Seroprevalence of Anaplasma phagocytophilum Antibodies Following Tick Bites: A Serosurvey in a Tertiary Care Hospital in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
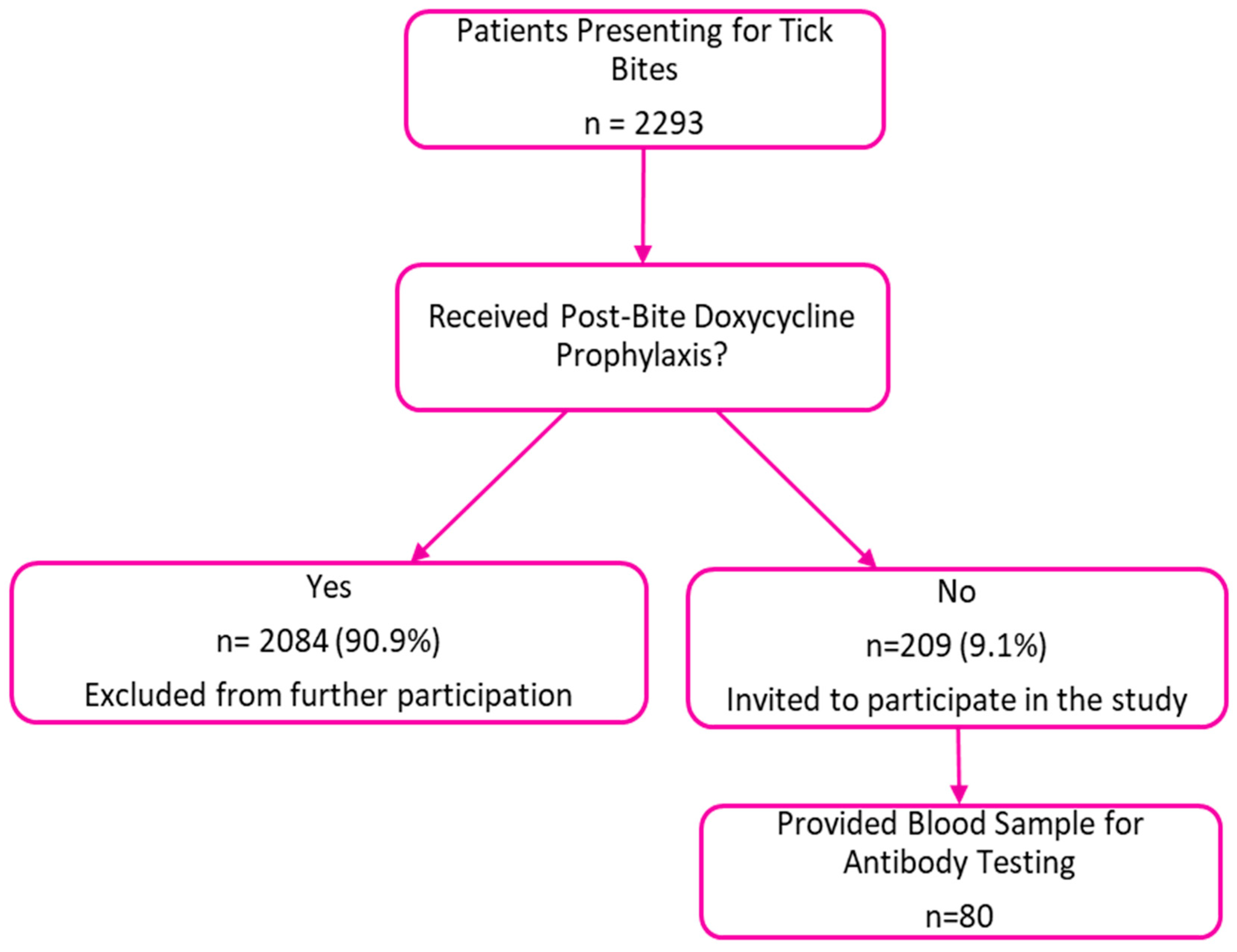
2.2. Collection of Blood Samples and Questionnaires
2.3. Detection of Antibodies Against A. phagocytophilum
2.4. Statistical Analysis
2.5. Ethical Committee Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| DNA | Deoxyribonucleic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HGA | Human granulocytic anaplasmosis |
| HGE | Human granulocytic ehrlichiosis |
| IDSA | Infectious Diseases Society of America |
| IgG | Immunoglobulin G |
| IQR | Interquartile Range |
| N/A | Not Applicable |
| OR | Odds Ratio |
| PCR | Polymerase chain reaction |
| s.l. | sensu lato |
| SD | Standard Deviation |
| SPSS | Statistical Package for the Social Sciences |
| SST | Serum Separator Tube |
References
- Andersson, M.O.; Tolf, C.; Tamba, P.; Stefanache, M.; Radbea, G.; Frangoulidis, D.; Tomaso, H.; Waldenström, J.; Dobler, G.; Chitimia-Dobler, L. Molecular survey of neglected bacterial pathogens reveals an abundant diversity of species and genotypes in ticks collected from animal hosts across Romania. Parasites Vectors 2018, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Vayssier-Taussat, M.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Karshima, S.N.; Ahmed, M.I.; Kogi, C.A.; Iliya, P.S. Anaplasma phagocytophilum infection rates in questing and host-attached ticks: A global systematic review and meta-analysis. Acta Trop. 2022, 228, 106299. [Google Scholar] [CrossRef] [PubMed]
- Mihalca, A.D.; Gherman, C.M.; Magdas, C.; Dumitrache, M.O.; Györke, A.; Sándor, A.D.; Domsa, C.; Oltean, M.; Mircean, V.; Mărcutan, D.I.; et al. Ixodes ricinus is the dominant questing tick in forest habitats in Romania: The results from a countrywide dragging campaign. Exp. Appl. Acarol. 2012, 58, 175–182. [Google Scholar] [CrossRef]
- Woldehiwet, Z. Anaplasma phagocytophilum in ruminants in Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 446–460. [Google Scholar] [CrossRef]
- Dumic, I.; Jevtic, D.; Veselinovic, M.; Nordstrom, C.W.; Jovanovic, M.; Mogulla, V.; Veselinovic, E.M.; Hudson, A.; Simeunovic, G.; Petcu, E.; et al. Human Granulocytic Anaplasmosis-A Systematic Review of Published Cases. Microorganisms 2022, 10, 1433. [Google Scholar] [CrossRef]
- Goodman, J.L.; Nelson, C.; Vitale, B.; Madigan, J.E.; Dumler, J.S.; Kurtti, T.J.; Munderloh, U.G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 1996, 334, 209–215. [Google Scholar] [CrossRef]
