Carbon Sink Potential of Sulfur-Oxidizing Bacteria in Groundwater at Petroleum-Contaminated Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
3. Results
3.1. Dynamic Characteristics of Hydrochemical Indicators
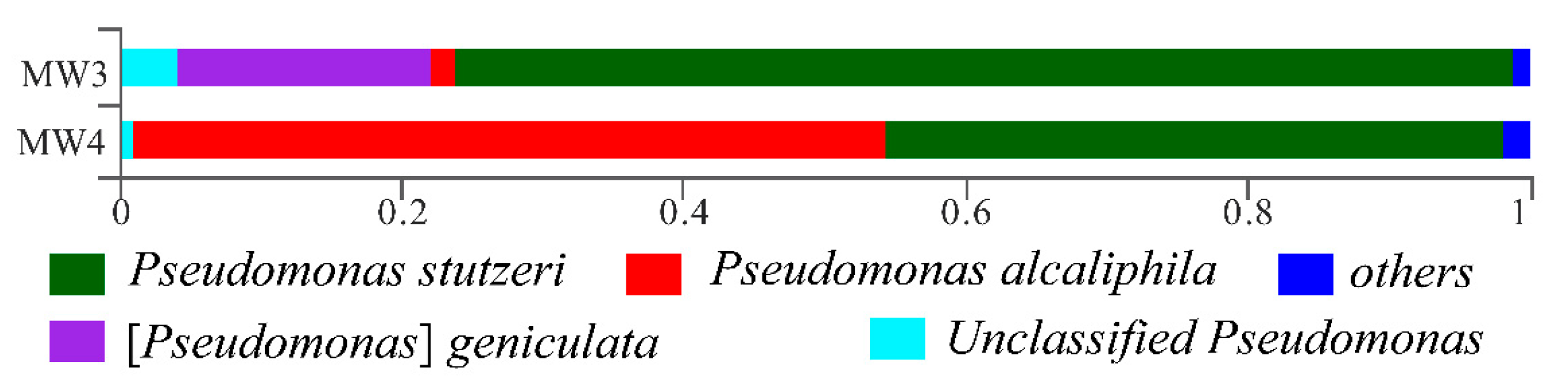
3.2. Microbial Identification
4. Discussions
4.1. Microbial and Biogeochemical Processes
4.2. Limitations Analysis and Future Prospects
4.3. Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolliger, C.; Hohener, P.; Hunkeler, D.; Haberli, K.; Zeyer, J. Intrinsic bioremediation of a petroleum hydrocarbon-contaminated aquifer and assessment of mineralization based on stable carbon isotopes. Biodegradation 1999, 10, 201–217. [Google Scholar] [CrossRef] [PubMed]
- McMahon, P.B.; Chapelle, F.H. Redox processes and water quality of selected principal aquifer systems. Ground Water 2008, 46, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; von Bromssen, M.; Scanlon, B.R.; Bhattacharya, P.; Fryar, A.E.; Hasan, M.A.; Ahmed, K.M.; Chatterjee, D.; Jacks, G.; Sracek, O. Hydrogeochemical comparison and effects of overlapping redox zones on groundwater arsenic near the western (Bhagirathi sub-basin, India) and eastern (Meghna sub-basin, Bangladesh) margins of the Bengal Basin. J. Contam. Hydrol. 2008, 99, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Ponsin, V. Natural Attenuation of Crude Oil in the La Crau Aquifer. Ph.D. Thesis, Aix-Marseille University, Aix-en-Provence, France, 2014. [Google Scholar]
- Jiménez, N.; Richnow, H.H.; Vogt, C.; Treude, T.; Krüger, M. Methanogenic hydrocarbon degradation: Evidence from field and laboratory studies. J. Mol. Microbiol. Biotechnol. 2016, 26, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.; Paradis, C.; Buscheck, T.; Daniels, E.; Hathaway, E.; de Sieyes, N.; Rasa, E.; Schmidt, R.; Peng, J. Methods to estimate source zone depletion of fuel releases by groundwater flow. Groundw. Monit. Remediat. 2018, 38, 26–41. [Google Scholar] [CrossRef]
- Suarez, M.P.; Rifai, H.S. Evaluation of btex remediation by natural attenuation at a coastal facility. Ground Water Monit. Remediat. 2010, 22, 62–77. [Google Scholar] [CrossRef]
- Maric, N.; Matic, I.; Papic, P.; Beskoski, V.P.; Ilic, M.; Gojgic-Cvijovic, G.; Miletic, S.; Nikic, Z.; Vrvic, M.M. Natural attenuation of petroleum hydrocarbons-a study of biodegradation effects in groundwater (Vitanovac, Serbia). Environ. Monit. Assess. 2018, 190, 89. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Liu, C. Hydrogeochemistry of rivers in Guizhou province, China: Constraints on crustal weathering in karst terrain. Adv. Earth Sci. 2005, 20, 394. [Google Scholar]
- ASTM E1943-98; Standard Guide for Remediation of Ground Water by Natural Attenuation at Petroleum Release Sites. ASTM International: West Conshohocken, PA, USA, 2010.
