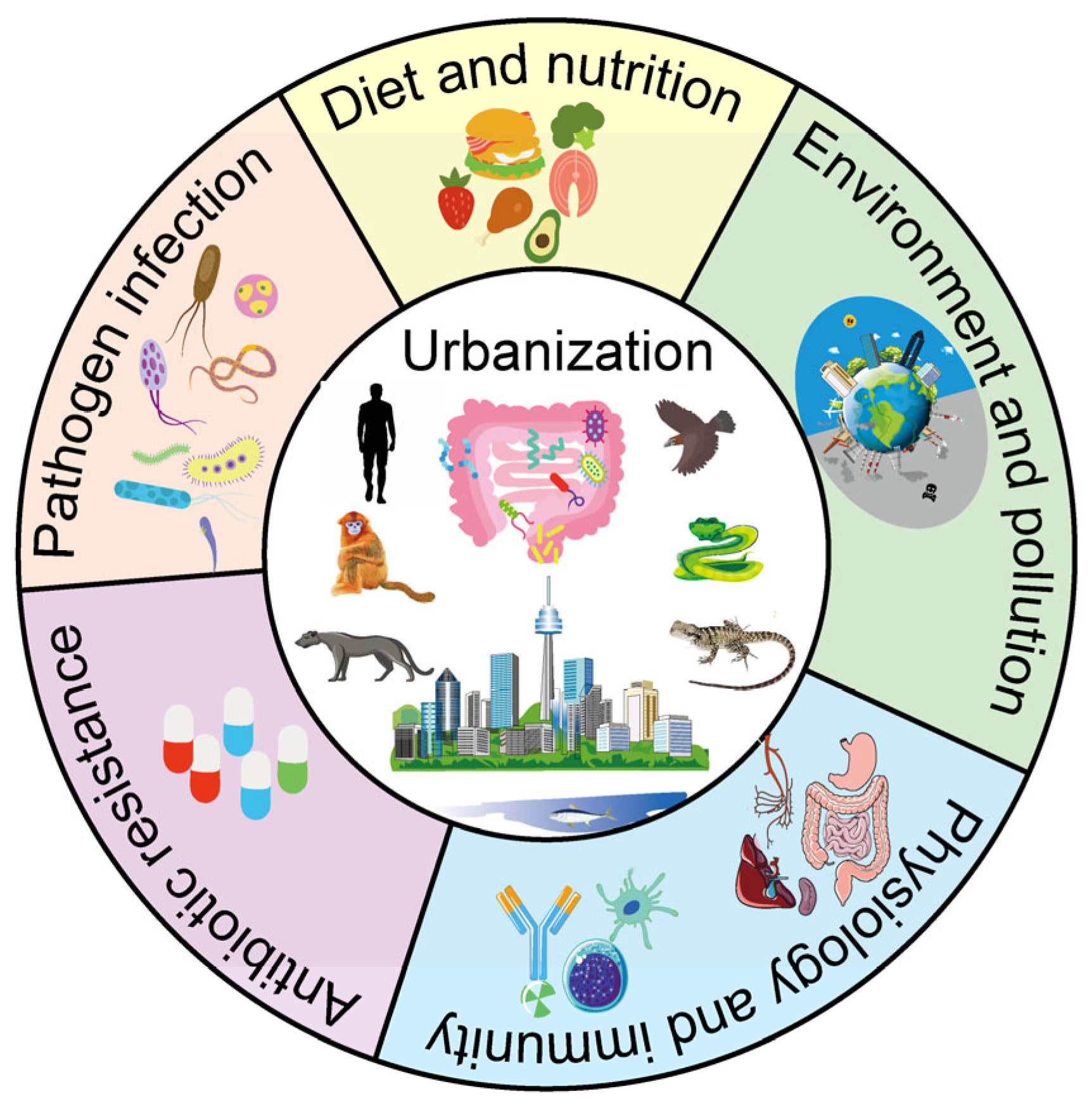
Double-Edged Sword: Urbanization and Response of Amniote Gut Microbiome in the Anthropocene
Abstract
1. Introduction
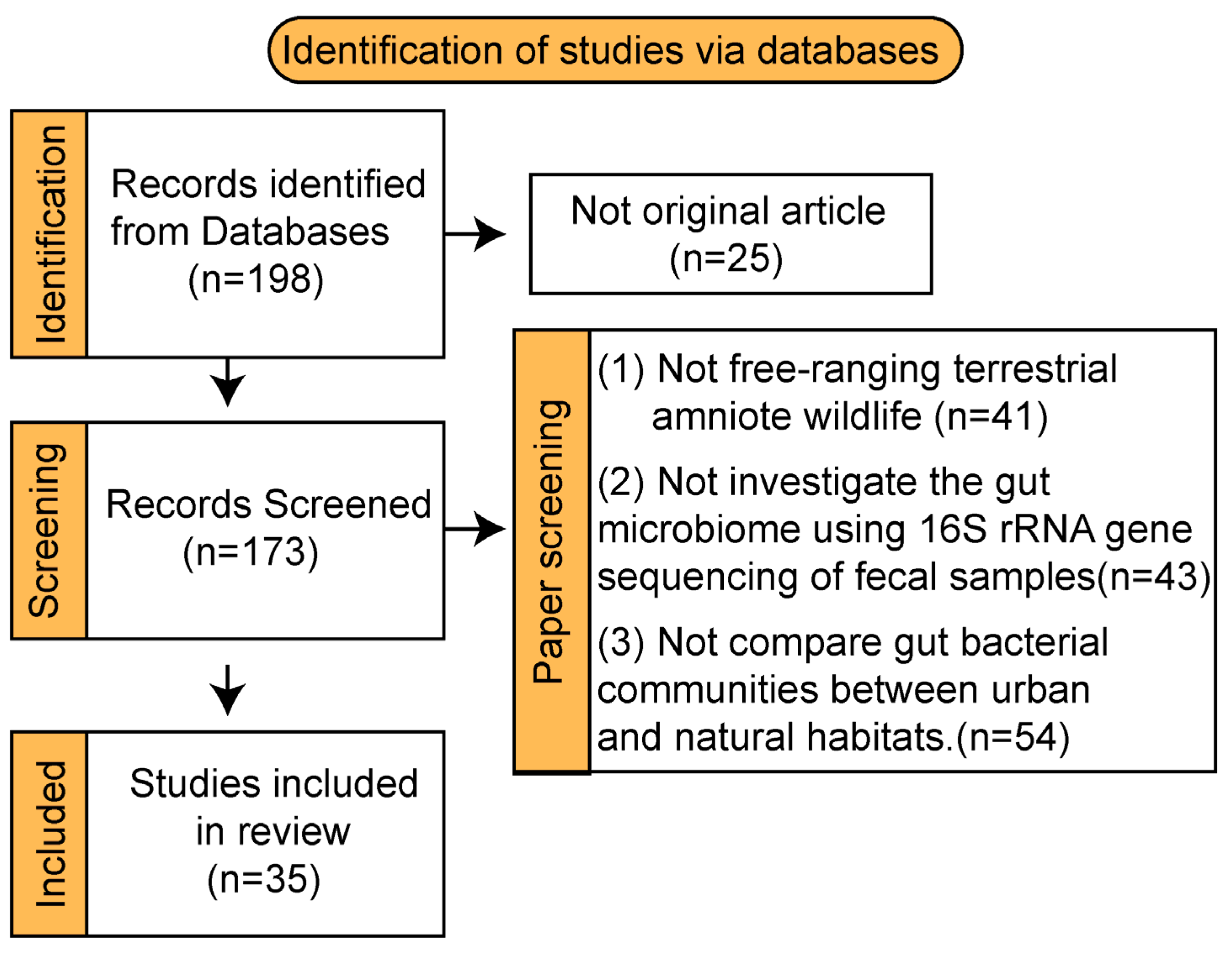
2. Methods
3. Impact of Urbanization on Amniote Gut Microbiota
3.1. Humans
3.2. Non-Human Primates
3.3. Carnivores
3.4. Birds
3.5. Reptiles
3.6. Summary
4. Key Factors Affecting Amniote Gut Microbiota in Urbanization
4.1. Impact of Diet and Nutrition on Amniote Gut Microbiota
4.1.1. Humans
4.1.2. Non-Human Primates
4.1.3. Carnivores
4.1.4. Birds
4.1.5. Reptiles
4.1.6. Summary
4.2. Environmental Pollution
5. Consequences of Urbanization-Induced Gut Microbiota Alterations
5.1. Physiological Alterations Drive Immunological Dysfunction and NCDs
5.2. Catalyzing the Spread of Zoonotic Pathogens
5.3. Microbiota-Mediated Behavioral Adaptations in Urban Wildlife
6. Suggestions for Improvement
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teyssier, A.; Rouffaer, L.O.; Saleh Hudin, N.; Strubbe, D.; Matthysen, E.; Lens, L.; White, J. Inside the guts of the city: Urban-induced alterations of the gut microbiota in a wild passerine. Sci. Total Environ. 2018, 612, 1276–1286. [Google Scholar] [CrossRef]
- Lewis, S.L.; Maslin, M.A. Defining the Anthropocene. Nature 2015, 519, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Aizpurua, O.; Bohmann, K.; Zepeda-Mendoza, M.L.; Gilbert, M.T.P. Do Vertebrate Gut Metagenomes Confer Rapid Ecological Adaptation? Trends Ecol. Evol. 2016, 31, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, K.; Gallo, T. Behavior Change in Urban Mammals: A Systematic Review. Front. Ecol. Evol. 2020, 8, 576665. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Rizwan, A.M.; Dennis, L.Y.C.; Liu, C. A review on the generation, determination and mitigation of Urban Heat Island. J. Environ. Sci. 2008, 20, 120–128. [Google Scholar] [CrossRef]
- Rodewald, A.D.; Kearns, L.J.; Shustack, D.P. Anthropogenic resource subsidies decouple predator–prey relationships. Ecol. Appl. 2011, 21, 936–943. [Google Scholar] [CrossRef]
- Quillfeldt, P.; Calegaro-Marques, C.; Amato, S.B. Urbanization Breaks Up Host-Parasite Interactions: A Case Study on Parasite Community Ecology of Rufous-Bellied Thrushes (Turdus rufiventris) along a Rural-Urban Gradient. PLoS ONE 2014, 9, e103144. [Google Scholar] [CrossRef]
- Adair, M.G.; Tolley, K.A.; van Vuuren, B.J.; da Silva, J.M. Anthropogenic reverberations on the gut microbiome of dwarf chameleons (Bradypodion). PeerJ 2025, 13, e18811. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.S.G.; McDonnell, M.J.; Phelan, G.K.; Keim, L.D.; Van Der Ree, R. Range expansion due to urbanization: Increased food resources attract Grey-headed Flying-foxes (Pteropus poliocephalus) to Melbourne. Austral Ecol. 2006, 31, 190–198. [Google Scholar] [CrossRef]
- Nguyen, H.K.D.; Jones, P.J.; Kendal, D.; Powell, S.M.; Flies, E.J. Wildlife microbiomes and the city: A systematic review of urban impacts on wildlife bacterial communities. Microbiota Host 2024, 2, e240003. [Google Scholar] [CrossRef]
- Alberti, M. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 2015, 30, 114–126. [Google Scholar] [CrossRef]
- Stofberg, M.; Cunningham, S.J.; Sumasgutner, P.; Amar, A. Juggling a “junk-food” diet: Responses of an urban bird to fluctuating anthropogenic-food availability. Urban. Ecosyst. 2019, 22, 1019–1026. [Google Scholar] [CrossRef]
- Lowry, H.; Lill, A.; Wong, B.B. Behavioural responses of wildlife to urban environments. Biol. Rev. Camb. Philos. Soc. 2013, 88, 537–549. [Google Scholar] [CrossRef]
- Derby Lewis, A.; Bouman, M.J.; Winter, A.M.; Hasle, A.F.; Stotz, D.F.; Johnston, M.K.; Klinger, K.R.; Rosenthal, A.; Czarnecki, C.A. Does Nature Need Cities? Pollinators Reveal a Role for Cities in Wildlife Conservation. Front. Ecol. Evol. 2019, 7, 220. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients 2025, 17, 948. [Google Scholar] [CrossRef]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Littleford-Colquhoun, B.L.; Weyrich, L.S.; Kent, N.; Frere, C.H. City life alters the gut microbiome and stable isotope profiling of the eastern water dragon (Intellagama lesueurii). Mol. Ecol. 2019, 28, 4592–4607. [Google Scholar] [CrossRef]
- Du, Y.; Ding, L.; Na, L.; Sun, T.; Sun, X.; Wang, L.; He, S.; Wang, Z.; Lu, Z.; Li, F.; et al. Prevalence of Chronic Diseases and Alterations of Gut Microbiome in People of Ningxia China During Urbanization: An Epidemiological Survey. Front. Cell Infect. Microbiol. 2021, 11, 707402. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Sonnenburg, E.D. Vulnerability of the industrialized microbiota. Science 2019, 366, eaaw9255. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Kamm, M.A.; Colombel, J.F.; Ng, S.C. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Qu, Q.; Wang, M.; Huang, M.; Zhou, W.; Wei, F. Global landscape of gut microbiome diversity and antibiotic resistomes across vertebrates. Sci. Total Environ. 2022, 838, 156178. [Google Scholar] [CrossRef]
- Koziol, A.; Odriozola, I.; Leonard, A.; Eisenhofer, R.; San Jose, C.; Aizpurua, O.; Alberdi, A. Mammals show distinct functional gut microbiome dynamics to identical series of environmental stressors. mBio 2023, 14, e0160623. [Google Scholar] [CrossRef]
- Huang, G.; Qi, D.; Yang, Z.; Hou, R.; Shi, W.; Zhao, F.; Li, Z.; Yan, L.; Wei, F. Gut microbiome as a key monitoring indicator for reintroductions of captive animals. Conserv. Biol. 2024, 38, e14173. [Google Scholar] [CrossRef]
- Dillard, B.A.; Chung, A.K.; Gunderson, A.R.; Campbell-Staton, S.C.; Moeller, A.H. Humanization of wildlife gut microbiota in urban environments. Elife 2022, 11, e76381. [Google Scholar] [CrossRef]
- Teyssier, A.; Matthysen, E.; Hudin, N.S.; de Neve, L.; White, J.; Lens, L. Diet contributes to urban-induced alterations in gut microbiota: Experimental evidence from a wild passerine. Proc. Biol. Sci. 2020, 287, 20192182. [Google Scholar] [CrossRef]
- Huang, G.; Wang, L.; Li, J.; Hou, R.; Wang, M.; Wang, Z.; Qu, Q.; Zhou, W.; Nie, Y.; Hu, Y.; et al. Seasonal shift of the gut microbiome synchronizes host peripheral circadian rhythm for physiological adaptation to a low-fat diet in the giant panda. Cell Rep. 2022, 38, 110203. [Google Scholar] [CrossRef]
- Petersen, C.; Hamerich, I.K.; Adair, K.L.; Griem-Krey, H.; Torres Oliva, M.; Hoeppner, M.P.; Bohannan, B.J.M.; Schulenburg, H. Host and microbiome jointly contribute to environmental adaptation. ISME J. 2023, 17, 1953–1965. [Google Scholar] [CrossRef]
- Fackelmann, G.; Gillingham, M.A.F.; Schmid, J.; Heni, A.C.; Wilhelm, K.; Schwensow, N.; Sommer, S. Human encroachment into wildlife gut microbiomes. Commun. Biol. 2021, 4, 800. [Google Scholar] [CrossRef]