- Karshima, S.N.; Ahmed, M.I.; Mohammed, K.M.; Pam, V.A.; Momoh-Abdullateef, H.; Gwimi, B.P. Worldwide meta-analysis on Anaplasma phagocytophilum infections in animal reservoirs: Prevalence, distribution and reservoir diversity. Vet. Parasitol. Reg. Stud. Rep. 2023, 38, 100830. [Google Scholar] [CrossRef]
- Karshima, S.N.; Ahmed, M.I.; Mohammed, K.M.; Pam, V.A. Global status of Anaplasma phagocytophilum infections in human population: A 50-year (1970–2020) meta-analysis. J. Vector Borne Dis. 2023, 60, 265–278. [Google Scholar] [CrossRef]
- Spec, A.; Escota, G.; Chrisler, C.; Davies, B. Comprehensive Review of Infectious Diseases, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wormser, G.P.; Zentmaier, L.; Liveris, D.; Schwartz, I.; Schneider, L.; Aguero-Rosenfeld, M.E. Antibodies to Anaplasma phagocytophilum in Patients with Human Granulocytic Anaplasmosis Confirmed by Both Polymerase Chain Reaction and Culture. Am. J. Med. 2025, 138, 669–672. [Google Scholar] [CrossRef]
- Aguero-Rosenfeld, M.E.; Zentmaier, L.; Liveris, D.; Visintainer, P.; Schwartz, I.; Dumler, J.S.; Wormser, G.P. Culture and other direct detection methods to diagnose human granulocytic anaplasmosis. Am. J. Clin. Pathol. 2025, 163, 313–319. [Google Scholar] [CrossRef]
- Zając, V.; Bell-Sakyi, L.; Wójcik-Fatla, A. Use of Tick Cell Lines in Co-Infection Studies with a Preliminary Study of Co-Culture of Borrelia burgdorferi and Anaplasma phagocytophilum. Pathogens 2025, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; Ivan, T.; Ionică, A.M.; D’Amico, G.; Deak, G.; Nadas, G.C.; Novac, C.S.; Gherman, C.M.; Mihalca, A.D. Anaplasma phagocytophilum in Multiple Tissue Samples of Wild Carnivores in Romania. J. Wildl. Dis. 2021, 57, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Matei, I.A.; D’aMico, G.; Ionică, A.M.; Kalmár, Z.; Corduneanu, A.; Sándor, A.D.; Fiţ, N.; Bogdan, L.; Gherman, C.M.; Mihalca, A.D. New records for Anaplasma phagocytophilum infection in small mammal species. Parasites Vectors 2018, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, A.M.; Ionita, M.; Mitrea, I.L. Serological Evidence of Natural Exposure to Tick-Borne Pathogens in Horses, Romania. Microorganisms 2021, 9, 373. [Google Scholar] [CrossRef]
- Raileanu, C.; Moutailler, S.; Pavel, I.; Porea, D.; Mihalca, A.D.; Savuta, G.; Vayssier-Taussat, M. Borrelia Diversity and Co-infection with Other Tick Borne Pathogens in Ticks. Front. Cell. Infect. Microbiol. 2017, 7, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matei, I.A.; Kalmár, Z.; Lupşe, M.; D’Amico, G.; Ionică, A.M.; Dumitrache, M.O.; Gherman, C.M.; Mihalca, A.D. The risk of exposure to rickettsial infections and human granulocytic anaplasmosis associated with Ixodes ricinus tick bites in humans in Romania: A multiannual study. Ticks Tick-Borne Dis. 2017, 8, 375–378. [Google Scholar] [CrossRef]