- Cai, P.; Ning, Z.; Zhang, M.; Guo, C.; Niu, M.; Shi, J. Autotrophic metabolism considered to extend the applicability of the carbon balances model for assessing biodegradation in petroleum-hydrocarbon-contaminated aquifers with abnormally low dissolved inorganic carbon. J. Clean. Prod. 2020, 261, 120738. [Google Scholar] [CrossRef]
- Ning, Z.; Cai, P.; Zhang, M.; Guo, C.; Shi, C.; He, Z. Abnormally low dissolved inorganic carbon anomaly in petroleum contaminated groundwater caused by microbiological geochemistry. Acta Sci. Circumstantiae 2019, 39, 1140–1147. [Google Scholar]
- Jia, W.; Cheng, L.; Tan, Q.; Liu, Y.; Dou, J.; Yang, K.; Yang, Q.; Wang, S.; Li, J.; Niu, G.; et al. Response of the soil microbial community to petroleum hydrocarbon stress shows a threshold effect: Research on aged realistic contaminated fields. Front. Microbiol. 2023, 14, 1188229. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wu, M.; Liu, H.; Xu, Y.; Liu, Z. Effect of petroleum hydrocarbon pollution levels on the soil microecosystem and ecological function. Environ. Pollut. 2022, 293, 118511. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; He, R.; Wang, L.a.; Liu, L.; Huang, X.; Ping, J.; Huang, C.; Wang, X.; Liu, Y. The dominant microbial metabolic pathway of the petroleum hydrocarbons in the soil of shale gas field: Carbon fixation instead of CO2 emissions. Sci. Total Environ. 2022, 807, 151074. [Google Scholar] [CrossRef] [PubMed]
- Kellermann, C.; Selesi, D.; Lee, N.; Hugler, M.; Esperschutz, J.; Hartmann, A.; Griebler, C. Microbial CO2 fixation potential in a tar-oil-contaminated porous aquifer. FEMS Microbiol. Ecol. 2012, 81, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, N.Y.; Cho, K.S.; Ryu, H.W. Removal of hydrogen sulfide by sulfate-resistant Acidithiobacillus thiooxidans AZ11. J. Biosci. Bioeng. 2006, 101, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Ning, Z.; Zhang, N.; Zhang, M.; Guo, C.; Niu, M.; Shi, J. Insights into biodegradation related metabolism in an abnormally low dissolved inorganic carbon (dic) petroleum-contaminated aquifer by metagenomics analysis. Microorganisms 2019, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.E.; Trussell, R.R.; Clesceri, L.S.; Association, A.W.W. Standard methods for the examination of water and wastewater: Supplement to the sixteenth edition. Am. J. Public Health Nations Health 2005, 56, 387. [Google Scholar]
- Ning, Z.; Zhang, M.; He, Z.; Cai, P.; Guo, C.; Wang, P. Spatial pattern of bacterial community diversity formed in different groundwater field corresponding to electron donors and acceptors distributions at a petroleum-contaminated site. Water 2018, 10, 842. [Google Scholar] [CrossRef]
- Xu, X.-J.; Chen, C.; Guo, H.-L.; Wang, A.-J.; Ren, N.-Q.; Lee, D.-J. Characterization of a newly isolated strain Pseudomonas sp. C27 for sulfide oxidation: Reaction kinetics and stoichiometry. Sci. Rep. 2016, 6, 21032. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, L.; Liu, Q.; Sikder, M.M.; Vestergård, M.; Zhou, K.; Wang, Q.; Yang, X.; Feng, Y. Pseudomonas fluorescens promote photosynthesis, carbon fixation and cadmium phytoremediation of hyperaccumulator Sedum alfredii. Sci. Total Environ. 2020, 726, 138554. [Google Scholar] [CrossRef] [PubMed]