- Bouilloud, M.; Galan, M.; Pradel, J.; Loiseau, A.; Ferrero, J.; Gallet, R.; Roche, B.; Charbonnel, N. Exploring the potential effects of forest urbanization on the interplay between small mammal communities and their gut microbiota. Anim. Microbiome 2024, 6, 16. [Google Scholar] [CrossRef]
- Stothart, M.R.; Palme, R.; Newman, A.E.M. It’s what’s on the inside that counts: Stress physiology and the bacterial microbiome of a wild urban mammal. Proc. Biol. Sci. 2019, 286, 20192111. [Google Scholar] [CrossRef]
- Sugden, S.; Sanderson, D.; Ford, K.; Stein, L.Y.; St Clair, C.C. An altered microbiome in urban coyotes mediates relationships between anthropogenic diet and poor health. Sci. Rep. 2020, 10, 22207. [Google Scholar] [CrossRef] [PubMed]
- Wasimuddin; Malik, H.; Ratovonamana, Y.R.; Rakotondranary, S.J.; Ganzhorn, J.U.; Sommer, S. Anthropogenic Disturbance Impacts Gut Microbiome Homeostasis in a Malagasy Primate. Front. Microbiol. 2022, 13, 911275. [Google Scholar] [CrossRef] [PubMed]
- Kuthyar, S.; Reesea, A.T. Variation in Microbial Exposure at the Human-Animal Interface and the Implications for Microbiome-Mediated Health Outcome. Msystems 2021, 6, e00567-21. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Fragiadakis, G.K.; Smits, S.A.; Sonnenburg, E.D.; Van Treuren, W.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Dominguez-Bello, M.G.; Leach, J.; et al. Links between environment, diet, and the hunter-gatherer microbiome. Gut Microbes 2019, 10, 216–227. [Google Scholar] [CrossRef]
- Martinez, I.; Stegen, J.C.; Maldonado-Gomez, M.X.; Eren, A.M.; Siba, P.M.; Greenhill, A.R.; Walter, J. The gut microbiota of rural papua new guineans: Composition, diversity patterns, and ecological processes. Cell Rep. 2015, 11, 527–538. [Google Scholar] [CrossRef]
- Afolayan, A.O.; Ayeni, F.A.; Moissl-Eichinger, C.; Gorkiewicz, G.; Halwachs, B.; Hogenauer, C. Impact of a Nomadic Pastoral Lifestyle on the Gut Microbiome in the Fulani Living in Nigeria. Front. Microbiol. 2019, 10, 2138. [Google Scholar] [CrossRef]
- Alencar, R.M.; Martinez, J.G.; Machado, V.N.; Alzate, J.F.; Ortiz-Ojeda, C.P.; Matias, R.R.; Benzaquem, D.C.; Santos, M.C.F.; Assuncao, E.N.; Lira, E.C.; et al. Preliminary profile of the gut microbiota from amerindians in the Brazilian amazon experiencing a process of transition to urbanization. Braz. J. Microbiol. 2024, 55, 2345–2354. [Google Scholar] [CrossRef]
- Ayeni, F.A.; Biagi, E.; Rampelli, S.; Fiori, J.; Soverini, M.; Audu, H.J.; Cristino, S.; Caporali, L.; Schnorr, S.L.; Carelli, V.; et al. Infant and Adult Gut Microbiome and Metabolome in Rural Bassa and Urban Settlers from Nigeria. Cell Rep. 2018, 23, 3056–3067. [Google Scholar] [CrossRef]
- Farinella, D.N.; Kaur, S.; Tran, V.; Cabrera-Mora, M.; Joyner, C.J.; Lapp, S.A.; Pakala, S.B.; Nural, M.V.; DeBarry, J.D.; Kissinger, J.C.; et al. Malaria disrupts the rhesus macaque gut microbiome. Front. Cell Infect. Microbiol. 2022, 12, 1058926. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Das, B.; Das, J. From the wild to the city: How domestication and urbanization reshape animal gut microbiome. bioRxiv 2023. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Tyakht, A.V.; Kostryukova, E.S.; Popenko, A.S.; Belenikin, M.S.; Pavlenko, A.V.; Larin, A.K.; Karpova, I.Y.; Selezneva, O.V.; Semashko, T.A.; Ospanova, E.A.; et al. Human gut microbiota community structures in urban and rural populations in Russia. Nat. Commun. 2013, 4, 2469. [Google Scholar] [CrossRef]
- Vinogradova, E.; Mukhanbetzhanov, N.; Nurgaziyev, M.; Jarmukhanov, Z.; Aipova, R.; Sailybayeva, A.; Bekbossynova, M.; Kozhakhmetov, S.; Kushugulova, A. Impact of urbanization on gut microbiome mosaics across geographic and diet contexts. mSystems 2024, 9, e0058524. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Zheng, H.M.; Li, P.; McDonald, D.; Sheng, H.F.; Chen, M.X.; Chen, Z.H.; Ji, G.Y.; Zheng, Z.D.; et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef]
- Kabwe, M.H.; Vikram, S.; Mulaudzi, K.; Jansson, J.K.; Makhalanyane, T.P. The gut mycobiota of rural and urban individuals is shaped by geography. BMC Microbiol. 2020, 20, 257. [Google Scholar] [CrossRef]
- Gonzalez Olmo, B.M.; Butler, M.J.; Barrientos, R.M. Evolution of the Human Diet and Its Impact on Gut Microbiota, Immune Responses, and Brain Health. Nutrients 2021, 13, 196. [Google Scholar] [CrossRef]
- Das, B.; Ghosh, T.S.; Kedia, S.; Rampal, R.; Saxena, S.; Bag, S.; Mitra, R.; Dayal, M.; Mehta, O.; Surendranath, A.; et al. Analysis of the Gut Microbiome of Rural and Urban Healthy Indians Living in Sea Level and High Altitude Areas. Sci. Rep. 2018, 8, 10104. [Google Scholar] [CrossRef] [PubMed]
- Sinsuebchuea, J.; Paenkaew, P.; Wutthiin, M.; Nantanaranon, T.; Laeman, K.; Kittichotirat, W.; Wattanachaisaereekul, S.; Dulsawat, S.; Nopharatana, M.; Vorapreeda, N.; et al. Characterization of the Gut Microbiota in Urban Thai Individuals Reveals Enterotype-Specific Signature. Microorganisms 2023, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Winglee, K.; Howard, A.G.; Sha, W.; Gharaibeh, R.Z.; Liu, J.; Jin, D.; Fodor, A.A.; Gordon-Larsen, P. Recent urbanization in China is correlated with a Westernized microbiome encoding increased virulence and antibiotic resistance genes. Microbiome 2017, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Tandon, D.; Haque, M.M.; Saravanan, R.; Shaikh, S.; Sriram, P.; Dubey, A.K.; Mande, S.S. A snapshot of gut microbiota of an adult urban population from Western region of India. PLoS ONE 2018, 13, e0195643. [Google Scholar] [CrossRef]
- Sun, S.; Wang, H.; Howard, A.G.; Zhang, J.; Su, C.; Wang, Z.; Du, S.; Fodor, A.A.; Gordon-Larsen, P.; Zhang, B. Loss of Novel Diversity in Human Gut Microbiota Associated with Ongoing Urbanization in China. mSystems 2022, 7, e0020022. [Google Scholar] [CrossRef]
- Verrelli, B.C.; Alberti, M.; Des Roches, S.; Harris, N.C.; Hendry, A.P.; Johnson, M.T.J.; Savage, A.M.; Charmantier, A.; Gotanda, K.M.; Govaert, L.; et al. A global horizon scan for urban evolutionary ecology. Trends Ecol. Evol. 2022, 37, 1006–1019. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, Y.; Huang, J.; Liu, Y.; Li, S. Comparative Study of Gut Microbiome in Urban and Rural Eurasian Tree Sparrows. Animals 2024, 14, 3497. [Google Scholar] [CrossRef]
- Tsuchida, S.; Ueda, A.; Kakooza, S.; Okubo, T.; Wampande, E.M.; Yamada, T.; Ushida, K. The fecal microbiomes analysis of Marabou storks (Leptoptilos crumenifer) reveals their acclimatization to the feeding environment in the Kampala urban areas, Uganda. J. Vet. Med. Sci. 2023, 85, 450–458. [Google Scholar] [CrossRef]
- Vasconcelos, D.S.; Harris, D.J.; Damas-Moreira, I.; Pereira, A.; Xavier, R. Factors shaping the gut microbiome of five species of lizards from different habitats. PeerJ 2023, 11, e15146. [Google Scholar] [CrossRef]
