Soil Salinity Drives the Arbuscular Mycorrhizal Fungal Generalists and Specialists Subcommunity Assembly in Extremely Dryland Forest in China
Abstract
1. Introduction
2. Materials and Methods
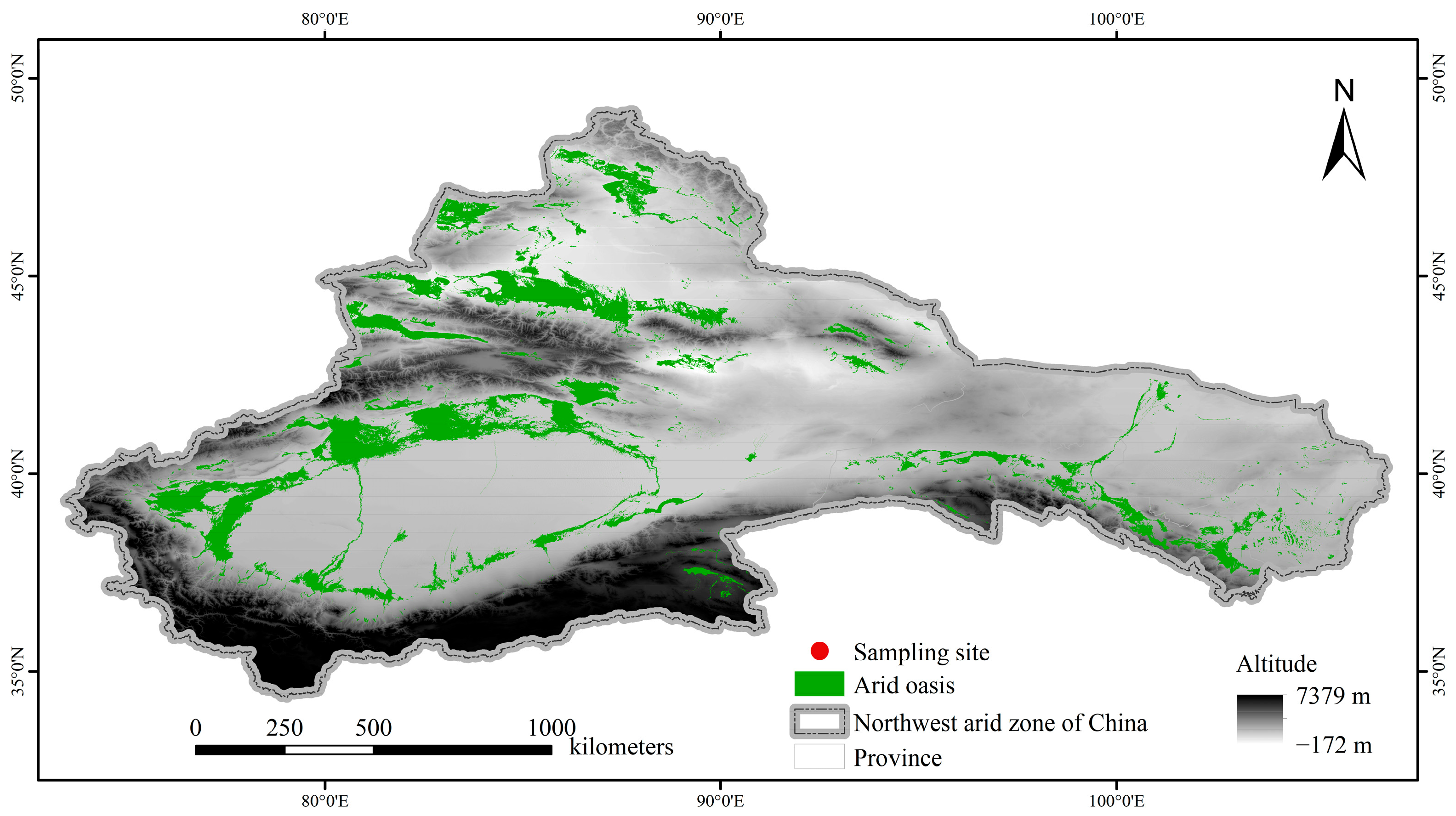
2.1. Study Region and Sample Collection
2.2. Soil, Climate, and Vegetation Factors
2.3. DNA Extraction and Sequencing
2.4. Analysis of Habitat Specialists and Generalists
2.5. Null Model and Neutral Model Analysis
2.6. AM Fungal Network Construction and Stability Analysis
2.7. Statistical Analysis
3. Results
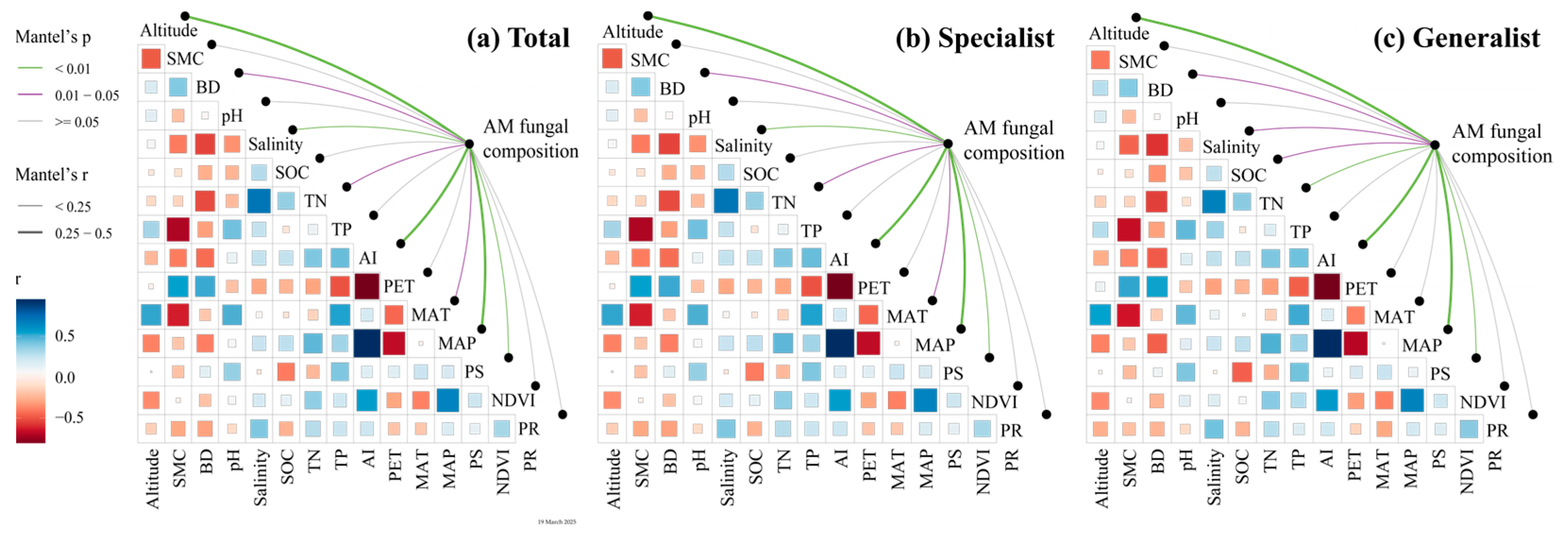
3.1. Composition of AM Fungal Community at Different Salinity Levels
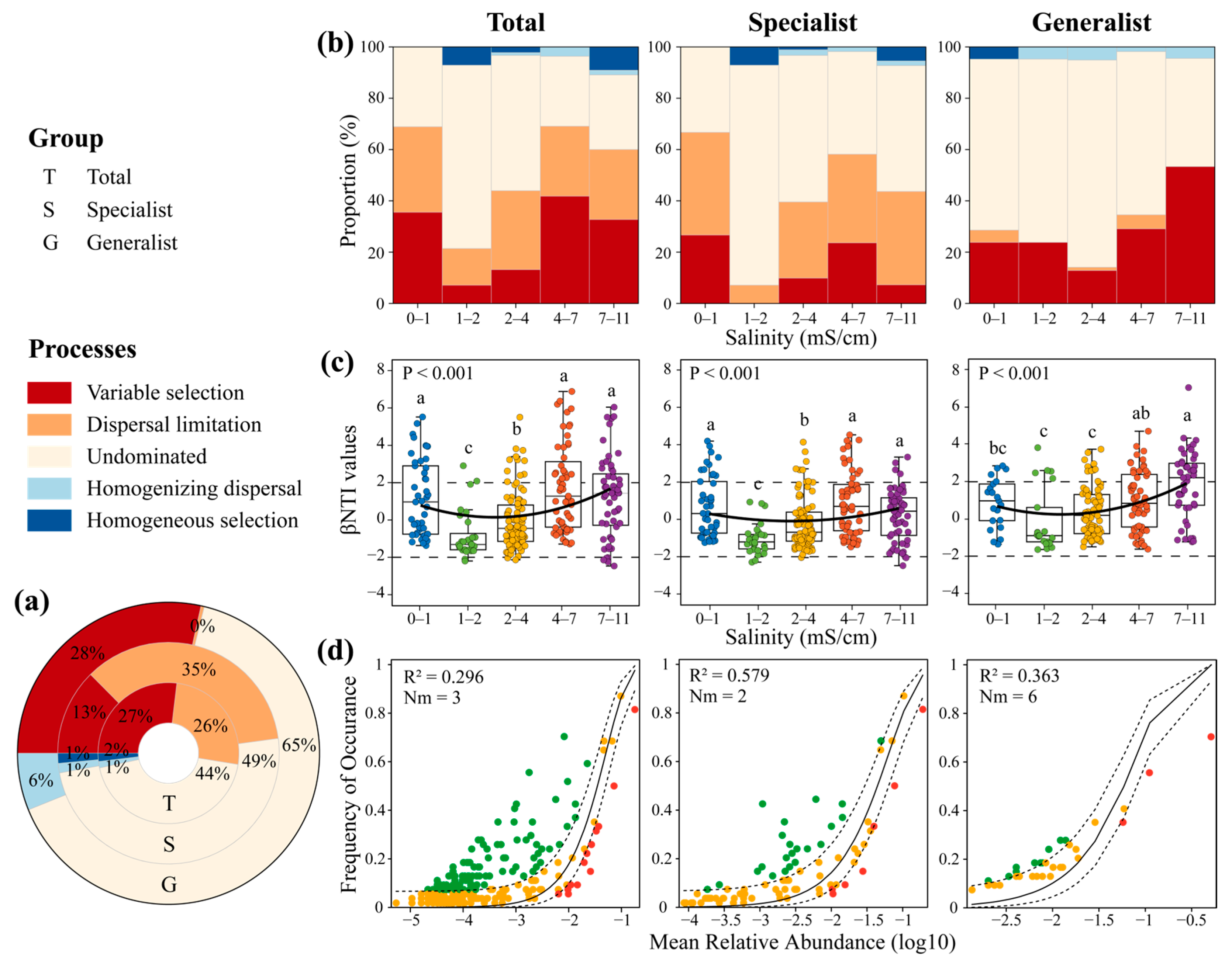
3.2. Ecological Assembly Processes of AM Fungal Community at Different Salinity Levels
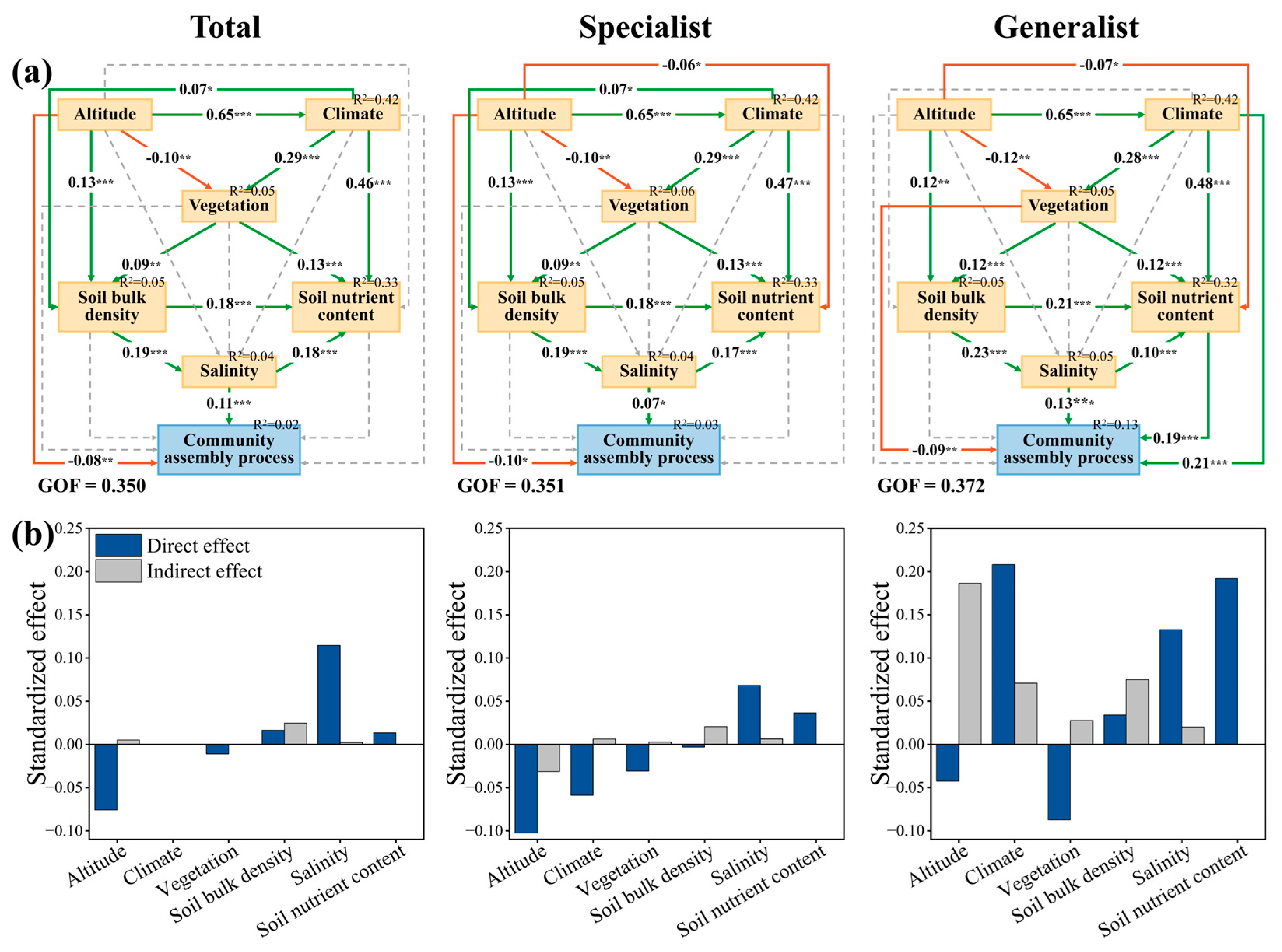
3.3. Direct and Indirect Effects of AM Fungal Community Processes
3.4. Network Patterns and Stability of AM Fungal Community at Different Salinity Levels
4. Discussion
4.1. AM Fungal Community Structure at Different Soil Salinity Levels
4.2. Dynamic Balance of Stochastic and Deterministic Processes Shapes the Assembly of AM Fungal Community
4.3. Stability of AM Fungal Network Under the Influence of Soil Salinity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Sosa, L.L.; Glanville, H.C.; Marshall, M.R.; Schnepf, A.; Cooper, D.M.; Hill, P.W.; Binley, A.; Jones, D.L. Stoichiometric constraints on the microbial processing of carbon with soil depth along a riparian hillslope. Biol. Fertil. Soils 2018, 54, 949–963. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Sexton, J.P.; Montiel, J.; Shay, J.E.; Stephens, M.R.; Slatyer, R.A. Evolution of Ecological Niche Breadth. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 183–206. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, L.; Yang, Y.; Cheng, K.; Li, K.; Zhou, Y.; Li, L.; Jin, Y.; He, X. Assembly, network and functional compensation of specialists and generalists in poplar rhizosphere under salt stress. Npj Biofilms Microbiomes 2025, 11, 28. [Google Scholar] [CrossRef]
- Vályi, K.; Mardhiah, U.; Rillig, M.C.; Hempel, S. Community assembly and coexistence in communities of arbuscular mycorrhizal fungi. ISME J. 2016, 10, 2341–2351. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, W.; Song, F.; Li, X. Diversity and composition of arbuscular mycorrhizal fungal communities in the cropland black soils of China. Glob. Ecol. Conserv. 2020, 22, e00964. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Liu, Z.; Fang, J.; Song, B.; Yang, Y.; Yu, Z.; Hu, J.; Dong, K.; Takahashi, K.; Adams, J.M. Stochastic processes dominate soil arbuscular mycorrhizal fungal community assembly along an elevation gradient in central Japan. Sci. Total Environ. 2023, 855, 158941. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Microbial mediation of plant competition and community structure. Funct. Ecol. 2013, 27, 865–875. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2014, 25, 13–24. [Google Scholar] [CrossRef]
- Li, M.; Cai, P.; Hou, S.; Cheng, Z.; Wu, F.; Lin, X.; Hu, J. Degradation of soil arbuscular mycorrhizal fungal diversity and functionality accompanied by the aggravation of pepper Phytophthora blight in a facility shed in Southwest China. Land Degrad. Dev. 2022, 33, 1337–1346. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y.; Chen, S.; Polle, A.; Rennenberg, H.; Luo, Z. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.; Bever, J.D. The missing link in grassland restoration: Arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession. J. Appl. Ecol. 2017, 54, 1301–1309. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—A meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zou, X.; Qu, M.; Li, J. Groundwater depth regulates assembly processes of abundant and rare bacterial communities across arid inland river basin. J. Environ. Manag. 2022, 319, 115767. [Google Scholar] [CrossRef]
- Jia, X.; Dini-Andreote, F.; Falcão Salles, J. Community Assembly Processes of the Microbial Rare Biosphere. Trends Microbiol. 2018, 26, 738–747. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Leibold, M.A.; Mcpeek, M.A. Coexistence of the niche and neutral perspectives in community ecology. Ecology 2006, 87, 1399–1410. [Google Scholar] [CrossRef]
- Mcgill, B.J.; Maurer, B.A.; Weiser, M.D. Empirical evaluation of neutral theory. Ecology 2006, 87, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zhang, Y.; Wang, H.; Zhu, X. Stochastic processes contribute to arbuscular mycorrhizal fungal community assembly in paddy soils along middle and lower Yangtze River. Appl. Soil Ecol. 2023, 183, 104759. [Google Scholar] [CrossRef]
- Powell, J.R.; Karunaratne, S.; Campbell, C.D.; Yao, H.; Robinson, L.; Singh, B.K. Deterministic processes vary during community assembly for ecologically dissimilar taxa. Nat. Commun. 2015, 6, 8444. [Google Scholar] [CrossRef]
- Xu, Q.; Vandenkoornhuyse, P.; Li, L.; Guo, J.; Zhu, C.; Guo, S.; Ling, N.; Shen, Q. Microbial generalists and specialists differently contribute to the community diversity in farmland soils. J. Adv. Res. 2022, 40, 17–27. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, Y.; Hu, A.; Wan, W.; Zhang, Z.; Liu, K. Distinct strategies of the habitat generalists and specialists in sediment of Tibetan lakes. Environ. Microbiol. 2022, 24, 4153–4166. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Cai, B.; Jie, W.; Lv, D. The arbuscular mycorrhizal symbiotic status of Populus euphratica, a drought resistant tree species from arid lands. Ecohydrology 2013, 6, 1001–1008. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Wang, J.-J.; Zhao, W.; Li, Q.-Q.; Chen, Y.; Yi, Z.-B.; Lyu, X.; Huang, Z.-Y. Structure of soil bacteria community and diversity in cotton field and Euphrates poplar forest in the middle and lower reaches of Tarim river basin. Microbiol. China 2019, 46, 2214–2230. [Google Scholar] [CrossRef]
- Nian, Y.; Wang, X.; Cai, D. Analysis on climate and ecological environment change in the ejin delta, the lower reaches of the heihe river. J. Arid Meteorol. 2015, 33, 28–37. [Google Scholar] [CrossRef]
- Liu, M.; Ye, L.; Chen, L.; Korpelainen, H.; Niinemets, Ü.; Li, C. Sex-specific phosphorus acquisition strategies and cycling in dioecious Populus euphratica forests along a natural water availability gradient. Plant Cell Environ. 2024, 47, 3266–3281. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; He, Y.; Xu, J.; Zhu, Z.; Korpelainen, H.; Li, C. Rhizosphere microbe populations but not root traits induced by drought in Populus euphratica males. Soil Ecol. Lett. 2023, 5, 220152. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.F. Influence of Vesicular-Arbuscular Mycorrhizae on Water Movement through Boutelouagracilis(H. B. K. ) Lag ex Steud. New Phytol. 1982, 91, 191–196. [Google Scholar] [CrossRef]
