Comprehensive Assessment of Health Risks Associated with Gram-Negative Bacterial Contamination on Healthcare Personnel Gowns in Clinical Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Temporal–Spatial Location, Study Population
2.2. Quantification of Gram-Negative Bacteria on Healthcare Personnel Gowns
2.3. Bacterial Identification by Mass Spectrometry MALDI-TOF
2.4. Antimicrobial Resistance Profiles
2.5. Carbapenemases and BLEE Production and Their Relationship with Genotypes
2.6. Screening to Detect Resistance Genes
2.7. Molecular Typing of Pantoea eucrina by ERIC-PCR
2.8. Risk Assessment by Using a Vester Matrix
3. Results
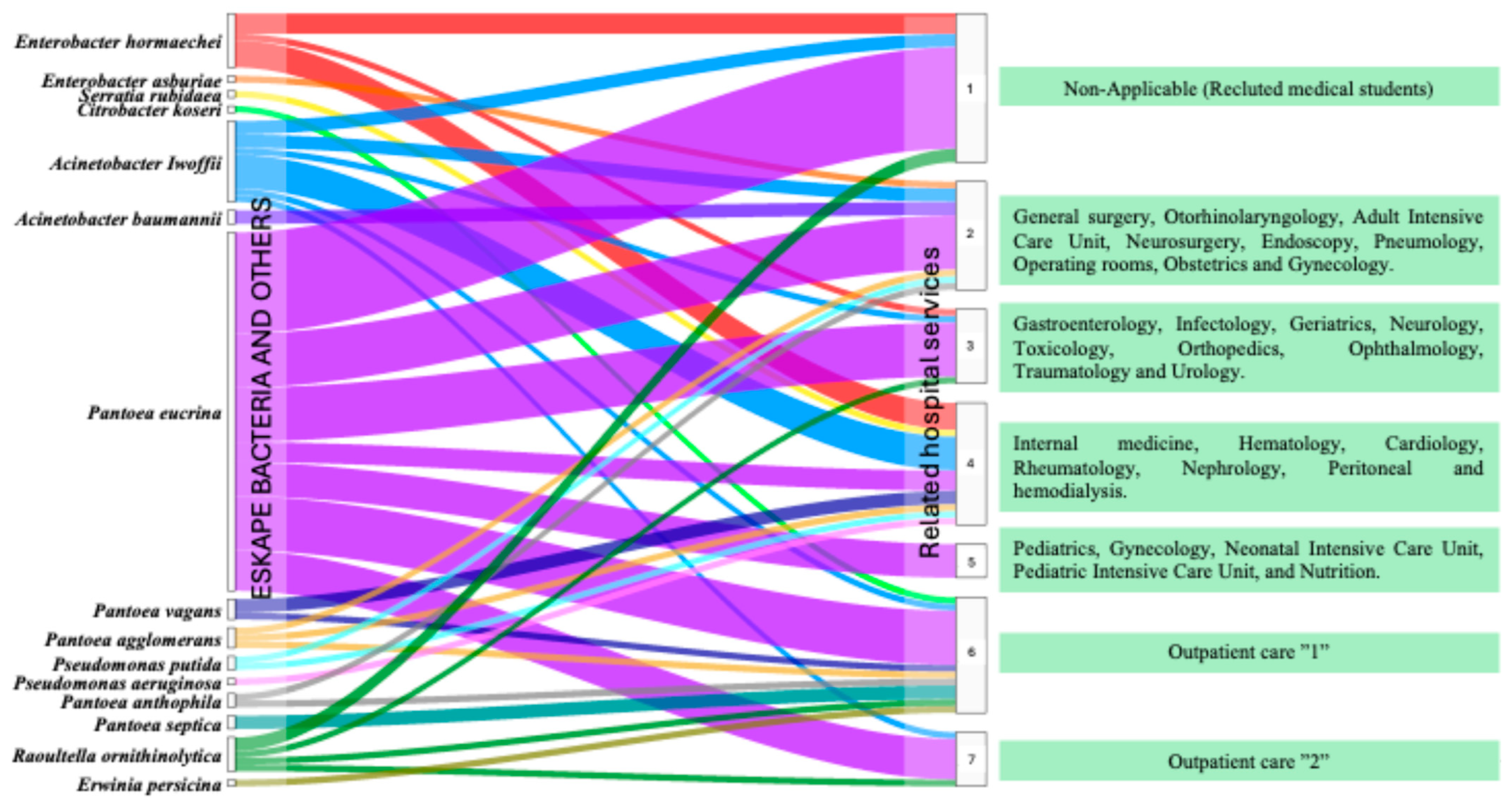
3.1. Study Population and Related Hospital Services
3.2. Analysis of Microbiological Contamination According to Sex
3.3. Comparison of Microbiological Contamination of Gowns by Hospital Care Area
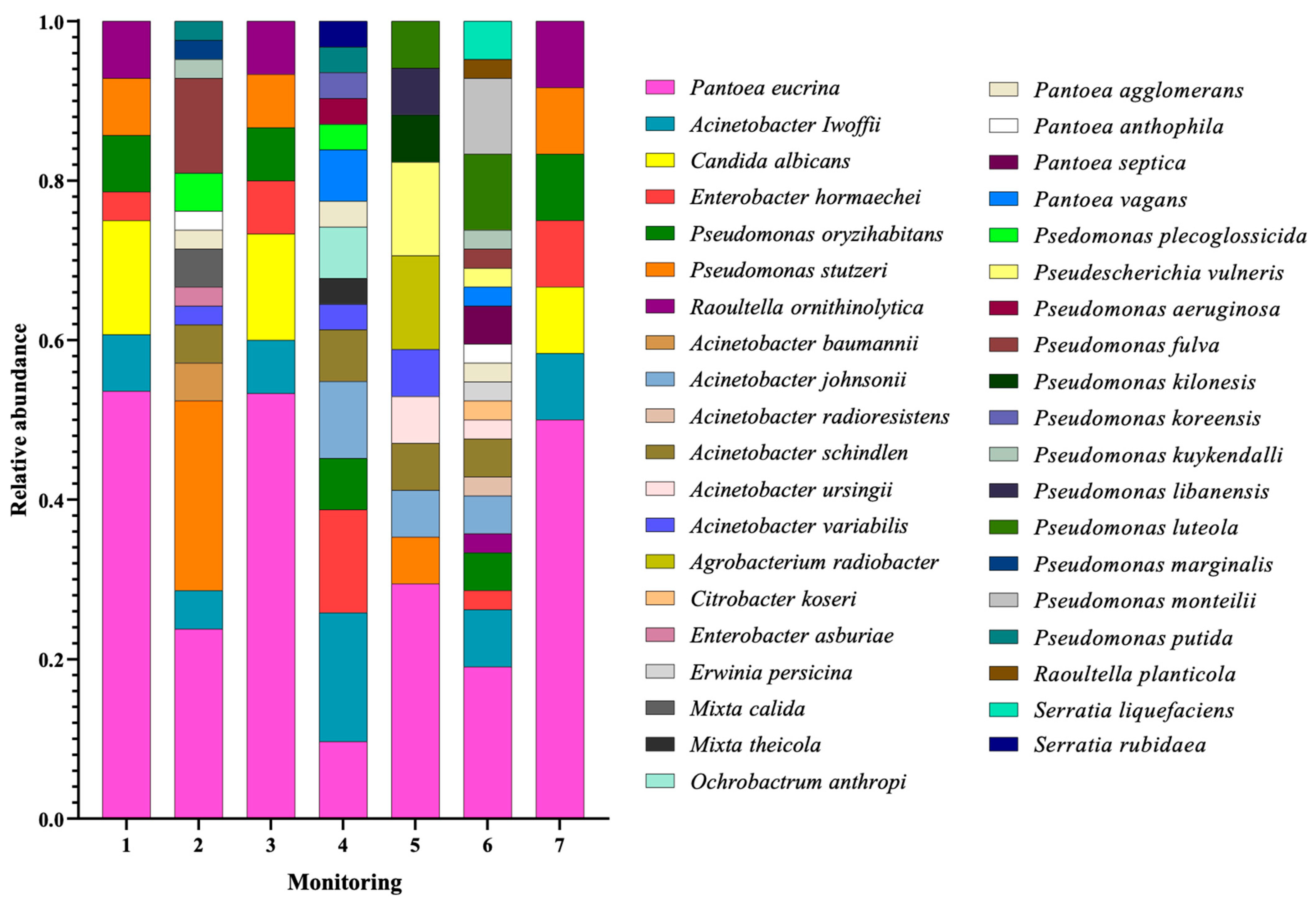
3.4. Taxonomic Characterization of Microorganisms Isolated from Healthcare Gowns
3.5. Flow of ESKAPE Bacteria on Healthcare Staff Gowns by Hospital Service
3.6. Resistance Phenotypes in Isolates from HJM Healthcare Staff Gowns
3.7. Resistance to Cephalosporins and Carbapenemics in ESKAPE Bacteria and Others
3.8. Analysis of Intergenic Consensus Reveals a Broad Diversity of P. eucrina
3.9. Identification of Problems Associated with Microbiological Contamination of Gowns
4. Discussion
| Genus | Species | Healthcare-Associated Infections | Reference |
|---|---|---|---|
| Pantoea | P. eucrina | Catheter-related bloodstream infection | [25] |
| Catheter-related bacteraemia | |||
| Sepsis | |||
| P. agglomerans | Sepsis | [43] | |
| Catheter-related bacteraemia | [44] | ||
| Ventilator-associated pneumonia | [45] | ||
| Urinary tract infection | [43] | ||
| Bloodstream infection | [46] | ||
| Soft tissue infection | [43] | ||
| P. anthophila | Bloodstream infection | [47] | |
| Urinary tract infection | [48] | ||
| P. vagans | Not reported | Non-applicable | |
| P. septica | Bloodstream infection | [49] | |
| Acinetobacter | A. baumannii | Ventilator-associated pneumonia | [50] |
| Infection of the skin and soft tissues | |||
| Bacteraemia | |||
| Catheter-related urinary tract infection | [51] | ||
| Bloodstream Infection | |||
| A. Iwoffii | Catheter-associated bacteraemia | [52] | |
| Sepsis | [53] | ||
| Pneumonia | |||
| Urinary tract infection | |||
| Skin and wound infections | |||
| Pseudomonas | P. putida | Urinary tract infection | [54] |
| Ventilator-associated pneumonia | [55] | ||
| Bacteraemia | [56] | ||
| Bloodstream infection | [57] | ||
| Urinary tract infection | [58] | ||
| Pneumonia | |||
| Sepsis | |||
| Wound infection | |||
| P. aeruginosa | Ventilator-associated pneumonia | [50] | |
| Wound infection | [59] | ||
| Bacteraemia | |||
| Catheter-related urinary tract infection | [60] | ||
| Bloodstream infection | |||
| Sepsis | [61] | ||
| Enterobacter | E. asburiae | Ventilator-associated pneumonia | [62] |
| Urinary tract infection | |||
| Sepsis | |||
| Bacteraemia | [63] | ||
| Infection of the skin and soft tissues | |||
| E. hormaechei | Bacteraemia | [64] | |
| Ventilator-associated pneumonia | |||
| Urinary tract infection | |||
| Sepsis | [65] | ||
| Bloodstream infection | [66] | ||
| Raoultella | R. ornithinolytica | Urinary tract infection | [67] |
| Infection of the skin and soft tissues | |||
| Bacteraemia | |||
| Ochrobactrum | O. anthropi | Bacteraemia | [68] |
| Ventilator-associated pneumonia | |||
| Sepsis | |||
| Bloodstream Infection | [69] | ||
| Serratia | S. Rubidaea | Bacteraemia | [70] |
| Sepsis | [71] | ||
| Citrobacter | C. koseri | Bacteraemia | [72] |
| Erwinia | E. persicina | Not reported | Non-applicable |
| Pseudomonas | P. stutzeri | Bacteraemia | [73] |
| Bloodstream infection | |||
| Sepsis | [74] | ||
| P. fulva | Bloodstream infection | [57] | |
| Bacteraemia | |||
| P. marginalis | Not reported | Non-applicable | |
| P. kuykendall | Not reported | Non-applicable | |
| P. oryzihabitans | Catheter-associated bacteraemia | [75] | |
| Wound infection | |||
| Ventilator-associated pneumonia | [76] | ||
| Sepsis | [23] | ||
| P. koreensis | Not reported | Non-applicable | |
| P. luteola | Ventilator-associated pneumonia | [77] | |
| Urinary tract infection | [78] | ||
| Bacteraemia | [79,80] | ||
| Bloodstream infection | [81] | ||
| P. libanensis | Not reported | Non-applicable | |