- DiaSorin Molecular Anaplasma phagocytophilum IFA IgG Assay (IF1450G) Package Insert—Laboratory Specifications—Available upon Request at MVZ Labor PD Dr. Volkmann und Kollegen GbR. Available online: https://laborvolkmann.de/analysenspektrum/HTML/index.html (accessed on 27 March 2023).
- Dascalu, E.F.; Ionita, M.; Mitrea, I.L. Study on Tick Infestations of Small Ruminants in Southern Romania. Sci. Work. Ser. C. Vet. Med. 2023, 69, 61–67. [Google Scholar]
- von Wissmann, B.; Hautmann, W.; Sing, A.; Hizo-Teufel, C.; Fingerle, V. Assessing the risk of human granulocytic anaplasmosis and lyme borreliosis after a tick bite in Bavaria, Germany. Int. J. Med. Microbiol. 2015, 305, 736–741. [Google Scholar] [CrossRef]
- Kocianová, E.; Kost’anová, Z.; Stefanidesová, K.; Spitalská, E.; Boldis, V.; Hucková, D.; Stanek, G. Serologic evidence of Anaplasma phagocytophilum infections in patients with a history of tick bite in central Slovakia. Wien. Klin. Wochenschr. 2008, 120, 427–431. [Google Scholar] [CrossRef]
- Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaerter, P.G.; Baldwin, K.; Bannuru, R.R.; Belani, K.K.; Bowie, W.R.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 Guidelines for the Prevention, Diagnosis, and Treatment of Lyme Disease. Arthritis Care Res. 2021, 73, 1–9. [Google Scholar] [CrossRef]
- Angulo, F.J.; Olsen, J.; Purdel, V.; Lupșe, M.; Hristea, A.; Briciu, V.; Colby, E.; Pilz, A.; Halsby, K.; Kelly, P.H.; et al. Incidence of symptomatic Borrelia burgdorferi sensu lato infection in Romania, 2018−2023. Parasites Vectors 2024, 17, 378. [Google Scholar] [CrossRef]
- Matei, I.A.; Kalmár, Z.; Magdaş, C.; Magdaş, V.; Toriay, H.; Dumitrache, M.O.; Ionică, A.M.; D’Amico, G.; Sándor, A.D.; Mărcuţan, D.I.; et al. Anaplasma phagocytophilum in questing Ixodes ricinus ticks from Romania. Ticks Tick-Borne Dis. 2015, 6, 408–413. [Google Scholar] [CrossRef]
- Dessau, R.; van Dam, A.; Fingerle, V.; Gray, J.; Hovius, J.; Hunfeld, K.-P.; Jaulhac, B.; Kahl, O.; Kristoferitsch, W.; Lindgren, P.-E.; et al. To test or not to test? Laboratory support for the diagnosis of Lyme borreliosis: A position paper of ESGBOR, the ESCMID study group for Lyme borreliosis. Clin. Microbiol. Infect. 2018, 24, 118–124. [Google Scholar] [CrossRef]
