Fatal Case of Viral Pneumonia Associated with Metapneumovirus Infection in a Patient with a Burdened Medical History
Abstract
1. Background
1.1. Case Presentation
1.2. Clinical History
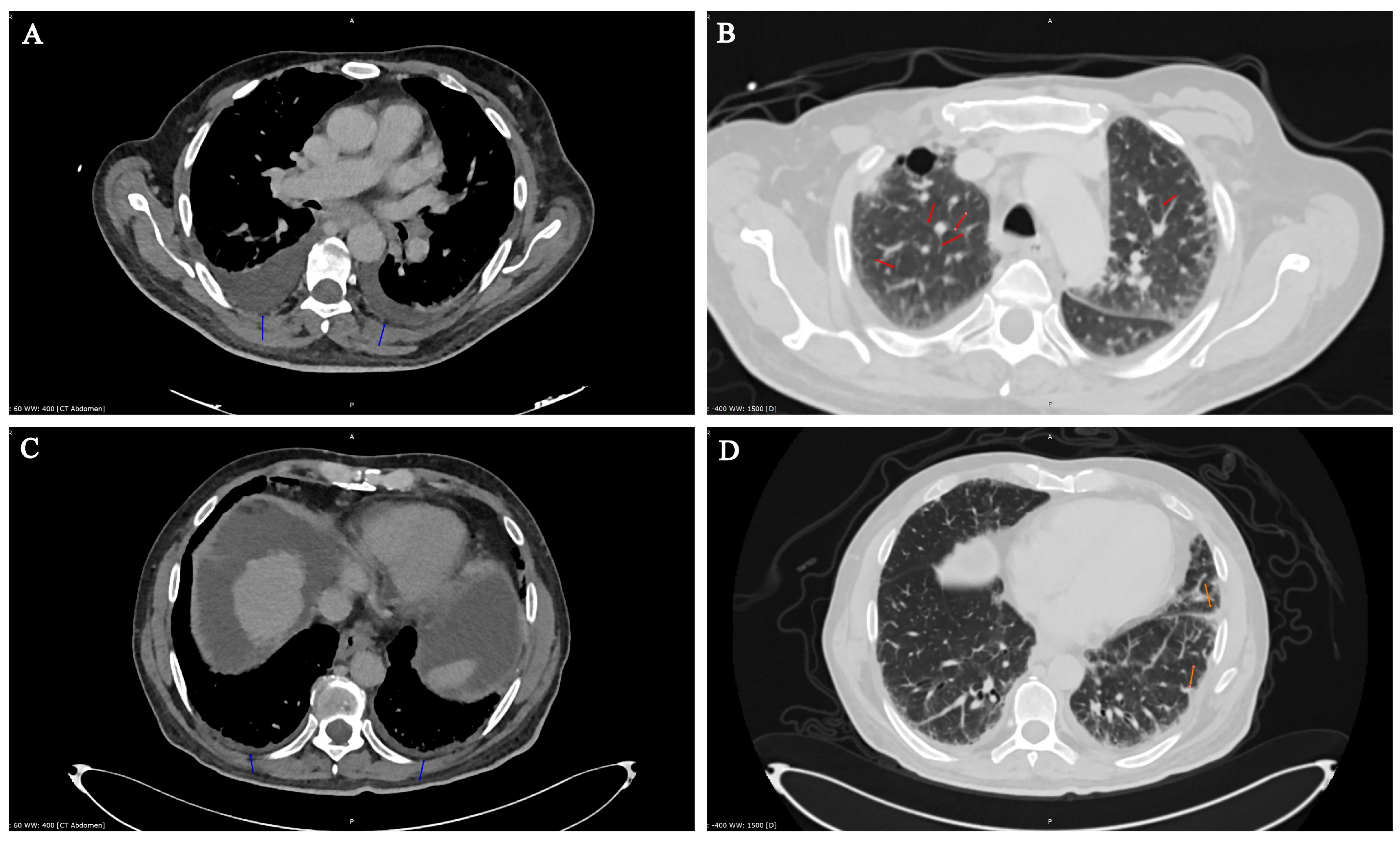
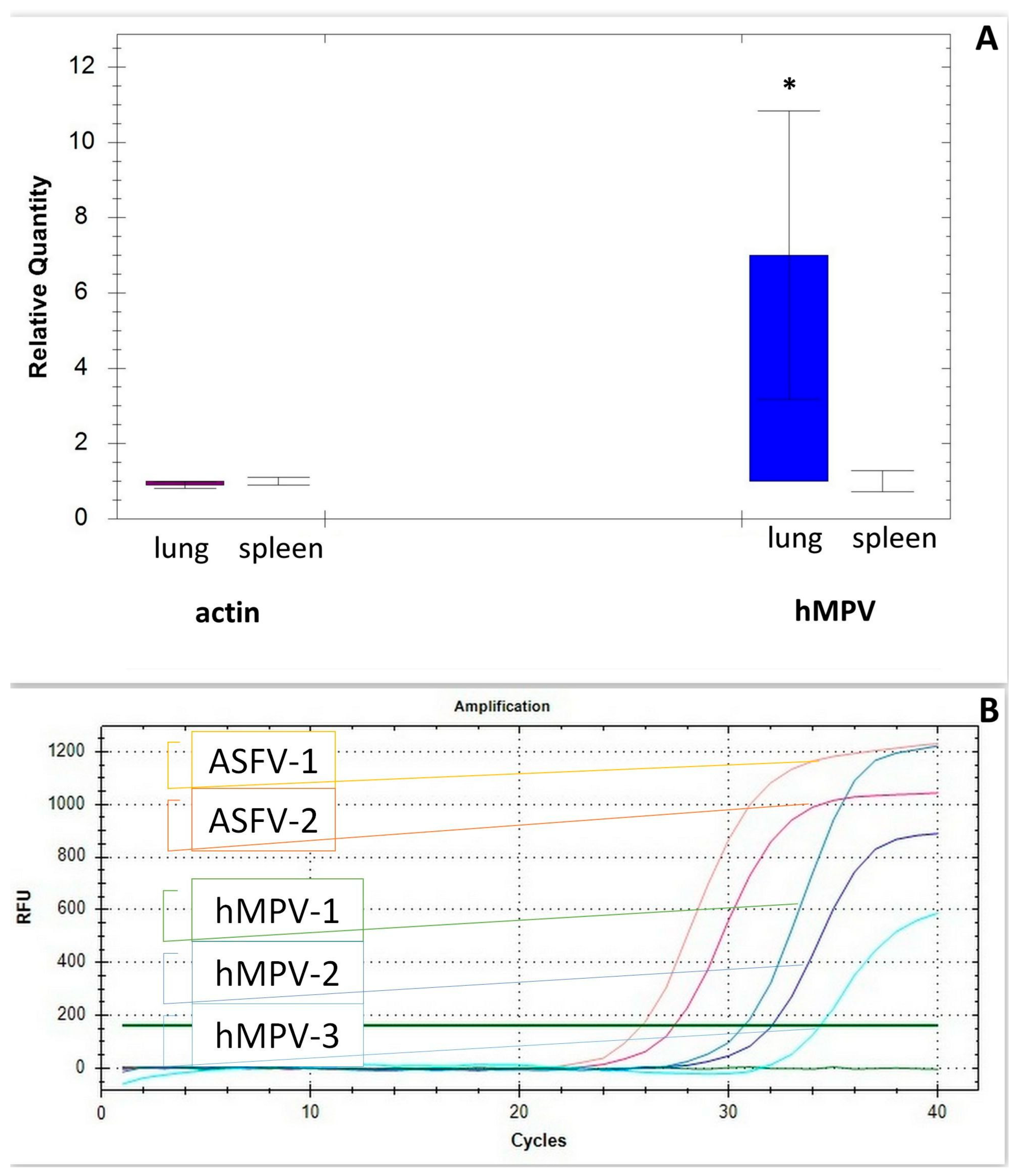
1.3. Laboratory Testing
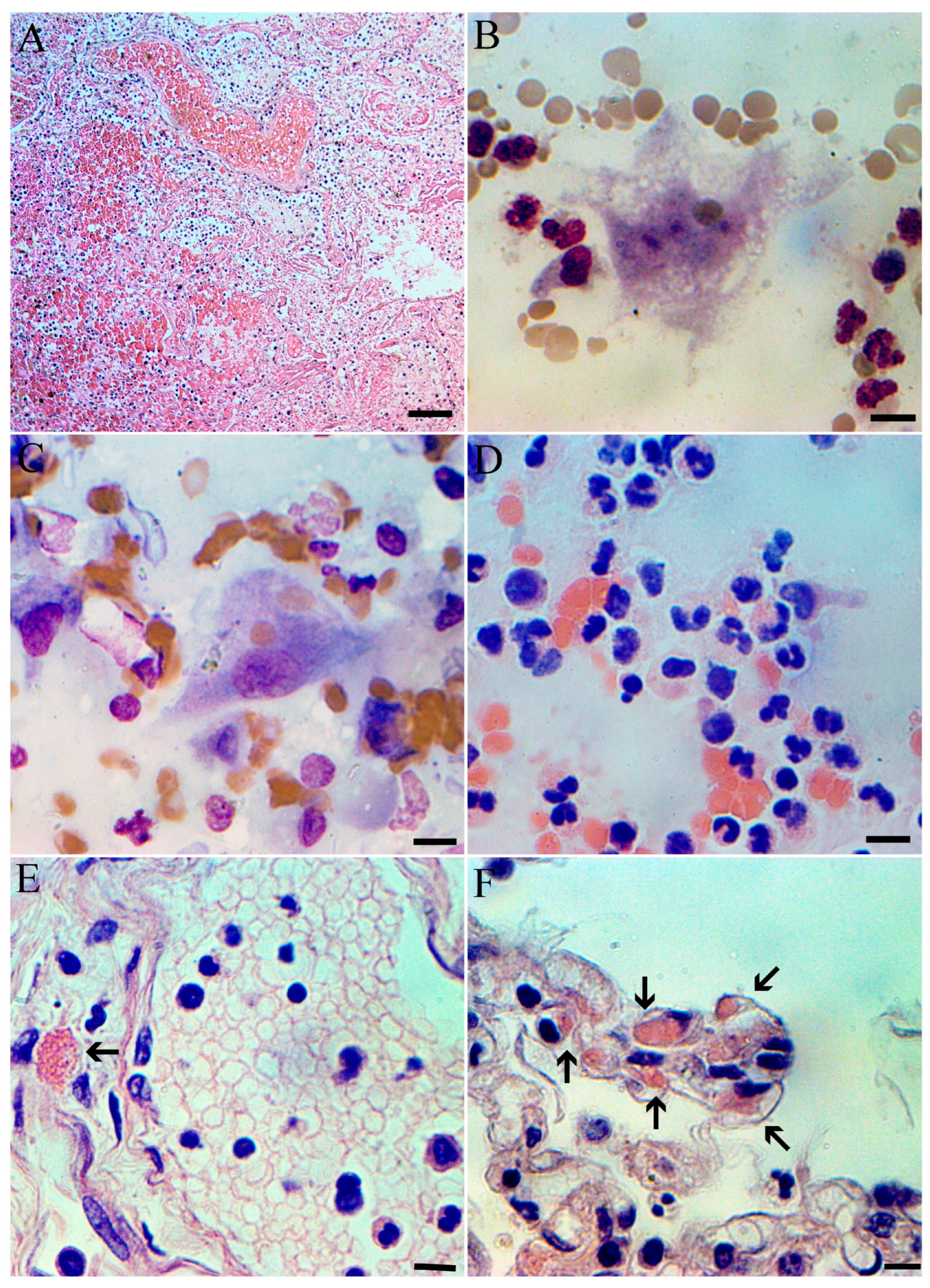
1.4. Pathological Findings
2. Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| hMPV | human metapneumovirus |
| RT-PCR | reverse transcription polymerase chain reaction |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| CT | computed tomographyomputed Tomography |
References
- Van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2017, 6, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Mohapatra, D.; Tripathy, M.; Mamidi, P.; Mohapatra, P.R. A re-emerging respiratory virus: Human metapneumovirus (hMPV). Cureus 2025, 17, e78354. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.V.; Martino, R.; Rabella, N.; Otegui, M.; Parody, R.; Heck, J.M.; Crowe, J.E., Jr. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J. Infect. Dis. 2005, 192, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, H. Lethal human metavirus pneumonia in a patient with chronic lymphocytic leukemia. Memo-Mag. Eur. Med. Oncol. 