| P. kilonesis | Not reported | Non-applicable | |
| P. monteilli | Bacteraemia | [82] | |
| Sepsis | |||
| Acinetobacter | A. schindleri | Bacteraemia | [83] |
| A. variabilis | Not reported | Non-applicable | |
| A. johnsonii | Bloodstream infection | [84] | |
| A. ursingii | Catheter-related bloodstream infection | [85] | |
| Bacteraemia | [86] | ||
| Sepsis | [87] | ||
| A. radioresistens | Bacteraemia | [88] | |
| Pneumonia | |||
| Mixta | M. calida | Bacteraemia | [89] |
| Sepsis | |||
| M. theicola | Not reported | Non-applicable | |
| Raoultella | R. planticola | Nosocomial pneumonia | [90] |
| Surgical site infection | |||
| Bloodstream infection | [91] | ||
| Agrobacterium | A. radiobacter | Sepsis | [92] |
| Pseudoescherichia | P. vulneris | Not reported | Non-applicable |
| Serratia | S. liquefaciens | Bloodstream infection | [93] |
| Urinary tract infection | [94] | ||
| Candida | C. albicans | Catheter-related urinary tract infection | [61] |
| Bloodstream infection | |||
| Ventilator-associated pneumonia |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gjerdingen, D.K.; Simpson, D.E.; Titus, S.L. Patients’ and physicians’ attitudes regarding the physician’s professional appearance. Arch. Intern. Med. 1987, 147, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.; Dornelles, A.C.; Hayek, G.; Deichmann, R.E. Patient Preferences for Doctor Attire: The White Coat’s Place in the Medical Profession. Ochsner J. 2013, 13, 334–342. [Google Scholar] [PubMed] [PubMed Central]
- Kazory, A. Physicians, their appearance, and the white coat. Am. J. Med. 2008, 121, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.; Phillips, K.; Pocotte, S.; Lee, C. The creation of a White Coat Ceremony. J. Prof. Nurs. 2020, 36, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Worboys, M. Joseph Lister and the performance of antiseptic surgery. Notes Rec. R Soc. Lond. 2013, 67, 199–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michaleas, S.N.; Laios, K.; Charalabopoulos, A.; Samonis, G.; Karamanou, M. Joseph Lister (1827–1912): A Pioneer of Antiseptic Surgery. Cureus 2022, 14, e32777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Treakle, A.M.; Thom, K.A.; Furuno, J.P.; Strauss, S.M.; Harris, A.D.; Perencevich, E.N. Bacterial contamination of health care workers’ white coats. Am. J. Infect. Control 2009, 37, 101–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishra, S.K.; Maharjan, S.; Yadav, S.K.; Sah, N.P.; Sharma, S.; Parajuli, K.; Sherchand, J.B. Bacteria on Medical Professionals’ White Coats in a University Hospital. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 5957284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsagkaris, C.; Rueger, M.; Tschudi, S.B.; Dreher, T. White Coats at a Crossroads: Hygiene, Infection Risk, and Patient Trust in Healthcare Attire-An Umbrella Review with Quantitative Synthesis and Stress, Weaknesses, Opportunities, and Threats Analysis. Microorganisms 2024, 12, 2659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uneke, C.J.; Ijeoma, P.A. The potential for nosocomial infection transmission by white coats used by physicians in Nigeria: Implications for improved patient-safety initiatives. World Health Popul. 2010, 11, 44–54. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; (WHO Reference Number: WHO/EMP/IAU/2017.12); World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- De Rosa, F.G.; Corcione, S.; Pagani, N.; Di Perri, G. From ESKAPE to ESCAPE, from KPC to CCC. Clin. Infect. Dis. 2015, 60, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Albericio, F. Antibiotic Resistance: From the Bench to Patients. Antibiotics 2019, 8, 129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Botelho, J.; Cazares, A.; Schulenburg, H. The ESKAPE mobilome contributes to the spread of antimicrobial resistance and CRISPR-mediated conflict between mobile genetic elements. Nucleic Acids Res. 2023, 51, 236–252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rock, C.; Thom, K.A.; Masnick, M.; Johnson, J.K.; Harris, A.D.; Morgan, D.J. Frequency of Klebsiella pneumoniae carbapenemase (KPC)-producing and non-KPC-producing Klebsiella species contamination of healthcare workers and the environment. Infect. Control Hosp. Epidemiol. 2014, 35, 426–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Munoz-Price, L.S.; Arheart, K.L.; Mills, J.P.; Cleary, T.; Depascale, D.; Jimenez, A.; Fajardo-Aquino, Y.; Coro, G.; Birnbach, D.J.; Lubarsky, D.A. Associations between bacterial contamination of health care workers’ hands and contamination of white coats and scrubs. Am. J. Infect. Control 2012, 40, e245–e248. [Google Scholar] [CrossRef] [PubMed]