- Lalucat, J.; Bennasar, A.; Bosch, R.; Garcia-Valdes, E.; Palleroni, N.J. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. MMBR 2006, 70, 510–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, P.; Hao, B.; Yu, Z. Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri yzn-001. Bioresour. Technol. 2011, 102, 9866–9869. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Teske, A.; Robertson, L.A.; Kuenen, J.G. Anaerobic oxidation of thiosulfate to tetrathionate by obligately heterotrophic bacteria, belonging to the Pseudomonas stutzeri group. FEMS Microbiol. Ecol. 1999, 30, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ridl, J.; Suman, J.; Fraraccio, S.; Hradilova, M.; Strejcek, M.; Cajthaml, T.; Zubrova, A.; Macek, T.; Strnad, H.; Uhlik, O. Complete genome sequence of Pseudomonas alcaliphila jab1 (=dsm 26533), a versatile degrader of organic pollutants. Stand. Genom. Sci. 2018, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Hung, Y.-T.; Shammas, N.K. Physicochemical Treatment Processes; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3. [Google Scholar]
- Kvartenko, A. Research into factors of mutual influence of ground waters quality parameters on choice of water cleansing technologies. Boда i Boдooчиснi Тexнoлoгiї Наyкoвo-Тexнiчнi Bicmi 2017, 21, 39–49. [Google Scholar] [CrossRef]
- Perruchoud, L.; Jones, M.; Sutrisno, A.; Zamble, D.; Simpson, A.; Zhang, X.-A. A ratiometric nmr pH sensing strategy based on a slow-proton-exchange (spe) mechanism. Chem. Sci. 2015, 6, 6305–6311. [Google Scholar] [CrossRef] [PubMed]
- Hädrich, A.; Heuer, V.B.; Herrmann, M.; Hinrichs, K.-U.; Küsel, K. Origin and fate of acetate in an acidic fen. FEMS Microbiol. Ecol. 2012, 81, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, W.; Dam, B. Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol. Rev. 2009, 33, 999–1043. [Google Scholar] [CrossRef] [PubMed]
- Emesh, S.; Rapson, T.D.; Rajapakshe, A.; Kappler, U.; Bernhardt, P.V.; Tollin, G.; Enemark, J.H. Intramolecular electron transfer in sulfite-oxidizing enzymes: Elucidating the role of a conserved active site arginine. Biochemistry 2009, 48, 2156–2163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caldararu, O.; Feldt, M.; Cioloboc, D.; van Severen, M.-C.; Starke, K.; Mata, R.A.; Nordlander, E.; Ryde, U. QM/MM study of the reaction mechanism of sulfite oxidase. Sci. Rep. 2018, 8, 4684. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, G.; Xian, W.; Li, W.; Jiang, H. Sulfur isotope fractionation mediated by microbial anoxygenic photosynthetic sulfur oxidation processes and its geological implications. Earth Sci. 2023, 48, 2837–2850. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Madigan, M.; Martinko, J.; Dunlap, P.; Clark, D. Brock Biology of Microorganisms; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Postgate, J.R. Nitrogen Fixation; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Jennings, M.E. Oxidative Stress Response in Archaea: Elucidation of Oxidant Sensing and Tolerance Mechanisms in Methanosarcina acetivorans. Ph.D. Thesis, University of Arkansas, Fayetteville, Arkansas, 2016. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, P.; Ning, Z.; Zhang, M. Carbon Sink Potential of Sulfur-Oxidizing Bacteria in Groundwater at Petroleum-Contaminated Sites. Microorganisms 2025, 13, 1688. https://doi.org/10.3390/microorganisms13071688
Cai P, Ning Z, Zhang M. Carbon Sink Potential of Sulfur-Oxidizing Bacteria in Groundwater at Petroleum-Contaminated Sites. Microorganisms. 2025; 13(7):1688. https://doi.org/10.3390/microorganisms13071688
Chicago/Turabian StyleCai, Pingping, Zhuo Ning, and Min Zhang. 2025. "Carbon Sink Potential of Sulfur-Oxidizing Bacteria in Groundwater at Petroleum-Contaminated Sites" Microorganisms 13, no. 7: 1688. https://doi.org/10.3390/microorganisms13071688
APA StyleCai, P., Ning, Z., & Zhang, M. (2025). Carbon Sink Potential of Sulfur-Oxidizing Bacteria in Groundwater at Petroleum-Contaminated Sites. Microorganisms, 13(7), 1688. https://doi.org/10.3390/microorganisms13071688