- Manara, S.; Asnicar, F.; Beghini, F.; Bazzani, D.; Cumbo, F.; Zolfo, M.; Nigro, E.; Karcher, N.; Manghi, P.; Metzger, M.I.; et al. Microbial genomes from non-human primate gut metagenomes expand the primate-associated bacterial tree of life with over 1000 novel species. Genome Biol. 2019, 20, 299. [Google Scholar] [CrossRef]
- Goldberg, T.L.; Gillespie, T.R.; Rwego, I.B.; Estoff, E.L.; Chapman, C.A. Forest fragmentation as cause of bacterial transmission among nonhuman primates, humans, and livestock, Uganda. Emerg. Infect. Dis. 2008, 14, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.H.; Shaw, G.M.; De Cock, K.M.; Sharp, P.M. AIDS as a zoonosis: Scientific and public health implications. Science 2000, 287, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Keesing, F.; Ostfeld, R.S. Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc. Natl. Acad. Sci. USA 2021, 118, e2023540118. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Stothart, M.R.; Newman, A.E.M. Shades of grey: Host phenotype dependent effect of urbanization on the bacterial microbiome of a wild mammal. Anim. Microbiome 2021, 3, 46. [Google Scholar] [CrossRef]
- Phillips, J.N.; Berlow, M.; Derryberry, E.P. The Effects of Landscape Urbanization on the Gut Microbiome: An Exploration Into the Gut of Urban and Rural White-Crowned Sparrows. Front. Ecol. Evol. 2018, 6, 148. [Google Scholar] [CrossRef]
- Berlow, M.; Phillips, J.N.; Derryberry, E.P. Effects of Urbanization and Landscape on Gut Microbiomes in White-Crowned Sparrows. Microb. Ecol. 2021, 81, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Fuirst, M.; Veit, R.R.; Hahn, M.; Dheilly, N.; Thorne, L.H. Effects of urbanization on the foraging ecology and microbiota of the generalist seabird Larus argentatus. PLoS ONE 2018, 13, e0209200. [Google Scholar] [CrossRef] [PubMed]
- Gadau, A.; Crawford, M.S.; Mayek, R.; Giraudeau, M.; McGraw, K.J.; Whisner, C.M.; Kondrat-Smith, C.; Sweazea, K.L. A comparison of the nutritional physiology and gut microbiome of urban and rural house sparrows (Passer domesticus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 237, 110332. [Google Scholar] [CrossRef]
- Murray, M.H.; Lankau, E.W.; Kidd, A.D.; Welch, C.N.; Ellison, T.; Adams, H.C.; Lipp, E.K.; Hernandez, S.M. Gut microbiome shifts with urbanization and potentially facilitates a zoonotic pathogen in a wading bird. PLoS ONE 2020, 15, e0220926. [Google Scholar] [CrossRef] [PubMed]
- Laforest-Lapointe, I.; Messier, C.; Kembel, S.W. Tree Leaf Bacterial Community Structure and Diversity Differ along a Gradient of Urban Intensity. mSystems 2017, 2, 10–1128. [Google Scholar] [CrossRef]
- Grond, K.; Sandercock, B.K.; Jumpponen, A.; Zeglin, L.H. The avian gut microbiota: Community, physiology and function in wild birds. J. Avian Biol. 2018, 49, e01788. [Google Scholar] [CrossRef]
- Sayed, Y.; Hassan, M.; Salem, H.M.; Al-Amry, K.; Eid, G.E. Prophylactic influences of prebiotics on gut microbiome and immune response of heat-stressed broiler chickens. Sci. Rep. 2023, 13, 13991. [Google Scholar] [CrossRef]
- Stanković, D.; Eira Pereira, H.J.; Raković, M.; Skorić, S.; Chakarov, N. Effects of urban life on the gut microbiota and the susceptibility to avian malaria infection in a population of the house sparrow Passer domesticus. J. Avian Biol. 2025, 2025, e03303. [Google Scholar] [CrossRef]
- Knutie, S.A.; Elderd, B. Food supplementation affects gut microbiota and immunological resistance to parasites in a wild bird species. J. Appl. Ecol. 2020, 57, 536–547. [Google Scholar] [CrossRef]
- Abjani, F.; Madhavan, P.; Chong, P.P.; Chinna, K.; Rhodes, C.A.; Lim, Y.A.L. Urbanisation and its associated factors affecting human gut microbiota: Where are we heading to? Ann. Hum. Biol. 2023, 50, 137–147. [Google Scholar] [CrossRef]
- Guo, J.; Lv, Q.; Ariff, A.; Zhang, X.; Peacock, C.S.; Song, Y.; Wen, X.; Saiganesh, A.; Melton, P.E.; Dykes, G.A.; et al. Western oropharyngeal and gut microbial profiles are associated with allergic conditions in Chinese immigrant children. World Allergy Organ. J. 2019, 12, 100051. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Diet Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term diet patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Partula, V.; Mondot, S.; Torres, M.J.; Kesse-Guyot, E.; Deschasaux, M.; Assmann, K.; Latino-Martel, P.; Buscail, C.; Julia, C.; Galan, P.; et al. Associations between usual diet and gut microbiota composition: Results from the Milieu Interieur cross-sectional study. Am. J. Clin. Nutr. 2019, 109, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Brushett, S.; Sinha, T.; Reijneveld, S.A.; de Kroon, M.L.A.; Zhernakova, A. The Effects of Urbanization on the Infant Gut Microbiota and Health Outcomes. Front. Pediatr. 2020, 8, 408. [Google Scholar] [CrossRef]
- Lee, W.; Hayakawa, T.; Kiyono, M.; Yamabata, N.; Hanya, G. Gut microbiota composition of Japanese macaques associates with extent of human encroachment. Am. J. Primatol. 2019, 81, e23072. [Google Scholar] [CrossRef]
- Chen, T.; Li, Y.; Liang, J.; Li, Y.; Huang, Z. Gut microbiota of provisioned and wild rhesus macaques (Macaca mulatta) living in a limestone forest in southwest Guangxi, China. Microbiologyopen 2020, 9, e981. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, Y.; Dong, M.; Xia, T.; Li, D.; Xie, M.; Wu, J.; Wen, A.; Wang, Q.; Zhu, G.; et al. Characterisation of the gut microbial community of rhesus macaques in high-altitude environments. BMC Microbiol. 2020, 20, 68. [Google Scholar] [CrossRef]
- Sugden, S.; St Clair, C.C.; Stein, L.Y. Individual and Site-Specific Variation in a Biogeographical Profile of the Coyote Gastrointestinal Microbiota. Microb. Ecol. 2021, 81, 240–252. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. Camb. Philos. Soc. 2012, 87, 545–562. [Google Scholar] [CrossRef]
- Alipio, C.; McCullah-Boozer, M.R.; Gaete, C.L.; Hall, L.K. Spatiotemporal partitioning between the endangered San Joaquin kit fox and a novel mesocarnivore community in the urban environment as revealed by camera traps. Glob. Ecol. Conserv. 2024, 54, e03184. [Google Scholar] [CrossRef]
- Fletcher, J.W.J.; Tollington, S.; Cox, R.; Tolhurst, B.A.; Newton, J.; McGill, R.A.R.; Cropper, P.; Berry, N.; Illa, K.; Scott, D.M. Utilisation of Anthropogenic Food by Red Foxes (Vulpes vulpes) in Britain as Determined by Stable Isotope Analysis. Ecol. Evol. 2025, 15, e70844. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Brun, A.; Magallanes, M.; Brinkerhoff, J.; Laspiur, A.; Acosta, J.C.; Bordenstein, S.R.; Caviedes-Vidal, E. Physiological and microbial adjustments to diet quality permit facultative herbivory in an omnivorous lizard. J. Exp. Biol. 2016, 219, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Gurbanov, R.; Kabaoglu, U.; Yagci, T. Metagenomic analysis of intestinal microbiota in wild rats living in urban and rural habitats. Folia Microbiol. (Praha) 2022, 67, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Anders, J.L.; Mychajliw, A.M.; Moustafa, M.A.M.; Mohamed, W.M.A.; Hayakawa, T.; Nakao, R.; Koizumi, I. Dietary niche breadth influences the effects of urbanization on the gut microbiota of sympatric rodents. Ecol. Evol. 2022, 12, e9216. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Murray, M.; Cembrowski, A.; Latham, A.D.M.; Lukasik, V.M.; Pruss, S.; St Clair, C.C. Greater consumption of protein-poor anthropogenic food by urban relative to rural coyotes increases diet breadth and potential for human–wildlife conflict. Ecography 2015, 38, 1235–1242. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, L.; Dong, Y.; Cheng, Y.; Song, Y. The gut microbiome of hooded cranes (Grus monacha) wintering at Shengjin Lake, China. Microbiologyopen 2017, 6, e00447. [Google Scholar] [CrossRef]