- Durán-Manuel, E.M.; Cruz-Cruz, C.; Ibáñez-Cervantes, G.; Bravata-Alcantará, J.C.; Sosa-Hernández, O.; Delgado-Balbuena, L.; León-García, G.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Castro-Escarpulli, G.; et al. Clonal dispersion of Acinetobacter baumannii in an intensive care unit designed to patients COVID-19. J. Infect. Dev. Ctries. 2021, 15, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ortíz, I.A.; Juárez-Gómez, J.C.; Cu-Quijano, C.; Flores-Paz, R.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Gutiérrez-Muñoz, V.H.; Sosa-Hernández, O.; Escobar-Escamilla, N.; Bravata-Alcántara, J.C.; et al. Klebsiella pneumoniae blaNDM-1 carrying a class 1 integron causing a hospital outbreak in a Mexican attention center. J. Infect. Dev. Ctries. 2021, 15, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Loyola-Cruz, M.Á.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Márquez-Valdelamar, L.M.; Bravata-Alcántara, J.C.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Ibáñez-Cervantes, G.; Fernández-Sánchez, V.; Castro-Escarpulli, G.; et al. ESKAPE bacteria characterization reveals the presence of Acinetobacter baumannii and Pseudomonas aeruginosa outbreaks in COVID-19/VAP patients. Am. J. Infect. Control 2023, 51, 729–737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durán-Manuel, E.M.; Loyola-Cruz, M.Á.; Cruz-Cruz, C.; Ibáñez-Cervantes, G.; Gaytán-Cervantes, J.; González-Torres, C.; Quiroga-Vargas, E.; Calzada-Mendoza, C.C.; Cureño-Díaz, M.A.; Fernández-Sánchez, V.; et al. Massive sequencing of the V3–V4 hypervariable region of bronchoalveolar lavage from patients with COVID-19 and VAP reveals the collapse of the pulmonary microbiota. J. Med. Microbiol. 2022, 71. [Google Scholar] [CrossRef] [PubMed]
- Quintero, R.; David, J.; Chavarriaga-Restrepo, A. Bacteriemia por Raoultella planticola de origen gastrointestinal. Iatreia 2017, 30, 67–71. [Google Scholar] [CrossRef][Green Version]
- Owusu, M.; Owusu-Dabo, E.; Acheampong, G.; Osei, I.; Amuasi, J.; Sarpong, N.; Annan, A.; Chiang, H.Y.; Kuo, C.H.; Park, S.E.; et al. Pseudomonas oryzihabitans sepsis in a 1-year-old child with multiple skin rashes: A case report. J. Med. Case Rep. 2017, 11, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adefila, W.O.; Osei, I.; Lamin, K.M.; Wutor, B.M.; Olawale, Y.A.; Molfa, M.; Barjo, O.; Omotosho, M.; Salaudeen, R.; Mackenzie, G. Ochrobactrum anthropi sepsis in a 15-month-old child: A case report. Clin. Case Rep. 2024, 12, e9042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kølle, I.S.; Vinter-Jensen, L.; Nielsen, M.E.; Nielsen, H.L. Pantoea eucrina: Catheter-Related Bloodstream Infection in a Woman With Short Bowel Syndrome. Clin. Case Rep. 2025, 13, e70103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. Assoc. Comput. Mach. 2017, 28, 1–5. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI Suppl. M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Pierce, V.M.; Simner, P.J.; Lonsway, D.R.; Roe-Carpenter, D.E.; Johnson, J.K.; Brasso, W.B.; Bobenchik, A.M.; Lockett, Z.C.; Charnot-Katsikas, A.; Ferraro, M.J.; et al. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among enterobacteriaceae. J. Clin. Microbiol. 2017, 55, 2321–2333. [Google Scholar] [CrossRef]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L.; Carrër, A.; Toleman, M.; Walsh, T. How to detect NDM-1 producers. J. Clin. Microbiol. 2011, 49, 718–721. [Google Scholar] [CrossRef]
- Versalovic, J.; Koeuth, T.; Lupski, J.R. Distribution of repetitive DNA sequences in eubacteria and. Application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef]
- Cole, A. The Influence Matrix Methodology: A Technical Report. Landcare Research Contract Report. New Zealand. 2006. Available online: https://icm.landcareresearch.co.nz/knowledgebase/publications/public/imatrix_tech_report.pdf (accessed on 11 January 2025).