- Wang, Y.D.; Sun, Q.; Liu, J.; Wang, L.; Wu, X.; Zhao, Z.; Wang, N.; Gao, Z. Suaeda salsa Root-Associated Microorganisms Could Effectively Improve Maize Growth and Resistance under Salt Stress. Microbiol. Spectr. 2022, 10, e0134922. [Google Scholar] [CrossRef]
- Li, H.; La, S.; Zhang, X.; Gao, L.; Tian, Y. Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress. ISME J. 2021, 15, 2865–2882. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Sun, C.; Xiang, Y.; Hao, H. Changes in terrestrial water storage and evaluation of oasis ecological security in the Tarim River Basin. Arid Land Geogr. 2024, 47, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.Q.; Xu, J.; Shen, Y.; Xing, X.; Xie, T.; Li, Z.; Yang, L.; Xi, H.; Zhu, C.; et al. Changes and protection suggestions in water resources and ecological environment in arid region of Northwest China. Bull. Chin. Acad. Sci. 2023, 38, 385–393. [Google Scholar] [CrossRef]
- Zomer, R.J.; Xu, J.; Trabucco, A. Version 3 of the Global Aridity Index and Potential Evapotranspiration Database. Sci. Data 2022, 9, 409. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, Y.; You, J. A 30-m Annual Maximum NDVI Dataset in China from 2000 to 2020. 2021. Available online: https://www.nesdc.org.cn/sdo/detail?id=60f68d757e28174f0e7d8d49 (accessed on 19 March 2025). [CrossRef]
- van Tuinen, D.; Jacquot, E.; Zhao, B.; Gollotte, A.; Gianinazzi-Pearson, V. Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol. Ecol. 1998, 7, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Gollotte, A.; van Tuinen, D.; Atkinson, D. Diversity of arbuscular mycorrhizal fungi colonising roots of the grass species Agrostis capillaris and Lolium perenne in a field experiment. Mycorrhiza 2004, 14, 111–117. [Google Scholar] [CrossRef]
