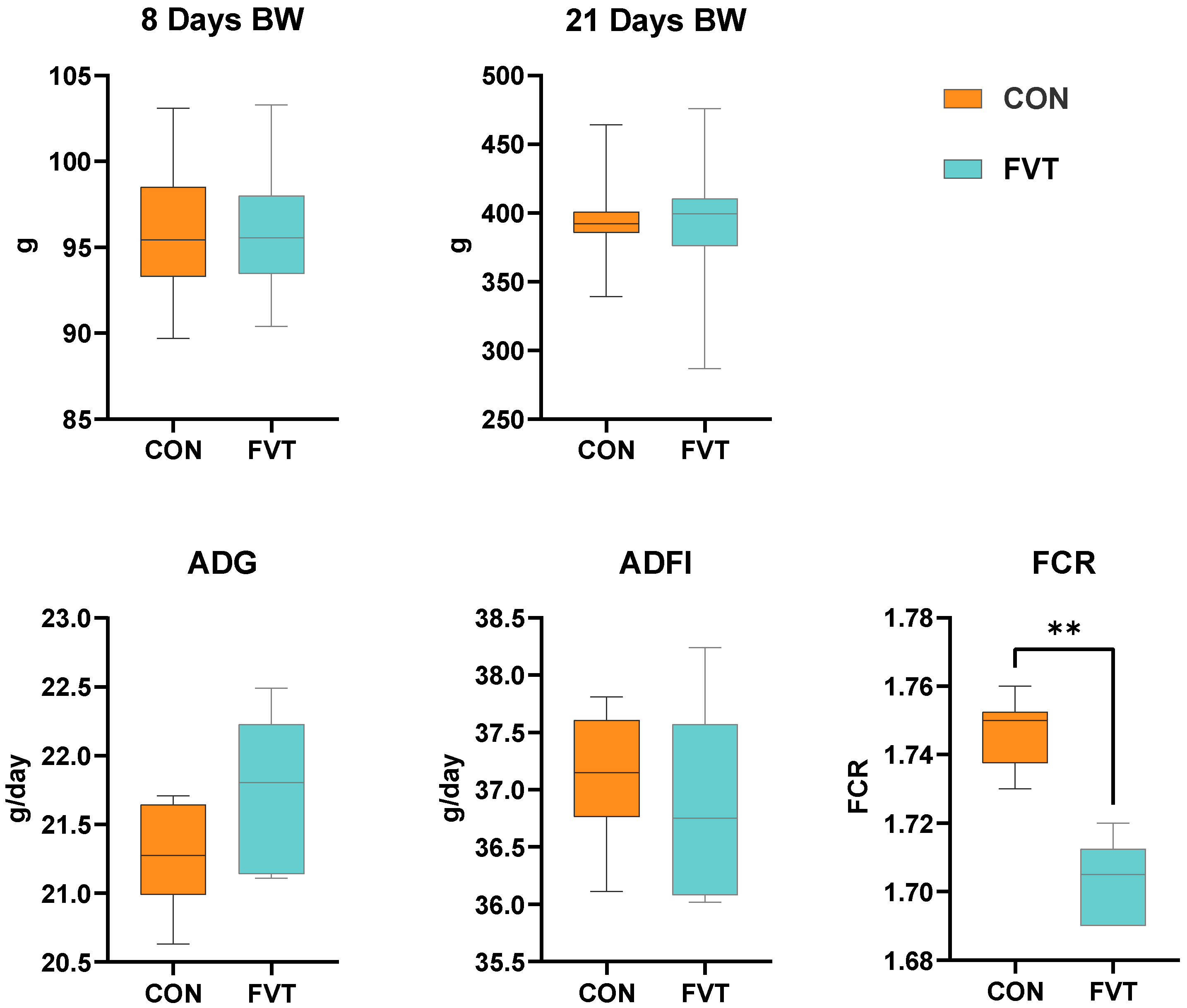
3.1. Growth Performance
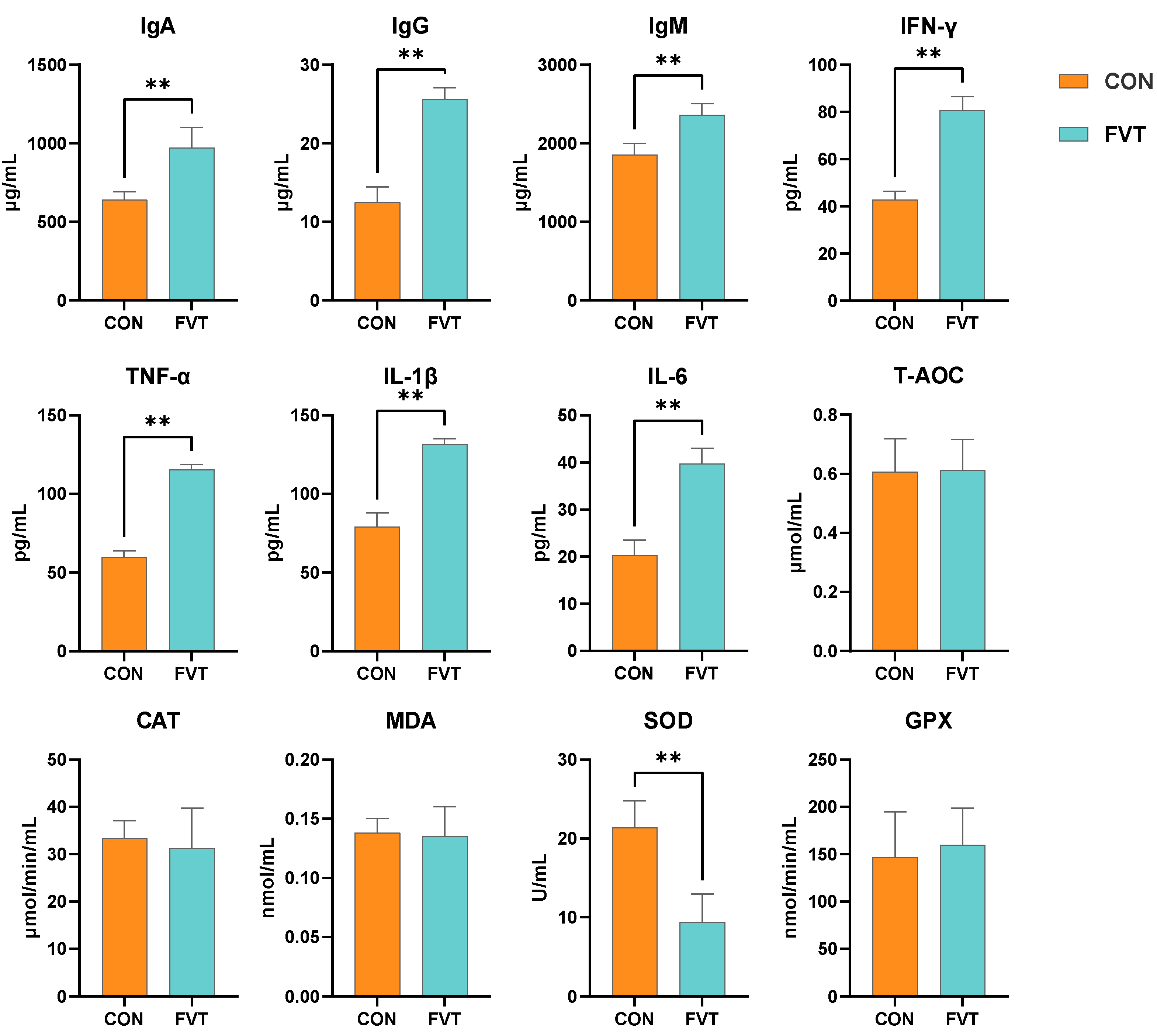
3.2. Blood Immunology and Antioxidation
3.3. Hepatic Immunology and Antioxidation
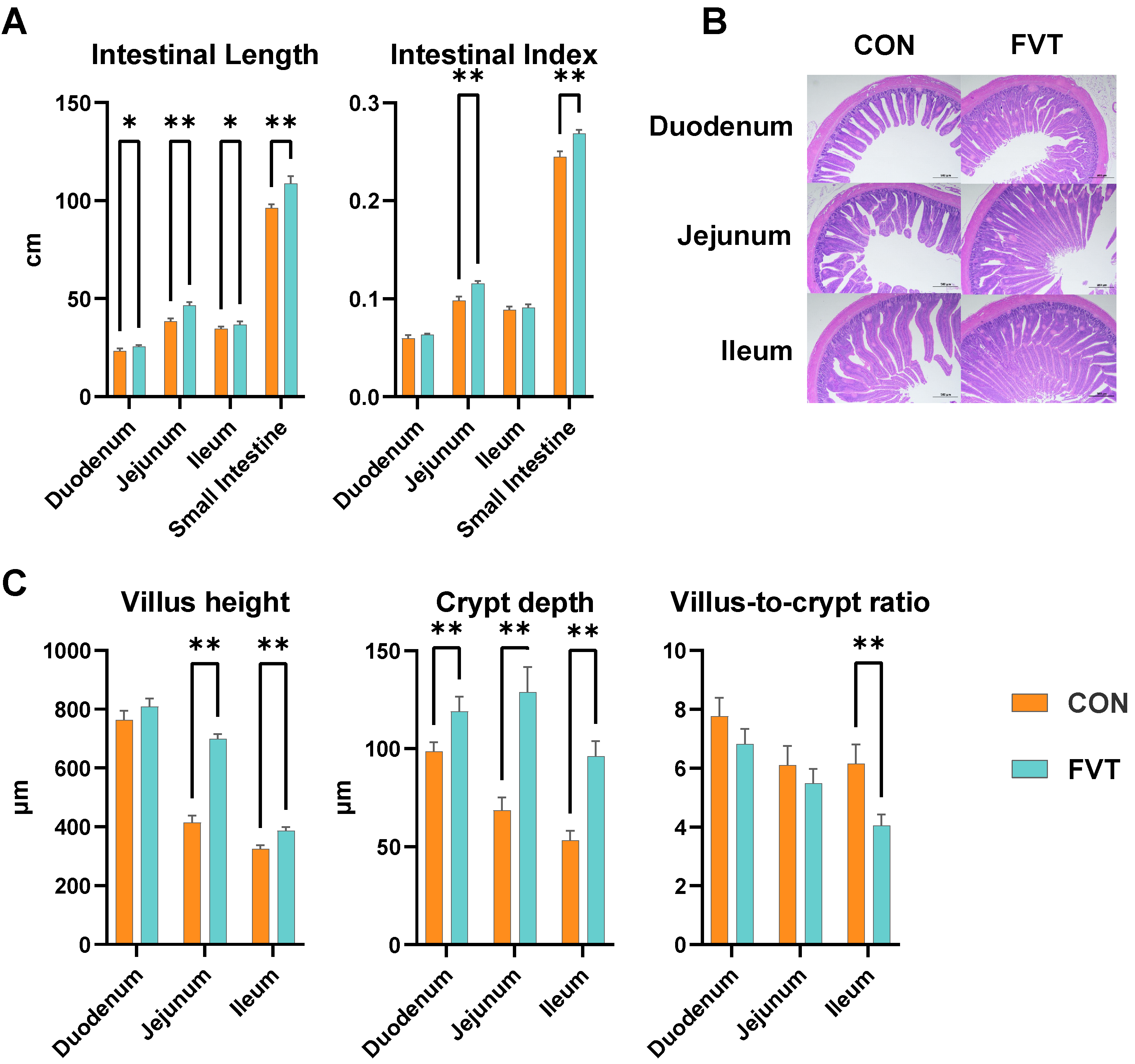
3.4. Intestinal Histomorphology
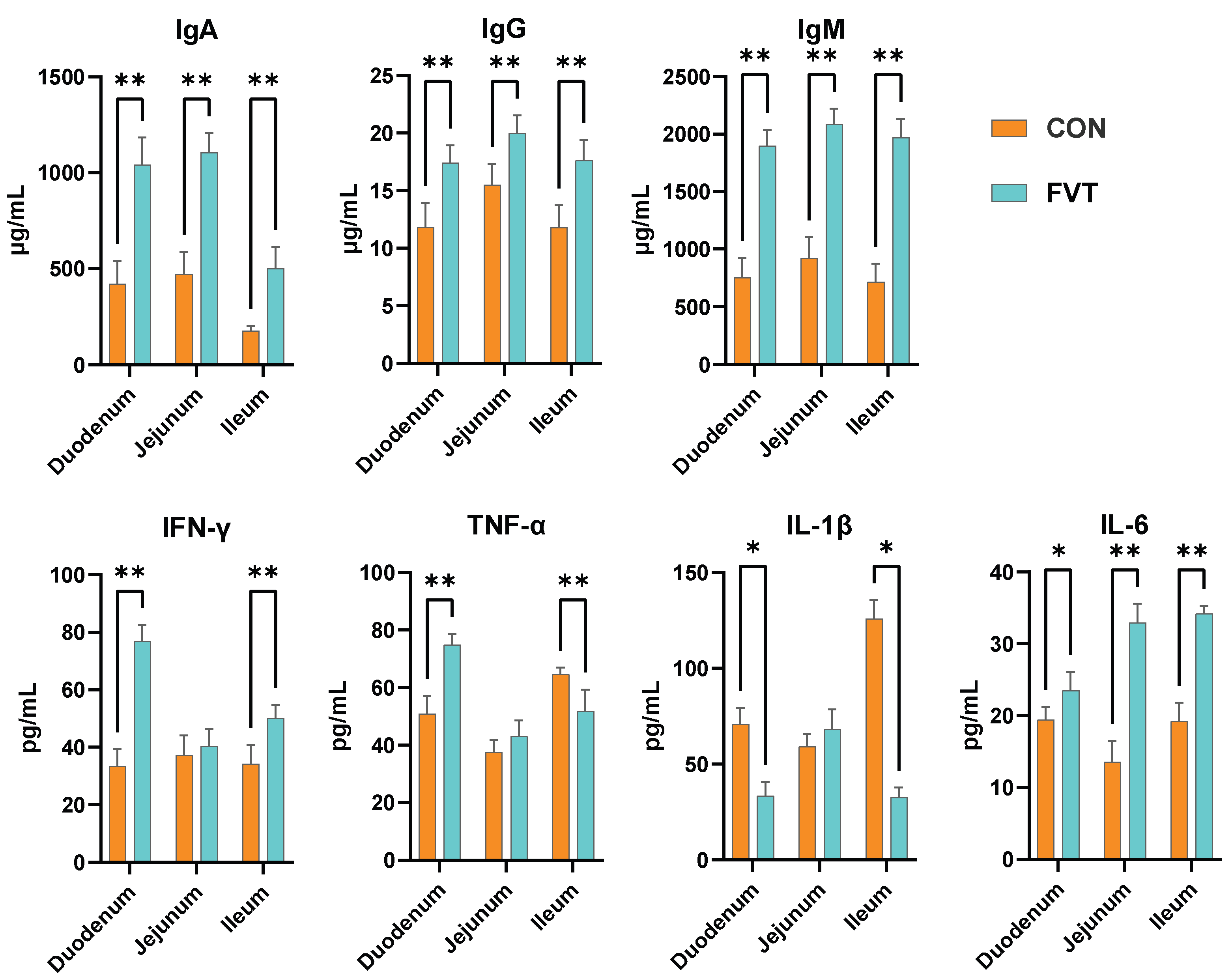
3.5. Intestinal Immunology
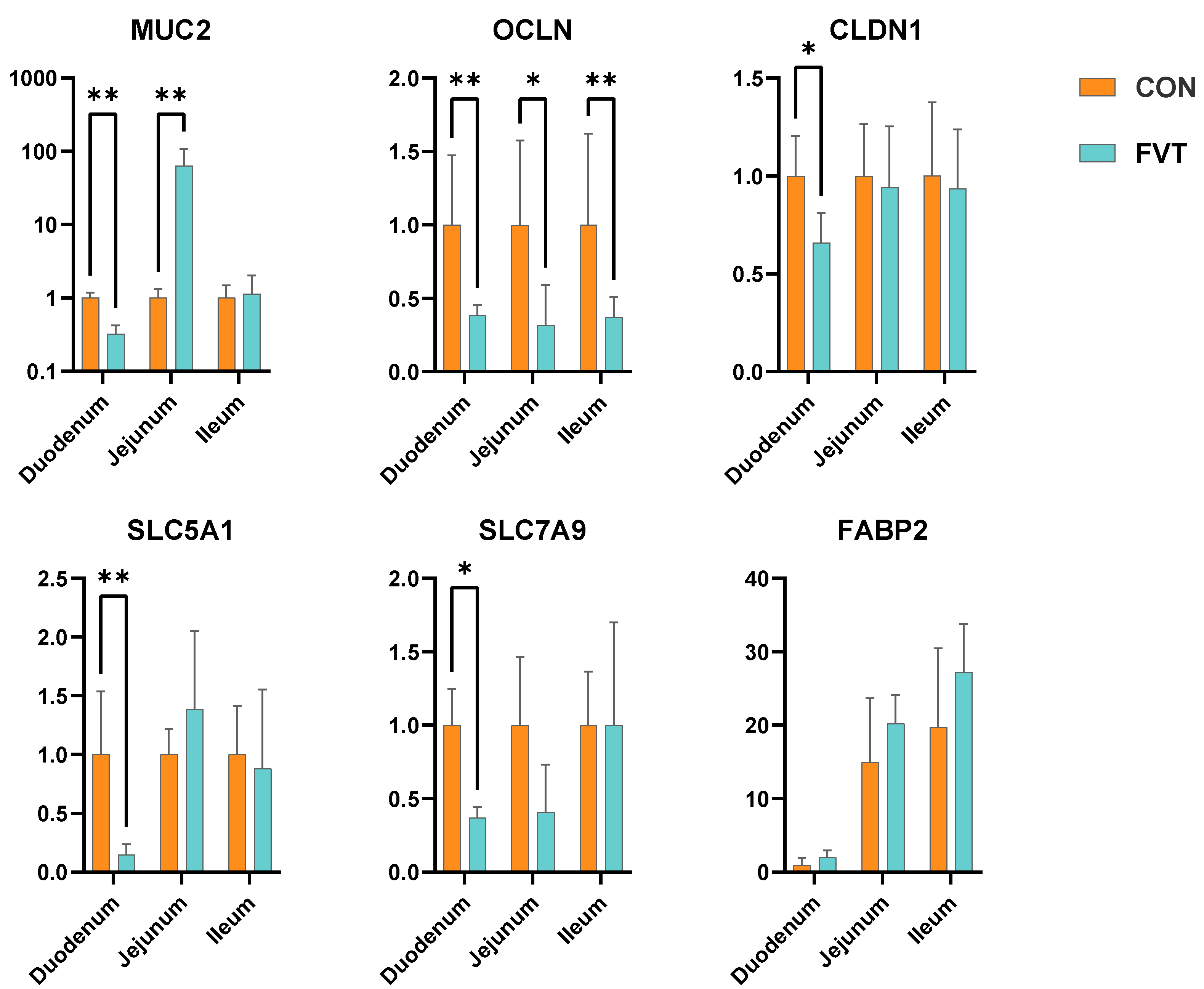
3.6. Expression of Genes Related to Intestinal Barrier and Nutrient Absorption
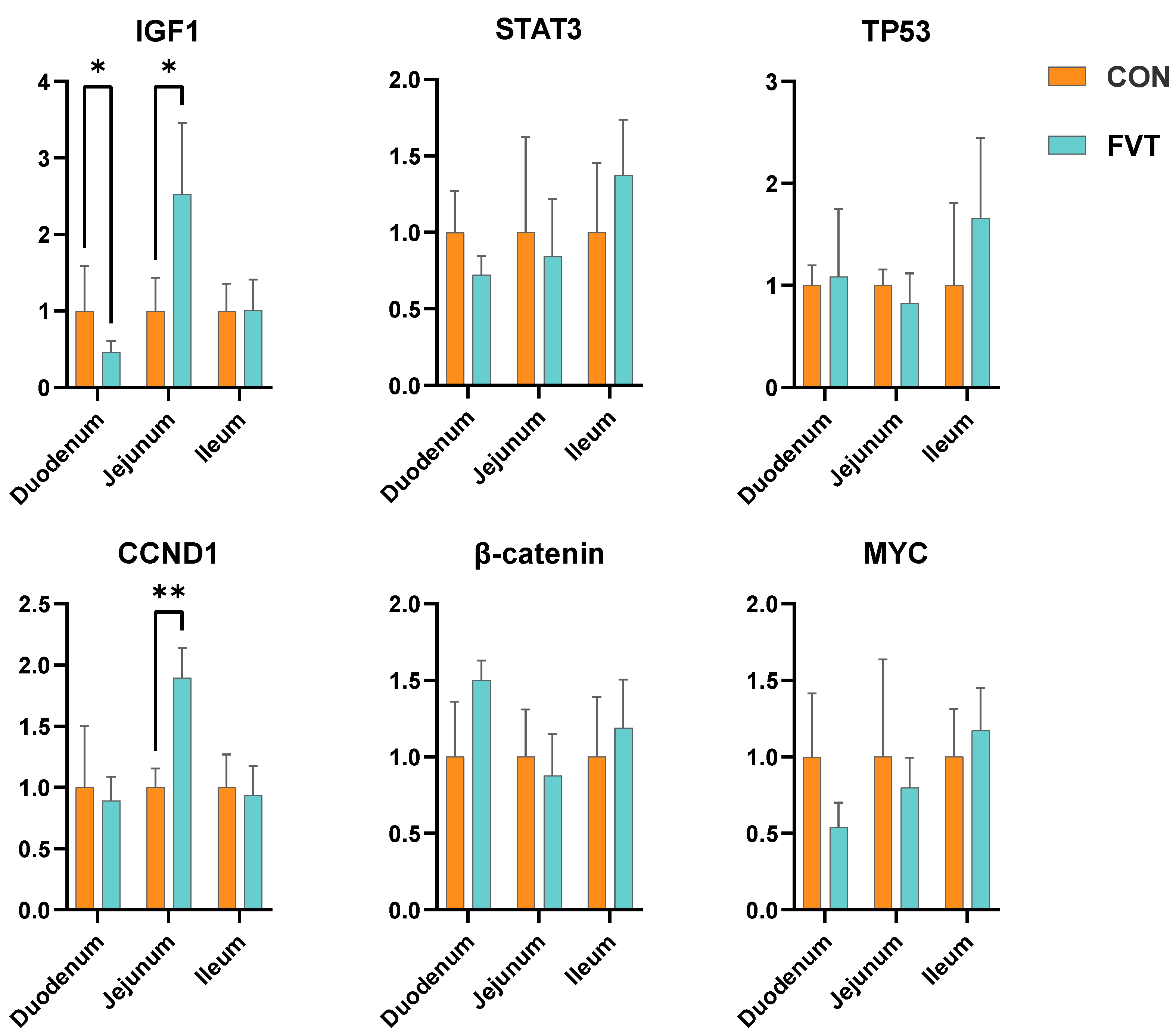
3.7. Expression of Genes Related to Intestinal Development and Repair
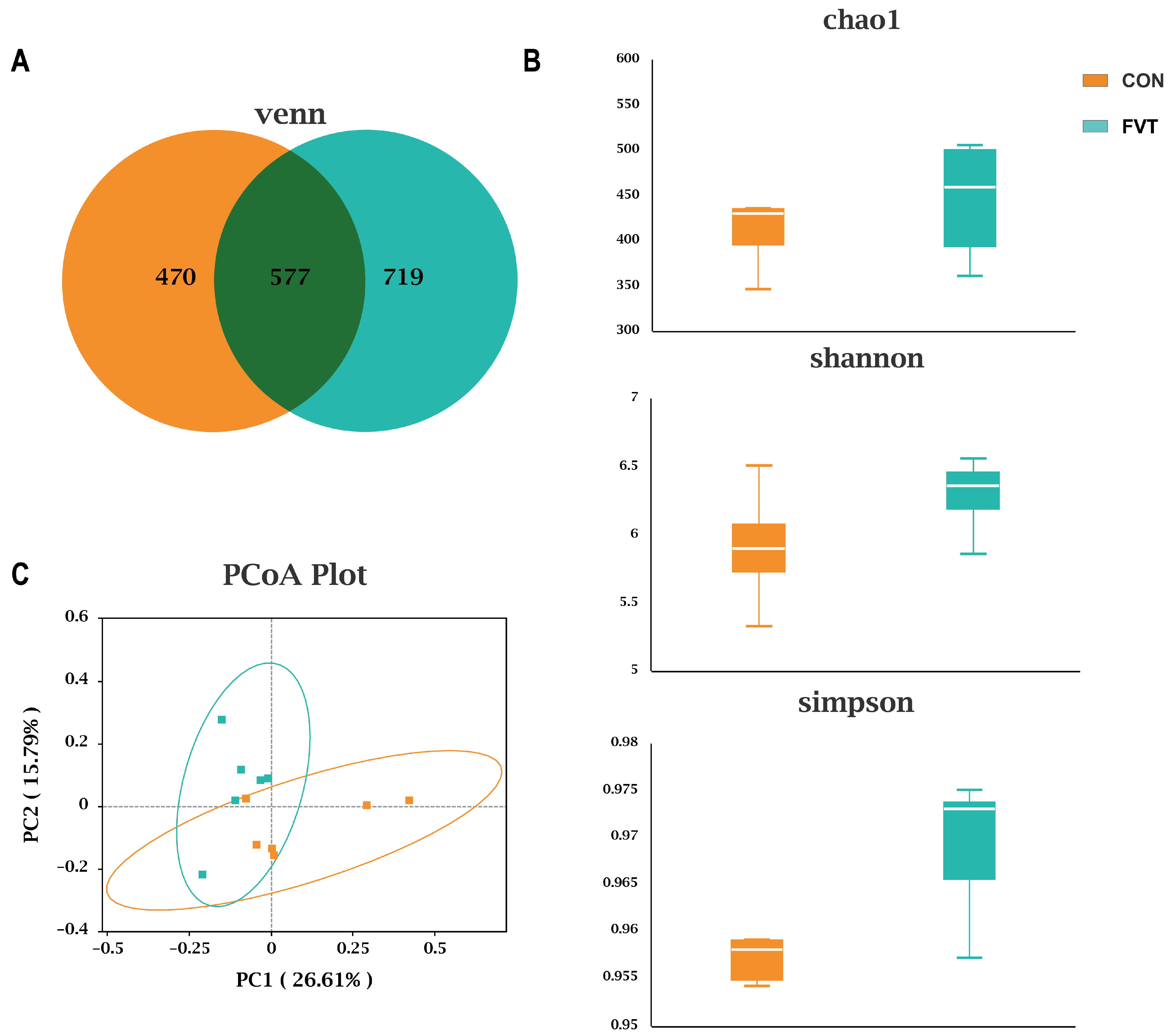
3.8. Cecal Microbial Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, P.; Luo, M.; Fan, J.; Xiong, L. Multiomics Analysis Reveals Gut Virome–Bacteria–Metabolite Interactions and Their Associations with Symptoms in Patients with IBS-D. Viruses 2024, 16, 1054. [Google Scholar] [CrossRef] [PubMed]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J.; Koci, M.D. Development of the chick microbiome: How early exposure influences future microbial diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Bian, G.; Zhang, K.; Liu, N.; Yin, Y.; Hou, Y.; Xie, F.; Zhu, W.; Mao, S.; Liu, J. Early-life