- Guillamet, M.C.V.; Vazquez, R.; Deaton, B.; Shroba, J.; Vazquez, L.; Mercier, R.C. Host-Pathogen-Treatment Triad: Host Factors Matter Most in Methicillin-Resistant Staphylococcus aureus Bacteremia Outcomes. Antimicrob. Agents Chemother. 2018, 62, e01902–e01917. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durán-Manuel, E.M.; Fiscal-Baxin, E.; Nolasco-Rojas, A.E.; Loyola-Cruz, M.Á.; Cruz-Cruz, C.; Paredes-Mendoza, M.; López-Ornelas, A.; Razo Blanco-Hernández, D.M.; Nieto-Velázquez, N.G.; Rodríguez-Tovar, A.V.; et al. Seasonal Characterization of the Aerobiome in Hematopoietic Stem Cell Transplant Rooms: Potential Risk for Immunosuppressed Patients. Microorganisms 2024, 12, 2352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cureño-Díaz, M.A.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Ibáñez-Cervantes, G.; Rojo-Gutiérrez, M.I.; Moncayo-Coello, C.V.; Loyola-Cruz, M.Á.; Castro-Escarpulli, G.; Hernández, D.M.R.; Bello-López, J.M. Impact of the modification of a cleaning and disinfection method of mechanical ventilators of COVID-19 patients and ventilator-associated pneumonia: One year of experience. Am. J. Infect. Control 2021, 49, 1474–1480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, A.P.; Chougale, R.A.; Sinduri, I. Bacterial Contamination of White Coats among Medical Personnel—A Cross Sectional Study in Kolhapur, India. J. Pure Appl. Microbiol. 2020, 14, 1405–1411. [Google Scholar] [CrossRef]
- Banu, A.; Anand, M.; Nagi, N. White coats as a vehicle for bacterial dissemination. J. Clin. Diagn. Res. 2012, 6, 1381–1384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akanbi, A.A.A.; Kareem, T.; Adedoja, A.; Nyamngee, A.; Muhammed, M.B.U.; Abdulkareem, K.; Atata, R.F. Bacterial contamination of medical doctors’ white coats as contributing factor to hospital acquired infections. Int. J. Biol. Chem. Sci. 2017, 11, 185–194. [Google Scholar] [CrossRef]
- Muhadi, S.A.; Aznamshah, N.A.; Jahanfar, S. A cross sectional study of microbial contamination of medical students’ white coat. Malays. J. Microbiol. 2007, 3, 35–38. [Google Scholar]
- Sosa-Hernández, O.; Matías-Téllez, B.; Estrada-Hernández, A.; Cureño-Díaz, M.A.; Bello-López, J.M. Incidence and costs of ventilator-associated pneumonia in the adult intensive care unit of a tertiary referral hospital in Mexico. Am. J. Infect. Control 2019, 47, e21–e25. [Google Scholar] [CrossRef] [PubMed]
- Mirtella, D.; Fedeli, P.; Scendoni, R.; Cannovo, N.; Cingolani, M. A Case of Nosocomial Outbreak of Pantoea agglomerans Related to Parenteral Nutrition Procedures. Healthcare 2021, 9, 684. [Google Scholar] [CrossRef]
- Mallick, A.; Sarkar, S.; Lopes, B.S.; Das, S. Drug-resistant Pantoea agglomerans Causing Bacteremia at a Tertiary Care Hospital in Kolkata, India: First Report of Carbapenem Resistance Mediated by OXA-181. Curr. Microbiol. 2024, 81, 389. [Google Scholar] [CrossRef]
- Kurşun, O.; Unal, N.; Cesur, S.; Altın, N.; Canbakan, B.; Argun, C.; Koldaş, K.; Sencan, I. A case of ventilator-associated pneumonia due to Pantoea agglomerans. Mikrobiyoloji Bul. 2012, 46, 295–298. [Google Scholar]
- Yablon, B.R.; Dantes, R.; Tsai, V.; Lim, R.; Moulton-Meissner, H.; Arduino, M.; Jensen, B.; Patel, M.T.; Vernon, M.O.; Grant-Greene, Y.; et al. Outbreak of Pantoea agglomerans Bloodstream Infections at an Oncology Clinic—Illinois, 2012–2013. Infect. Control Hosp. Epidemiol. 2016, 38, 314–319. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Y.; Hu, L. Hemodialysis Catheter-Related Bloodstream Infection Caused by Pantoea: Report of Two Cases and Literature Review. Infect. Drug Resist. 2024, 17, 4167–4173. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Y.; Zhan, Y.; Wang, H.; Li, X.; Wang, H.; Feng, T.; Shi, L.; Wang, J.; Wang, H.; et al. Genomic characterization of Pantoea anthophila strain UI705 causing urinary tract infections in China. Front. Cell. Infect. Microbiol. 2023, 13, 1208473. [Google Scholar] [CrossRef]
- Casale, R.; Boattini, M.; Bianco, G.; Comini, S.; Corcione, S.; Garazzino, S.; Silvestro, E.; De Rosa, F.G.; Cavallo, R.; Costa, C. Bloodstream Infections by Pantoea Species: Clinical and Microbiological Findings from a Retrospective Study, Italy, 2018–2023. Antibiotics 2023, 12, 1723. [Google Scholar] [CrossRef]
- Loyola-Cruz, M.Á.; Gonzalez-Avila, L.U.; Martínez-Trejo, A.; Saldaña-Padilla, A.; Hernández-Cortez, C.; Bello-López, J.M.; Castro-Escarpulli, G. ESKAPE and Beyond: The Burden of Coinfections in the COVID-19 Pandemic. Pathogens 2023, 12, 743. [Google Scholar] [CrossRef]