- Gamez, S.; Potts, A.; Mills, K.L.; Allen, A.A.; Holman, A.; Randon, P.M.; Linson, O.; Harris, N.C. Downtown diet: A global meta-analysis of increased urbanization on the diets of vertebrate predators. Proc. Biol. Sci. 2022, 289, 20212487. [Google Scholar] [CrossRef]
- Muegge, B.D.; Kuczynski, J.; Knights, D.; Clemente, J.C.; Gonzalez, A.; Fontana, L.; Henrissat, B.; Knight, R.; Gordon, J.I. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 2011, 332, 970–974. [Google Scholar] [CrossRef]
- Coogan, S.C.P.; Machovsky-Capuska, G.E.; Senior, A.M.; Martin, J.M.; Major, R.E.; Raubenheimer, D. Macronutrient selection of free-ranging urban Australian white ibis (Threskiornis moluccus). Behav. Ecol. 2017, 28, 1021–1029. [Google Scholar] [CrossRef]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F., Jr.; Davis, C.D.; Solano-Aguilar, G. Flavanol-Enriched Cocoa Powder Alters the Intestinal Microbiota, Tissue and Fluid Metabolite Profiles, and Intestinal Gene Expression in Pigs. J. Nutr. 2016, 146, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Mhete, M.; Eze, P.N.; Rahube, T.O.; Akinyemi, F.O. Soil properties influence bacterial abundance and diversity under different land-use regimes in semi-arid environments. Sci. Afr. 2020, 7, e00246. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Li, Y.; Song, J.; Liang, Y.; Chen, F.; Wei, X.; Li, C.; Liu, W.; Rensing, C.; et al. Linking bacterial life strategies with the distribution pattern of antibiotic resistance genes in soil aggregates after straw addition. J. Hazard. Mater. 2024, 471, 134355. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, J.; Chen, B.; Jin, W.; Wang, M.; Chen, X.; Jian, M.; Sun, L.; Piao, C. Bile acids as a key target: Traditional Chinese medicine for precision management of insulin resistance in type 2 diabetes mellitus through the gut microbiota-bile acids axis. Front. Endocrinol. (Lausanne) 2024, 15, 1481270. [Google Scholar] [CrossRef]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Xu, Z.; Knight, R. Dietary effects on human gut microbiome diversity. Br. J. Nutr. 2015, 113 (Suppl. S1), S1–S5. [Google Scholar] [CrossRef]
- Ducatez, S.; Sayol, F.; Sol, D.; Lefebvre, L. Are Urban Vertebrates City Specialists, Artificial Habitat Exploiters, or Environmental Generalists? Integr. Comp. Biol. 2018, 58, 929–938. [Google Scholar] [CrossRef]
- Mohr, A.E.; Basile, A.J.; Sweazea, K.L. An urban diet differentially alters the gut microbiome and metabolomic profiles compared with a seed diet in mourning doves. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R385–R396. [Google Scholar] [CrossRef]
- Hao, Y.; Ji, Z.; Shen, Z.; Xue, Y.; Zhang, B.; Yu, D.; Liu, T.; Luo, D.; Xing, G.; Tang, J.; et al. Increase Dietary Fiber Intake Ameliorates Cecal Morphology and Drives Cecal Species-Specific of Short-Chain Fatty Acids in White Pekin Ducks. Front. Microbiol. 2022, 13, 853797. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Armougom, F.; Carriere, F.; Bachar, D.; Laugier, R.; Lagier, J.C.; Robert, C.; Michelle, C.; Henrissat, B.; Raoult, D. A Metagenomic Investigation of the Duodenal Microbiota Reveals Links with Obesity. PLoS ONE 2015, 10, e0137784. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, F.; Li, L.; Wang, A.; Sharshov, K.; Druzyaka, A.; Lancuo, Z.; Wang, S.; Shi, Y. Characterization of the gut microbiome of black-necked cranes (Grus nigricollis) in six wintering areas in China. Arch. Microbiol. 2020, 202, 983–993. [Google Scholar] [CrossRef]
- Li, F.; Armet, A.M.; Korpela, K.; Liu, J.; Quevedo, R.M.; Asnicar, F.; Seethaler, B.; Rusnak, T.B.S.; Cole, J.L.; Zhang, Z.; et al. Cardiometabolic benefits of a non-industrialized-type diet are linked to gut microbiome modulation. Cell 2025, 188, 1226–1247.e1218. [Google Scholar] [CrossRef]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Cui, B.; Gai, Z.; She, X.; Wang, R.; Xi, Z. Effects of chronic noise on glucose metabolism and gut microbiota-host inflammatory homeostasis in rats. Sci. Rep. 2016, 6, 36693. [Google Scholar] [CrossRef]
- Kish, L.; Hotte, N.; Kaplan, G.G.; Vincent, R.; Tso, R.; Ganzle, M.; Rioux, K.P.; Thiesen, A.; Barkema, H.W.; Wine, E.; et al. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS ONE 2013, 8, e62220. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, Y.; Gao, Y.; Zhou, R.; Hu, Q.; He, Z.; Lv, Y.; Wang, X.; Chen, W.; Deng, Y.; et al. Gut microbiota mediate melatonin signalling in association with type 2 diabetes. Diabetologia 2022, 65, 1627–1641. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Xu, Y.; Wu, W.; Tang, W.; Chen, Z.; Ji, G.; Peng, J.; Jiang, Q.; Xiao, J.; et al. Gut microbiota partially mediates the effects of fine particulate matter on type 2 diabetes: Evidence from a population-based epidemiological study. Environ. Int. 2019, 130, 104882. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Park, H.; Ho, M.; Bhardwaj, K.; Sugimura, N.; Lee, H.W.; Meng, H.; Ebert, M.P.; Chao, K.; et al. Unveiling and harnessing the human gut microbiome in the rising burden of non-communicable diseases during urbanization. Gut Microbes 2023, 15, 2237645. [Google Scholar] [CrossRef] [PubMed]
- Price, N.D.; Magis, A.T.; Earls, J.C.; Glusman, G.; Levy, R.; Lausted, C.; McDonald, D.T.; Kusebauch, U.; Moss, C.L.; Zhou, Y.; et al. A wellness study of 108 individuals using personal, dense, dynamic data clouds. Nat. Biotechnol. 2017, 35, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Leclaire, S.; Pineaux, M.; Blanchard, P.; White, J.; Hatch, S.A. Microbiota composition and diversity of multiple body sites vary according to reproductive performance in a seabird. Mol. Ecol. 2023, 32, 2115–2133. [Google Scholar] [CrossRef]
- Moeller, A.H.; Ivey, K.; Cornwall, M.B.; Herr, K.; Rede, J.; Taylor, E.N.; Gunderson, A.R. The Lizard Gut Microbiome Changes with Temperature and Is Associated with Heat Tolerance. Appl. Environ. Microbiol. 2020, 86, e01181-20. [Google Scholar] [CrossRef]
- Navidshad, B.; Royan, M. Peroxisome Proliferator-Activated Receptor Alpha (PPARalpha), a Key Regulator of Lipid Metabolism in Avians. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 303–308. [Google Scholar] [CrossRef]