2021, 14, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Poghosyan, A.; Hakobyan, S.; Avagyan, H.; Avetisyan, A.; Bayramyan, N.; Hakobyan, L.; Abroyan, L.; Davtyan, A.; Poghosyan, D.; Baghdasaryan, B.; et al. The role of gastropods in African swine fever virus ecology. Virol. J. 2024, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Côté, S.; Abed, Y.; Boivin, G. Comparative evaluation of real-time PCR assays for detection of the human metapneumovirus. J. Clin. Microbiol. 2003, 41, 3631–3635. [Google Scholar] [CrossRef] [PubMed]
- Ponce, C.A.; Bustamante, R.I.; Gallo, M.; Vargas, S.L. Diagnosis of the primary infection by pneumocystis in autopsy specimens from two infants using lung impression smears (touch preps). Med. Mycol. Case Rep. 2014, 5, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Cloonan, S.M.; Haspel, J.A.; Choi, A.M.K. The emerging importance of autophagy in pulmonary diseases. Chest 2012, 142, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S. Diffuse alveolar hemorrhage. Tuberc. Respir. Dis. 2013, 74, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nava, G.; Shrestha, E.; Upadhyay, B.; Morante, A.; Joseph, D.; Suhail, A.; Trelles-Garcia, D.P.; Yanez-Bello, M.A. Bleeding pneumonia: Diffuse alveolar hemorrhage due to human metapneumovirus. IDCases 2020, 21, e00894. [Google Scholar] [CrossRef] [PubMed]