- Zhang, J.L. spaa: Species Association Analysis. R Package Version 0.2.2. 2016. Available online: https://CRAN.R-project.org/package=spaa (accessed on 19 March 2025).
- Salazar, G. EcolUtils: Utilities for Community Ecology Analysis. R Package Version 0.1. 2025. Available online: https://github.com/GuillemSalazar/EcolUtils (accessed on 19 March 2025).
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous. R Package Version 5.2-3. 2025. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 19 March 2025).
- Csárdi, G.; Nepusz, T.; Traag, V.; Horvát, S.; Zanini, F.; Noom, D.; Müller, K. igraph: Network Analysis and Visualization. R Package Version 2.1.4. 2025. Available online: https://CRAN.R-project.org/package=igraph (accessed on 19 March 2025).
- Wu, H.; Yang, J.; Fu, W.; Rillig, M.C.; Cao, Z.; Zhao, A.; Hao, Z.; Zhang, X.; Chen, B.; Han, X. Identifying thresholds of nitrogen enrichment for substantial shifts in arbuscular mycorrhizal fungal community metrics in a temperate grassland of northern China. New Phytol. 2023, 237, 279–294. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Herren, C.M.; McMahon, K.D. Cohesion: A method for quantifying the connectivity of microbial communities. ISME J. 2017, 11, 2426–2438. [Google Scholar] [CrossRef]
- Wu, L.; Yang, P.; Luo, L.; Zhu, W.; Hong, Y.; Tong, C.; Peñuelas, J. Conversion of mangrove forests to shrimp ponds in southeastern China destabilizes sediment microbial networks. Geoderma 2022, 421, 115907. [Google Scholar] [CrossRef]
- Sanchez, G.; Trinchera, L.; Russolillo, G. plspm: Tools for Partial Least Squares Path Modeling (PLS-PM). R Package Version 0.4.9. 2015. Available online: https://github.com/gastonstat/plspm (accessed on 19 March 2025).