- Oyefiade, A.; Erdman, L.; Goldenberg, A.; Malkin, D.; Bouffet, E.; Taylor, M.D.; Ramaswamy, V.; Scantlebury, N.; Law, N.; Mabbott, D.J. PPAR and GST polymorphisms may predict changes in intellectual functioning in medulloblastoma survivors. J. Neurooncol 2019, 142, 39–48. [Google Scholar] [CrossRef]
- Djouadi, F.; Weinheimer, C.J.; Saffitz, J.E.; Pitchford, C.; Bastin, J.; Gonzalez, F.J.; Kelly, D.P. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator- activated receptor alpha- deficient mice. J. Clin. Investig. 1998, 102, 1083–1091. [Google Scholar] [CrossRef]
- Lima-Camara, T.N. Emerging arboviruses and public health challenges in Brazil. Rev. Saude Publica 2016, 50, 36. [Google Scholar] [CrossRef]
- Al-Yasiri, M.H.; Normand, A.C.; Piarroux, R.; Ranque, S.; Mauffrey, J.F. Gut yeast communities in Larus michahellis from various breeding colonies. Med. Mycol. 2017, 55, 436–444. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, L.; Zhao, F.; Zhang, L.; Lu, Z.; Chu, T.; Wang, S.; Liu, Z.; Sun, Y.; Chen, M.; et al. Cryptococcus neoformans, a global threat to human health. Infect. Dis. Poverty 2023, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Strazar, M.; Temba, G.S.; Vlamakis, H.; Kullaya, V.I.; Lyamuya, F.; Mmbaga, B.T.; Joosten, L.A.B.; van der Ven, A.; Netea, M.G.; de Mast, Q.; et al. Gut microbiome-mediated metabolism effects on immunity in rural and urban African populations. Nat. Commun. 2021, 12, 4845. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C.; et al. Microbial bile acid metabolites modulate gut RORgamma(+) regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef]
- Visekruna, A.; Luu, M. The Role of Short-Chain Fatty Acids and Bile Acids in Intestinal and Liver Function, Inflammation, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 703218. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Maclean, P.; Thomas, D.G.; Cave, N.J.; Young, W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. PeerJ 2017, 5, e3019. [Google Scholar] [CrossRef]
- Schmidt, M.; Unterer, S.; Suchodolski, J.S.; Honneffer, J.B.; Guard, B.C.; Lidbury, J.A.; Steiner, J.M.; Fritz, J.; Kolle, P. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS ONE 2018, 13, e0201279. [Google Scholar] [CrossRef]
- Chen, L.; Wilson, J.E.; Koenigsknecht, M.J.; Chou, W.C.; Montgomery, S.A.; Truax, A.D.; Brickey, W.J.; Packey, C.D.; Maharshak, N.; Matsushima, G.K.; et al. Corrigendum: NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat. Immunol. 2017, 18, 1270. [Google Scholar] [CrossRef]
- Martinez, I.; Lattimer, J.M.; Hubach, K.L.; Case, J.A.; Yang, J.; Weber, C.G.; Louk, J.A.; Rose, D.J.; Kyureghian, G.; Peterson, D.A.; et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. 2013, 7, 269–280. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Jin, Y.; Zhao, L.; Zhao, F.; Feng, J.; Li, A.; Wei, Y. Splenectomy Leads to Amelioration of Altered Gut Microbiota and Metabolome in Liver Cirrhosis Patients. Front. Microbiol. 2018, 9, 963. [Google Scholar] [CrossRef]
- Blaser, M.J. The theory of disappearing microbiota and the epidemics of chronic diseases. Nat. Rev. Immunol. 2017, 17, 461–463. [Google Scholar] [CrossRef] [PubMed]
- He, K.; An, F.; Zhang, H.; Yan, D.; Li, T.; Wu, J.; Wu, R. Akkermansia muciniphila: A Potential Target for the Prevention of Diabetes. Foods 2024, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.A.; Hegazy, S.M.; Aziz, R.K. The curious case of Prevotella copri. Gut Microbes 2023, 15, 2249152. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, J.; Feng, S.; Huang, C.; Wang, H.; Huo, F.; Liu, H. Akkermansia muciniphila, which is enriched in the gut microbiota by metformin, improves cognitive function in aged mice by reducing the proinflammatory cytokine interleukin-6. Microbiome 2023, 11, 120. [Google Scholar] [CrossRef]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef]
- Kobayashi, C.C.; Souza, L.K.; Fernandes Ode, F.; Brito, S.C.; Silva, A.C.; Sousa, E.D.; Silva Mdo, R. Characterization of Cryptococcus neoformans isolated from urban environmental sources in Goiania, Goias State, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2005, 47, 203–207. [Google Scholar] [CrossRef]
- Mourkas, E.; Valdebenito, J.O.; Marsh, H.; Hitchings, M.D.; Cooper, K.K.; Parker, C.T.; Szekely, T.; Johansson, H.; Ellstrom, P.; Pascoe, B.; et al. Proximity to humans is associated with antimicrobial-resistant enteric pathogens in wild bird microbiomes. Curr. Biol. 2024, 34, 3955–3965. [Google Scholar] [CrossRef]
- Alirol, E.; Getaz, L.; Stoll, B.; Chappuis, F.; Loutan, L. Urbanisation and infectious diseases in a globalised world. Lancet Infect. Dis. 2011, 11, 131–141. [Google Scholar] [CrossRef]
- Bradley, C.A.; Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef]
- Solomon, G.; Love, A.C.; Vaziri, G.J.; Harvey, J.; Verrett, T.; Chernicky, K.; Simons, S.; Albert, L.; Chaves, J.A.; Knutie, S.A. Effect of urbanization and parasitism on the gut microbiota of Darwin’s finch nestlings. Mol. Ecol. 2023, 32, 6059–6069. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Rouffaer, L.O.; Strubbe, D.; Teyssier, A.; Salleh Hudin, N.; Van den Abeele, A.M.; Cox, I.; Haesendonck, R.; Delmee, M.; Haesebrouck, F.; Pasmans, F.; et al. Effects of urbanization on host-pathogen interactions, using Yersinia in house sparrows as a model. PLoS ONE 2017, 12, e0189509. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.R.; Brans, K.I.; Des Roches, S.; Donihue, C.M.; Diamond, S.E. Adaptive Evolution in Cities: Progress and Misconceptions. Trends Ecol. Evol. 2021, 36, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Ma, J.E.; Li, J.; Zhang, X.J.; Li, L.M.; He, N.; Liu, H.Y.; Luo, S.Y.; Wu, Z.J.; Han, R.C.; et al. Diets Alter the Gut Microbiome of Crocodile Lizards. Front. Microbiol. 2017, 8, 2073. [Google Scholar] [CrossRef]
- Bestion, E.; Jacob, S.; Zinger, L.; Di Gesu, L.; Richard, M.; White, J.; Cote, J. Climate warming reduces gut microbiota diversity in a vertebrate ectotherm. Nat. Ecol. Evol. 2017, 1, 161. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Byrne, L.B.; Chies, J.A.B. Examining the paradox of urban disease ecology by linking the perspectives of Urban One Health and Ecology with Cities. Urban. Ecosyst. 2022, 25, 1735–1744. [Google Scholar] [CrossRef]
- Trott, D.J.; Abraham, S.; Adler, B. Antimicrobial Resistance in Leptospira, Brucella, and Other Rarely Investigated Veterinary and Zoonotic Pathogens. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Madsen, A.M.; White, J.K.; Nielsen, J.L.; Keskin, M.E.; Tendal, K.; Frederiksen, M.W. A cross sectional study on airborne inhalable microorganisms, endotoxin, and particles in pigeon coops—Risk assessment of exposure. Environ. Res. 2022, 204, 112404. [Google Scholar] [CrossRef]