- Hasvold, J.; Sjoding, M.; Pohl, K.; Cooke, C.; Hyzy, R.C. The role of human metapneumovirus in the critically ill adult patient. J. Crit. Care 2016, 31, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Scheuerman, O.; Barkai, G.; Mandelboim, M.; Mishali, H.; Chodick, G.; Levy, I. Human metapneumovirus (hMPV) infection in immunocompromised children. J. Clin. Virol. 2016, 83, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Biggs, H.M.; Van Beneden, C.A.; Kurkjian, K.; Kobayashi, M.; Peret, T.C.T.; Watson, J.T.; Schneider, E.; Gerber, S.I.; Ravishankar, J. Severe Human Metapneumovirus and Group A Streptococcus Pneumonia in an Immunocompetent Adult. Clin. Infect. Dis. 2020, 70, 2712–2714. [Google Scholar] [CrossRef] [PubMed]
- Kawataki, M.; Ito, A.; Koyama, T.; Ishida, T. Lobar pneumonia due to human metapneumovirus: A case report. Int. J. Infect. Dis. 2024, 146, 107162. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Lee, H.N.; Choi, S.H.; Sung, H.; Kim, H.J.; Do, K.H. Clinical and Radiologic Characteristics of Human Metapneumovirus Infections in Adults, South Korea. Emerg. Infect. Dis. 2019, 25, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Jobe, F.; Simpson, J.; Hawes, P.; Guzman, E.; Bailey, D. Respiratory Syncytial Virus Sequesters NF-κB Subunit p65 to Cytoplasmic Inclusion Bodies To Inhibit Innate Immune Signaling. J. Virol. 2020, 94, e01380-20. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes-Muñoz, N.; Branttie, J.; Slaughter, K.B.; Dutch, R.E. Human Metapneumovirus Induces Formation of Inclusion Bodies for Efficient Genome Replication and Transcription. J. Virol. 