- Michalopoulos, A.; Falagas, M.E. Treatment of Acinetobacter infections. Expert Opin. Pharmacother. 2010, 11, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Tega, L.; Raieta, K.; Ottaviani, D.; Russo, G.L.; Blanco, G.; Carraturo, A. Catheter-related Bacteremia and Multidrug-resistant Acinetobacter lwoffii. Emerg. Infect. Dis. 2007, 13, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Regalado, N.G.; Martin, G.; Antony, S.J. Acinetobacter lwoffii: Bacteremia associated with acute gastroenteritis. Travel Med. Infect. Dis. 2009, 7, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, G.; Luzzaro, F.; Docquier, J.; Riccio, M.L.; Perilli, M.; Coli, A.; Amicosante, G.; Rossolini, G.M.; Toniolo, A. Nosocomial Infections Caused by Multidrug-Resistant Isolates of Pseudomonas putida Producing VIM-1 Metallo-β-Lactamase. J. Clin. Microbiol. 2002, 40, 4051–4055. [Google Scholar] [CrossRef]
- Fernández, M.; Porcel, M.; De la Torre, J.; Molina-Henares, M.A.; Daddaoua, A.; Llamas, M.A.; Roca, A.; Carriel, V.; Garzón, I.; Ramos, J.L.; et al. Analysis of the pathogenic potential of nosocomial Pseudomonas putida strains. Front. Microbiol. 2015, 6, 871. [Google Scholar] [CrossRef]
- Kim, S.E.; Park, S.; Park, H.B.; Park, K.; Kim, S.; Jung, S.; Shin, J.; Jang, H.; Kang, S.J. Nosocomial Pseudomonas putida Bacteremia: High Rates of Carbapenem Resistance and Mortality. Chonnam Med. J. 2012, 48, 91. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, K.; Yu, X.; Li, B.; Cao, B. Identification and control of a Pseudomonas spp. (P. fulva and P. putida) bloodstream infection outbreak in a teaching hospital in Beijing, China. Int. J. Infect. Dis. 2014, 23, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.A.G.; Sampedro, M.M.; Macazaga, J.A.C.; Orbáiz, C.G. Infección nosohusial por Pseudomonas putida. An. De Med. Interna 2005, 22, 201–202. [Google Scholar] [CrossRef][Green Version]
- Juan, C.; Peña, C.; Oliver, A. Host and Pathogen Biomarkers for Severe Pseudomonas aeruginosa Infections. J. Infect. Dis. 2017, 215 (Suppl. 1), S44–S51. [Google Scholar] [CrossRef]
- Pierce, G.E. Pseudomonas aeruginosa, Candida albicans, and device-related nosocomial infections: Implications, trends, and potential approaches for control. J. Ind. Microbiol. Biotechnol. 2005, 32, 309–318. [Google Scholar] [CrossRef]
- Balandin, B.; Ballesteros, D.; De Luna, R.R.; López-Vergara, L.; Pintado, V.; Sancho-González, M.; Soriano-Cuesta, C.; Pérez-Pedrero, M.J.; Asensio-Martín, M.J.; Fernández-Simón, I.; et al. Multicenter study of ceftolozane/tazobactam for treatment of Pseudomonas aeruginosa infections in critically ill patients. Int. J. Antimicrob. Agents 2021, 57, 106270. [Google Scholar] [CrossRef]
- Mattioni Marchetti, V.; Kuka, A.; Piazza, A.; Gaiarsa, S.; Merla, C.; Sottosanti, M.; Cambieri, P.; Migliavacca, R.; Baldanti, F. Enterobacter asburiae ST229: An emerging carbapenemases producer. Sci. Rep. 2024, 14, 6220. [Google Scholar] [CrossRef]
- Horinouchi, N.; Shiota, S.; Takakura, T.; Yoshida, A.; Kikuchi, K.; Nishizono, A.; Miyazaki, E. Bacteremia caused by Enterobacter asburiae misidentified biochemically as Cronobacter sakazakii and accurately identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: A case report. J. Med. Case Rep. 2022, 16, 19. [Google Scholar] [CrossRef]
- Silva, F.; Martínez, O.P. Complejo Enterobacter cloacae. Rev. Chil. De Infectol. Organo Off. De La Soc. Chil. De Infectol. 2018, 35, 297–298. [Google Scholar] [CrossRef]
- Townsend, S.M.; Hurrell, E.; Caubilla-Barron, J.; Loc-Carrillo, C.; Forsythe, S.J. Characterization of an extended-spectrum beta-lactamase Enterobacter hormaechei nosocomial outbreak, and other Enterobacter hormaechei misidentified as Cronobacter (Enterobacter) sakazakii. Microbiology 2008, 154, 3659–3667. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.L.P.; Miranda, L.E.V.; Moreira, B.M.; Rebello, D.; Carson, L.; Kellum, M.; de Almeida, M.C.L.; Sampaio, J.L.M.; O’Hara, C.M. Infección sanguínea por Enterobacter hormaechei en tres unidades de cuidados intensivos neonatales de Brasil. Rev. De Enfermedades Infecc. Pediátricas 2002, 21, 175–177. [Google Scholar]
- Seng, P.; Boushab, B.M.; Romain, F.; Gouriet, F.; Bruder, N.; Martin, C.; Paganelli, F.; Bernit, E.; Treut, Y.P.L.; Thomas, P.; et al. Emerging role of Raoultella ornithinolytica in human infections: A series of cases and review of the literature. Int. J. Infect. Dis. 2016, 45, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Chao, Z.; Fayyaz, A.; Antony, S. Ochrobactrum Anthropi; an Unusual Cause of Bacteremia and Pneumonia: A Case Report and a Brief Review of the Literature. Infect. Disord. Drug Targets 2023, 24, 8–11. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, X.; Zhu, Q.; Zhang, Z.; Dai, Y.; Chen, L.; Liang, Z. Clinical characteristics of patients with Ochrobactrum anthropi bloodstream infection in a Chinese tertiary-care hospital: A 7-year study. J. Infect. Public Health 2018, 11, 873–877. [Google Scholar] [CrossRef]