- Gupta, P.; Malik, S.; Khare, V.; Banerjee, G.; Mehrotra, A.; Mehrotra, S.; Singh, M. A fatal case of meningitis caused by Cryptococcus neoformans var. grubii in an immunocompetent male. J. Infect. Dev. Ctries. 2011, 5, 71–74. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Cao, L.; Yin, H.; Xu, M.; Wang, Z.; Liu, Y.; Wang, X.; Deng, Y. Habitat environments impacted the gut microbiome of long-distance migratory swan geese but central species conserved. Sci. Rep. 2018, 8, 13314. [Google Scholar] [CrossRef] [PubMed]
- Stecher, B.; Robbiani, R.; Walker, A.W.; Westendorf, A.M.; Barthel, M.; Kremer, M.; Chaffron, S.; Macpherson, A.J.; Buer, J.; Parkhill, J.; et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007, 5, 2177–2189. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Fumagalli, S.; Ghisleni, G.; Labra, M. The Microbiome of the Built Environment: The Nexus for Urban Regeneration for the Cities of Tomorrow. Microorganisms 2022, 10, 2311. [Google Scholar] [CrossRef] [PubMed]
- The MetaSUB International Consortium. The Metagenomics and Metadesign of the Subways and Urban Biomes (MetaSUB) International Consortium inaugural meeting report. Microbiome 2016, 4, 24. [Google Scholar] [CrossRef]
- Jin, M.K.; Zhang, Q.; Zhao, W.L.; Li, Z.H.; Qian, H.F.; Yang, X.R.; Zhu, Y.G.; Liu, H.J. Fluoroquinolone antibiotics disturb the defense system, gut microbiome, and antibiotic resistance genes of Enchytraeus crypticus. J. Hazard. Mater. 2022, 424, 127509. [Google Scholar] [CrossRef]
- Jin, M.K.; Zhang, Q.; Yang, Y.T.; Zhao, C.X.; Li, J.; Li, H.; Qian, H.; Zhu, D.; Zhu, Y.G. Exposure to cypermethrin pesticide disturbs the microbiome and disseminates antibiotic resistance genes in soil and the gut of Enchytraeus crypticus. J. Hazard. Mater. 2023, 449, 131026. [Google Scholar] [CrossRef]
- Lefebvre, L. The opening of milk bottles by birds: Evidence for accelerating learning rates, but against the wave-of-advance model of cultural transmission. Behav. Process. 1995, 34, 43–53. [Google Scholar] [CrossRef]
- Stanton, L.A.; Cooley-Ackermann, C.; Davis, E.C.; Fanelli, R.E.; Benson-Amram, S. Wild raccoons demonstrate flexibility and individuality in innovative problem-solving. Proc. Biol. Sci. 2024, 291, 20240911. [Google Scholar] [CrossRef]
- Fu, Y.; Dou, Q.; Smalla, K.; Wang, Y.; Johnson, T.A.; Brandt, K.K.; Mei, Z.; Liao, M.; Hashsham, S.A.; Schaffer, A.; et al. Gut microbiota research nexus: One Health relationship between human, animal, and environmental resistomes. mLife 2023, 2, 350–364. [Google Scholar] [CrossRef]
- Hahs, A.K.; Fournier, B.; Aronson, M.F.J.; Nilon, C.H.; Herrera-Montes, A.; Salisbury, A.B.; Threlfall, C.G.; Rega-Brodsky, C.C.; Lepczyk, C.A.; La Sorte, F.A.; et al. Urbanisation generates multiple trait syndromes for terrestrial animal taxa worldwide. Nat. Commun. 2023, 14, 4751. [Google Scholar] [CrossRef]
- Ramaboli, M.C.; Ocvirk, S.; Khan Mirzaei, M.; Eberhart, B.L.; Valdivia-Garcia, M.; Metwaly, A.; Neuhaus, K.; Barker, G.; Ru, J.; Nesengani, L.T.; et al. Diet changes due to urbanization in South Africa are linked to microbiome and metabolome signatures of Westernization and colorectal cancer. Nat. Commun. 2024, 15, 3379. [Google Scholar] [CrossRef]
- Kortman, G.A.M.; Timmerman, H.M.; Schaafsma, A.; Stoutjesdijk, E.; Muskiet, F.A.J.; Nhien, N.V.; van Hoffen, E.; Boekhorst, J.; Nauta, A. Mothers’ Breast Milk Composition and Their Respective Infant’s Gut Microbiota Differ between Five Distinct Rural and Urban Regions in Vietnam. Nutrients 2023, 15, 4802. [Google Scholar] [CrossRef]
- Watanabe, M.; Sianoya, A.; Mishima, R.; Therdtatha, P.; Rodriguez, A.; Ramos, D.C.; Lee, Y.K.; Dalmacio, L.M.; Nakayama, J. Gut microbiome status of urban and rural Filipino adults in relation to diet and metabolic disorders. FEMS Microbiol. Lett. 2021, 368, fnab149. [Google Scholar] [CrossRef] [PubMed]
- Oduaran, O.H.; Tamburini, F.B.; Sahibdeen, V.; Brewster, R.; Gomez-Olive, F.X.; Kahn, K.; Norris, S.A.; Tollman, S.M.; Twine, R.; Wade, A.N.; et al. Gut microbiome profiling of a rural and urban South African cohort reveals biomarkers of a population in lifestyle transition. BMC Microbiol. 2020, 20, 330. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Quinto, A.; Cerqueda-Garcia, D.; Falcon, L.I.; Gaona, O.; Martinez-Correa, S.; Nieto, J.; G-Santoyo, I. Gut Microbiome in Children from Indigenous and Urban Communities in Mexico: Different Subsistence Models, Different Microbiomes. Microorganisms 2020, 8, 1592. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Turroni, S.; Rampelli, S.; Soverini, M.; D’Amico, F.; Biagi, E.; Brigidi, P.; Troiani, E.; Candela, M. Gut microbiome response to a modern Paleolithic diet in a Western lifestyle context. PLoS ONE 2019, 14, e0220619. [Google Scholar] [CrossRef]
- Hansen, M.E.B.; Rubel, M.A.; Bailey, A.G.; Ranciaro, A.; Thompson, S.R.; Campbell, M.C.; Beggs, W.; Dave, J.R.; Mokone, G.G.; Mpoloka, S.W.; et al. Population structure of human gut bacteria in a diverse cohort from rural Tanzania and Botswana. Genome Biol. 2019, 20, 16. [Google Scholar] [CrossRef]
- Ruggles, K.V.; Wang, J.; Volkova, A.; Contreras, M.; Noya-Alarcon, O.; Lander, O.; Caballero, H.; Dominguez-Bello, M.G. Changes in the Gut Microbiota of Urban Subjects during an Immersion in the Traditional Diet and Lifestyle of a Rainforest Village. mSphere 2018, 3, e00193-18. [Google Scholar] [CrossRef]
- Kisuse, J.; La-Ongkham, O.; Nakphaichit, M.; Therdtatha, P.; Momoda, R.; Tanaka, M.; Fukuda, S.; Popluechai, S.; Kespechara, K.; Sonomoto, K.; et al. Urban Diets Linked to Gut Microbiome and Metabolome Alterations in Children: A Comparative Cross-Sectional Study in Thailand. Front. Microbiol. 2018, 9, 1345. [Google Scholar] [CrossRef]
- Turroni, S.; Fiori, J.; Rampelli, S.; Schnorr, S.L.; Consolandi, C.; Barone, M.; Biagi, E.; Fanelli, F.; Mezzullo, M.; Crittenden, A.N.; et al. Fecal metabolome of the Hadza hunter-gatherers: A host-microbiome integrative view. Sci. Rep. 2016, 6, 32826. [Google Scholar] [CrossRef]
- Anwesh, M.; Kumar, K.V.; Nagarajan, M.; Chander, M.P.; Kartick, C.; Paluru, V. Elucidating the richness of bacterial groups in the gut of Nicobarese tribal community—Perspective on their lifestyle transition. Anaerobe 2016, 39, 68–76. [Google Scholar] [CrossRef]
- Obregon-Tito, A.J.; Tito, R.Y.; Metcalf, J.; Sankaranarayanan, K.; Clemente, J.C.; Ursell, L.K.; Xu, Z.Z.; Van Treuren, W.; Knight, R.; Gaffney, P.M.; et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat. Commun. 2015, 6, 6505. [Google Scholar] [CrossRef]
- Maraci, O.; Corsini, M.; Antonatou-Papaioannou, A.; Junemann, S.; Sudyka, J.; Di Lecce, I.; Caspers, B.A.; Szulkin, M. Changes to the gut microbiota of a wild juvenile passerine in a multidimensional urban mosaic. Sci. Rep. 2022, 12, 6872. [Google Scholar] [CrossRef]