ruminal microbiome-derived indole-3-carboxaldehyde and prostaglandin D2 are effective promoters of rumen development. Genome Biol. 2024, 25, 64. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Mu, A.; Wallace, M.; Gengatharan, J.; Furst, A.; Bode, L.; Metallo, C.; Ayres, J. Microbiota control of maternal behavior regulates early postnatal growth of offspring. Sci. Adv. 2021, 7, eabe6563. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, K.; Tofazzal Hossain, M.; Ahmed, R.; Hasan, M.M.; Islam, R.; Hossen, M.I.; Shaha, S.N.; Islam, M.R. Role of different growth enhancers as alternative to in-feed antibiotics in poultry industry. Front. Vet. Sci. 2022, 8, 794588. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, J.; Yao, Z.; Li, M. A review on the alternatives to antibiotics and the treatment of antibiotic pollution: Current development and future prospects. Sci. Total Environ. 2024, 926, 171757. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; An, F.; Zhang, T.; Lou, M.; Guo, J.; Liu, K.; Zhu, Y.; Wu, J.; Wu, R. Antimicrobial peptides: An alternative to traditional antibiotics. Eur. J. Med. Chem. 2024, 265, 116072. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-F.; Yao, W.-Q.; Chen, Q.-W.; Zheng, D.; Han, Z.-Y.; Zhang, X.-Z. In situ bioorthogonal conjugation of delivered bacteria with gut inhabitants for enhancing probiotics colonization. ACS Cent. Sci. 2022, 8, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiao, L.; Zhou, M.; Zhang, H. The microbiota: A crucial mediator in gut homeostasis and colonization resistance. Front. Microbiol. 2024, 15, 1417864. [Google Scholar] [CrossRef] [PubMed]
- Elbediwi, M.; Rolff, J. Metabolic pathways and antimicrobial peptide resistance in bacteria. J. Antimicrob. Chemother. 2024, 79, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A. Targeting the microbiome: From probiotics to fecal microbiota transplantation. Genome Med. 2018, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Rodrigues, D.; Briggs, W.; Duff, A.; Chasser, K.; Bielke, L. Evaluation of the impact of in ovo administered bacteria on microbiome of chicks through 10 days of age. Poult. Sci. 2019, 98, 5949–5960. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.M.; Paswan, V.K.; Attia, Y.A.; Abdel-Moneim, A.-M.E.; Abougabal, M.S.; Sharaf, M.; Elmazoudy, R.; Alghafari, W.T.; Osman, M.A.; Farag, M.R. Managing gut microbiota through in ovo nutrition influences early-life programming in broiler chickens. Animals 2021, 11, 3491. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Akhtar, M.; Pan, H.; Liu, Q.; Chen, Y.; Zhou, X.; You, Y.; Shi, D.; Liu, H. Fecal microbiota transplantation improves chicken growth performance by balancing jejunal Th17/Treg cells. Microbiome 2023, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Akhtar, M.; Kong, N.; Zhang, R.; Liang, Y.; Gu, Y.; Yang, D.; Nafady, A.A.; Shi, D.; Ansari, A.R. Early fecal microbiota transplantation continuously improves chicken growth performance by inhibiting age-related Lactobacillus decline in jejunum. Microbiome 2025, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, W.; Zhang, F. The next generation fecal microbiota transplantation: To transplant bacteria or virome. Adv. Sci. 2023, 10, 2301097. [Google Scholar] [CrossRef] [PubMed]
- Podlacha, M.; Gaffke, L.; Grabowski, Ł.; Mantej, J.; Grabski, M.; Pierzchalska, M.; Pierzynowska, K.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage DNA induces an interrupted immune response during phage therapy in a chicken model. Nat. Commun. 2024, 15, 2274. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.; Mirakzehi, M.T.; Bidokhti, H.M.; Kazemi, M. Evaluation of the effect of bacteriophages and organic acids as a feed additive to reduce Salmonella enteritidis in challenged chickens. J. Anim. Physiol. Anim. Nutr. 2025, 109, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.C.; Zablocki, O.; Zayed, A.A.; Howell, A.; Bolduc, B.; Sullivan, M.B. The gut virome database reveals age-dependent patterns of virome diversity in the human gut. Cell Host Microbe 2020, 28, 724–740.e8. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Duan, Y.; Fouts, D.E.; Schnabl, B. Intestinal virome and therapeutic potential of bacteriophages in liver disease. J. Hepatol. 2021, 75, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Draper, L.A.; Ryan, F.J.; Dalmasso, M.; Casey, P.G.; McCann, A.; Velayudhan, V.; Ross, R.P.; Hill, C. Autochthonous faecal viral transfer (FVT) impacts the murine microbiome after antibiotic perturbation. BMC Bio. 2020, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, H.; Noori, M.; Azimirad, M.; Mohebbi, S.R.; Asadzadeh Aghdaei, H.; Yadegar, A.; Zali, M.R. Emerging applications of phage therapy and fecal virome transplantation for treatment of Clostridioides difficile infection: Challenges and perspectives. Gut Pathog. 2023, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xiong, J.; Liang, S.; Wang, Y.; Zhu, Y.; Hou, Q.; Yang, X.; Yang, X. Fecal virus transplantation has more moderate effect than fecal microbiota transplantation on changing gut microbial structure in broiler chickens. Poult. Sci. 2024, 103, 103282. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liang, S.; Du, X.; Xiao, J.; Feng, H.; Ren, Z.; Yang, X.; Yang, X. Effects of fecal microbiota transplantation and fecal virome transplantation on LPS-induced intestinal injury in broilers. Poult. Sci. 2024, 103, 103316. [Google Scholar] [CrossRef]
- NY/T 3645-2020; Nutrient Requirements of Yellow-feathered Broilers. China Agricultural Press: Beijing, China, 2020.