- Panciu, A.M.; Cheran, C.A.; Militaru, E.D.; Rîciu, C.D.; Hristea, A. Serosurvey of Tick-Borne Encephalitis Virus Infection in Romania. Pathogens 2024, 13, 231. [Google Scholar] [CrossRef]
| Characteristic | All Participants (N = 80) | A. phagocytophilum IgG Positive (n = 8) | A. phagocytophilum IgG Negative (n = 72) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age (years) Median (IQR) | 46 (35–57) | 46.5 (24.7–66.7) | 46 (35–57) | ||
| <46 years | 40 (50) | 4 (50) | 36 (50) | 1 (0.2–4.3) | 1 |
| Male | 28 (35) | 4 (50) | 24 (33.3) | 0.5 (0.1–2.1) | 0.4 |
| Tick bite occurring in rural areas | 43 (53.8) | 4 (50) | 39 (54.2) | 1.2 (0.3–5.4) | 1 |
| History of prior tick bites | 27 (33.8) | 3 (37.5) | 24 (33.3) | 1.2 (0.3–5.4) | 1 |
| Frequent outdoor activities associated with possible tick exposure | 53 (66.3) | 5 (62.5) | 48 (66.7) | 0.8 (0.1–3.7) | 1 |
| Clinical manifestations reported by the participants after the tick bite | |||||
| Erythema migrans Rash non-erythema migrans Fever Headaches Fatigue Muscle pain Nausea/vomiting | 35 (43.8) | 5 (62.5) | 30 (41.6) | 2.3 (0.5–10.5) | 0.3 |
| 18 (22.5) | 0 | 18 (25) | N/A | ||
| 15 (18.8) 25 (31.3) 31 (38.8) 20 (25) 7 (8.8) | 0 3 (37.5) 5 (62.5) 4 (50) 0 | 15 (20.8) 22 (30.5) 26 (36.1) 16 (22.2) 7 (9.7) | N/A 1.37 (0.3–6.2) 2.9 (0.6–13.3) 5.8 (1.2–27.1) N/A | 0.7 0.2 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheran, C.A.; Iacob, D.G.; Neagu, G.; Panciu, A.M.; Hristea, A. Seroprevalence of Anaplasma phagocytophilum Antibodies Following Tick Bites: A Serosurvey in a Tertiary Care Hospital in Romania. Microorganisms 2025, 13, 1758. https://doi.org/10.3390/microorganisms13081758
Cheran CA, Iacob DG, Neagu G, Panciu AM, Hristea A. Seroprevalence of Anaplasma phagocytophilum Antibodies Following Tick Bites: A Serosurvey in a Tertiary Care Hospital in Romania. Microorganisms. 2025; 13(8):1758. https://doi.org/10.3390/microorganisms13081758
Chicago/Turabian StyleCheran, Cristina Alexandra, Diana Gabriela Iacob, Georgiana Neagu, Andreea Madalina Panciu, and Adriana Hristea. 2025. "Seroprevalence of Anaplasma phagocytophilum Antibodies Following Tick Bites: A Serosurvey in a Tertiary Care Hospital in Romania" Microorganisms 13, no. 8: 1758. https://doi.org/10.3390/microorganisms13081758
APA StyleCheran, C. A., Iacob, D. G., Neagu, G., Panciu, A. M., & Hristea, A. (2025). Seroprevalence of Anaplasma phagocytophilum Antibodies Following Tick Bites: A Serosurvey in a Tertiary Care Hospital in Romania. Microorganisms, 13(8), 1758. https://doi.org/10.3390/microorganisms13081758