- Kolp, M.; Marcello, M.; Holt, A.; Rossi, K.; Zurawski, C.; Cancelliere, K.; Telemeco, S.; Swift, J.F.; Purple, K.; Faulkner, C. Evidence of canine intestinal parasites and associated fecal bacteria among urban and rural dog parks in central Appalachia U.S. Vet. Parasitol. Reg. Stud. Rep. 2025, 62, 101280. [Google Scholar] [CrossRef]
| Species | Time | Area | Country | Region | Food | Sample No. | Character of Gut Microbiome | Reason | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Diversity | |||||||||
| Kazakh Population | - | Urban | Kazakhstan | Astana, Karagandy, Kostanay, Ridder | Salt ↑, cholesterol ↑, protein ↑ | 502 | Firmicutes/Bacteroidetes Ratios ↑, Coprococcus spp. ↑, Parasutterella spp. ↑ | α, β ↓ | 1. Diet 2. Environment 3. Antibiotics 4. Lifestyle | [49] |
| Rural | Torgay, Akzhar, Zhansary, Novodolinka | Carbohydrates, fiber | 149 | Ligilactobacillus spp., Sutterella spp., Paraprevotella spp. | ||||||
| Yanomami | - | Urban | Brazil | Manaus | Industrialized foods | 12 | Bacteroides spp. ↑ | ND | 1. Antibiotics 2. Diets. 3. Lifestyle. | [43] |
| Rural | Roraima, Amazonas | Seeds, roots, fruits, fish | 18 | Prevotella spp., Lactobacillus spp., Treponema spp. *, Succinivibrio spp. * | ||||||
| Amaxhosa | November 2019–February 2020 | Urban | South Africa | Cape Town | Energy, fat, animal protein diet | 20 | Bacteroidota ↑, Proteobacteria ↑, Prevotella spp. ↓ Faecalibacterium spp. ↓ | α ↓ | 1. Diet 2. Lifestyle | [171] |
| Rural | Zithulele | Fiber, plant protein, polyphenols | 24 | Prevotella spp., Faecalibacterium spp., Dialister spp., Treponema spp. | ||||||
| Vietnamese | July 2013–2023 | Urban | Vietnam | Hanoi | Westernized diet | 40 | Pathogenic, Bacteria (Enterobacteriaceae) ↑, Bifidobacterium spp. ↓. | α ↓ | 1. Diet 2. Breast milk composition 3. Environment 4. Lifestyle | [172] |
| Rural | Tien Giang, Phu Tho, Ha Long Bay. | Carbohydrates, plant-based fat, animal protein | 60 | Bifidobacterium spp., Pathogenic Bacteria (Enterobacteriaceae) | ||||||
| Malay | January 2019–October 2019 | Urban | Philippines | Manila | Pasta, pizza, French fries, processed meat, mayonnaise, butter. | 25 | Clostridiales ↑ | - | 1. Diet | [173] |
| Rural | Albay | Rice, starchy roots, green leafy vegetables, smoked fish, coconut milk | 67 | Prevotella-Driven Microbiome, Bacteroidetes, Proteobacteria | ||||||
| Mongoloid | 2018–2019 | Urban | China | Ningxia Hui Autonomous | Meat ↑, rice ↑, potatoes ↓. | 1204 | Blautia spp. ↑, Klebsiella spp. ↑ | α ↓ | 1. Urbanization level 2. Diet | [22] |
| Rural | Potatoes, whole grains | 1303 | Faecalibacterium spp., Prevotella spp., Pseudobutyrivibrio spp. | |||||||
| Black Race | 2016–2017 | Urban | South Africa | Soweto | Westernized diet | 51 | Bacteroides spp. ↑ | α ↓ | 1. Environment 2. Diet 3. Lifestyle 4. Epidemiological | [174] |
| Rural | Bushbuckridge | Traditional plant-based diet | 119 | Prevotella spp., Vampirovibrio spp. | ||||||
| Amerindians | - | Urban | Mexico. | México City | Animal protein ↑, fiber ↓ | 13 | Saccharibacteria * | α ↓ | 1. Diet 2. Lifestyle 3. Antibiotics 4. Hygiene | [175] |
| Rural | Me’Phaa | Agricultural crops | 29 | Deinococcus-Thermus *, Chloroflexi *, Verrucomicrobia * | ||||||
| Nigeria | - | Urban | Nigeria | Jos City | High-fiber foods | 22 | Bacteroidetes ↑, Spirochaetes ↑, Prevotellaceae spp. ↑; | α ↑ | 1. Diet 2. Lifestyle 3. Pathogens. | [42] |
| Rural | Jengre Town | Low-fiber processed foods | 28 | Firmicutes, Ruminococcaceae spp., Lachnospiraceae spp., Christensenellaceae spp., Blautia spp. | ||||||
| Caucasian | March 2017–April 2017 | Urban | Italy | - | Mpd: vegetables, fruit, nuts, seeds, eggs, fish, lean meat Md: Mediterranean diet | 158 | Bile-Tolerant (Bacteroides spp., Collinsella spp., Dorea spp.) ↑, Fat-Loving Microbes (Bilophila spp.) ↑, SCFA Producers (Lachnospira spp., Coprococcus spp.) ↑ | - | 1. Diet 2. Environment 3. Lifestyle | [176] |
| Rural | Tanzania, Peru, Canada | - | Hadza/Matses: High-fiber plant foods Inuit: Animal fat/protein | 73 | Prevotella spp. | |||||
| Tanzania and Botswana: African Black Usa: White, African American | Tanzania: March Botswana: April (No year) | Urban | USA | - | Industrial diet, fiber ↓. | 12 | Bacteroidaceae ↑ | α ↓ | 1. Diet 2. Environment 3. Genetic relatedness 4. Lifestyle | [177] |
| Rural | Tanzania, Botswana | - | Fiber. | 114 | Prevotellaceae | |||||
| Amerindian | January 2015 | Urban | Venezuela | Caracas | Traditional rural diet. | 7 | Bacteroides spp. ↑, Blautia spp. ↑, Faecalibacterium spp. ↑ | α ↓ | 1. Diet 2. Lifestyle 3. Environment 4. Exposure 5. Age difference: | [178] |
| Rural | Bolivar, Venezuela | Mainly cassava, fish, various fruits, meat | 38 | Treponema spp., Succinivibrio spp., Ruminobacter spp. | ||||||
| Thai People | - | Urban | Thailand | Bangkok | High-fat modern diet | 17 | Bacteroidales ↑, Selenomonadales ↑, Clostridiales ↓ | α ↓ | 1. Diet 2. Lifestyle | [179] |
| Rural | Buriram | Traditional vegetable-based diet | 28 | Clostridiales | ||||||
| Leh:Mons, Mongols, Dards Ballabhgarh: Aryan Descendants. | - | Urban | India | Ballabhgarh | Processed foods ↑ | 24 | Firmicutes ↑, Proteobacteria ↑, Lactobacillus spp. ↑ | α ↓ | 1. Diet 2. Geographical locations 3. Lifestyle | [53] |
| Rural | Ballabhgarh, Leh | (Ballabhgarh): Vegetarian (Leh): Non-vegetarian, low dairy intake. | 60 | Bacteroidetes, Parabacteroides spp., Blautia spp., Prevotella spp. | ||||||
| Nigerian Ethnic Groups | July 2015–September 2015 | Urban | Nigeria | Ilorin, Abeokuta, Ado Ekiti, Ibadan, Nigeria. | Traditional Nigerian foods and Western diet | 30 (12 Infants, 18 Adults) | Firmicutes ↑, Proteobacteria ↑, Firmicutes\Bacteroidetes ↑. Bacteroides spp. ↑, Bifidobacterium spp. ↑, Oscillospira spp. ↑ | ND | 1. Environmental 2. Microbial dispersal 3. Diet 4. Lifestyle and healthcare practices | [44] |
| Rural | Bassa | Self-sufficient diet of tubers, grains, untreated river water. | 18 (9 Infants, 9 Adults) | Bacteroidetes, Spirochaetes | ||||||
| Han Chinese | - | Urban | China. | Hunan Province | Westernized diet | 20 | Archaea ↓, Escherichia spp. ↑, Shigella spp. ↑ | α ↓ | 1. Westernized diet 2. Hygiene practices 3. Antibiotics 4. Lifestyle | [55] |
| Rural | Fiber, processed foods | 20 | Beneficial Bacteria (Ruminococcus spp.) | |||||||
| - | 2013 | Urban | Italy | Bologna | Westernized diet | 12 | Bifidobacterium spp. ↑, Bacteroide ↑ | - | 1. Diet 2. Lifestyle 3. Environment | [180] |
| Rural | Tanzania | - | Primarily plants (tubers) | 17 | Prevotella spp., Succinivibrio spp., Treponema spp., Bulleidia spp., Bifidobacterium spp., Bacteroides spp., Blautia spp., Dorea spp. | |||||
| Mongoloid | - | Urban | India | Port Blair | Carbohydrates, proteins | 12 | Bifidobacterium spp. ↑ | α ↓ | 1. Diet 2. Lifestyle | [181] |
| Rural | Nancowry | Agriculture, forests | 12 | Bacteroidetes | ||||||