- Gentille, D.; Pérez, M.; Centelles, M.J. Bacteriemia por Serratia rubidaea con fenotipo atípico de resistencia a quinolonas. Rev. Chil. De Infectología 2014, 31, 351–352. [Google Scholar] [CrossRef]
- Okada, T.; Yokota, E.; Matsumoto, I. Community Acquired Sepsis by Serratia rubidaea. Kansenshogaku Zasshi 2002, 76, 109–112. [Google Scholar] [CrossRef]
- Kanzawa, Y.; Ishimaru, N.; Seto, H.; Tsutsumi, T.; Kinami, S. Liver abscess with Citrobacter koseri bacteremia. Le Infez. Med. Riv. Period. Di Eziologia Epidemiol. Diagn. Clin. E Ter. Delle Patol. Infett. 2018, 26, 266–269. [Google Scholar]
- Horcajada, J.P.; Edwards, F.; Fonio, S.; Montero, M.; Harris, P.; Paterson, D.L.; Laupland, K.B. Pseudomonas stutzeri bloodstream infection is a prevailing community-onset disease with important mortality rates: Results from a retrospective observational study in Australia. Infect. Dis. 2024, 56, 606–615. [Google Scholar] [CrossRef]
- Bello, C.S. Pseudomonas stutzeri: A rare cause of neonatal septicaemia. East. Mediterr. Health J. 2007, 13, 731–734. [Google Scholar] [PubMed]
- Silva, F. Pseudomonas (Flavimonas) oryzihabitans. Rev. Chil. De Infectología 2015, 32, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Bustos, D.C.; Hernández Marín, A.M.; Corrales Ramírez, L.C. Pseudomonas oryzihabitans: Un microorganismo de creciente interés científico. Nova 2009, 7, 103–112. [Google Scholar] [CrossRef]
- Dharmayanti, A.; Astrawinata, D. Ventilator-associated pneumonia (VAP) in a patient with Guillain-Barre Syndrome. Acta Medica Indones. 2017, 49, 151–157. [Google Scholar]
- Ali, M.A.; Aljanaby, A.A.J. First case report of Pseudomonas Luteola isolated from urinary tract infection in Babylon City, Iraq. E3S Web Conf. 2023, 381, 01102. [Google Scholar] [CrossRef]
- Bayhan, G.I.; Senel, S.; Tanir, G.; Ozkan, S. Bacteremia Caused by Pseudomonas luteola in Pediatric Patients. Jpn. J. Infect. Dis. 2014, 68, 50–54. [Google Scholar] [CrossRef]
- Freney, J.; Hansen, W.; Etienne, J.; Vandenesch, F.; Fleurette, J. Postoperative infant septicemia caused by Pseudomonas luteola (CDC group Ve-1) and Pseudomonas oryzihabitans (CDC group Ve-2). J. Clin. Microbiol. 1998, 26, 1241–1243. [Google Scholar] [CrossRef]
- Wen, A.Y.; Weiss, I.K.; Kelly, R.B. Chryseomonas luteola bloodstream infection in a pediatric patient with pulmonary arterial hypertension receiving intravenous treprostinil therapy. Infection 2013, 41, 719–722. [Google Scholar] [CrossRef]
- Lee, D.; Jung, K.H.; Son, H.-J.; Moon, I.T. Pseudomonas monteilii bacteremia and sepsis following insertable cardiac monitor implantation: A case report. J. Med. Case Rep. 2024, 18, 480. [Google Scholar] [CrossRef]
- Montaña, S.; Palombarani, S.; Carulla, M.; Kunst, A.; Rodriguez, C.H.; Nastro, M.; Vay, C.; Ramirez, M.S.; Almuzara, M. First case of bacteraemia due to Acinetobacter schindleri harbouring blaNDM-1 in an immunocompromised patient. New Microbes New Infect. 2018, 21, 28–30. [Google Scholar] [CrossRef]
- Seifert, H.; Strate, A.; Schulze, A.; Pulverer, G. Vascular Catheter-Related Bloodstream Infection Due to Acinetobacter johnsonii (Formerly Acinetobacter calcoaceticus var lwoffii): Report of 13 Cases. Clin. Infect. Dis. 1993, 17, 632–636. [Google Scholar] [CrossRef]
- Daniel, A.M.; Garzón, D.; Vivas, A.; Viviana, T.M.; Cubides-Diaz, D.A.; Fabian, Y.M. Catheter-related bloodstream infection due to Acinetobacter ursingii in a hemodialysis patient: Case report and literature review. Pan Afr. Med. J. 2021, 39, 208. [Google Scholar]
- Yakut, N.; Kepenekli, E.K.; Karaaslan, A.; Atici, S.; Akkoc, G.; Demir, S.O.; Soysal, A.; Bakir, M. Bacteremia due to Acinetobacter ursingii in infants: Reports of two cases. Pan Afr. Med. J. 2016, 23, 193. [Google Scholar] [CrossRef]
- González-Carrera, E.; Rodríguez-Lorenzo, P.; Méndez-Sánchez, A.; De la Iglesia Martínez, P.; Pérez-Méndez, C. Acinetobacter ursingii: An Unusual Infectious Agent of Early-onset Neonatal Sepsis. Pediatr. Infect. Dis. J. 2024, 44, e179. [Google Scholar] [CrossRef]