- Oehl, F.; Sieverding, E.; Ineichen, K.; Ris, E.-A.; Boller, T.; Wiemken, A. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 2005, 165, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, X.; Zhang, Z.; Zhao, Y.; Yang, J.; Zhu, Y. Species diversity and drivers of arbuscular mycorrhizal fungal communities in a semi-arid mountain in China. PeerJ 2017, 5, e4155. [Google Scholar] [CrossRef]
- Baki, Z.I. Molecular genetic characterization of arbuscular mycorrhizal fungi associated with upland rice in Bangladesh. Rhizosphere 2021, 18, 100357. [Google Scholar] [CrossRef]
- An, Y.; Yang, X.; Zhang, L.; Zhang, J.; Du, B.; Yao, L.; Li, X.; Guo, C. Alfalfa MsCBL4 enhances calcium metabolism but not sodium transport in transgenic tobacco under salt and saline–alkali stress. Plant Cell Rep. 2020, 39, 997–1011. [Google Scholar] [CrossRef]
- Zhao, K.; Li, F.; Zhang, F. Chinese Salt-Bearing Plants, 2nd ed.; Science Press: Beijing, China, 2013. [Google Scholar]
- Zhang, G.; Bai, J.; Tebbe, C.C.; Zhao, Q.; Jia, J.; Wang, W.; Wang, X.; Yu, L. Salinity controls soil microbial community structure and function in coastal estuarine wetlands. Environ. Microbiol. 2021, 23, 1020–1037. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, E.; Yang, X.; Yang, J. Shifts in Microbial Community Structure and Co-occurrence Network along a Wide Soil Salinity Gradient. Microorganisms 2024, 12, 1268. [Google Scholar] [CrossRef]
- Van Horn, D.J.; Okie, J.G.; Buelow, H.N.; Gooseff, M.N.; Barrett, J.E.; Takacs-Vesbach, C.D. Soil microbial responses to increased moisture and organic resources along a salinity gradient in a polar desert. Appl. Environ. Microbiol. 2014, 80, 3034–3043. [Google Scholar] [CrossRef]
- Rath, K.M. Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: A review. Soil Biol. Biochem. 2015, 81, 108–123. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P. Response of microbial activity and biomass to increasing salinity depends on the final salinity, not the original salinity. Soil Biol. Biochem. 2012, 53, 50–55. [Google Scholar] [CrossRef]
- Yuan, B.-C.; Li, Z.-Z.; Liu, H.; Gao, M.; Zhang, Y.-Y. Microbial biomass and activity in salt affected soils under arid conditions. Appl. Soil Ecol. 2007, 35, 319–328. [Google Scholar] [CrossRef]
- Zhou, X.; Tahvanainen, T.; Malard, L.; Chen, L.; Pérez-Pérez, J.; Berninger, F. Global analysis of soil bacterial genera and diversity in response to pH. Soil Biol. Biochem. 2024, 198, 109552. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Bannar-Martin, K.H.; Kremer, C.T.; Ernest, S.K.M.; Leibold, M.A.; Auge, H.; Chase, J.; Declerck, S.A.J.; Eisenhauer, N.; Harpole, S.; Hillebrand, H.; et al. Integrating community assembly and biodiversity to better understand ecosystem function: The Community Assembly and the Functioning of Ecosystems (CAFE) approach. Ecol. Lett. 2017, 21, 167–180. [Google Scholar] [CrossRef]
- Xu, X.; Qiu, Y.; Zhang, K.; Yang, F.; Chen, M.; Luo, X.; Yan, X.; Wang, P.; Zhang, Y.; Chen, H.; et al. Climate warming promotes deterministic assembly of arbuscular mycorrhizal fungal communities. Glob. Change Biol. 2022, 28, 1147–1161. [Google Scholar] [CrossRef]
- Chaudhary, V.B.; Nolimal, S.; Sosa-Hernández, M.A.; Egan, C.; Kastens, J. Trait-based aerial dispersal of arbuscular mycorrhizal fungi. New Phytol. 2020, 228, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.; Moora, M.; Öpik, M.; Adholeya, A.; Ainsaar, L.; Bâ, A.; Burla, S.; Diedhiou, A.G.; Hiiesalu, I.; Jairus, T.; et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 2014, 349, 970–973. [Google Scholar] [CrossRef]
- Xun, W.; Li, W.; Xiong, W.; Ren, Y.; Liu, Y.; Miao, Y.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Diversity-triggered deterministic bacterial assembly constrains community functions. Nat. Commun. 2019, 10, 3833. [Google Scholar] [CrossRef]