2017, 91, e01282-17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khachatryan, P.; Karalyan, N.; Petunts, H.; Hakobyan, S.; Avagyan, H.; Ter-Pogossyan, Z.; Karalyan, Z. Fatal Case of Viral Pneumonia Associated with Metapneumovirus Infection in a Patient with a Burdened Medical History. Microorganisms 2025, 13, 1790. https://doi.org/10.3390/microorganisms13081790
Khachatryan P, Karalyan N, Petunts H, Hakobyan S, Avagyan H, Ter-Pogossyan Z, Karalyan Z. Fatal Case of Viral Pneumonia Associated with Metapneumovirus Infection in a Patient with a Burdened Medical History. Microorganisms. 2025; 13(8):1790. https://doi.org/10.3390/microorganisms13081790
Chicago/Turabian StyleKhachatryan, Parandzem, Naira Karalyan, Hasmik Petunts, Sona Hakobyan, Hranush Avagyan, Zarine Ter-Pogossyan, and Zaven Karalyan. 2025. "Fatal Case of Viral Pneumonia Associated with Metapneumovirus Infection in a Patient with a Burdened Medical History" Microorganisms 13, no. 8: 1790. https://doi.org/10.3390/microorganisms13081790
APA StyleKhachatryan, P., Karalyan, N., Petunts, H., Hakobyan, S., Avagyan, H., Ter-Pogossyan, Z., & Karalyan, Z. (2025). Fatal Case of Viral Pneumonia Associated with Metapneumovirus Infection in a Patient with a Burdened Medical History. Microorganisms, 13(8), 1790. https://doi.org/10.3390/microorganisms13081790