- Motie, I.; Burns, K.; Thompson, R.; Friar, E.; Bermingham, I.; Ranasinghe, U.; Wiese-Rometsch, W. Acinetobacter radioresistens and Enterococcus casseliflavus co-infection with endocarditis, bacteremia, and pneumonia. IDCases 2022, 30, e01622. [Google Scholar] [CrossRef]
- Blairon, L.; Everaert, J.; Baillon, B.; Collignon, S.; Coenen, F.; Cupaiolo, R.; Beukinga, I.; Tré-Hardy, M. Mixta calida, not only an environmental bacterium, but a potential opportunistic threat: Case report of an osteitis with skin necrosis and mini review of the literature. New Microbes New Infect. 2024, 62, 101524. [Google Scholar] [CrossRef] [PubMed]
- Tufa, T.B.; Fuchs, A.; Feldt, T.; Galata, D.T.; Mackenzie, C.R.; Pfeffer, K.; Häussinger, D. CTX-M-9 group ESBL-producing Raoultella planticola nosocomial infection: First report from sub-Saharan Africa. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Demiray, T.; Koroglu, M.; Ozbek, A.; Altindis, M. A rare cause of infection, Raoultella planticola: Emerging threat and new reservoir for carbapenem resistance. Infection 2016, 44, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, R.; Nanetti, A.; Ferri, M.; Mastroianni, A.; Coronado, O.V.; Chiodo, F. Emerging gram-negative pathogens in the immunocompromised host: Agrobacterium radiobacter septicemia during HIV disease. New Microbiol. 1999, 22, 375–382. [Google Scholar]
- Grohskopf, L.A.; Roth, V.R.; Feikin, D.R.; Arduino, M.J.; Carson, L.A.; Tokars, J.I.; Holt, S.C.; Jensen, B.J.; Hoffman, R.E.; Jarvis, W.R. Serratia liquefaciens Bloodstream Infections from Contamination of Epoetin Alfa at a Hemodialysis Center. N. Engl. J. Med. 2001, 344, 1491–1497. [Google Scholar] [CrossRef]
- Serruys-Schoutens, E.; Rost, F.; Depré, G. A nosocomial epidemic of Serratia liquefaciens urinary tract infection after cystometry. Eur. J. Clin. Microbiol. 1984, 3, 316–317. [Google Scholar] [CrossRef]
- Sah, R.K.; Bhattarai, A.; Khadka, P.; Sharma, S.; Mishra, S.K.; Rai, J.R.; Raut, S. Current Antibiotic Resistance Profile of ESKAPE Pathogens in a Nepalese Hospital: A Cross-Sectional Study. Can. J. Infect. Dis. Med. Microbiol. 2025, 2025, 4426596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kazemi Najafabadi, M.; Alikiaei, B.; Khorvash, F.; Shafiee, F.; Soltani, R. The Effectiveness of Colistin/Rifampin Compared to Colistin/Meropenem in the Treatment of Ventilator-associated Pneumonia Caused by Carbapenem-resistant Acinetobacter baumannii: A Randomized Controlled Clinical Trial. J. Res. Pharm. Pract. 2025, 13, 65–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, W.R.; Arias, C.A. ESKAPE pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Merla, C.; Mileto, I.; Gaiarsa, S.; Achille, C.; Ghirardello, S.; Corbella, M.; Baldanti, F.; Cambieri, P. Surveillance in a Neonatal Intensive Care Unit Allowed the Isolation of a Strain of VIM-Producing Pantoea brenneri. Antibiotics 2023, 12, 98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, G.; Choudhary, D.; Stice, S.P.; Myers, B.K.; Gitaitis, R.D.; Venter, S.N.; Kvitko, B.H.; Dutta, B. Pan-Genome-Wide Analysis of Pantoea ananatis Identified Genes Linked to Pathogenicity in Onion. Front. Microbiol. 2021, 12, 684756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hortaç İştar, E.; Alışkan, H.E.; Göçmen, J.S. Diverse efficacy of CarbaNP test among OXA-48 carbapenemase producing Enterobacterales in an endemic region. Acta Microbiol. Immunol. Hung. 2021, 68, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Nolasco-Rojas, A.E.; Cruz-Del-Agua, E.; Cruz-Cruz, C.; Loyola-Cruz, M.Á.; Ayil-Gutiérrez, B.A.; Tamayo-Ordóñez, M.C.; Tamayo-Ordoñez, Y.d.J.; Rojas-Bernabé, A.; Tamayo-Ordoñez, F.A.; Durán-Manuel, E.M.; et al. Microbiological Risks to Health Associated with the Release of Antibiotic-Resistant Bacteria and β-Lactam Antibiotics Through Hospital Wastewater. Pathogens 2025, 14, 402. [Google Scholar] [CrossRef]
- BBC News. 16 December 2008. Doctor’s White Coat to be Banned. Available online: http://news.bbc.co.uk/1/hi/scotland/7784552.stm (accessed on 10 July 2025).
- Dancer, S.J. Pants, policies and paranoia. J. Hosp. Infect. 2010, 74, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.G.; Duerden, B.I. Changes to clinician attire have done more harm than good. J. R. Coll. Physicians Edinb. 2014, 44, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Reglamento de la Ley General de Salud en Materia de Investigación para la Salud. DOF, México. 1987. Available online: https://www.diputados.gob.mx/LeyesBiblio/regley/Reg_LGS_MIS.pdf (accessed on 20 May 2025).