- Chen, W.; Wen, D. Archaeal and bacterial communities assembly and co-occurrence networks in subtropical mangrove sediments under Spartina alterniflora invasion. Environ. Microbiome 2021, 16, 10. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- Wani, O.A.; Sharma, V.; Kumar, S.S.; Malik, A.R.; Pandey, A.; Devi, K.; Kumar, V.; Gairola, A.; Yadav, D.; Valente, D.; et al. Geostatistical modelling of soil properties towards long-term ecological sustainability of agroecosystems. Ecol. Indic. 2024, 166, 112540. [Google Scholar] [CrossRef]
- Deng, Y.; Li, X.; Wang, Z.; Shi, F.; Zhao, S.; Hu, G. Natural seasonal freeze-thaw processes influenced soil quality in alpine grasslands: Insights from soil functions. Soil Biol. Biochem. 2025, 200, 109642. [Google Scholar] [CrossRef]
- Ren, L.; Huo, J.; Xiang, X.; Pan, Y.; Li, Y.; Wang, Y.; Meng, D.; Yu, C.; Chen, Y.; Xu, Z.; et al. Environmental conditions are the dominant factor influencing stability of terrestrial ecosystems on the Tibetan plateau. Commun. Earth Environ. 2023, 4, 196. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, J.; Wang, S.; Jin, T.; Wang, Y. Shifts bidirectional dependency between vegetation greening and soil moisture over the past four decades in China. Sci. Total Environ. 2023, 897, 166388. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Ma, Q.; Marsden, K.A.; Chadwick, D.R.; Luo, Y.; Kuzyakov, Y.; Wu, L.; Jones, D.L. Microbial community succession in soil is mainly driven by carbon and nitrogen contents rather than phosphorus and sulphur contents. Soil Biol. Biochem. 2023, 180, 109019. [Google Scholar] [CrossRef]
- Schlüter, S.; Henjes, S.; Zawallich, J.; Bergaust, L.; Horn, M.; Ippisch, O.; Vogel, H.-J.; Dörsch, P. Denitrification in Soil Aggregate Analogues-Effect of Aggregate Size and Oxygen Diffusion. Front. Environ. Sci. 2018, 6, 17. [Google Scholar] [CrossRef]
- Mo, L.; Yang, H.; Luo, P.; Mou, C.-X.; Wang, J. The joint effects of resource stress and grazing on plant–plant interactions in alpine meadows on the eastern Qinghai-Tibetan Plateau. J. Plant Ecol. 2023, 16, rtac062. [Google Scholar] [CrossRef]
- Borer, B.; Tecon, R.; Or, D. Spatial organization of bacterial populations in response to oxygen and carbon counter-gradients in pore networks. Nat. Commun. 2018, 9, 769. [Google Scholar] [CrossRef]
- Michalet, R.; Brooker, R.W.; Cavieres, L.A.; Kikvidze, Z.; Lortie, C.J.; Pugnaire, F.I.; Valiente-Banuet, A.; Callaway, R.M. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 2006, 9, 767–773. [Google Scholar] [CrossRef]
- Soliveres, S.; García-Palacios, P.; Castillo-Monroy, A.P.; Maestre, F.T.; Escudero, A.; Valladares, F. Temporal dynamics of herbivory and water availability interactively modulate the outcome of a grass–shrub interaction in a semi-arid ecosystem. Oikos 2011, 120, 710–719. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, M.; Wang, J.; Wang, Y.; Zou, X.; Lei, X.; Luo, M.; Wang, W.; Li, J. Soil Salinity Drives the Arbuscular Mycorrhizal Fungal Generalists and Specialists Subcommunity Assembly in Extremely Dryland Forest in China. Microorganisms 2025, 13, 1742. https://doi.org/10.3390/microorganisms13081742
Qu M, Wang J, Wang Y, Zou X, Lei X, Luo M, Wang W, Li J. Soil Salinity Drives the Arbuscular Mycorrhizal Fungal Generalists and Specialists Subcommunity Assembly in Extremely Dryland Forest in China. Microorganisms. 2025; 13(8):1742. https://doi.org/10.3390/microorganisms13081742
Chicago/Turabian StyleQu, Mengjun, Jianming Wang, Yin Wang, Xuge Zou, Xun Lei, Meiwen Luo, Wenkai Wang, and Jingwen Li. 2025. "Soil Salinity Drives the Arbuscular Mycorrhizal Fungal Generalists and Specialists Subcommunity Assembly in Extremely Dryland Forest in China" Microorganisms 13, no. 8: 1742. https://doi.org/10.3390/microorganisms13081742
APA StyleQu, M., Wang, J., Wang, Y., Zou, X., Lei, X., Luo, M., Wang, W., & Li, J. (2025). Soil Salinity Drives the Arbuscular Mycorrhizal Fungal Generalists and Specialists Subcommunity Assembly in Extremely Dryland Forest in China. Microorganisms, 13(8), 1742. https://doi.org/10.3390/microorganisms13081742