- Lv, X.; Zhang, M.; Ji, K.; Zhou, C.; Hua, J. Evaluation of ginger straw as a forage source for goats: Effects on performance, ruminal fermentation, meat quality and immunity. Anim. Nutr. 2025, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ta, N.; Zhang, J.; Liu, X.; Ding, H.; Zhang, X. Nettle supplementation improves antioxidant status and modulates inflammatory response by altering lysophosphatidylcholines and enterolactone metabolism in dairy cows. Anim. Nutr. 2025, 21, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Obianwuna, U.E.; Yang, W.; Zhang, Z.; An, K.; Shi, B.; Dong, Y.; Wu, S.; Xia, Z. Astaxanthin supplementation mitigated intestinal damage and immunity in overfed Pekin ducks by regulating gut morphology, intestinal inflammation, and antioxidant balance. Anim. Nutr. 2025, 21, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Li, J.; Xing, Y.; Zhou, X.; Ge, J.; Geng, T.; Gong, D.; Zhao, M. Glucose deposited during goose fatty liver formation inhibits inflammation in goose fatty liver by reducing the interaction between PKA and IκB. Poult. Sci. 2025, 104, 105515. [Google Scholar] [CrossRef]
- Yang, L.; Cai, M.; Zhong, L.; Yin, Y.; Xie, Y.; Xie, S.; Hu, Y.; Zhang, J. Yellow mealworm (Tenebrio molitor) meal in diets of grass carp (Ctenopharyngodon idellus): Effects on growth performance, antioxidant capacity, immunity, intestinal morphology, and intestinal microbiota. Anim. Nutr. 2025, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Majerczyk, D.; Ayad, E.G.; Brewton, K.L.; Saing, P.; Hart, P.C. Systemic maternal inflammation promotes ASD via IL-6 and IFN-γ. Biosci. Rep. 2022, 42, BSR20220713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef]
- Yang, Y.; Palm, N.W. Immunoglobulin A and the microbiome. Curr. Opin. Microbiol. 2020, 56, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Huchzermeyer, B.; Menghani, E.; Khardia, P.; Shilu, A. Metabolic pathway of natural antioxidants, antioxidant enzymes and ROS providence. Antioxidants 2022, 11, 761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, L.; Sun, Z.; Huang, Y.; Liu, N. An atmospheric pollutant (inorganic nitrogen) alters the response of evergreen broad-leaved tree species to extreme drought. Ecotoxicol. Environ. Saf. 2020, 187, 109750. [Google Scholar] [CrossRef]
- Beyaz Coşkun, A.; Sağdiçoğlu Celep, A.G. Therapeutic modulation methods of gut microbiota and gut-liver axis. Crit. Rev. Food Sci. Nutr. 2022, 62, 6505–6515. [Google Scholar] [CrossRef]
- Luo, M.J.; Wang, A.; Wang, Y.; Che, Y.; Wan, X.; Peng, Q.; Gao, X.; Gao, M.X. Discussion of the correlation between HIV-1 gp120-induced intestinal dysbiosis and intestinal barrier loss. Int. J. Infect. Dis. 2025, 152, 107558. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Banerjee, N.; Liang, Y.; Du, X.; Boor, P.J.; Hoffman, K.L.; Khan, M.F. Aberrant gut microbiome contributes to intestinal oxidative stress, barrier dysfunction, inflammation and systemic autoimmune responses in MRL/lpr mice. Front. Immunol. 2021, 12, 651191. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Wang, X.; Che, H.; Zhang, Y. Piperine improves obesity by inhibiting fatty acid absorption and repairing intestinal barrier function. Plant Foods Hum. Nutr. 2021, 76, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ge, L.; Wen, S.; Sun, J. Dysfunction of the intestinal physical barrier in the intestinal inflammation of tongue sole, Cynoglossus semilaevis, induced by Shewanella algae infection. Fish Shellfish Immunol. 2023, 139, 108900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, C.; Rosa, D.P.; Dalmoro, Y.K.; Segatto, A.L.; Vieira, M.S.; Moraes, M.L.; Santin, E. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci. 2020, 6, 491. [Google Scholar] [CrossRef]
- Brosch, P.K.; Korsa, T.; Taban, D.; Eiring, P.; Kreisz, P.; Hildebrand, S.; Neubauer, J.; Zimmermann, H.; Sauer, M.; Shirakashi, R. Glucose and inositol transporters, SLC5A1 and SLC5A3, in glioblastoma cell migration. Cancers 2022, 14, 5794. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, X.; Fu, L.; Gao, J. n-3 PUFA reduction caused by fabp2 deletion interferes with triacylglycerol metabolism and cholesterolhomeostasis in fish. Appl. Microbiol. Biotechnol. 2020, 104, 2149–2161. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F.; Closs, E.I.; Wagner, C.A.; Palacin, M.; Endou, H.; Kanai, Y. CATs and HATs: The SLC7 family of amino acid transporters. Pflügers Arch. 2004, 447, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Pan, J.; Song, Y.; Liu, S.; Sun, P.; Zheng, X. Effect of inulin supplementation in maternal fecal microbiota transplantation on the early growth of chicks. Microbiome 2025, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, J.; Chen, Q.; Yang, N.; Bao, Z.; Hu, S.; Chen, Y.; Wu, X. A treatment combination of IGF and EGF promotes hair growth in the angora rabbit. Genes 2020, 12, 24. [Google Scholar] [CrossRef]