| Primer | Molecular Target | Sequence (5′→3′) | Size (bp) | Reference |
|---|---|---|---|---|
| IMP-F | blaIMP | TTGACACTCCATTTACDG | 139 | [30] |
| IMP-R | GATYGAGAATTAAGCCACYCT | |||
| VIM-F | blaVIM | GATGGTGTTTGGTCGCATA | 390 | |
| VIM-R | CGAATGCGCAGCACCAG | |||
| KPC-F | blaKPC | CATTCAAGGGCTTTCTTGCTGC | 538 | |
| KPC-R | ACGACGGCATAGTCATTTGC | |||
| OXA-48F | blaOXA-48 | GCACTTCTTTTGTGATGGC | 281 | |
| OXA-48R | GAGCACTTCTTTTGTGATGGC | |||
| SHV-F | blaSHV | AGCCGCTTGAGCAAATTAAAC | 713 | |
| SHV-R | ATCCCGCAGATAAATCACCAC | |||
| CTX-M-1-F | blaCTX-1 | TTAGGAARTGTGCCGCTGYA | 688 | |
| CTX-M-1-R | CGATATCGTTGGTGGTRCCAT | |||
| NDM-F | blaNDM | GGTTTGGCGAT CTGGTTTTC | 621 | [31] |
| NDM-R | CGGAATGGCTCATCACGATC | |||
| ERIC1R | Intergenic consensus | ATGTAAGCTCCTGGGGATTCA | Variable | [32] |
| ERIC2 | AGTAAGTGACTGGGGTGAGC |
| Monitoring/Areas | Health Personnel n (%) | Sex n (%) | Total | ||||
|---|---|---|---|---|---|---|---|
| Resident Physicians | Medical Students | Assigned Physicians | Male | Female | |||
| 1 | NA | 0(0) | 91(100) | 0(0) | 41(28.7) | 50(28.1) | 45(28.3) |
| 2 | a | 11(45.8) | 13(54.2) | 0(0) | 13(9.1) | 11(6.2) | 24(7.50) |
| 3 | b | 31(64.6) | 14(29.2) | 3(6.3) | 21(14.7) | 27(15.2) | 48(15.0) |
| 4 | c | 20(66.7) | 10(33.3) | 0(0) | 18(12.6) | 12(6.7) | 30(9.30) |
| 5 | d | 28(59.6) | 18(38.3) | 1(2.1) | 13(9.1) | 34(19.1) | 47(14.60) |
| 6 | e | 41(85.4) | 5(10.4) | 2(4.2) | 28(19.6) | 20(11.2) | 48(15.0) |
| 7 | f | 27(81.8) | 5(15.2) | 1(3.0) | 9(6.3) | 24(13.5) | 33(10.30) |
| Totals | 157(100) | 156(100) | 8(100) | 143(100) | 178(100) | 321(100) | |
| ESKAPE Bacteria and Others | Phenotype or Producer n (%) | Genotype n (%) | ||
|---|---|---|---|---|
| Carbapenemases (C) | BLEE (B) | C + BLEE | ||
| Pantoea eucrina (n = 9) | 4 (44.4) | 6 (66.6) | 1 (11.1) | blaKPC = 1 (11.1) blaCTX-1 = 2 (22.2) |
| Pseudomonas putida (n = 2) | 2 (100) | 0 | 0 | blaKPC = 2 (100) |
| Enterobacter hormaechei (n = 1) | 1 (100) | 1 | 1 | ND |
| Enterobacter asburiae (n = 1) | 1 (100) | 0 | 0 | |
| Citrobacter koseri (n = 1) | 1 (100) | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Torres, D.; Jiménez-Zamarripa, C.A.; Munguía-Mogo, S.M.; Calzada-Mendoza, C.C.; Cruz-Cruz, C.; Durán-Manuel, E.M.; Gutiérrez-Ramírez, A.; Castro-Escarpulli, G.; Vélez-Cruz, M.E.; Sosa-Hernández, O.; et al. Comprehensive Assessment of Health Risks Associated with Gram-Negative Bacterial Contamination on Healthcare Personnel Gowns in Clinical Settings. Microorganisms 2025, 13, 1687. https://doi.org/10.3390/microorganisms13071687
Moreno-Torres D, Jiménez-Zamarripa CA, Munguía-Mogo SM, Calzada-Mendoza CC, Cruz-Cruz C, Durán-Manuel EM, Gutiérrez-Ramírez A, Castro-Escarpulli G, Vélez-Cruz ME, Sosa-Hernández O, et al. Comprehensive Assessment of Health Risks Associated with Gram-Negative Bacterial Contamination on Healthcare Personnel Gowns in Clinical Settings. Microorganisms. 2025; 13(7):1687. https://doi.org/10.3390/microorganisms13071687
Chicago/Turabian StyleMoreno-Torres, Daniela, Carlos Alberto Jiménez-Zamarripa, Sandy Mariel Munguía-Mogo, Claudia Camelia Calzada-Mendoza, Clemente Cruz-Cruz, Emilio Mariano Durán-Manuel, Antonio Gutiérrez-Ramírez, Graciela Castro-Escarpulli, Madeleine Edith Vélez-Cruz, Oscar Sosa-Hernández, and et al. 2025. "Comprehensive Assessment of Health Risks Associated with Gram-Negative Bacterial Contamination on Healthcare Personnel Gowns in Clinical Settings" Microorganisms 13, no. 7: 1687. https://doi.org/10.3390/microorganisms13071687
APA StyleMoreno-Torres, D., Jiménez-Zamarripa, C. A., Munguía-Mogo, S. M., Calzada-Mendoza, C. C., Cruz-Cruz, C., Durán-Manuel, E. M., Gutiérrez-Ramírez, A., Castro-Escarpulli, G., Vélez-Cruz, M. E., Sosa-Hernández, O., Rojas-Bernabé, A., Leal-Escobar, B., García-Hernández, O. A., Vásquez-Jiménez, E., Lugo-Zamudio, G. E., Tamayo-Ordóñez, M. C., Tamayo-Ordóñez, Y. d. J., Razo Blanco-Hernández, D. M., Hernández-Castellanos, B., ... Bello-López, J. M. (2025). Comprehensive Assessment of Health Risks Associated with Gram-Negative Bacterial Contamination on Healthcare Personnel Gowns in Clinical Settings. Microorganisms, 13(7), 1687. https://doi.org/10.3390/microorganisms13071687