| Matses, Tunapuco, Residents Of Norman, USA | - | Urban | Peru | Norman, Oklahoma, USA | Processed foods, bread, dairy products | 56 | Bifidobacterium spp. ↑, Ruminococcus spp. ↑, Blautia spp. ↑ | α ↓ | 1. Diet 2. Lifestyle | [182] |
| Rural | Matses, Tunapuco | Tubers, fish, game meat, rare dairy, processed foods | 23 | Spirochaetes, Proteobacteria | ||||||
| Hadza Hunter-Gatherers | January 2013–April 2013 | Urban | Italy | Bologna | Mediterranean diet | 16 | Bifidobacterium spp. ↑ | α ↓ | 1. Diet 2. Lifestyle 3. Environment | [38] |
| Rural | Tanzania | - | Wild foods | 27 | Firmicutes, Bacteroidetes |
| Species | Time | Area | Country | Region | Food | Sample No. | Character of Gut Microbiome | Reason | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Diversity | |||||||||
| Birds | ||||||||||
| Anser cygnoides | Poyang Lake: January 2015 | Urban | China | Poyang Lake | Stems of submerged macrophytes | 20 | Proteobacteria ↑, Clostridium spp. ↑, Lactobacilli ↑, Basidiomycota ↑ | α ↓ | 1. Environment 2. Human activities | [161] |
| Khukh Lake: August 2014 | Natural | Mongolia | Khuvsgul Lake | 20 | Turicibacter spp., Solibacillus spp., Ascomycota | |||||
| Zonotrichia leucophrys | 2021 | Urban | USA | California | Diets similar to humans | 87 | Similar to humans (Bacteroides spp. ↑) | α ↓ | 1. Bacterial spillover 2. Diet convergence 3. Environmental pressures | [28] |
| Natural | Natural foods | Differ significantly from humans | ||||||||
| Geospiza fuliginosa | February 2018–May 2019 | Urban | Ecuador | Provincia De Galápagos | More diverse diet, processed foods | 44 | Firmicutes spp. ↑, Arthromitus spp. ↑ | α ↓ | 1. Diet 2. Urban physiological stress 3. Vertical transmission | [150] |
| Natural | Insects | 14 | Peptostreptococcus spp. ↑, Klebsiella spp., Erysipelatoclostridium spp. ↑. | |||||||
| Leptoptilos crumeniferus | September 2019 | Urban | Uganda | Kampala | City garbage | 80 | Lactobacilli ↑ | α ↑ | 1. Diet 2. Environmental adaptation | [60] |
| Natural | Pig waste | 20 | Peptostreptococcus spp. | |||||||
| Parus major | May 2018–July 2018 | Urban | Poland. | Warsaw | Anthropogenic food | 76 | Enterobacteriaceae ↑ | α ↓ | 1. Reduced tree cover density 2. Sound pollution 3. Distance to city center | [183] |
| Natural | Natural foods | - | Catellicoccus spp., Microbacteriaceae, Pseudonocardiaceae, Carnobacteriaceae, Sphingomonadaceae | |||||||
| Eudocimus albus | October 2015–March 2017 | Urban | USA | Florida | Human-provided food | 82 | Proteobacteria ↑, Bacteroidetes ↑. | α ↓ | 1. Habitat 2. Diet 3. Environment | [74] |
| Natural | Natural foods | Firmicutes, Cyanobacteria. | ||||||||
| Passer domesticus | October 2016–December 2016 | Urban | USA | Arizona | Human-derived food waste | 7 | Proteobacteria ↑, Pseudomonadales ↑, Pseudomonas ↑ | α ↓ | 1. Diet 2. Physiological adjustment 3. Microbiota shift 4. Environment | [73] |
| Natural | Human-produced grains | 13 | Proteobacteria, Pseudomonadales, Pseudomonas | |||||||
| Passer montanus | November 2021–January 2022 | Urban | China. | Hubei | Human food residues | 10 | Proteobacteria ↑ | α ↑ | 1. Diet variations 2. Environmental exposures 3. Microbiota adaptation | [59] |
| Natural | Natural foods | 10 | Firmicutes | |||||||
| Non-human primates | ||||||||||
| Microcebus griseorufus | 2013–2015 | Urban | Madagascar | Miarintsoa | - | 47 | Veillonellaceae ↑ | α ↓ | 1. Diet shifts 2. Habitat 3. Fragmentation, human–livestock contact | [36] |
| Natural | Andranovao | Gum, fruits, insects. | 113 | Bacteroidaceae, Verrucomicrobia | ||||||
| Carnivore | ||||||||||
| Canis lupus | February 2023–November 2024 | Urban | USA | Tennessee | - | 211 | Fusobacteria ↑ | α ↓ | 1. Management 2. Environment 3. Hygiene | [184] |
| Natural | 135 | Ancylostoma spp. | ||||||||
| Canis latrans | 2017–2018 2018–2019 winter | Urban | Canada | Alberta | Anthropogenic food | 30 | Streptococcus spp. ↑, Enterococcus spp. ↑ | α ↓ | 1. Diet 2. Immune system stress 3. Activity range | [35] |
| Natural | Natural prey, fruits | 65 | Fusobacteria, Sutterella spp., Anaerobiospirillum spp. | |||||||
| Reptile | ||||||||||
| Bradypodion melanocephalum Bradypodion thamnobates Bradypodion setaroi | - | Urban | South Africa | Ethekwini, Stlucia, Howick | Insectivorous animal | 10 | Firmicutes ↑, Desulfovibrionaceae ↑, Ruminococcaceae, Christensenellaceae ↑ | ND | 1. Similarities between urban and natural habitat vegetation | [10] |
| Natural | 10 | Proteobacteria, Bacteroidota | ||||||||
| Rural: Podarcis bocagei, podarcis lusitanicus Urban: Podarcis siculus, podarcis virescens | September 2020 | Urban | Portugal | Lisbon | Hemiptera, coleoptera, Diptera, Hymenoptera, and Araneae | 41 | Odoribacter spp. ↑, Corynebacterium spp. ↑ | α ↑ | 1. Species influenced gut bacterial community structure only in lizards from the urbanized environment | [61] |
| Natural | Moledo | 61 | Corynebacterium spp. | |||||||
| Urban: Anolis cristatellus, Rural: Anolis cristatellus | July 2019 | Urban | USA, Canada | Edmonton, San Francisco, Mayagüez | Podarcis virescens: more versatile diet, fruits and nectar, class Arachnida, and orders Hymenoptera, Hemiptera, Coleoptera, and Diptera Teira dugesii: insects, small fruits | 127 | Bacteroides spp. ↑, Firmicutes/Bacteroidetes ratio ↑ | α ↓ | 1. Acquire GM associated with urban humans 2. Convergence of urban GM | [28] |
| Natural | Maricao, Quemado, Leduc | Bacteroides spp. | ||||||||
| Intellagama lesueurii | November 2014–April 2015 | Urban | Australia | Queensland | Insects, native vegetation, and small reptiles | 41 | Ruminococcus spp. ↑, Lactobacillus spp. ↑ | α ↑ | 1. Diverse diet 2. Higher in fat | [21] |
| Natural | 25 | Blautia spp. ↑, Citrobacter spp. ↑ | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Huang, M.; Sun, X.; Ling, W.; Hao, X.; Huang, G.; Wu, X.; Chen, Z.; Tang, X. Double-Edged Sword: Urbanization and Response of Amniote Gut Microbiome in the Anthropocene. Microorganisms 2025, 13, 1736. https://doi.org/10.3390/microorganisms13081736
Peng Y, Huang M, Sun X, Ling W, Hao X, Huang G, Wu X, Chen Z, Tang X. Double-Edged Sword: Urbanization and Response of Amniote Gut Microbiome in the Anthropocene. Microorganisms. 2025; 13(8):1736. https://doi.org/10.3390/microorganisms13081736
Chicago/Turabian StylePeng, Yi, Mengyuan Huang, Xiaoli Sun, Wenqing Ling, Xiaoye Hao, Guangping Huang, Xiangdong Wu, Zheng Chen, and Xiaoli Tang. 2025. "Double-Edged Sword: Urbanization and Response of Amniote Gut Microbiome in the Anthropocene" Microorganisms 13, no. 8: 1736. https://doi.org/10.3390/microorganisms13081736
APA StylePeng, Y., Huang, M., Sun, X., Ling, W., Hao, X., Huang, G., Wu, X., Chen, Z., & Tang, X. (2025). Double-Edged Sword: Urbanization and Response of Amniote Gut Microbiome in the Anthropocene. Microorganisms, 13(8), 1736. https://doi.org/10.3390/microorganisms13081736


_Di_Marco.png)