- Hov, H.k.; Våtsveen, T.K.; Waage, A.; Sundan, A.; Borset, M. Induction of Cyclin D1 by HGF, IGF-1 and IL-6 in a Human Myeloma Cell Line with at (11:14) Translocation. Blood 2006, 108, 5055. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, X.; He, H.; Guo, X.; Chen, J. Targeting the STAT3 pathway with STAT3 degraders. Trends Pharmacol. Sci. 2024, 45, 811–823. [Google Scholar] [CrossRef]
- Bisso, A.; Filipuzzi, M.; Gamarra Figueroa, G.P.; Brumana, G.; Biagioni, F.; Doni, M.; Ceccotti, G.; Tanaskovic, N.; Morelli, M.J.; Pendino, V. Cooperation between MYC and β-Catenin in liver tumorigenesis requires Yap/Taz. Hepatology 2020, 72, 1430–1443. [Google Scholar] [CrossRef]
- Joerger, A.C.; Stiewe, T.; Soussi, T. TP53: The unluckiest of genes? Cell Death Differ. 2025, 32, 219–224. [Google Scholar] [CrossRef]
- Noohi, N.; Papizadeh, M.; Rohani, M.; Talebi, M.; Pourshafie, M.R. Screening for probiotic characters in lactobacilli isolated from chickens revealed the intra-species diversity of Lactobacillus brevis. Anim. Nutr. 2021, 7, 119–126. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Xu, D.; Ma, K.; Zhang, C.; Wang, G.; Dong, M.; Li, W. Structural and prebiotic activity analysis of the polysaccharide produced by Lactobacillus helveticus SNA12. Carbohydr. Polym. 2022, 296, 119971. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Guo, J.; Shen, J.; Jiang, S.; Han, S.; Li, L. Butyrate ameliorated the intestinal barrier dysfunction and attenuated acute pancreatitis in mice fed with ketogenic diet. Life Sci. 2023, 334, 122188. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V. Microbial metabolite-receptor interactions in the gut microbiome. Curr. Opin. Chem. Biol. 2024, 83, 102539. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef] [PubMed]


| Item | Content |
|---|---|
| Ingredients, % | |
| Maize | 40.85 |
| Soybean meal | 43.35 |
| Soybean oil | 9.05 |
| Premix 1 | 5.00 |
| L-Lysine HCl | 0.10 |
| DL-Methionine | 0.20 |
| Calcium carbonate | 0.30 |
| Calcium hydrogen phosphate | 1.15 |
| Total | 100 |
| Nutrient levels 2 | |
| Crude protein, % | 21.51 |
| Crude fat, % | 11.22 |
| Crude ash, % | 6.97 |
| ME, MJ/kg | 12.38 |
| Lysine, % | 1.29 |
| Methionine, % | 0.50 |
| Calcium, % | 1.02 |
| Total phosphorus, % | 0.74 |
| Gene | Primer Sequences (5′ to 3′) | Accession No. |
|---|---|---|
| β-actin | F_ATTGTCCACCGCAAATGCTTC R_AAATAAAGCCATGCCAATCTCGTC | NM_205518.2 |
| MUC2 | F_TTCATGATGCCTGCTCTTGTG R_CCTGAGCCTTGGTACATTCTTGT | XM_040673064.2 |
| OCLN | F_GGCGGAGGGCCACCA R_GTCGTCCACGTAGTAGGAGC | NM_205128.1 |
| CLDN1 | F_TACTCCTGGGTCTGGTTGGT R_GTGCTGACAGACCTGCAATG | NM_001013611.2 |
| SLC5A1 | F_TCCACCGCCATAAGGATCAA R_GGTTGGTTGAGTACATAGCCCAT | NM_001293240.2 |
| SLC7A9 | F_TGGCACCAATTATCACCGCA R_GTGCATAGTGATGGGCTCTGAT | XM_046925531.1 |
| FABP2 | F_TCATGGAAGCAATGGGCGTG R_TTCGATGTCGATGGTACGGAAG | NM_001007923.2 |
| IGF-1 | F_TGCCAAAAGCACAAAAGGAAGT R_AAGTACCCTGCAGATGGCAC | NM_001004384.3 |
| STAT3 | F_CTCAGGTAGTGCTGCTCCGT R_GGGCAGGTCAATGGTATTGC | XM_040653164.2 |
| TP53 | F_CGCCGTGGCCGTCTATAA R_GTACAGTCAGAGCCCACCTCG | NM_205264.1 |
| CCND1 | F_ACCCGACGAGTTACTGCAAA R_TAGCGCACAGAGCCACAAAA | NM_001396513.1 |
| β-catenin | F_ATTGTCCACCGCAAATGCTTC R_AAATAAAGCCATGCCAATCTCGTC | NM_205518.2 |
| MYC | F_CAGCAGCGACTCGGAAGAAGAA R_AACTTTAGCCTCTTGGCGGC | NM_001030952.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Liu, T.; Chen, J.; Shen, H.; Wang, J. Fecal Virome Transplantation Confirms Non-Bacterial Components (Virome and Metabolites) Participate in Fecal Microbiota Transplantation-Mediated Growth Performance Enhancement and Intestinal Development in Broilers with Spatial Heterogeneity. Microorganisms 2025, 13, 1795. https://doi.org/10.3390/microorganisms13081795
Chen S, Liu T, Chen J, Shen H, Wang J. Fecal Virome Transplantation Confirms Non-Bacterial Components (Virome and Metabolites) Participate in Fecal Microbiota Transplantation-Mediated Growth Performance Enhancement and Intestinal Development in Broilers with Spatial Heterogeneity. Microorganisms. 2025; 13(8):1795. https://doi.org/10.3390/microorganisms13081795
Chicago/Turabian StyleChen, Shuaihu, Tingting Liu, Junyao Chen, Hong Shen, and Jungang Wang. 2025. "Fecal Virome Transplantation Confirms Non-Bacterial Components (Virome and Metabolites) Participate in Fecal Microbiota Transplantation-Mediated Growth Performance Enhancement and Intestinal Development in Broilers with Spatial Heterogeneity" Microorganisms 13, no. 8: 1795. https://doi.org/10.3390/microorganisms13081795
APA StyleChen, S., Liu, T., Chen, J., Shen, H., & Wang, J. (2025). Fecal Virome Transplantation Confirms Non-Bacterial Components (Virome and Metabolites) Participate in Fecal Microbiota Transplantation-Mediated Growth Performance Enhancement and Intestinal Development in Broilers with Spatial Heterogeneity. Microorganisms, 13(8), 1795. https://doi.org/10.3390/microorganisms13081795






